Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Detection of nitro-aromatics using C5N2 as an electrochemical sensor: a DFT approach†
Nabeela a,
Muhammad Ali Hashmi
a,
Muhammad Ali Hashmi *ab,
Ahmad Nauman Shah Saqib
*ab,
Ahmad Nauman Shah Saqib a,
Aqsa Kamrana and
Ahmed Lakhani
a,
Aqsa Kamrana and
Ahmed Lakhani *c
*c
aDepartment of Chemistry, Division of Science & Technology, University of Education, Lahore, 54770, Pakistan. E-mail: muhammad.hashmi@ue.edu.pk
bSchool of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, 6012, New Zealand
cDepartment of Biomedical and Health Sciences, Calumet College of St. Joseph, 2400, New York Ave, Whiting, IN 46394, USA. E-mail: alakhani@ccsj.edu
First published on 23rd September 2024
Abstract
Nitroaromatics impose severe health problems and threats to the environment. Therefore, the detection of such hazardous substances is essential to save the whole ecosystem. Herein, the C5N2 sheet is used as an electrochemical sensor for the detection of 1,3-dinitrobenzene (1,3-DNB), trinitrotoluene (TNT), and picric acid (PA) using the PBE0/def2SVP level of theory as implemented in Gaussian 16. The highest interaction energy was observed for the picric acid@C5N2 complex. The trend in interaction energies for the studied system is PA@C5N2 >TNT@C5N2 >1,3-DNB@C5N2. The studied systems were further analysed by qualitative and quantitative analyses to determine the interactions between the nitroaromatic analytes and the C5N2 sheet. Electronic properties of all analytes@C5N2 complexes have been examined by NBO, EDD, FMO and DOS analysis. QTAIM analysis depicts the stronger non-covalent interactions for the PA@C5N2, which shows consistency with interaction energy and NCI analysis. Furthermore, NBO and FMO analyses show that the C5N2 substrate exhibits high sensitivity and selectivity towards the picric acid compared to TNT and 1,3-DNB nitroaromatics. EDD and DOS analyses are in agreement with NBO and FMO analyses. Furthermore, the recovery time of the studied system has been computed to determine the efficiency of C5N2 material as an electrochemical sensor. Overall, the results show that carbon nitride can be a good sensor for the detection of nitroaromatics.
1 Introduction
In recent years, extensive use of explosives in defence systems has raised serious concerns as they have potential negative impacts on human health and the whole ecosystem.1 Among these, nitroaromatics such as 1,3-dinitrobenzene (DNB), 2,4,6-trinitrophenol, also known as picric acid (PA), and trinitrotoluene (TNT), have been widely used due to their highly explosive nature. Besides, these nitroaromatics have also gained popularity in the synthetic industry, including pharmaceuticals, pigments, dyes, pesticides, wood preservatives, and rubber chemicals.2–5 Release of these compounds, intentionally or unintentionally, contaminates the environment and deteriorates human health by causing problems such as skin irritation, cataracts, cancer, anaemia, cyanosis, and male infertility, damaging the liver, kidney, and blood cells in humans.6–8 Therefore, detection of such toxic compounds is crucial to overcome their potential threats.Various techniques such as HPLC, gas chromatography,9 ion mobility spectrometry,10–12 and capillary electrophoresis13,14 have already been used for detection purposes. However, their non-specificity, difficulty in handling the instruments, pre-sample processes, expensive instrumentations, and portability hinder their applicability as detectors.15,16 The demand for rapid, responsive, portable, and flexible detecting systems marked the electrochemical sensors as a potential alternative for efficient detection.17
Porous materials have gained significant importance as electrochemical sensors due to their exceptional sensing properties18 and various 2D and 3D substrates have been evaluated both experimentally and theoretically for this purpose. Such substances include fullerene,19 metal–organic frameworks (MOFs),20 aluminium nitride,21 silicon carbide,22 ZnO nanotubes,23 silver–graphene quantum dots,24 metal-doped graphene,25,26 and covalent organic frameworks.27 In addition to these, graphene–polyaniline,28 polyaniline/ZnO,29 single-walled nanotubes,30 multi-walled nanotubes,31 polypyrrole,32 and h-BN nanoclusters33 have been used for detecting the chemical warfare agents (CWAs) including nitro explosives. Although these adsorbents are used to sense toxic agents, they lack certain characteristics, such as large surface area coupled with more active sites for adsorption, high porosity and reproducible results, required for the efficient performance of the adsorbents.34
Recently, carbon nitride became the heart of sensors due to its specific surface area and highly porous and chemically stable structure. Moreover, the intrinsic bandgap of carbon nitride proves its efficacy as a catalyst in water-splitting reactions, carbon dioxide reduction reactions, and degradation of organic pollutants.35,36 Numerous experimental and theoretical studies have been performed on the sensing capability of carbon nitride having different ratios of carbon and nitrogen, such as C2N, C3N4, and C4N nanoflakes.37 These materials show excellent sensing performance in the detection of toxic agents containing ammonia, hydrogen sulphides,38 pesticides,39 nitrogen halides,40 neurotoxin hydrazine derivatives,41 CWAs,42,43 and nitro-explosives.44,45
An innovative form of carbon nitride, i.e. C5N2, has not yet been explored for sensing. The C5N2 material is synthesised through a condensation reaction between 1,2,4,5-benzene tetramine and hexaketocyclohexane.46,47 Theoretical studies showed that C5N2 is used as a catalyst to determine its stability and activity for the synthesis of hydrogen peroxide (H2O2).48 Moreover, C5N2 is superior to the other carbon nitride forms due to its nanoporous crystalline structure with a narrow band gap (1.10 eV).49 These findings motivated us to explore the sensing capability of the C5N2 toward the nitroaromatic compounds, i.e., PA, 1,3-DNB and TNT. DFT calculations have been performed to determine the adsorption of the analytes on the C5N2 surface. The selectivity and sensitivity of the analytes on the C5N2 surface have been investigated by the FMO, NBO and DOS analyses. Furthermore, the nature of interactions is explored through the QTAIM and NCI analyses.
2 Computational methodology
Quantum chemistry computational calculations have been performed using Gaussian 16 B.01.50 Structures of analystes, C5N2 and complexes have been modelled using Gauss View software.51 Geometries of the substrate C5N2 and nitro-containing analytes were optimised at the PBE0-D3BJ/def2-SVP level of theory. PBE0 is a hybrid DFT functional, which outperforms the pure DFT approach in computing molecular structures and properties throughout the periodic table.52 The literature has also backed the selection of PBE0 functional as it is widely applicable for molecular systems, providing accurate results for atomic energies, structural geometry, dissociation energies, and electronic and magnetic properties53 and sensing interactions.54 PBE0/def2XVP has been used for adsorption studies of CO2,55 metformin,56 and different gases including H2, N2, CO, H2S, NH3, SO2, and CH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 over graphitic carbon nitride and to study the non-covalent interactions between the decavanadate, arginine, and lysine side chains in proteins.58 Similarly, PBE0 has also been studied with different basis set combinations to study Se-doped graphitic carbon nitride (Se@gC3N4) nanostructures used for the smart therapeutic delivery of zidovudine,59 for the efficient sensing of glucose towards borophene,53 functionalized carbon quantum dots for sensing curcumin,60 and in studying the sensing of telomerase through semiconducting carbon nitrides.61 In a study about adsorption behaviour for efficient sensing of melamine through Mg12O11–X nanostructured materials, different hybrid functionals have been employed, and PBE0 has been reported to be ranked at the top in describing the sensing interactions.54 Considering the long-range non-covalent interactions, Grimme's empirical D3 correction62 with Becke–Johnston damping (D3BJ)63 has been applied in all calculations. Multiwfn (version 3.80)64 and VMD software65 have been used for qualitative and quantitative analyses. CYLview66 has been employed to obtain high-resolution visualisation of modelled structures.
57 over graphitic carbon nitride and to study the non-covalent interactions between the decavanadate, arginine, and lysine side chains in proteins.58 Similarly, PBE0 has also been studied with different basis set combinations to study Se-doped graphitic carbon nitride (Se@gC3N4) nanostructures used for the smart therapeutic delivery of zidovudine,59 for the efficient sensing of glucose towards borophene,53 functionalized carbon quantum dots for sensing curcumin,60 and in studying the sensing of telomerase through semiconducting carbon nitrides.61 In a study about adsorption behaviour for efficient sensing of melamine through Mg12O11–X nanostructured materials, different hybrid functionals have been employed, and PBE0 has been reported to be ranked at the top in describing the sensing interactions.54 Considering the long-range non-covalent interactions, Grimme's empirical D3 correction62 with Becke–Johnston damping (D3BJ)63 has been applied in all calculations. Multiwfn (version 3.80)64 and VMD software65 have been used for qualitative and quantitative analyses. CYLview66 has been employed to obtain high-resolution visualisation of modelled structures.
Analytes with different orientations on the C5N2 sheet have been optimised to obtain the lowest energy structure of the complexes. These optimised structures are further validated via the vibrational frequency analysis, ensuring they have no imaginary frequency. The interaction energies of analytes with the sheet have been calculated through the following formula:
| ΔEint = [Eanalyte@C5N2 − (Eanalyte + EC5N2)] | (1) |
The interactions between the C5N2 substrate and the nitro analytes have been thoroughly analyzed using Non-Covalent Interactions (NCI) analysis. This method primarily utilizes electron density (ρ) and the Reduced Density Gradient (RDG) to probe these interactions. The RDG is calculated using the following equation:67
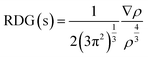 | (2) |
This equation indicates that the RDG value is inversely proportional to the electron density at a point. A higher electron density generally leads to a lower RDG value, suggesting a stronger potential for non-covalent bonding at that location.
Additionally, the electron density magnitude influences these non-covalent interactions' strength. In contrast, the interaction's specific nature—whether repulsive or attractive—is determined by the Laplacian of the electron density (∇2ρ). The Laplacian is expressed as the sum of its three eigenvalues from the Hessian matrix of electron density:24
| ∇2ρ = λ1 + λ2 + λ3 | (3) |
Among the eigenvalues derived from the Hessian matrix, the second eigenvalue (λ2) is especially important for determining the nature of the interactions within the molecule. Specifically, a negative λ2 indicates strong, attractive interactions, such as those seen in hydrogen bonds, which are critical for molecular stability and function. Conversely, a positive λ2 points to repulsive interactions, which might indicate regions of molecular strain or steric clashes. Additionally, when λ2 is near zero, it typically signifies the presence of weaker interactions, like van der Waals forces, which are essential for subtler molecular phenomena.
To visually capture and illustrate these different types of non-covalent interactions, we employ 2D RDG graphs and 3D isosurfaces generated using Multiwfn Software (version 3.80).64,68 These visualizations are color-coded to enhance understanding and interpretation: red represents repulsive interactions, blue denotes strong attractive interactions (notably hydrogen bonds), and green highlights the van der Waals interactions. This color-coding aids in quickly identifying the nature of interactions at a glance, providing an intuitive map of molecular interaction landscapes.69
Furthermore, Quantum Theory of Atoms in Molecules (QTAIM) analysis has been performed to recognise the intramolecular and intermolecular interactions, particularly the non-covalent interactions.70,71 The nature and strength of intermolecular interactions between the nitroaromatic analytes and C5N2 substrate are determined by the various topological parameters such as electron density (ρ), kinetic and potential energy density (G and V), total electron energy density (H), and Laplacian of the electron density (∇2ρ), which exist at bond critical points (BCP) between nitroaromatic analytes and the substrate.69
| H(r) = V(r) + G(r) | (4) |
Generally, the values of potential energy density and kinetic energy density are always negative and positive, respectively. The sum of these two parameters (V and G) gives the total energy density (H). The values of H and ∇2ρ depict the type of interactions either covalent or non-covalent. If the value is H > 0 < ∇2ρ, then it shows the non-covalent interactions, while covalent interactions are depicted by the H < 0 > ∇2ρ.71,72 Electronic density values further specify the strength of non-covalent interactions. If the value of electronic density is greater than 0.1, then it indicates the electrostatic attractive forces, while the presence of van der Waals interactions is indicated by the value of electronic density lower than 0.1.73
In addition, the electronic properties of the analytes@C5N2 complexes have been determined at the same level of theory through frontier molecular orbital (FMO), electron density distribution (EDD), density of states (DOS) and natural bond orbital (NBO) analysis. The density of states validates the FMO analysis by better understanding the energy gap after the adsorption of analytes. EDD plots of electronic density were obtained using Chemcraft.74 Moreover, NBO charges are confirmed by providing visual aids through EDD plots to understand charge transfer upon complexation.
3 Results and discussion
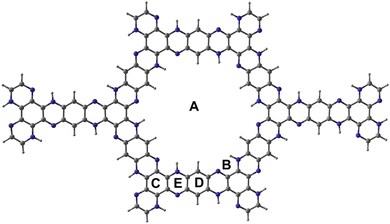
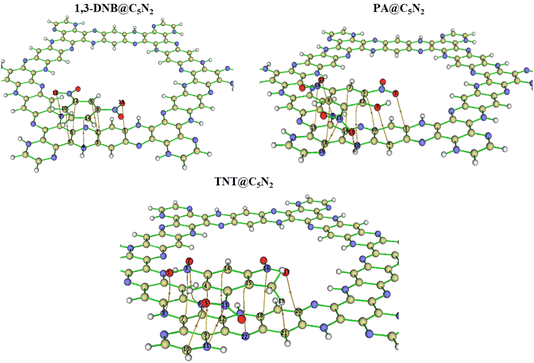
Fig. 1 shows the optimised structure of C5N2 used as an adsorbent. C5N2 sheet consists of five fused benzene rings, bridged by a pyrazine ring, consisting of four different kind of bonds, depending on the type of atoms and ring involved. The C–N bond length in the pyrazine ring is 1.36 Å whereas the bond length of three types of C–C bonds is 1.4 Å, 1.42 Å and 1.38 Å present in pyrazine, benzene and hydrogenated benzene rings, respectively. The analytes (1,3-DNB, TNT and PA) were placed on five possible binding sites with different orientations to get the most stable ones. Such sites are labelled as A (in the central cavity of the sheet), B (forming a triangle with nitrogen atoms at the edges), C (top of the benzene ring), D (top of the pyrazine ring) and E (top of hydrogenated benzene ring). Analytes were positioned with possible orientations on the mentioned sites of the C5N2 sheet to get the most stable geometry of the complex.Among the possible orientations of the 1,3-DNB complex (see Fig. S1†), the most stable geometry occurred at the top of the pyrazine ring with an interaction energy of −23.21 kcal mole−1. The benzene ring of the 1,3-DNB analyte was located on the site D while the two nitro groups were situated on the site B and E of the C5N2 surface, respectively. For the TNT@C5N2 complex, the analyte was placed in five possible orientations on the C5N2 sheet (see Fig. S3†). Among these, the most stable geometry was obtained with an interaction energy of −31.64 kcal mole−1 where the analyte was oriented in between the C and D sites of the surface. The oxygen of TNT formed a hydrogen bond with the hydrogen pyrazine ring of C5N2 substrate (2.49 Å). The interaction energies of the possible orientation of analytes at C5N2 are also presented in Table S1.† Stable geometries of 1,3-DNB@C5N2, TNT@C5N2 and PA@C5N2 have been shown in Fig. 2.
 | ||
| Fig. 2 The stable optimised structure of 1,3-DNB@C5N2, TNT@C5N2 and picric acid@C5N2 at PBE0-D3BJ/def2SVP level of theory. Interacting atoms of analytes and substrate have been shown by dotted lines. | ||
Among all the three complex systems, picric acid interacts strongly with the surface of the substrate. For PA@C5N2 complexes, the most stable geometry is obtained when the analyte is located on the top of the D site (pyrazine ring) with −34.37 kcal mole−1 of interaction energy. The other unstable optimized structures are presented in Fig. S2.† One of the reasons for such a high interaction energy is the formation of a hydrogen bond between the oxygen of picric acid and hydrogen of the pyrazine ring. The interaction energies of TNT@C5N2 and PA@C5N2 are stronger than those of the 1,3-DNB@C5N2 complex due to the presence of hydrogen bond formation between the analyte and the substrate. Trends in interaction energies for the considered complexes are PA@C5N2 > TNT@C5N2 > 1,3-DNB@C5N2. The interaction energies along with bond lengths of all three complex systems have been presented in Table 1.
| Analyte@C5N2 sheet complex | Interacting atoms of analytes and C5N2 | Bond lengths (Å) | Interaction energies (kcal mol−1) |
|---|---|---|---|
| PA@C5N2 | N7–N8 | 3.05 | −34.37 |
| O5–H6 | 2.67 | ||
| C3–N4 | 3.06 | ||
| C9–C10 | 2.99 | ||
| N11–C12 | 3.06 | ||
| O1–C2 | 3.01 | ||
| TNT@C5N2 | N5–N6 | 2.91 | −31.64 |
| C7–C8 | 2.92 | ||
| O11–N12 | 3.03 | ||
| N1–C2 | 3.09 | ||
| O9–N10 | 3.15 | ||
| O3–H4 | 2.49 | ||
| 1,3-DNB@C5N2 | C7–C8 | 3.17 | −23.21 |
| C9–C10 | 3.09 | ||
| C5–C6 | 3.11 | ||
| C3–C4 | 3.15 | ||
| O1–C2 | 2.99 | ||
| O11–N12 | 3.01 |
3.1 Quantum theory of atoms in molecules analysis
The nature of intermolecular interactions in nitroaromatics@C5N2 complexes is analysed through the quantum theory of atoms in molecules (QTAIM) analysis. For the 1,3-DNB@C5N2 complex, seven BCPs with four C–C, one C–N, one C–O, and one N–O were identified between the 1,3-DNB and C5N2 substrate, as shown in Fig. 3. The value of Laplacian of electron density ranges from 0.019 to 0.033, signifying the non-covalent interactions, while electron density exhibits a value less than 0.1 (0.008 to 0.011), depicting the van der Waals interactions between the 1,3-DNB and C5N2 sheet. Additionally, the remaining topological parameter values, i.e., H, G and V of 1,3-DNB@C5N2 also indicate the presence of non-covalent interactions (Table S2†). For the TNT@C5N2, eleven BCPs with two C–C, three C–N, two C–O, one C–H, one N–N, and two N–O were observed, as shown in Fig. 3. Topological parameters at the mentioned BCPs show non-covalent interactions between the TNT and C5N2 substrate. The values of Laplacian and electron density range from 0.023 to 0.037 and 0.007 to 0.014, respectively. The remaining topological parameters, i.e., H, G and V of TNT@C5N2 also indicated the presence of non-covalent interactions (see Table S1†).In the case of PA@C5N2, ten BCPs are obtained with one O–H, two C–O, three C–C, two C–N, one N–N, and one N–O, between the picric acid and substrate (see Fig. 3). The topological parameters of PA@C5N2 were similar to the TNT@C5N2 except for the one O–H BCP. For all the BCPs, values of Laplacian and electron density range from 0.023 to 0.033 and 0.005 to 0.012, respectively. These topological values were in good agreement with the non-covalent interactions, particularly the van der Waals interactions. The value of electron density was highest for BCPs between the C8 of the picric acid and C18 of the C5N2 surface which depicted the strong non-covalent interactions. The rest of topological parameters (H, G, V) also showed consistency with values of Laplacian and electron density as reported in Table 2.
| Analyte–C5N2 | ∇2ρ | ρ | V(r) | H | G(r) |
|---|---|---|---|---|---|
| PA@C5N2 | |||||
| N11–N19 | 0.027 | 0.0082 | −0.005 | 0.001 | 0.006 |
| C8–C18 | 0.033 | 0.0121 | −0.006 | 0.001 | 0.007 |
| O2–C1 | 0.031 | 0.0101 | −0.006 | 0.001 | 0.007 |
| O13–H6 | 0.023 | 0.0055 | −0.004 | 0.001 | 0.005 |
| O17–N9 | 0.024 | 0.0069 | −0.005 | 0.001 | 0.005 |
| N16–C15 | 0.030 | 0.0085 | −0.005 | 0.001 | 0.006 |
| C12–N20 | 0.028 | 0.0099 | −0.005 | 0.001 | 0.006 |
| O3–C4 | 0.025 | 0.0084 | −0.005 | 0.001 | 0.005 |
| C7–C14 | 0.026 | 0.0091 | −0.004 | 0.001 | 0.005 |
| C5–C10 | 0.027 | 0.0096 | −0.005 | 0.001 | 0.006 |
Topological parameters for all considered complexes indicated the presence of non-covalent interaction between the analytes and the C5N2 substrate. Values of electron density and Laplacian for PA@C5N2 complexes showed van der Waals interactions along with hydrogen bond interactions. These QTAIM results are consistent with the interaction energies.
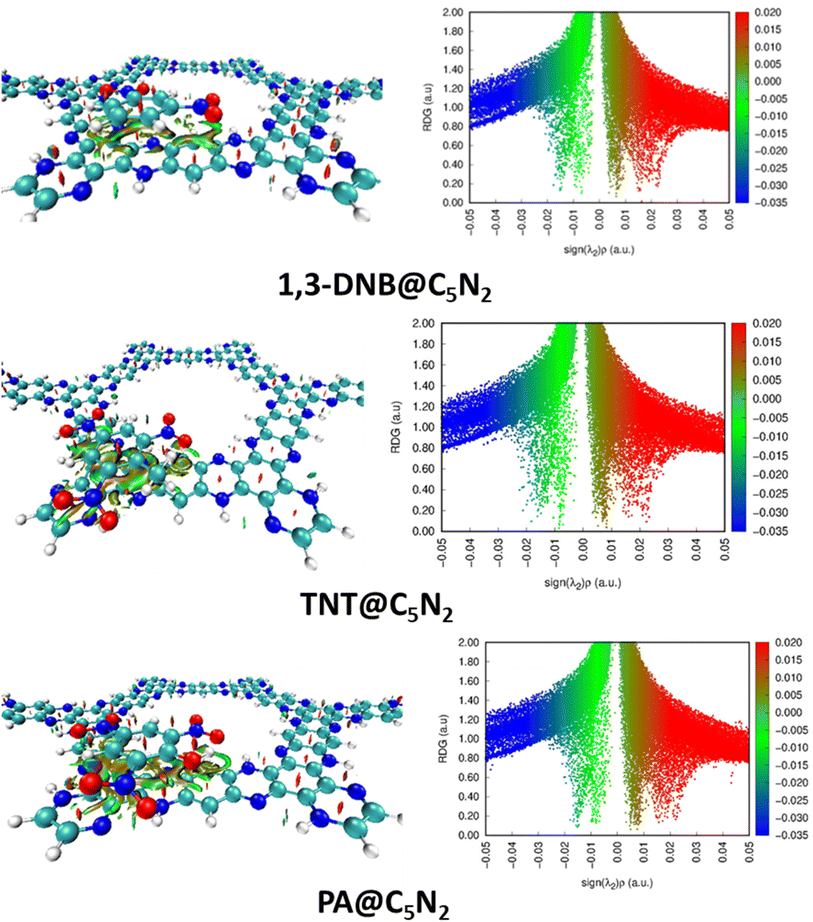
3.2 Non-covalent interaction analysis
The intermolecular interactions have been further characterised by the NCI analysis in the complexes of analytes–C5N2. It comprises of 2D RDG graph and 3D isosurfaces. Three color schemes have been used in 3D isosurface which describes the non-covalent interactions i.e., blue, green and red for attractive, weak van der Waals, and dispersive interactions between the analytes and substrate. Similarly, the 2D RDG graph shows attractive interactions in the form of hydrogen bonding (blue spikes), repulsive force (red spikes) and weak van der Waals forces (green spikes). Whereas the size of a particular patch is directly related to the strength of interactions. The larger the size of a particular patch, the greater will be the strength of that interaction. Similarly, values of electron density on the x-axis vary directly with the nature of interactions between the analytes and the substrate.In the 3D isosurface, the presence of green isosurface between the analytes (1,3-DNB, TNT and PA) and the C5N2 substrate confirms the weak van der Waals interactions consistent with the 2D RDG graph. Conversely, there is no hydrogen bond interaction between the analytes and substrate as revealed by the absence of clear blue patches.75 However, dispersed bluish-green spots in the case of TNT@C5N2 and PA@C5N2 showed more van der Waals interactions. 2D RDG and 3D isosurface are presented in Fig. 4. Moreover, the presence of red spikes (0.01 to 0.03) in the 2D RDG graph represents the repulsive interaction that mostly exists between the atoms of aromatic rings of the C5N2 substrate, also depicted in the 3D isosurface.76 In the case of picric acid@C5N2 complex, the green isosurface and spikes in 3D and 2D graphs respectively, are dense and wide as compared to 1,3-DNB and TNT complexes, reflecting more van der Waals interactions between the picric acid and C5N2 which is also consistent with the interaction energies and QTAIM topological results.
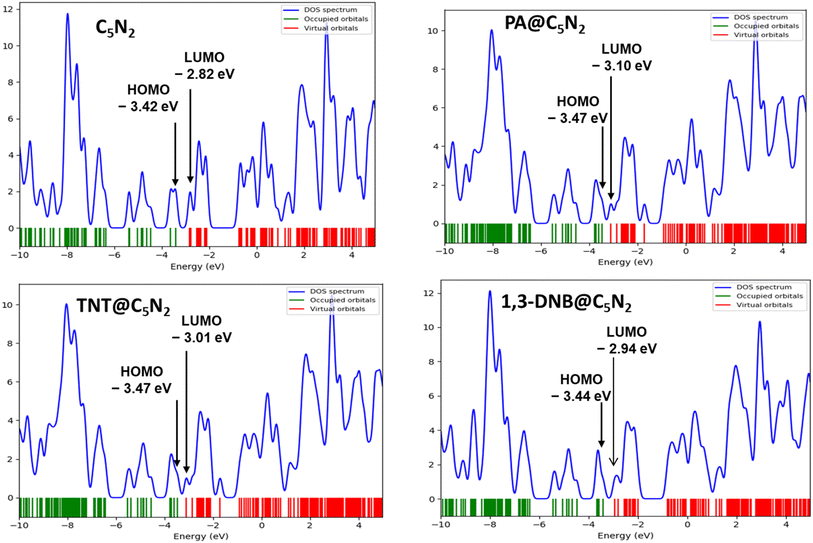
3.3 Electronic properties: FMO and DOS analysis
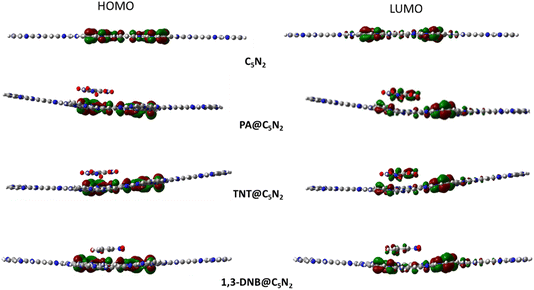
Electronic properties of the substrate upon complexation have been studied through FMO analysis. After the analytes' adsorption, the substrate's conductivity significantly changed, which influenced its sensing capability. FMO analysis for the considered analytes@C5N2 complexes has been performed. The energy values of HOMO, LUMO and their energy gaps have been reported in Table 3. Orbital density isosurface of all the analytes@complexes are shown in Fig. 5. Analysis shows that HOMO and LUMO values for pristine C5N2 substrate are −3.42 eV and −2.82 eV respectively, which results in an energy gap of 0.60 eV. The energy gap of the pristine C5N2 has been changed after complexation with the analytes (1,3-DNB, TNT and PA). HOMO and LUMO energy values of 1,3-DNB@C5N2 have been increased to −3.41 eV and −2.94 eV, respectively which results in decreased value of EH–L gap (0.47 eV). After adsorption of TNT analytes upon C5N2, the HOMO–LUMO energy gap reduces to 0.46 eV similar to the 1,3-DNB@C5N2 complex. In this case, the decrease in EH–L gap is now attributed to the decreased value of both HOMO (−3.47 eV) and LUMO (−3.01 eV) as compared to the pristine C5N2 substrate. Among all the studied systems, a significant reduction in the HOMO–LUMO energy gap is observed in the case of PA@C5N2. The value of HOMO is reduced to −3.47 eV, comparable to the HOMO value of the pristine C5N2. However, pronounced shift in EH–L is mainly due to the reduction in the value of LUMO (−3.10 eV). This noticeable reduction in energy gap (0.37 eV) results in excellent conductivity and sensitivity of C5N2 substrate towards the picric acid as compared to the other analytes (1,3-DNB and TNT).77| Analyte@C5N2 | Pristine C5N2 | PA@C5N2 | TNT@C5N2 | 1,3-DNB @C5N2 |
|---|---|---|---|---|
| HOMO (eV) | −3.42 | −3.47 | −3.47 | −3.41 |
| LUMO (eV) | −2.82 | −3.10 | −3.01 | −2.94 |
| EH–L gap (eV) | 0.60 | 0.37 | 0.46 | 0.47 |
| NBO charge (e−) | −0.426 | −0.406 | −0.228 |
Orbital density patterns for considered analytes@C5N2 complexes obtained through FMO analysis are shown in Fig. 5. Orbital density distribution shows similar patterns in all three analytes@C5N2 complex systems. For all three systems (1,3-DNB@C5N2, TNT@C5N2, PA@C5N2), the HOMO isosurface lies entirely on the sheet depicting the transfer of charge density from the C5N2 surface after adsorption of analytes. The LUMO density orbital is found half on the analyte and half on the substrate which shows a significant reduction in the HOMO–LUMO energy gap. Among the 1,3-DNB@C5N2 and TNT@C5N2 complexes, PA@C5N2 shows larger orbitals density isosurface, depicting the major shift in electronic density between C5N2 and picric acid. This visual representation is analogous to the reduced HOMO–LUMO energy gap resulting in excellent sensitivity of picric acid toward the C5N2 as compared to the rest of the analytes.
Electronic properties of considered complexes are further executed by the DOS analysis. The spectra of DOS are plotted in Fig. 6 for a better understanding of interactions after adsorptions of analytes. Density of state spectra reveals that after adsorption of analytes, virtual orbitals are shifted more negatively, resulting in a reduced energy gap. In the DOS spectra, it is observed that HOMO appeared at −3.47 eV for PA@C5N2 and TNT@C5N2 as compared to pristine C5N2 sheet (−3.42 eV) while the LUMO virtual orbitals are shifted to −3.10 eV and −3.01 eV from −2.82 eV of C5N2, respectively. The formation of new orbitals of HOMO and LUMO results in a reduction of energy gap as compared to pristine substrate. A similar pattern is also observed for the 1,3-DNB@C5N2 complex. Among the studied systems, the more pronounced shift is observed for the PA@C5N2 system, thus lowering the energy gap. The shifting of orbitals and their peak intensities reflect the conductivity and sensitivity of C5N2 towards analytes, validating FMO results.76
3.4 NBO and EDD analysis
Natural bond orbital (NBO) analysis provides a deeper evaluation of the interaction mechanism by determining the amount of charge transfer between the nitroaromatic analytes and C5N2 substrate upon complexation. The NBO results of nitroaromatics@C5N2 complexes are presented in Table 3. The values of NBO charges are negative on the 1,3-DNB, TNT and PA in nitroaromatics@C5N2 complexes (presented in Table 3), which shows that charges are transferred from the sheet towards the nitroaromatics analytes. For the TNT@C5N2 complex, 0.406 e charge is shifted from the C5N2 substrate to TNT. Similarly, 0.426 e charge is being transferred from substrate to PA in case of PA@C5N2 complex while for 1,3-DNB@C5N2 complex, the amount of charge transferred from substrate to the analyte is only 0.208 e. From these results, it is deduced that PA extracted the highest amount of charge from the C5N2 substrate as compared to TNT and 1,3-DNB.78Electron density difference (EDD) analysis helps to visualize the electronic density distribution among the nitroaromatics analytes and substrate upon complexation. The difference in the electronic density of the nitroaromatics@C5N2 complex and the sum of the electronic density of the nitroaromatic analytes and C5N2 substrate, taken separately, gives the EDD results.44 The EDD plots are given in the Fig. 7. In EDD plots, the transfer of electronic density is depicted by the orange (electronic density accumulated region) and pink (electronic density depleted region) colored isosurface, at the interacting sites of nitroaromatics and C5N2 substrate. EDD results of 1,3-DNB@C5N2 and TNT@C5N2 show that nitroaromatics are mainly covered with orange isosurface, indicating the analytes withdraw electron density from C5N2 substrate. In the EDD plot of picric acid@C5N2 complex, the analyte is covered primarily with orange isosurface which depicts the shifting of electron density from pyrazine and benzene ring of C5N2, making them depleted region shown by pink color isosurface. The orange isosurface covering the phenolic group of picric acid along with three nitro groups signifies strong extraction of electrons from the benzene ring of C5N2 substrate as compared to TNT@C5N2 and 1,3-DNB@C5N2. These results show consistency with NBO analysis.
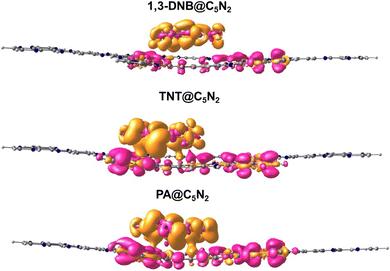 | ||
| Fig. 7 EDD plots of nitroaromatics@C5N2 complexes. The orange color isosurface shows the charge-accumulated region and the pink color isosurface represents the charge-depleted region. | ||
3.5 Recovery time
Recovery time is one of the parameters to determine the reproducibility of C5N2 material as a sensor. Generally, thermal effects are applied to calculate the recovery time through transition state theory.79 The equation for calculating recovery time is as follows:| τ = ν−1e−Eads/KT | (5) |
The τ stands for the recovery time, ν represents the attempt frequency (1014 s−1),45,80,81 Eads express the adsorption energy, K denotes Boltzmann constant (1.99 × 10−3 kcal mol−1 K−1), while T represents the temperature. For calculating the recovery time for the nitroaromatics@C5N2, different temperatures are employed. Generally, recovery time is directly proportional to interaction energies. Therefore, the recovery times for 1,3-DNB@C5N2, TNT@C5N2 and PA@C5N2 are, 5.24 × 10−4 s, 4.10 s and 74.74 s, respectively at 473 K. The recovery time of the nitroaromatics@C5N2 at different temperatures is presented in the Table S3.† It is observed that the systems' recovery time decreases with an increase in temperature.81
4 Conclusion
DFT calculations have been employed to determine the sensing capability of C5N2 substrate towards the lethal nitroaromatic compounds including 1,3-dinitrobenzene, trinitrotoluene and picric acid. Structures of nitroaromatics, C5N2 sheet, and their complexes have been optimised at the PBE0-D3BJ/def2SVP level of theory. Nitroaromatics are placed at five positions with different orientations to get the most stable geometry. Among studied systems, PA@C5N2 is observed with high interaction energy of −34.37 kcal mol−1. The trend in interaction is as follows: PA@C5N2 > TNT@C5N2 > 1,3-DNB@C5N2. Geometrical and electronic properties are determined for better understanding of the nature and type of interactions. QTAIM and NCI analyses confirm the existence of non-covalent interactions. The presence of denser green isosurface in 3D, along with bluish-green spots in the 2D RDG graph, shows the strong non-covalent interactions in the PA@C5N2 complex. These NCI results are verified by small and positive values of Laplacian and electronic density obtained through topological parameters. The conductivity of C5N2 has been increased after the adsorption of analytes due to a reduction in the HOMO–LUMO gap. More significant change is observed for PA@C5N2 (0.37) which shows selectivity of C5N2 towards the PA. FMO results are also confirmed by DOS analysis, which shows the prominent shifting of virtual orbital after the adsorption of PA. Charge transfer (NBO) analysis also shows that among the studied systems, significant charge transfer is observed for the PA@C5N2 system. Furthermore, EDD analysis confirms the NBO analysis by providing visual illustrations. Recovery time for all the studied complexes has been computed using the transition state theory equation. The results of recovery time follow the interaction energies; however, recovery time could be appreciably reduced by increasing temperature. These results show that the C5N2 substrate could be an efficient electrochemical sensor towards toxic nitroaromatics.Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its ESI.† Any further information will also be available upon request from the authorsConflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Computational facilities were provided by the Hope College high-performance computing cluster Curie.References
- D. T. McQuade, A. E. Pullen and T. M. Swager, Conjugated Polymer-Based Chemical Sensors, Chem. Rev., 2000, 100(7), 2537–2574 CrossRef.
- G. Awasthi, et al., Progressive Trends in Hybrid Material-Based Chemiresistive Sensors for Nitroaromatic Compounds, Polymers, 2022, 14(21), 4643 CrossRef.
- K. Milligan, et al., Detection of Multiple Nitroaromatic Explosives via Formation of a Janowsky Complex and SERS, Anal. Chem., 2020, 92(4), 3253–3261 CrossRef.
- P. Ghosh, et al., Recognition of an Explosive and Mutagenic Water Pollutant, 2,4,6-Trinitrophenol, by Cost-Effective Luminescent MOFs, Eur. J. Inorg. Chem., 2015, 2015(17), 2851–2857 CrossRef.
- V. Anand and V. Srivastava, Photocatalytic Degradation of Nitrobenzene and Azo Dye Using Zinc Oxide Nanoparticles Prepared by Electrochemical Method, J. Sci. Ind. Res., 2016, 75, 632–637 CAS.
- K.-S. Ju and R. E. Parales, Nitroaromatic Compounds, from Synthesis to Biodegradation, Microbiol. Mol. Biol. Rev., 2010, 74(2), 250–272 CrossRef PubMed.
- R. K. Jain, J. H. Dreisbach and J. C. Spain, Biodegradation of p-nitrophenol via 1,2,4-benzenetriol by an Arthrobacter sp, Appl. Environ. Microbiol., 1994, 60(8), 3030–3032 CrossRef.
- M. Kaur, S. K. Mehta and S. K. Kansal, A fluorescent probe based on nitrogen doped graphene quantum dots for turn off sensing of explosive and detrimental water pollutant, TNP in aqueous medium, Spectrochim. Acta, Part A, 2017, 180, 37–43 CrossRef PubMed.
- F. Rowell, et al., Detection of nitro-organic and peroxide explosives in latent fingermarks by DART- and SALDI-TOF-mass spectrometry, Forensic Sci. Int., 2012, 221(1), 84–91 CrossRef PubMed.
- Q. Zhou, et al., Detection of Nitro-Based and Peroxide-Based Explosives by Fast Polarity-Switchable Ion Mobility Spectrometer with Ion Focusing in Vicinity of Faraday Detector, Sci. Rep., 2015, 5(1), 10659 CrossRef PubMed.
- M. Tabrizchi and V. Ilbeigi, Detection of explosives by positive corona discharge ion mobility spectrometry, J. Hazard. Mater., 2010, 176(1), 692–696 CrossRef PubMed.
- A. E. Akmalov, et al., A laser desorption ion-mobility increment spectrometer for detection of ultralow concentrations of nitro compounds, Instrum. Exp. Tech., 2013, 56(3), 309–316 CrossRef CAS.
- Q. Lu, et al., Sensitive capillary electrophoresis microchip determination of trinitroaromatic explosives in nonaqueous electrolyte following solid phase extraction, Anal. Chim. Acta, 2002, 469(2), 253–260 CrossRef CAS.
- A. Hilmi, J. H. T. Luong and A.-L. Nguyen, Development of Electrokinetic Capillary Electrophoresis Equipped with Amperometric Detection for Analysis of Explosive Compounds, Anal. Chem., 1999, 71(4), 873–878 CrossRef CAS.
- S. Singh, Sensors—An effective approach for the detection of explosives, J. Hazard. Mater., 2007, 144(1), 15–28 CrossRef CAS PubMed.
- M. Marshall and J. C. Oxley, Aspects of Explosives Detection, Elsevier Science, 2011 Search PubMed.
- A. J. Bandodkar, et al., Solid-state Forensic Finger sensor for integrated sampling and detection of gunshot residue and explosives: towards ‘Lab-on-a-finger’, Analyst, 2013, 138(18), 5288–5295 RSC.
- L. Benny, et al., Waste elimination to porous carbonaceous materials for the application of electrochemical sensors: Recent developments, J. Cleaner Prod., 2021, 290, 125759 CrossRef.
- M. R. Jalali Sarvestani and Z. Doroudi, Fullerene (C20) as a potential sensor for thermal and electrochemical detection of amitriptyline: A DFT study, J. Chem. Lett., 2020, 1(2), 63–68 Search PubMed.
- L. E. Kreno, et al., Metal–Organic Framework Materials as Chemical Sensors, Chem. Rev., 2012, 112(2), 1105–1125 CrossRef.
- A. J. González Fá, R. Faccio and I. López-Corral, Detection of SOF2 and SO2F2 through aluminium nitride nanosheets: A DFT study, Appl. Surf. Sci., 2021, 538, 147899 CrossRef.
- S. S. Dindorkar and A. Yadav, Monolayered Silicon Carbide for Sensing Toxic Gases: a Comprehensive Study Based on the First-principle Density Functional Theory, Silicon, 2022, 14(17), 11771–11779 CrossRef.
- F. Li and H. Asadi, DFT study of the effect of platinum on the H2 gas sensing performance of ZnO nanotube: Explaining the experimental observations, J. Mol. Liq., 2020, 309, 113139 CrossRef CAS.
- T. Jadoon, T. Mahmood and K. Ayub, Silver-graphene quantum dots based electrochemical sensor for trinitrotoluene and p-nitrophenol, J. Mol. Liq., 2020, 306, 112878 CrossRef CAS.
- A. S. Rad, Al-doped graphene as modified nanostructure sensor for some ether molecules: Ab-initio study, Synth. Met., 2015, 209, 419–425 CrossRef CAS.
- Y. Li, Y. Xu and X. Li, The sensing mechanism of HCHO gas sensor based on transition metal doped graphene: Insights from DFT study, Sens. Actuators, A, 2022, 338, 113460 CrossRef CAS.
- H. Sajid, et al., Novel microporous B6N6 covalent organic framework (COF) as an electrochemical sensor for the ultra-selective detection of nitroaniline isomers; a DFT outcome, Surf. Interfaces, 2021, 27, 101587 CrossRef CAS.
- B. A. Farooqi, et al., Graphene-polyaniline composite as superior electrochemical sensor for detection of cyano explosives, Eur. Polym. J., 2020, 138, 109981 CrossRef CAS.
- D. Sharma and T. Singh, A DFT study of polyaniline/ZnO nanocomposite as a photocatalyst for the reduction of methylene blue dye, J. Mol. Liq., 2019, 293, 111528 CrossRef.
- M. F. Fellah, Pt doped (8,0) single wall carbon nanotube as hydrogen sensor: A density functional theory study, Int. J. Hydrogen Energy, 2019, 44(49), 27010–27021 CrossRef.
- U. Kumar and B. C. Yadav, Development of humidity sensor using modified curved MWCNT based thin film with DFT calculations, Sens. Actuators, B, 2019, 288, 399–407 CrossRef.
- A. S. Rad, et al., Ab-initio study of interaction of some atmospheric gases (SO2, NH3, H2O, CO, CH4 and CO2) with polypyrrole (3PPy) gas sensor: DFT calculations, Sens. Actuators, B, 2015, 220, 641–651 CrossRef.
- M. Noei, Different electronic sensitivity of BN and AlN nanoclusters to SO2 gas: DFT studies, Vacuum, 2017, 135, 44–49 CrossRef.
- T. Wang, et al., A Review on Graphene-Based Gas/Vapor Sensors with Unique Properties and Potential Applications, Nano-Micro Lett., 2016, 8(2), 95–119 CrossRef.
- F. K. Kessler, et al., Functional carbon nitride materials — design strategies for electrochemical devices, Nat. Rev. Mater., 2017, 2(6), 17030 CrossRef CAS.
- G. Zhang, et al., Overall water splitting by Pt/gC 3 N 4 photocatalysts without using sacrificial agents, Chem. Sci., 2016, 7(5), 3062–3066 RSC.
- L. Tan, et al., Novel two-dimensional crystalline carbon nitrides beyond gC3N4: structure and applications, J. Mater. Chem. A, 2021, 9(1), 17–33 RSC.
- M. Yar, M. A. Hashmi and K. Ayub, Nitrogenated holey graphene (C2N) surface as highly selective electrochemical sensor for ammonia, J. Mol. Liq., 2019, 296, 111929 CrossRef CAS.
- M. Asif, et al., A first principles study on electrochemical sensing of highly toxic pesticides by using porous C4N nanoflake, J. Phys. Chem. Solids, 2022, 160, 110345 CrossRef CAS.
- M. Yar, M. A. Hashmi and K. Ayub, The C2N surface as a highly selective sensor for the detection of nitrogen iodide from a mixture of NX3 (X= Cl, Br, I) explosives, RSC Adv., 2020, 10(53), 31997–32010 RSC.
- S. Qureshi, et al., First-principles study for electrochemical sensing of neurotoxin hydrazine derivatives via h-g-C3N4 quantum dot, Surf. Interfaces, 2022, 30, 101913 CrossRef CAS.
- S. Qureshi, et al., Electrochemical sensing of heptazine graphitic C3N4 quantum dot for chemical warfare agents; a quantum chemical approach, Mater. Sci. Semicond. Process., 2022, 148, 106753 CrossRef CAS.
- M. Yar, et al., Adsorption and sensor applications of C2N surface for G-series and mustard series chemical warfare agents, Microporous Mesoporous Mater., 2021, 317, 110984 CrossRef CAS.
- S. Sarfaraz, et al., DFT investigation of adsorption of nitro-explosives over C2N surface: Highly selective towards trinitro benzene, J. Mol. Liq., 2022, 352, 118652 CrossRef CAS.
- M. Yar, et al., Selective detection and removal of picric acid by C2N surface from a mixture of nitro-explosives, New J. Chem., 2020, 44(43), 18646–18655 RSC.
- Y. Kou, et al., Supercapacitive Energy Storage and Electric Power Supply Using an Aza-Fused π-Conjugated Microporous Framework, Angew. Chem., Int. Ed., 2011, 50(37), 8753–8757 CrossRef CAS PubMed.
- S. Kim and H. C. Choi, Light-promoted synthesis of highly-conjugated crystalline covalent organic framework, Commun. Chem., 2019, 2(1), 60 CrossRef.
- Y. Cao, et al., Hydrogen peroxide synthesis on porous graphitic carbon nitride using water as a hydrogen source, J. Mater. Chem. A, 2020, 8(1), 124–137 RSC.
- Z.-D. Yang, W. Wu and X. C. Zeng, Electronic and transport properties of porous graphenes: two-dimensional benzo-and aza-fused π-conjugated-microporous-polymer sheets and boron–nitrogen co-doped derivatives, J. Mater. Chem. C, 2014, 2(16), 2902–2907 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, et al., Gaussian 16, Revision C. 01. 2016 Search PubMed.
- A. Yaghoubi and A. Ramazani, Using Gaussian and GaussView software for effective teaching of chemistry by drawing molecules, Res. Chem. Educ., 2024, 6(1), 69–90 Search PubMed.
- C. Adamo and V. Barone, Toward reliable density functional methods without adjustable parameters: The PBE0 model, J. Chem. Phys., 1999, 110(13), 6158–6170 CrossRef CAS.
- M. Ibarra-Rodríguez, P. Horley and M. Sánchez, Metal-adorned borophene for efficient glucose adsorption, Comput. Theor. Chem., 2024, 1231, 114403 CrossRef.
- H. Louis, et al., Modeling of Mg12O11–X (X = B, N, P and S) Nanostructured Materials as Sensors for Melamine (C3H6N6), J. Comput. Biophys. Chem., 2022, 21(08), 999–1021 CrossRef.
- M. Ibarra-Rodríguez and M. Sánchez, Graphitic carbon nitride functionalized with four boron atoms for adsorption and separation of CO2/CH4: DFT calculations, Adsorption, 2020, 26(4), 597–605 CrossRef.
- M. Ibarra-Rodríguez and M. Sánchez, Adsorption of metformin on graphitic carbon nitride functionalized with metals of group 1–3 (Li, Na, K, Be, Mg, Ca, B, Al, and Ga), DFT calculations, Comput. Theor. Chem., 2022, 1207, 113532 CrossRef.
- M. Ibarra-Rodríguez and M. Sánchez, Adsorption of H2, N2, CO, H2S, NH3, SO2 and CH4 on Li-functionalized graphitic carbon nitride investigated by density functional theory, Bull. Mater. Sci., 2020, 43(1), 144 CrossRef.
- L. F. Paredes-Pérez, et al., Guanidinium and spermidinium decavanadates: as small biomimetic models to understand non-covalent interactions between decavanadate and arginine and lysine side chains in proteins, Front. Chem. Biol., 2024, 3, 1451167 CrossRef.
- F. A. Nelson, et al., The iron group transition-metal (Fe, Ru, Os) coordination of Se-doped graphitic carbon (Se@ gC 3 N 4) nanostructures for the smart therapeutic delivery of zidovudine (ZVD) as an antiretroviral drug: a theoretical calculation perspective, RSC Adv., 2023, 13(48), 34078–34096 RSC.
- L. Yang, et al., DFT-D3 and TD-DFT studies on the sensor performance of the functionalized carbon quantum dots for curcumin, Surf. Interfaces, 2024, 45, 103870 CrossRef CAS.
- K. Wu, et al., Multifunctional semiconducting carbon nitrides enabling sequential fluorescent sensing of telomerase activity and internal self-checking, Sens. Actuators, B, 2023, 378, 133170 CrossRef CAS.
- S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction, J. Comput. Chem., 2006, 27(15), 1787–1799 CrossRef CAS.
- S. Grimme, et al., A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132(15), 154104 CrossRef PubMed.
- T. Lu and F. Chen, Multiwfn: A multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33(5), 580–592 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, VMD: Visual molecular dynamics, J. Mol. Graphics, 1996, 14(1), 33–38 CrossRef.
- C. Legault, CYLview, 1.0b, Université de Sherbrooke Canada, 2009 Search PubMed.
- S. Pan, et al., Selectivity in Gas Adsorption by Molecular Cucurbit[6]uril, J. Phys. Chem. C, 2016, 120(26), 13911–13921 CrossRef.
- T. Lu and F. Chen, Bond Order Analysis Based on the Laplacian of Electron Density in Fuzzy Overlap Space, J. Phys. Chem. A, 2013, 117(14), 3100–3108 CrossRef PubMed.
- N. S. Venkataramanan, A. Suvitha and Y. Kawazoe, Unravelling the nature of binding of cubane and substituted cubanes within cucurbiturils: A DFT and NCI study, J. Mol. Liq., 2018, 260, 18–29 CrossRef.
- S. A. Bhadane, D. N. Lande and S. P. Gejji, Understanding Binding of Cyano-Adamantyl Derivatives to Pillar[6]arene Macrocycle from Density Functional Theory, J. Phys. Chem. A, 2016, 120(43), 8738–8749 CrossRef PubMed.
- N. L. Marana, S. M. Casassa and J. R. Sambrano, Adsorption of NH3 with Different Coverages on Single-Walled ZnO Nanotube: DFT and QTAIM Study, J. Phys. Chem. C, 2017, 121(14), 8109–8119 CrossRef CAS.
- M. Aetizaz, S. Sarfaraz and K. Ayub, Interaction of imidazolium based ionic liquid electrolytes with carbon nitride electrodes in supercapacitors; a step forward for understanding electrode–electrolyte interaction, J. Mol. Liq., 2023, 369, 120955 CrossRef CAS.
- D. Cremer and E. Kraka, Chemical Bonds without Bonding Electron Density — Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond?, Angew Chem. Int. Ed. Engl., 1984, 23(8), 627–628 CrossRef.
- G. Zhurko, Chemcraft, 2014, vol. 22, https://www.chemcraftprog.com Search PubMed.
- W.-j. Hu, et al., Theoretical investigation on the intermolecular interactions between 3-nitro-1,2,4-triazol-5-one and 2,6-diamino-3,5-dinitropyrazine-1-oxide using DFT methods, Chem. Pap., 2022, 76(5), 2747–2758 Search PubMed.
- H. Sajid, et al., Effective adsorption of A-series chemical warfare agents on graphdiyne nanoflake: a DFT study, J. Mol. Model., 2021, 27(4), 117 Search PubMed.
- H. Sajid, et al., High selectivity of cyclic tetrapyrrole over tetrafuran and tetrathiophene toward toxic chemicals; A first-principles study, Microporous Mesoporous Mater., 2020, 299, 110126 Search PubMed.
- A. S. Rad and K. Ayub, O3 and SO2 sensing concept on extended surface of B12N12 nanocages modified by Nickel decoration: A comprehensive DFT study, Solid State Sci., 2017, 69, 22–30 Search PubMed.
- M. Rakib Hossain, et al., First-principles study of the adsorption of chlormethine anticancer drug on C24, B12N12 and B12C6N6 nanocages, Comput. Theor. Chem., 2021, 1197, 113156 Search PubMed.
- Z. Zhao, et al., Gas-Sensing Properties of the SiC Monolayer and Bilayer: A Density Functional Theory Study, ACS Omega, 2020, 5(21), 12364–12373 Search PubMed.
- S. Peng, et al., Ab initio study of CNT NO2 gas sensor, Chem. Phys. Lett., 2004, 387(4), 271–276 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05600k |
| This journal is © The Royal Society of Chemistry 2024 |