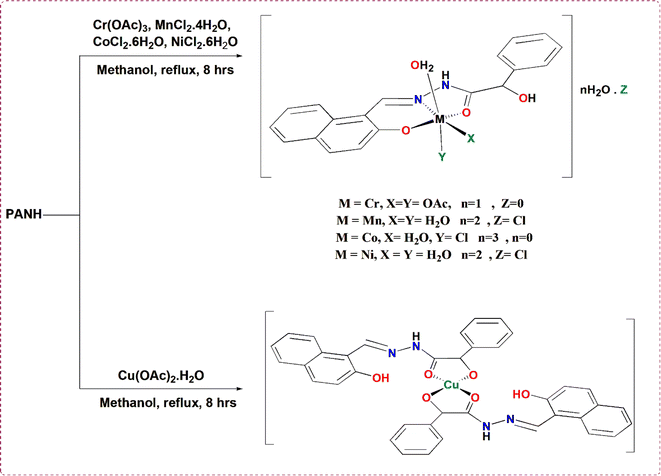
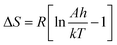Open Access Article
Open Access ArticleInsight into the synthesis, structure affirmation and catalytic efficiency of divalent and trivalent metal chelates of mandelic acid hydrazone derivative†
Hoda A. El-Ghamry *a,
Mohamed Gabera,
Fatmah M. Alkhatibb,
Hossa F. Al Shareefb,
Khadiga M. Takronib and
Shaimaa K. Fathallac
*a,
Mohamed Gabera,
Fatmah M. Alkhatibb,
Hossa F. Al Shareefb,
Khadiga M. Takronib and
Shaimaa K. Fathallac
aChemistry Department, Faculty of Science, Tanta University, Tanta, Egypt. E-mail: hoda.elghamri@science.tanta.edu.eg
bChemistry Department, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia
cChemistry Department, Faculty of Science, Taif University, Taif, Saudi Arabia
First published on 25th September 2024
Abstract
The current work reports the synthesis of Cr(III), Mn(II), Co(II), Ni(II) and Cu(II) chelates of the Schiff base ligand named hydroxy-phenyl-acetic acid (2-hydroxy-naphthalen-1-ylmethylene)-hydrazide with multi-chelation centre toward metal ions. The spectral tools, 1H-NMR, FTIR, mass, UV-vis spectra, and the analytical elemental and thermal analysis, in addition to magnetic moment and conductivity measurements all combined have been applied to conclude the structure and geometry of the synthesized metal complexes. The formed metal chelates have been assured to be formed with the molar compositions of 1 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M for PANH-Cr, PANH-Mn, PANH-Co, PANH-Ni and 2 L
1 M for PANH-Cr, PANH-Mn, PANH-Co, PANH-Ni and 2 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M for PANH-Cu. All the complexes have been confirmed to be non-electrolytic except the PANH-Mn and PANH-Ni which are 1
1 M for PANH-Cu. All the complexes have been confirmed to be non-electrolytic except the PANH-Mn and PANH-Ni which are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolytes. FTIR spectral analysis assured the ligand to act as mono basic bi or tridentate ligand leading to the formation of octahedral complexes with all metals except Cu(II) complex which assured to has square planar structure. Except PANH-Cr, all the synthesized metal chelates exhibited phenoxazinone synthase like efficacy with varying activity with dramatically high activity for PANH-Mn complex with TOF number of 169.89 h−1.
1 electrolytes. FTIR spectral analysis assured the ligand to act as mono basic bi or tridentate ligand leading to the formation of octahedral complexes with all metals except Cu(II) complex which assured to has square planar structure. Except PANH-Cr, all the synthesized metal chelates exhibited phenoxazinone synthase like efficacy with varying activity with dramatically high activity for PANH-Mn complex with TOF number of 169.89 h−1.
1. Introduction
Hydrazones are considered flexible ligands that are classified as azomethine compounds, having the structure R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NNR2. They are distinguished from other members of this class, such as oximes, imines, and others, by the presence of linked nitrogen atoms.1
NNR2. They are distinguished from other members of this class, such as oximes, imines, and others, by the presence of linked nitrogen atoms.1
Hydrazone-based ligands have gained interest due to their numerous coordination characteristics and capacity to coordinate in both protonated and deprotonated forms.2,3 A dominant contribution in the advancement of coordination chemistry was achieved by hydrazone ligands formed by the condensation reaction of o-hydroxyaldehydes and ketones with hydrazides due to the formed conceivable poly-cleating ligands that pass amide, azomethine and phenol functionalities and provide a variety of bonding options to metal centres in metal chelates.4 Benzoylhydrazones of salicylaldehyde,5 2-hydroxy-1-naphthaldehyde6 and their analogies compounds7,8 are considered the most important hydrazone ligands since this structure building provide a class of ligands with bi-or tridentate cavity coordinating to the metal centres through the carbonyl oxygen, azomethine nitrogen and hydroxyl oxygen resulting in the formation of five and/or six membered chelate ring surrounding the metal centre. Condensation of 2-hydroxy-1-naphthaldehyde with mandelic acid hydrazide resulting in the formation of analogies ligand structure.
The great importance of hydrazone compounds and their metal chelates steam from their wide range of applications since they are applied in biological system as antimicrobial, antibiotic, antimalarial and antitumor9–12 beside their uses in analytical,13 catalytic14–16 applications and also as molecular sensors17 and luminescent probes.18
Among the transition elements, Mn element is considered a significant element for human healthiness, metabolism, antioxidant system and fundamental in photosystem for O2 evolution.19–22 Recently, great attention has been allocated for the design and synthesis of Mn coordination compounds due to their efficiently catalytic performance23–26 specifically with Schiff base ligands incorporating N and O atoms. The reason is that in such complexes the Mn(II) centers are subjected to aerial oxidation to form complexes in which Mn ion is oxidized to higher oxidation states (i.e. Mn(III) and/or Mn(IV)).27 Among the important catalytic reaction for Mn complexes is bio-mimicking oxidase (e.g. catecholase and phenoxazinone synthase) and oxygenase (e.g. epoxidation) reactions.27–30
Phenoxazinone synthase is one of the enzymes whose behavior and effects have been experimentally and widely mimicked experimentally. This enzyme is called poly nuclear copper oxidase. This enzyme acts as a catalyst for the oxidative condensation of o-aminophenol and its derivatives to phenoxazinone chromophore and is found naturally in the bacteria Streptomyces antibioticus. This phenoxazinone chromophore is used therapeutically to treat Wilm's tumor curing, gestational choriocarcinoma, and other tumor types. Its function is to stop DNA intercalation from synthesizing RNA based on DNA in these tumor kinds.31 The major condition for a metal chelate to be as promising as phenoxazinone synthase is the availability of labile positions on the metal centers. Metal complexes incorporating other metals such as Cu(II), Co(II) and Ru(III) are also reported to catalyse such reactions.31–35
All the previous facts motivated us to design and prepare a number of metal complexes of the hydrazone ligand named hydroxy-phenyl-acetic acid (2-hydroxy-naphthalen-1-ylmethylene)-hydrazide. Structure assertion of the synthesized metal ion chelates (Cr(III), Mn(II), Co(II), Ni(II) and Cu(II) chelates) were performed using the analytical and spectral methods. The interested complexes have been tested and evaluated for their catalytic activity as phenoxazinone synthase.
2. Experimental
2.1. Preparation of the Schiff base ligand (PANH)
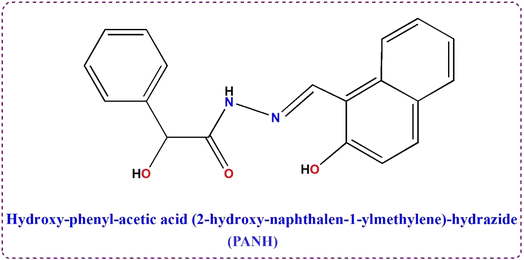
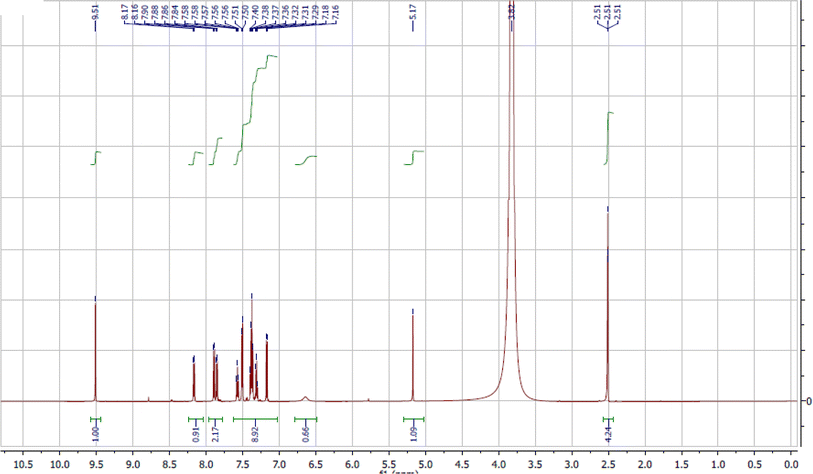
The target Schiff base has been synthesised as previously reported.8 0.01 mol of 2-hydroxy-1-naphthaldehyde and mandelic acid hydrazide (1.72 g and 1.66 g, respectively) were separately solubilized in the least sufficient amount of hot MeOH followed by slow addition of one solution to the other. The final solution was allowed for reflux at 60 °C for 4 h within which yellowish crystalline needles have been appeared. The hot reaction solution was filtered to separate the formed compound which rinsed several times using hot methanol and eventually with ether. The product was kept drying in vacuum desiccator (Fig. 1). Purity of the ligand has been assured by 1H-NMR, FTIR, mass and elemental analysis.1H-NMR (600 MHz, DMSO-d6, δ, ppm): δ: 3.81 (s, 1H, CH–OH), 5.17 (s, 1H, CH–OH), 6.65 (s, br, 1H, NH), 7.16–7.9 (d, m, 11H, Ar–H), 8.17 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 9.51 (s, 1H, phenolic OH).
N), 9.51 (s, 1H, phenolic OH).
2.2. Synthesis of metal chelates
The above mentioned multidentate ligand (i.e. PANH) has been used for the synthesis of the Cr(III), Mn(II), Co(II), Ni(II) and Cu(II) chelates through dissolving 0.001 mol of each of Cr(CH3COO)3 (0.232 g) MnCl2·4H2O (0.197 g), CoCl2·6H2O (0.237 g), NiCl2·6H2O (0.237 g) and Cu(CH3COO)·H2O (0.199 g) in the least sufficient quantity of hot MeOH and added separately to a heated methanolic solution that included 0.32 g (0.001 mol of PANH). The resultant mixtures were refluxed for eight hours at boiling temperature, during which time colorful compounds developed. After being filtered out, the solid products were repeatedly washed with hot methanol and then with ether. The product was maintained in a vacuum desiccator for drying out.2.3. Measurements instrumentation and OAP catalytic oxidation
The Sigma-Aldrich company is the supplier for all of the analytical grade reagents used in the current study, which were used exactly as they were given. Information regarding the tools and techniques used for structure assertion and implementation is provided from the previously reported study.31 Using o-aminophenol as the substrate, the methods and strategies employed to assign the complexes under investigation's phenoxazinone synthase-like activity are generally as previously mentioned34 and as described in details in the ESI file.†3. Results and discussion
3.1. 1H NMR spectra of PANH ligand
Fig. 2 represents the 1H-NMR spectrum of PANH ligand measured in DMSO-d6 using the internal standard tetramethylsilane (TMS). The signal appearing at 9.51 ppm has been appointed to the phenolic proton whereas the one appearing at 8.17 ppm is assigned to the azomethine proton. The broad signal that appeared at 6.6 ppm has been attributed to the NH proton.36 Aromatic protons of the phenyl and naphthyl rings featured as doublet and multiple signals inside 7.9–7.16 ppm range. The aliphatic CH and OH protons appeared at 5.17 and 3.81 ppm, respectively.37,38 The OH proton signal at 3.81 ppm is overlapped with the solvent signal as it is appearing in the same range.3.2. Complexes composition and conductivity
As concluded from the results of the contained elements percent, the isolated metal chelates have been formed in the molar composition 1 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M for PANH-Cr, PANH-Mn, PANH-Co, PANH-Ni. For the Cu(II) complex, PANH-Cu, although it has been synthesized in the molar ratio 1 L
1 M for PANH-Cr, PANH-Mn, PANH-Co, PANH-Ni. For the Cu(II) complex, PANH-Cu, although it has been synthesized in the molar ratio 1 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M, the isolated complex formed in 2 L
1 M, the isolated complex formed in 2 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M ratio. The molecular formulas of all complexes were concluded to be [Cr(PANH)(AOc)2H2O]·H2O, [Mn(PANH)(H2O)3]·Cl·2H2O, [Co(PANH)Cl(H2O)2]·3H2O, [Ni(PANH)(H2O)3]·Cl·2H2O and [Cu(PANH)2]; Table 1. The solubility test of the highly stable complexes indicated that all complexes have no solubility in all non-polar solvents, partially soluble in MeOH and EtOH and extremely soluble in MeCN, DMSO and DMF. The molar conductivity values as measured from 10−3 M solution of each compound dissolved in DMSO were found to be 17.2, 69.5, 18.6, 71.4 and 15.7 Ω−1 cm2 mol−1 for PANH-Cr, PANH-Mn, PANH-Co, PANH-Ni and PANH-Cu, successively, which is in good matching with the non-electrolytic features of PANH-Cr, PANH-Co, and PANH-Cu complexes and 1
1 M ratio. The molecular formulas of all complexes were concluded to be [Cr(PANH)(AOc)2H2O]·H2O, [Mn(PANH)(H2O)3]·Cl·2H2O, [Co(PANH)Cl(H2O)2]·3H2O, [Ni(PANH)(H2O)3]·Cl·2H2O and [Cu(PANH)2]; Table 1. The solubility test of the highly stable complexes indicated that all complexes have no solubility in all non-polar solvents, partially soluble in MeOH and EtOH and extremely soluble in MeCN, DMSO and DMF. The molar conductivity values as measured from 10−3 M solution of each compound dissolved in DMSO were found to be 17.2, 69.5, 18.6, 71.4 and 15.7 Ω−1 cm2 mol−1 for PANH-Cr, PANH-Mn, PANH-Co, PANH-Ni and PANH-Cu, successively, which is in good matching with the non-electrolytic features of PANH-Cr, PANH-Co, and PANH-Cu complexes and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolyte for and PANH-Mn and PANH-Ni as compared by reported compounds.39,40
1 electrolyte for and PANH-Mn and PANH-Ni as compared by reported compounds.39,40
| Comp. symbol | Ligand/complex (molecular formula; M. wt) | Colour (m.p. °C) | Elemental analysisb | Λm | |||
|---|---|---|---|---|---|---|---|
| % C | % H | % N | % M | ||||
| a Decomposition temperature.b Calculated (Found). | |||||||
| PANH | (C19H16N2O3; 320.34) | Yellow (242) | 71.24 (71.32) | 5.03 (5.14) | 8.47 (8.53) | — | — |
| PANH-Cr | [Cr(PANH)(AOc)2H2O]·H2O (C23H25CrN2O9; 525.45) | Yellowish green (294) | 52.57 (52.69) | 4.80 (4.68) | 5.33 (5.21) | 9.90 (9.74) | 17.2 |
| PANH-Mn | [Mn(PANH)(H2O)3]·Cl·2H2O (C19H25ClMnN2O8, 499.80) | Brown (275)a | 45.66 (45.76) | 5.04 (5.24) | 5.60 (5.73) | 10.99 (11.77) | 69.5 |
| PANH-Co | [Co(PANH)Cl(H2O)2]·3H2O (C19H23ClCoN2O7; 485.78) | Brown (252)a | 46.98 (47.22) | 4.77 (4.81) | 5.77 (5.92) | 12.13 (12.93) | 18.6 |
| PANH-Ni | [Ni(PANH)(H2O)3]·Cl·2H2O (C19H25ClNiN2O8; 503.56) | Dark yellow (>300) | 45.32 (45.45) | 5.00 (5.23) | 5.56 (5.71) | 11.66 (11.40) | 71.4 |
| PANH-Cu | [Cu(PANH)2] (C38H30CuN4O6; 702.21) | Olive green (265)a | 65.00 (65.17) | 4.31 (4.39) | 7.98 (8.26) | 9.05 (9.49) | 15.7 |
3.3 EI-mass spectra
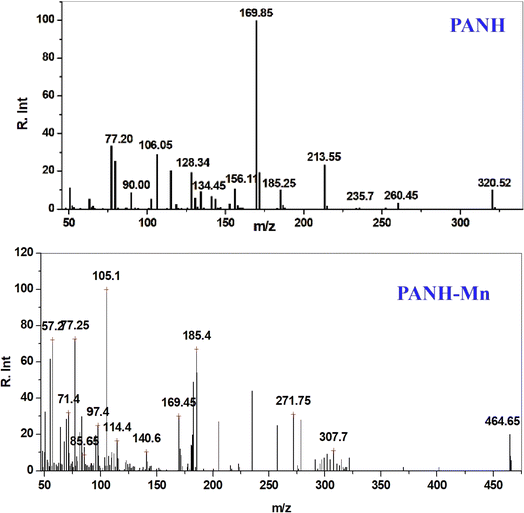
The prospected molecular weights of the ligand, PANH, and its chelates, PANH-Cr, PANH-Mn, PANH-Co, PANH-Ni and PANH-Cu, have been assured through comparison of the mass spectral results. The molecular weight of the ligand and PANH-Mn complex were found to be 320.52 and 464.65 matching with the theoretical molecular weight of the ligand (i.e. 320.34) and M − 1 for PANH-Mn (without hydration water molecules); Fig. 3. The mass spectra of complexes PANH-Cr, PANH-Co, PANH-Ni and PANH-Cu (Fig. S1–S4†) gave the molecular ion peaks at 509.25 (M + 2 − 2H2O), 449.92 (M − 2H2O), 466.58 (M − 1 − 2H2O) and 705.62 (M + 3), respectively, which is in large agreement with the concluded molecular weights.3.4. IR spectra and mode of bonding
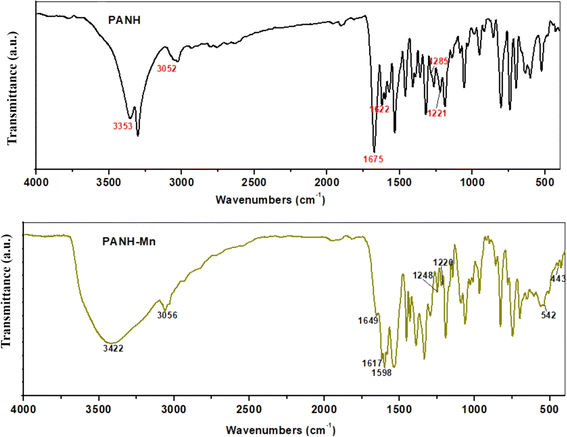
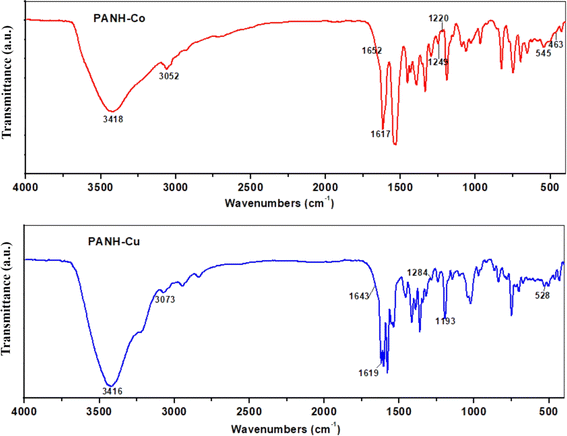
The coordinating function groups in the ligand to the metal centers can be identified by comparing the FTIR spectra of the metal complexes with those of the free Schiff base, PANH. Changes in position and/or intensity of spectral bands are common in coordinating function groups. It's also possible for some bands to disappear during chelation. The most important infrared bands of PANH and its complexes are shown in Table 2, along with their respective assignments, the spectra are shown in Fig. 4, 5 & S5.†| Comp. | νOH | νNH | νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
νC–Oa,b | νC–O | νM–N |
|---|---|---|---|---|---|---|---|
| a Phenolic.b 2° alcohol. | |||||||
| PANH | 3353 | 3052 | 1675 | 1622 | 1285 | — | — |
| 1221 | |||||||
| PANH-Cr | 3415 | 3054 | 1649 | 1613 | 1250 | 536 | 448 |
| 1220 | |||||||
| PANH-Mn | 3422 | 3056 | 1649 | 1617 | 1248 | 542 | 443 |
| 1220 | |||||||
| PANH-Co | 3418 | 3052 | 1652(sh) | 1617 | 1249 | 545 | 463 |
| 1220 | |||||||
| PANH-Ni | 3414 | 3060 | 1656 | 1617 | 1247 | 529 | 460 |
| 1192 | |||||||
| PANH-Cu | 3416 | 3073 | 1643(sh) | 1619 | 1284 | 528 | — |
| 1193 | |||||||
The bands in the spectra of PANH that appeared at 3353 and 3052 cm−1 were assigned to the stretching vibrations of the OH and NH groups, respectively, according to an analysis of the results shown in Table 2. While the band at 1622 cm−1 was linked to v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) groups, the band at 1675 cm−1 was related to the stretching vibration of C
N) groups, the band at 1675 cm−1 was related to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Two bands have been assigned to phenolic and secondary alcoholic C–O stretching at 1285 and 1221 cm−1, respectively.41,42
O. Two bands have been assigned to phenolic and secondary alcoholic C–O stretching at 1285 and 1221 cm−1, respectively.41,42
In the spectra of metal complexes PANH-Cr, PANH-Mn, PANH-Co and PANH-Ni, the three bands corresponding to the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), ν(C
O), ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and ν(C–Ophenolic) appeared within the ranges 1649–1656, 1613–1617 and 1247–1250 cm−1, respectively, affording a shift in their position in the ligand spectrum by 19–26, 5–9 and 35–38 cm−1 which are large enough to confirm the involvement of C
N) and ν(C–Ophenolic) appeared within the ranges 1649–1656, 1613–1617 and 1247–1250 cm−1, respectively, affording a shift in their position in the ligand spectrum by 19–26, 5–9 and 35–38 cm−1 which are large enough to confirm the involvement of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–O (phenolic) in ligation to the metal centre. Nearly no shift in the position of secondary alcoholic C–O band and hence no probable involvement of such band in coordination to the metal centre.42
N and C–O (phenolic) in ligation to the metal centre. Nearly no shift in the position of secondary alcoholic C–O band and hence no probable involvement of such band in coordination to the metal centre.42
In the spectrum of PANH-Cu complex, although the band corresponding to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) afforded a shift in its position by 32 cm−1 relative to its position in the ligand spectrum, the band corresponding to C
O) afforded a shift in its position by 32 cm−1 relative to its position in the ligand spectrum, the band corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–O (phenolic) bonds appeared almost at the same position relative to the ligand spectrum and hence these two bands are not involved in coordination to the Cu centre. On the other hand, the secondary alcoholic C–O band that appeared in ligand spectrum at 1221 cm−1 underwent a shift in its position appearing at 1193 cm−1 in PANH-Cu complex's spectrum confirming its involvement in ligation to Cu centre through proton removal. The appearance of new bands within the ranges 443–463 cm−1 (νM–N) and 528–545 cm−1 (νM–O) confirms the participation of azomethine nitrogen and phenolic, carbonyl and/or alcoholic O atoms in formation of the complexes.
N and C–O (phenolic) bonds appeared almost at the same position relative to the ligand spectrum and hence these two bands are not involved in coordination to the Cu centre. On the other hand, the secondary alcoholic C–O band that appeared in ligand spectrum at 1221 cm−1 underwent a shift in its position appearing at 1193 cm−1 in PANH-Cu complex's spectrum confirming its involvement in ligation to Cu centre through proton removal. The appearance of new bands within the ranges 443–463 cm−1 (νM–N) and 528–545 cm−1 (νM–O) confirms the participation of azomethine nitrogen and phenolic, carbonyl and/or alcoholic O atoms in formation of the complexes.
The two non-ligand bands appearing in the spectrum of PANH-Cr at 1582 and 1353 cm−1 have been assigned to νasy(COO−) and νsym(COO−), respectively, with Δν separation value of 229 cm−1 confirming the coordination of the carboxylate group to the Cr ion in a monodentate fashion.43
All complexes' spectra have bands in the ranges 3414–3422 cm−1 that are attributed to coordinated and lattice water molecules, whereas the bands in the range 3054–3073 cm−1 are attributed to (NH). Although the NH group is not involved in the formation of complexes, H-bond formation is likely responsible for shifting the position of the NH band in the complexes' spectra relative to the free ligand.44
3.5. Thermal gravimetric analytical results and kinetic parameters
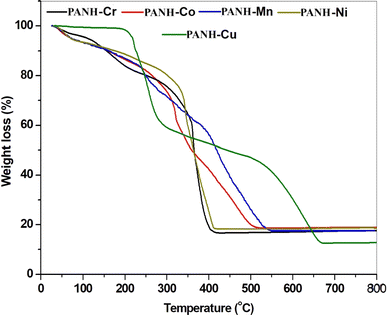
One of the most helpful methods for predicting the molecular structure and stability of compounds is thermal analysis, which provides vital information on the thermal properties of compounds, the phases of thermal degradation, the kinds of intermediates, and the residual products of thermal degradation. Through such examination, the amount and kind of water and/or organic solvent molecules, as well as the other anions attached to the metal center, can be allocated. Thermogravimetric thermograms of the metal complexes are given in Fig. 6 and their analysis are given in details in Table 3. Investigation of the obtained results featured that PANH-Cr, PANH-Co, PANH-Ni and PANH-Cu complexes afforded 3-stages thermograms whereas PANH-Mn complex has 4-stages thermogram. Analysis of these thermograms showed that, except PANH-Cu complex, all the studied compounds started their decomposition from room temperature and the first decomposition stage extended up to 113 °C in which lattice water molecules included in complexes' structures got lost. The following decomposition step started at 91 °C and ended at 341 °C inside which the coordinated water and anion molecules (chloride or acetate) are lost. The successive 1 or 2 stages which started 255 °C and extended to 800 °C are analysed to degradation of the organic ligand molecule incorporated in the complexes' structure and lead to the formation of metallic oxide in addition to small percent of remaining carbon content. For PANH-Cu complex with 3-stages thermogram, this complex exhibited thermal stability up to 184 °C and the first stage of decomposition occurred in the range 184–298 °C within which the organic ligand partially decomposed with the loss of 2 molecules of hydroxy naphthyl groups. The second and third stages of decomposition occurred in 298–483 °C and 483–678 °C range and assigned to the loss of C2H4N4 and C16H12O3 fragments, respectively, leading to the formation of CuO residue with percent of 11.89% which is in accordance the calculated percent that is 11.33%.| Symbol molecular formula (M. wt) | Temp. Range (°C) | Mass loss% | Assignment | |
|---|---|---|---|---|
| Found | Calc. | |||
| PANH-Cr [[CrL(AOc)2H2O]]·H2O 525.45 | 25–91 | 3.65 | 3.43 | Loss of hydration H2O |
| 91–330 | 25.54 | 25.88 | Loss of coordination H2O and 2 acetate molecules | |
| 330–430 | 54.32 | 53.86 | Loss of (C18H15N2O1.5) | |
| >430 | 16.49 | 16.75 | Formation of 0.5 Cr2O3 + 1C as residue | |
| PANH-Mn [MnL(H2O)3]·Cl·2H2O 499.80 | 26–105 | 7.13 | 7.20 | Loss of 2 hydration H2O |
| 105–255 | 13.85 | 14.20 | Loss of 3 coordination H2O and OH group | |
| 255–399 | 22.66 | 22.51 | Loss of Cl− anion and dissociation of the organic ligand (C6H5) | |
| 399–568 | 38.60 | 39.41 | Further dissociation of the organic ligand (C12H9N2O) | |
| >568 | 17.76 | 16.60 | Formation of MnO + 1C as residue | |
| PANH-Co [CoLCl(H2O)2]·3H2O 485.78 | 29–113 | 7.11 | 7.41 | Loss of 2 hydration H2O |
| 113–324 | 30.00 | 30.57 | Loss of coordination H2O, Cl− anion and C6H5. | |
| 324–535 | 43.42 | 44.05 | Further dissociation of the organic ligand (C12H10N2O2) | |
| >535 | 19.07 | 17.89 | Formation of CoO + 1C as residue | |
| PANH-Ni [NiL(H2O)3]·Cl·2H2O 503.56 | 29–107 | 7.01 | 7.15 | Loss of 2 hydration H2O |
| 107–341 | 21.44 | 21.15 | Loss of 3 coordination H2O, Cl− anion and OH group | |
| 341–426 | 52.37 | 52.03 | Decomposition of the organic ligand (C17H14N2O) formation of NiO + 2C as residue | |
| >426 | 19.18 | 19.62 | ||
| PANH-Cu [Cu(L)2] 702.21 | 29–184 | — | — | Thermal stability |
| 184–298 | 40.70 | 40.77 | Degradation of the ligand moiety and loss of (C20H14O2) | |
| 298–483 | 11.69 | 11.97 | Loss of (C2H4N4) | |
| 483–678 | 35.72 | 35.92 | Loss of (C16H12O3) | |
| >678 | 11.89 | 11.33 | Formation of CuO as residue | |
Application of Coats–Redfern equations for the successive decomposition stages enables the computation of the kinetic parameters “order of reaction (n), pre-exponential factor (A), and energy of activation (E)”45 which have been obtained from the plots as shown in Fig. 7 and S6–S8† (Coats–Redfern plot for PANH-Mn and PANH-Ni complex, as illustrative examples) and the results are depicted in Table 4. The thermodynamic parameters, ΔH, ΔS, and ΔG, were also calculated applying the previous reported equations:
| ΔH = ΔE − RT |
| ΔG = ΔH − TΔS |
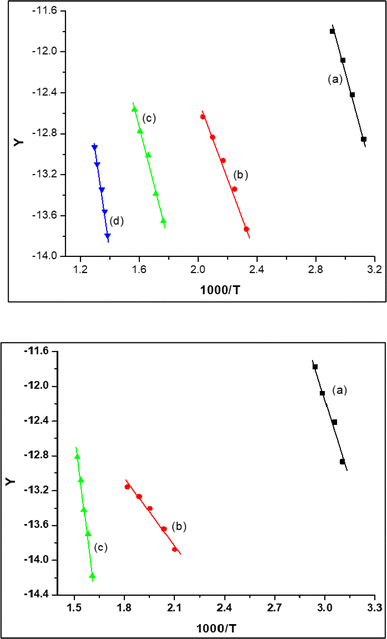 | ||
| Fig. 7 Coats–Redfern plot for PANH-Mn (up) and PANH-Ni (down). (a) first step (b) second step (c) third step where, Y = [−ln(1 − α)]/T2 for n = 1. | ||
| Complex | n | Step | r | T (K) | ΔH* | A | −ΔS* | ΔG* | |
|---|---|---|---|---|---|---|---|---|---|
| PANH-Cr | 1 | 1st | 0.96834 | 327.25 | 65.904 | 63.6355 | 0.358![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 874 874 |
0.2640 | 149.95 |
| 1 | 2nd | 0.99910 | 440 | 27.254 | 23.5305 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 392 392![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423.9 423.9 |
0.1222 | 77.34 | |
| 1 | 3rd | 0.98761 | 647.2 | 194.344 | 188.946 | 2.30388 × 10−5 | 0.3484 | 414.47 | |
| PANH-Mn | 1 | 1st | 0.99581 | 331.625 | 41.269 | 38.5123 | 3365.103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 219 219 |
0.1865 | 100.38 |
| 1 | 2nd | 0.99512 | 460.8 | 30.451 | 26.6200 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 337 337![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 456.085 456.085 |
0.1266 | 84.97 | |
| 1 | 3rd | 0.99669 | 602.8 | 46.489 | 41.4031 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 461 461![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 953.223 953.223 |
0.1368 | 125.11 | |
| 1 | 4th | 0.99617 | 745.8 | 79.119 | 72.8698 | 169![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515.6068 515.6068 |
0.1608 | 193.75 | |
| PANH-Co | 1 | 1st | 0.99352 | 329.125 | 40.103 | 37.3675 | 4752.113![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441 441 |
0.1836 | 97.81 |
| 1 | 2nd | 0.98847 | 469.7 | 23.166 | 19.1924 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 716 716![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 377.5 377.5 |
0.1063 | 70.01 | |
| 0.5 | 3rd | 0.99849 | 705 | 34.466 | 28.5238 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 813 813![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121.51 121.51 |
0.1095 | 106.81 | |
| PANH-Ni | 1 | 1st | 0.98937 | 331 | 52.182 | 49.4307 | 78.27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 469 469![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713 713 |
0.2178 | 121.53 |
| 1 | 2nd | 0.98788 | 511.7 | 21.0727 | 16.7442 | 124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 782 782![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 239.6 239.6 |
0.1028 | 70.29 | |
| 1 | 3rd | 0.99575 | 640.5 | 128.334 | 122.970 | 3.584![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 854 854![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 628 628 |
0.2490 | 283.62 | |
| PANH-Cu | — | 1st | — | — | — | — | — | — | — |
| 0.66 | 2nd | 0.99266 | 529.9 | 73.858 | 69.4294 | 1507.203![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925 |
0.1972 | 174.49 | |
| 0.66 | 3rd | 0.99829 | 693.6 | 31.448 | 25.5912 | 138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 476![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 513.3 513.3 |
0.1045 | 99.22 | |
| 1 | 4th | 0.99763 | 894.1 | 152.693 | 145.201 | 183.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464 464![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653 653 |
0.2190 | 342.62 |
Table 4 findings demonstrate the −ve ΔS* implication that either the activated complex is more ordered than its reactants or that the complexes' normal breakdown process is taking longer than usual.46 Positive ΔH values signify endothermic breakdown processes.47 Additionally, it has been found that the vast majority of the time, the heat decomposition stages of all complexes follow zero- or first-order kinetics.
3.6. UV-vis spectra and magnetic moment
DMSO solutions of PANH and its metal chelates were synthesized for recording UV-vis spectra which done in the range of 200–800 nm (Fig. S9 & S10†). The free ligand PANH showed the absorption band at 276 nm that assigned to π → π* of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group. The bands that appeared at and 310 and 428 nm assigned to n → π* transitions of C
N group. The bands that appeared at and 310 and 428 nm assigned to n → π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, successively.43,48 The appearance of such bands in complexes' spectra shifted from these values is evidence for the attachment of both azomethine nitrogen and carbonyl oxygen to the metal ion in complex formation.
O groups, successively.43,48 The appearance of such bands in complexes' spectra shifted from these values is evidence for the attachment of both azomethine nitrogen and carbonyl oxygen to the metal ion in complex formation.
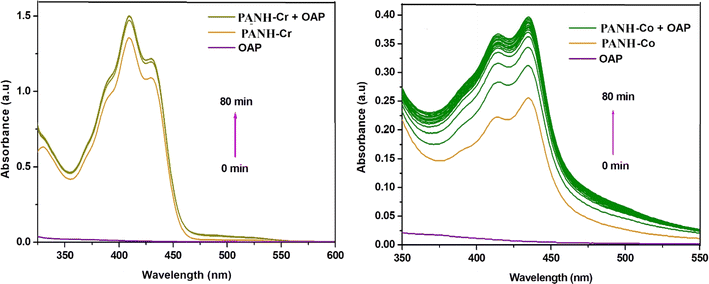
For PANH-Cr complex, the three observed bands centred at 390, 431 and 523 nm allocated to 4A2g → 4T1g (P) (ν3), 4A2g → 4T1g (F) (ν2), and 4A2g → 4T2g (F) (ν1) transitions, respectively, and confirmed octahedral architecture of the complex.49,50 The room temperature magnetic moment value of this complex was calculated and found to be of the complex was measured to be 3.82 B. M. that is approaching to the spin only value of three unpaired electrons (i.e. 3.88 B. M.) in octahedral Cr(III) chelates and hence confirming the predicted geometry.50
In the spectrum of PANH-Mn chelate, the three transitions appeared in the spectrum at 586, 464 and 433 nm, respectively, were assigned to 6A1g → 4T1g(G), 6A1g → 4T2g(G) and 6A1g → 4Eg(G) reported for Mn(II) chelates.51,52 Magnetic moment value of 6.12 B. M. was calculated for this complex which assigned to high spin Mn(II) chelates.
The Co(II) chelate, PANH-Co, afforded its spectral bands at 572 and 475 nm that correspond to the transitions 4T1g(F) → 4A2g(F) and 4T1g(F) → 4T1g(p), successively, familiar to octahedral Co(II) complexes.52 Octahedral geometry for this complex is further supported by the magnetic moment value which calculated to be 4.63 B. M.
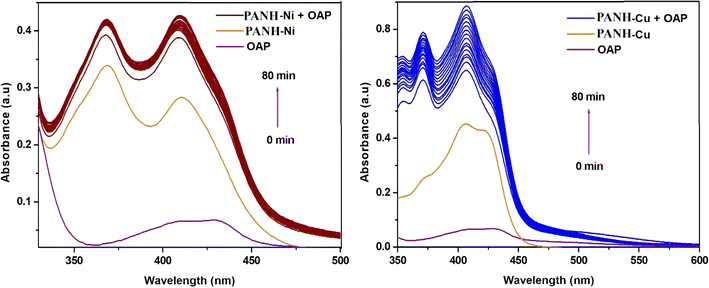
Octahedral arrangement around the Ni(II) centre in PANH-Ni complex was concluded by the appearance of the spectral peaks centred at 520 and 438 nm that attributed to the transitions of the type 3A2g(F) → 3T1g(F) (ν3) and 3A2g(F) → 3T1g(P) (ν2) specified for octahedral Ni(II) complexes.53 Magnetic moment value of 3.32 B. M. was found to PANH-Ni complex.
The spectrum of PANH-Cu complex represented a broad low-intensity absorption band cantered at 728 nm that assigned to the transition of the type 2B1g → 2A1g reported for square planar Cu(II) complexes in addition to the peak appearing to 417 nm characteristic for LMCT.54 Magnetic moment value of 1.84 B. M. was observed for this complex.
Collection of the results obtained above led to the prospected complexes' structures shown in Scheme 1.
3.7. Phenoxazinone synthase effectiveness and kinetic investigation
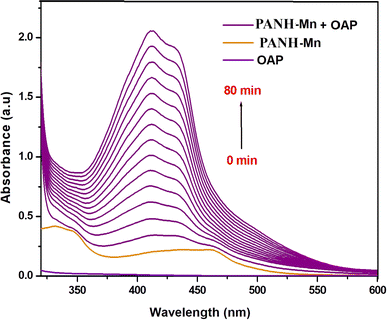
The existence of the metal complexes interested in this study in a solution containing OAP (o-aminophenol) led to its oxidation and generation of APX (2-aminophenoxazine-3-one). Such behaviour was assured by the generation and then increase in the absorbance of APX peak with time. Such band emerged in complexes' spectra at 430, 435, 433 and 428 nm for PANH-Mn, PANH-Co, PANH-Ni and PANH-Cu complexes, successively. Thus, the appearance of such band is evidence for the complexes phenoxazinone synthase catalytic efficacy. For PANH-Cr, the growing up of such band was not observed with time ensuring that it is not active as a catalyst for such catalytic reaction. The time-dependent spectra of complexes PANH-Mn, PANH-Co, PANH-Ni and PANH-Cu with complexes concentration of 3 × 10−5 M and 10−2 M concentration of OAP in DMF medium and aerobic conditions are given in Fig. 8–10. For these experiments, time began when OAP was added to the complexes' solutions, and the compounds' spectra were taken every five minutes up to seventy minutes after that. There was no peak observed for (blank experiment). The observed rate constant (kobs) and the initial rate of response (Vo) were determined by using the initial rate method upon using ε(APX) equals 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−155 (Table 5).
300 M−1 cm−155 (Table 5).
| Comp. code | Kobs (min−1) | V (M min−1) | Vmax (M min−1) | KM (M) | kcat (h−1) |
|---|---|---|---|---|---|
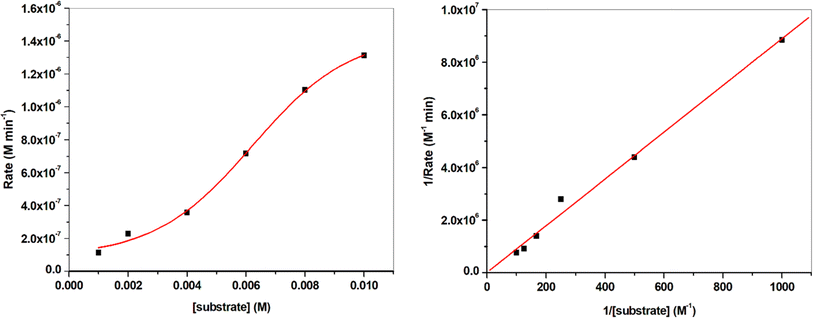
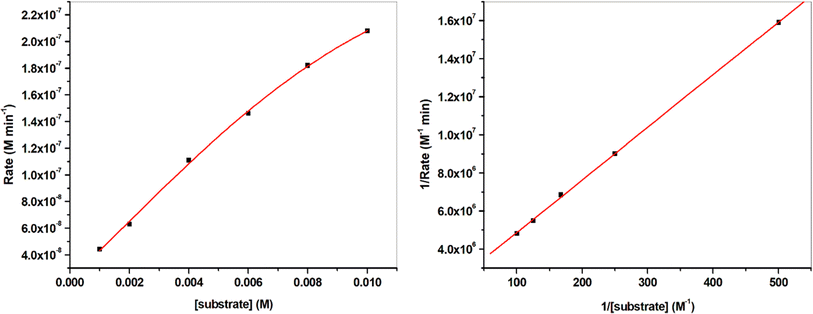
| PANH-Mn | 0.02401 | 1.31 × 10−6 | 8.49 × 10−5 | 0.0754 | 169.89 |
| PANH-Co | 0.0038 | 2.07 × 10−7 | 4.74 × 10−7 | 1.31 × 10−2 | 0.948 |
| PANH-Ni | 4.241 × 10−4 | 2.32 × 10−8 | 2.398 × 10−8 | 6.73 × 10−4 | 0.048 |
| PANH-Cu | 0.0066 | 3.61 × 10−7 | 4.461 × 10−7 | 2.704 × 10−3 | 0.892 |
Kinetics studies were implemented in order to investigate the extent of activity of the metal chelates under study with respect to each other and with respect to reported compounds. This study was done using a concentration of 3 × 10−5 M of the catalyst. To this concentration of the catalyst, alternative substrate concentrations in the range of (1–8) × 10−3 M were separately added. The generates mixtures were subjected to UV-vis spectral recording once the catalyst and the substrate were mixed and after each 3 minutes until the time reached 21 minutes. The absorbances of the generated APX were recorded to calculate the initial rate for the catalytic mixtures. Saturation kinetics were deduced from the shape of the plot of substrate concentrations ([S]) against initial rate (Vo) as obvious in Fig. 11 & 12 demonstrating the pre-equilibrium formation of the complex-substrate intermediate. The rate-determining stage for the catalytic cycle is the irreversible substrate oxidation that developed APX as the product. Such mechanism is well described by the equation:
Since the tested catalysts led to saturation kinetics, so, the most suitable equation for treating these reactions is the Michaelis–Menten equation.34 Application of this equation lead to the calculation of the kinetic parameters Vmax, KM and the turnover number (kcat, h−1); Table 5.
Comparison of the results collected in Table 5 demonstrated that the Mn(II) complex PANH-Mn exhibited dramatically high activity in comparison with the rest of examined complexes.
Comparison of the activity of the applied complexes based on the obtained results demonstrated that PANH-Cr chelate did not show any activity for catalytic the interested reaction (i.e. oxidation dimerization of OAP). On the other hand, PANH-Mn complex afforded dramatically high activity in comparison with the rest of examined complexes as observed form its kcat value of 169.89 h−1. The other investigated complexes afforded relatively low activity with kcat in the range 0.048–0.948 h−1.
It is noted that for APX to be produced by the oxidation of OAP by the catalytic action of metal complexes, molecular dioxygen must be present because there almost ever arises a peak of APX in a similar pattern of behavior in the published work.56,57 So, the prospected mechanism is shown in Scheme 2, taking the structure of the most efficient PANH-Mn complex as a model.28
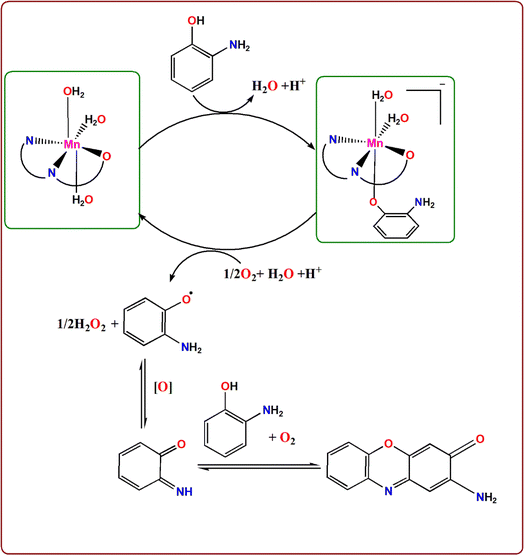 | ||
| Scheme 2 proposed route for the conversion of OAP to APX by oxidative dimerization catalysed by PANH-Mn. | ||
Since all the investigated complexes are formed with the same ligand, so the key factors affecting their activity are related to the metal centres incorporated in the complexes' structure, their type and oxidation state. Likening the efficacy of the complexes with respect to each other revealed that Cr(III) complex afforded no activity toward oxidation of OAP while the highest activity was exhibited by Mn(II) chelate. For the rest of complexes, the order of activity was PANH-Co > PANH-Cu > PANH-Ni. Precise realization of the complexes' structures also led to notice that all the active complexes contained chloro ligand in their structure while the inactive chromium complex contained acetate ligand in its coordination sphere leading to conclude that the coordinated anion type is also among the factors with dominant the catalysts' efficiency. This behavior can be explained by the accessibility of the first coordination sphere's labile sites, which is preferable for chloro anion over acetate group.34 Also, the steric influence caused by the CH3COO− group's bulkiness relative to Cl− affect the complexes' reactivity.
4. Conclusion
The trivalent Cr and the divalent Mn, Co, Ni and Cu chelates of the designed Schiff base ligand hydroxy-phenyl-acetic acid (2-hydroxy-naphthalen-1-ylmethylene)-hydrazide have been synthesized and investigated. Successful structure confirmation of the ligand and its metal chelates was achieved by the aid of 1H-NMR, molar conductivity, FTIR, mass, UV-vis spectra, elemental analysis and magnetic moment in addition to analytical results. The empirical formulas of the target metal complexes have been assured to be: [Cr(PANH)(AOc)2H2O]·H2O, [Mn(PANH)(H2O)3]·Cl·2H2O, [Co(PANH)Cl(H2O)2]·3H2O, [Ni(PANH)(H2O)3]·Cl·2H2O and [Cu(PANH)] in which the complexes PANH-Cr, PANH-Mn, PANH-Co, PANH-Ni have been formed with the molar ratio 1 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M whereas PANH-Cu complex was formed in the ratio 2 L
1 M whereas PANH-Cu complex was formed in the ratio 2 L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 M. Molar conductivity values showed that all the complexes have non-electrolytic nature except PANH-Mn and PANH-Ni complexes are 1
1 M. Molar conductivity values showed that all the complexes have non-electrolytic nature except PANH-Mn and PANH-Ni complexes are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolyte. From the results of FTIR spectra, the ligand has been confirmed to act as monobasic bidentate in PANH-Cu complex and monobasic tridentate in the structure of the rest complexes. Octahedral geometry was confirmed to all the complexes except PANH-Cu complex which assured to has square planar geometry as concluded from UV-vis spectral results and magnetic moment. The complexes were applied to estimate their activity in oxidation coupling of o-aminophenol (OAP) to 2-aminophenoxazine-3-one (APX) revealing an extremely high activity for the Mn(II) complex PANH-Mn with kcat value of 169.89 h−1. The other complexes afforded low efficiency with TOF numbers within 0.048–0.948 h−1 range while PANH-Cr complex afforded no catalytic activity. The other examined complexes afforded relatively low activity in comparison to Mn(II) complex. The uneven catalytic behavior of the metal complex can be explained on the basis of different metal center included in the complexes' structure (i.e., Cr, Mn, Co, Ni and Cu), different oxidation states of metal centers (i.e. +2 or +3) and the different surrounding environments concerned with different coordinating anions (i.e. chloro or acetate).
1 electrolyte. From the results of FTIR spectra, the ligand has been confirmed to act as monobasic bidentate in PANH-Cu complex and monobasic tridentate in the structure of the rest complexes. Octahedral geometry was confirmed to all the complexes except PANH-Cu complex which assured to has square planar geometry as concluded from UV-vis spectral results and magnetic moment. The complexes were applied to estimate their activity in oxidation coupling of o-aminophenol (OAP) to 2-aminophenoxazine-3-one (APX) revealing an extremely high activity for the Mn(II) complex PANH-Mn with kcat value of 169.89 h−1. The other complexes afforded low efficiency with TOF numbers within 0.048–0.948 h−1 range while PANH-Cr complex afforded no catalytic activity. The other examined complexes afforded relatively low activity in comparison to Mn(II) complex. The uneven catalytic behavior of the metal complex can be explained on the basis of different metal center included in the complexes' structure (i.e., Cr, Mn, Co, Ni and Cu), different oxidation states of metal centers (i.e. +2 or +3) and the different surrounding environments concerned with different coordinating anions (i.e. chloro or acetate).
Conflicts of interest
There are no conflicts to declare.References
- M. M. E. Shakdofa, M. H. Shtaiwi and N. Morsy, Metal complexes of hydrazones and their biological, analytical and catalytic applications: A review, Main Group Chem., 2014, 13, 187–218 CAS.
- Y. S. Moroz, K. Kulon, M. Haukka, E. Gumienna-Kontecka, H. Kozłowski, F. Meyer and I. O. Fritsky, Synthesis and structure of [2 x 2] molecular grid copper(II) and nickel(II) complexes with a new polydentate oxime-containing Schiff base ligand, Inorg. Chem., 2008, 47, 5656–5665 CrossRef CAS PubMed.
- M. D. K. Hossain, M. O. Plutenko, J. A. Schachner, M. Haukka, N. C. Mosch-Zanetti, I. O. Fritsky and E. Nordlander, Dioxomolybdenum(VI) complexes of hydrazone phenolate ligands - syntheses and activities in catalytic oxidation reactions, J. Indian Chem. Soc., 2021, 98, 100006 CrossRef CAS.
- B. Oinam Chanu, A. Kumar, A. Ahmed and R. A. Lal, Synthesis and characterisation of heterometallic trinuclear copper(II) and zinc(II) complexes derived from bis(2-hydroxy-1-naphthaldehyde) oxaloyldihydrazone, J. Mol. Struct., 2012, 1007, 257–274 CrossRef.
- S. A. Yasrebi, H. Mobasheri, I. Sheikhshoaie and M. Rahban, DNA-binding studies of two dioxomolybdenum(VI) complexes of salicylaldehyde benzoylhydrazone ligands, Inorg. Chim. Acta, 2013, 400, 222–227 CrossRef CAS.
- A. A. R. Despaigne, F. B. Da Costa, O. E. Piro, E. E. Castellano, S. R. W. Louro and H. Beraldo, Complexation of 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones to copper(II) as an effective strategy for antimicrobial activity improvement, Polyhedron, 2012, 38(1), 285–290 CrossRef.
- Q. C. Ton, M. Bolte and E. Egerta, Structural similarities among eight benzoylhydrazones, Acta Crystallogr., 2014, C70, 912–919 Search PubMed.
- R. M. Issa, S. A. Abdel-Latif and H. A. Abdel-Salam, Synthesis and characterization of new Cu(II) complexes derived from benzilic and mandelic hydrazones, Synth. React. Inorg. Met.-Org. Chem., 2001, 31(1), 95–105 CrossRef CAS.
- M. Padmaja, J. Pragathi and C. G. Kumari, Synthesis, spectral characterization, molecular modeling and biological activity of first row transition metal complexes with Schiff base ligand derived from chromone-3-carbaldehyde and o-amino benzoic acid, J. Chem. Pharm. Res., 2011, 3, 602–613 CAS.
- K. Kiranmai, Y. Prashanthi and N. Subhashini, Synthesis, characterization and biological activity of metal complexes of 3-amino-5-methyl isoxazole Schiff bases, J. Chem. Pharm. Res., 2010, 2, 375–384 CAS.
- A. D. M. Mohamad, M. J. A. Abualreish and A. M. Abu-Dief, Antimicrobial and anticancer activities of cobalt (III)-hydrazone complexes: Solubilities and chemical potentials of transfer in different organic co-solvent-water mixtures, J. Mol. Liq., 2019, 290, 111162 CrossRef CAS.
- A. M. Abu-Dief, R. M. El-khatib, S. M. El Sayed, S. Alzahrani, F. Alkhatib, G. El-Sarrag and M. Ismael, Tailoring, structural elucidation, DFT calculation, DNA interaction and pharmaceutical applications of some aryl hydrazone Mn(II), Cu(II) and Fe(III) complexes, J. Mol. Struct., 2021, 1244, 131017 CrossRef CAS.
- M. A. Jabeen, Comprehensive Review on Analytical Applications of Hydrazone Derivatives, J. Turk. Chem. Soc., Sect. A, 2022, 9(3), 663–698 CrossRef CAS.
- M. Bagherzadeh, M. Zare, V. Amani, A. Ellern and L. K. Woob, Dioxo and oxo-peroxo molybdenum(VI) complexes bearing salicylidene 2-picoloyl hydrazone: Structures and catalytic performances, Polyhedron, 2013, 53, 223–229 CrossRef CAS.
- O. Pouralimardan, A. C. Chamayou, C. Janiak and H. Hosseini-Monfared, Hydrazone Schiff base-manganese(II) complexes: Synthesis, crystal structure and catalytic reactivity, Inorg. Chim. Acta, 2007, 360, 1599–1608 CrossRef CAS.
- S. Balgotra, P. K. Verma, R. A. Vishwakarma and S. D. Sawant, Catalytic advances in direct functionalizations using arylated hydrazines as the building blocks, Catal. Rev., 2019, 62, 406–479 CrossRef.
- W. Xiao, Z. L. Lu, C. Y. Su, K. B. Yu, L. R. Deng, H. Q. Liu and B. S. Kang, Bivalent transition metal complexes of 4,5-diazafluorene-9-one benzoylhydrazone (HL) and the characterization of weak interaction in CoL2(H2O)2, J. Mol. Struct., 2000, 553, 91–99 CrossRef CAS.
- J. Patole, U. Sandbhor, S. Padhye, D. N. Deobagkar, C. E. Anson and A. Powell, Structural chemistry and In vitro antitubercular activity of acetylpyridine benzoyl hydrazone and its copper complex against Mycobacterium smegmatis, Bioorg. Med. Chem. Lett., 2003, 13, 51–55 CrossRef CAS PubMed.
- M. Yamamoto, K. Sakurai, A. Eguchi, S. Yamazaki, S. F. Nakayama, T. Isobe, A. Takeuchi, T. Sato, A. Hata, C. Mori and H. Nitta, Association between blood manganese level during pregnancy and birth size: The Japan environment and children's study (JECS), Environ. Res., 2019, 172, 117–126 CrossRef CAS PubMed.
- S. Vaddypally, S. K. Kondaveeti and M. J. Zdilla, Synthesis of a high-valent, four-coordinate manganese cubane cluster with a pendant Mn atom: photosystem II-inspired manganese–nitrogen clusters, Inorg. Chem., 2012, 51, 3950–3952 CrossRef CAS PubMed.
- M. M. Najafpour, B. Pashaeia and Z. Zanda, Photodamage of the manganese–calcium oxide: a model for UV-induced photodamage of the water oxidizing complex in photosystem II, Dalton Trans., 2013, 42, 4772–4776 RSC.
- N. Schuth, Z. Liang, M. Schönborn, A. Kussicke, R. Assunção, I. Zaharieva, Y. Zilliges and H. Dau, Inhibitory and Non-Inhibitory NH3 Binding at the Water-Oxidizing Manganese Complex of Photosystem II Suggests Possible Sites and a Rearrangement Mode of Substrate Water Molecules, Biochemistry, 2017, 56, 6240–6256 CrossRef CAS PubMed.
- S. Vaddypally, S. K. Kondaveeti, J. H. Roudebush, R. J. Cava and M. J. Zdilla, Formation of the tetranuclear, tetrakis-terminal-imido Mn 4 IV (N t Bu) 8 cubane cluster by four-electron reductive elimination of t BuN [double bond, length as m-dash] N t Bu. The role of the s-block ion in stabilization of high-oxidation state intermediates, Chem. Commun., 2014, 50, 1061–1063 RSC.
- S. Vaddypally, S. K. Kondaveeti, S. Karki, M. M. V. Vliet, R. J. Levis and M. J. Zdilla, Reactive Pendant Mn
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in a Synthetic Structural Model of a Proposed S4 State in the Photosynthetic Oxygen Evolving Complex, J. Am. Chem. Soc., 2017, 139, 4675–4681 CrossRef CAS PubMed.
O in a Synthetic Structural Model of a Proposed S4 State in the Photosynthetic Oxygen Evolving Complex, J. Am. Chem. Soc., 2017, 139, 4675–4681 CrossRef CAS PubMed. - S. Vaddypally, D. J. Jovinelli, I. G. McKendry and M. J. Zdilla, Covalent Metal–Metal-Bonded Mn4 Tetrahedron Inscribed within a Four-Coordinate Manganese Cubane Cluster, As Evidenced by Unexpected Temperature-Independent Diamagnetism, Inorg. Chem., 2017, 56, 3733–3737 CrossRef CAS PubMed.
- Y. Kim, S. Das, S. Bhattacharya, S. Hong, M. G. Kim, M. Yoon, S. Natarajan and K. Kim, Metal-Ion Metathesis in Metal–Organic Frameworks: A Synthetic Route to New Metal–Organic Frameworks, Chem.–Eur. J., 2012, 18, 16642–16648 CrossRef CAS PubMed.
- A. Das, M. Chakraborty, S. Maity and A. Ghosh, The catalytic activities and magnetic behaviours of rare μ3-chlorido and μ1,1,1-azido bridged defective dicubane tetranuclear Mn(II) complexes, Dalton Trans., 2019, 48, 9342–9356 RSC.
- S. Banerjee, P. Brandão, A. Bauzá, A. Frontera, M. Barceló-Oliver, A. Panja and A. Saha, Nuclearity versus oxidation state in the catalytic efficiency of MnII/III azo Schiff base complexes: computational study on supramolecular interactions and phenoxazinone synthase-like activity, New J. Chem., 2017, 41, 11607–11618 RSC.
- S. Ganguly, P. Kar, M. Chakraborty and A. Ghosh, The First Alternating MnII-MnIII 1D Chain: Structure, Magnetic Properties and Catalytic Oxidase Activities, New J. Chem., 2018, 42, 9517–9529 RSC.
- A. Panja, Metal ionic size directed complexation in manganese(II) coordination chemistry: efficient candidates showing phenoxazinone synthase mimicking activity, RSC Adv., 2014, 4, 37085–37094 RSC.
- S. K. Fathalla, H. A. El-Ghamry and M. Gaber, Ru(III) complexes of triazole based Schiff base and azo dye ligands: An insight into the molecular structure and catalytic role in oxidative dimerization of 2-aminophenol, Inorg. Chem. Commun., 2021, 129, 108616 CrossRef CAS.
- A. Panja, M. Shyamal, A. Saha and T. K. Mandal, Methylene bridge regulated geometrical preferences of ligands in cobalt(III) coordination chemistry and phenoxazinone synthase mimicking activity, Dalton Trans., 2014, 43, 5443–5452 RSC.
- S. Dasgupta, J. Adhikary, S. Giri, A. Bauza, A. Frontera and D. Das, Unveiling the effects of in situ generated arene anion radical and imine radical on catecholase like activity: A DFT supported experimental investigation, Dalton Trans., 2017, 46, 5888–5900 RSC.
- H. A. El-Ghamry, B. K. Alharbi, K. M. Takroni and A. M. Khedr, A series of nanosized Cu(II) complexes based on sulfonamide azo dye ligands: An insight into complexes molecular structures, antimicrobial, antitumor and catalytic performance for oxidative dimerization of 2-aminophenol, Appl. Organomet. Chem., 2023, 37, e6978 CrossRef CAS.
- A. Panja, Exclusive selectivity of multidentate ligands independent on the oxidation state of cobalt: influence of steric hindrance on dioxygen binding and phenoxazinone synthase activity, Dalton Trans., 2014, 43, 7760–7770 RSC.
- Y. M. Ahn, L. Vogeti, C.-J. Liu, H. K. R. Santhapuram, J. M. White, V. Vasandani, L. A. Mitscher, G. H. Lushington, P. R. Hanson, P. R. Hanson, D. R. Powell, R. H. Himes, K. F. Roby, Q. Ye and G. I. Georg, Design, synthesis, and antiproliferative and CDK2-cyclin a inhibitory activity of novel flavopiridol analogues, Bioorg. Med. Chem., 2007, 15, 702–713 CrossRef CAS PubMed.
- T. Akhtar, S. Hameed, K. M. Khan, A. Khan and M. I. Choudhary, Design, synthesis, and urease inhibition studies of some 1,3,4-oxadiazoles and 1,2,4-triazoles derived from mandelic acid, J. Enzyme Inhib. Med. Chem., 2010, 25(4), 572–576 CrossRef CAS PubMed.
- S. M. Sparks, C.-L. Chen and S. F. Martin, Tandem intramolecular benzyne–furan cycloadditions. Total synthesis of vineomycinone B2 methyl ester, Tetrahedron, 2007, 63, 8619–8635 CrossRef CAS PubMed.
- W. J. Geary, The use of conductivity measurements in organic solvents for the characterisation of coordination compounds, Coord. Chem. Rev., 1971, 7, 81–122 CrossRef CAS.
- I. A. Waseem, A. Wani and K. Saleem, Empirical formulae to molecular structures of metal complexes by molar conductance, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2013, 43, 1162–1170 CrossRef.
- K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1986 Search PubMed.
- H. A. El-ghamry, K. M. Takroni, D. O. AL-Rashidi, E. S. Alfear and R. A. Alsaedi, Design, spectral, thermal decomposition, antimicrobial, docking simulation and DNA binding tendency of sulfisoxazole azo dye derivative and its metal chelates with Mn2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+ and Cd2+, Appl. Organomet. Chem., 2022, 36, 1 CrossRef.
- M. Gaber, S. K. Fathalla and H. A. El-Ghamry, 2,4-Dihydroxy-5-[(5-mercapto-1H -1,2,4-triazole-3-yl)diazenyl]benzaldehyde acetato, chloro and nitrato Cu(II) complexes: Synthesis, structural characterization, DNA binding and anticancer and antimicrobial activity, Appl. Organomet. Chem., 2019, 33, e4707 Search PubMed.
- K. El-Baradie, R. El-Sharkawy, H. El-Ghamry and K. Sakai, Synthesis and characterization of Cu(II), Co(II) and Ni(II) complexes of a number of sulfadrug azodyes and their application for wastewater treatment, Spectrochim. Acta, Part A, 2014, 121, 180–187 CrossRef CAS PubMed.
- A. W. Coats and J. Redfern, Kinetic Parameters from Thermogravimetric Data, Nature, 1964, 201, 68–69 Search PubMed.
- A. A. Frost, R. G. Pearson, Kinetics and Mechanisms, Inc., New York, 1961, vol. 19 Search PubMed.
- U. El-Ayaan, I. Kenawy and Y. A. El-Reash, Synthesis, thermal and spectral studies of first-row transition metal complexes with Girard P reagent-based ligand, Spectrochim. Acta, Part A, 2007, 68, 211–219 CrossRef PubMed.
- N. H. Yarkandi, H. A. El-Ghamry and M. Gaber, Synthesis, spectroscopic and DNA binding ability of CoII, NiII, CuII and ZnII complexes of Schiff base ligand (E)-1-(((1H-benzo[d]imidazole-2-yl)methylimino)methyl)naphthalen-2-ol. X-ray crystal structure determination of cobalt (II) complex, Mater. Sci. Eng., C, 2017, 75, 1059 CrossRef CAS PubMed.
- G. B. Deacon and R. J. Phillips, Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination, Coord. Chem. Rev., 1980, 33, 227–250 CrossRef CAS.
- M. S. Refata, T. Altalhia, S. B. Bakareb, G. H. Al-Hazmic and K. Alam, New Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Zn(II), Cd(II), and Hg(II) Gibberellate Complexes: Synthesis, Structure, and Inhibitory Activity Against COVID-19 Protease, Russ. J. Gen. Chem., 2021, 91(5), 890–896 CrossRef PubMed.
- F. A. Cotton, G. Wilkinson, C. A. Murillo, M. Bochmann, Advanced Inorganic Chemistry, Wiley, New York, 6th edn, 1999 Search PubMed.
- M. Gaber, H. A. El-Ghamry and S. K. Fathalla, Synthesis, structural identification, DNA interaction and biological studies of divalent Mn, Co and Ni chelates of 3-amino-5-mercapto-1,2,4-triazole azo ligand, Appl. Organomet. Chem., 2020, 34, e5678 CrossRef CAS.
- A. B. P. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 2nd edn, 1984 Search PubMed.
- G. I. Mohammed, H. A. El-Ghamry and A. L. Saber, Rapid, sensitive, and selective copper (II) determination using sensitive chromogenic azo dye based on sulfonamide, Spectrochim. Acta, Part A, 2021, 247, 119103 CrossRef CAS PubMed.
- L. Gasque, A. Mendieta and G. Ferrer-Sueta, Comment on “Mixed azido/phenoxido bridged trinuclear Cu(ii) complexes of Mannich bases: synthesis, structures, magnetic properties and catalytic oxidase activities”, Dalton Trans., 2018, 47, 9385–9399 Search PubMed; L. Gasque, A. Mendieta and G. Ferrer-Sueta, Tri- and hexa-nuclear NiII–MnII complexes of a N2O2 donor unsymmetrical ligand: synthesis, structures, magnetic properties and catalytic oxidase activities, Dalton Trans., 2018, 47, 13957–13971 (Dalton Trans., 2020, 49, 3365–3368) RSC.
- W. P. Sohtun, S. Muthuramalingam, M. Sankaralingam, M. Velusamy and R. Mayilmurugan, Copper(II) complexes of tripodal ligand scaffold (N3O) as functional models for phenoxazinone synthase, J. Inorg. Biochem., 2021, 216, 111313 CrossRef CAS PubMed.
- A. Mandal, A. Sarkar, A. Adhikary, D. Samanta and D. Das, Structure and Synthesis of Copper Based Schiff base and Reduced Schiff base Complex: A Combined Experimental and Theoretical Investigation of Biomimetic Catalytic Activity, Dalton Trans., 2020, 49, 15461–15472 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05769d |
| This journal is © The Royal Society of Chemistry 2024 |