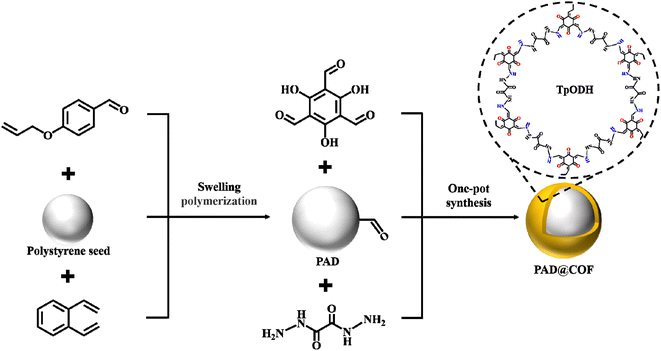
Open Access Article
Open Access ArticleFabrication of monodisperse micron-sized and aldehyde-functionalized microspheres coating with covalent organic framework for efficient and rapid removal of copper ions from wastewater†
Xiaoqiong Wanga,
Qingyan Baia,
Mingjia Yana,
Yashuai Zhaoa,
Shujuan Mac,
Chunmiao Bo *a and
Junjie Ou
*a and
Junjie Ou *bc
*bc
aSchool of Chemistry and Chemical Engineering, Ningxia Key Laboratory of Solar Chemical Conversion Technology, Key Laboratory for Chemical Engineering and Technology, State Ethnic Affairs Commission, North Minzu University, Yinchuan, 750021, China. E-mail: bocm-001@163.com; Fax: +86-0951-2067917; Tel: +86-0951-2067917
bCollege of Chemistry and Materials Science, Northwest University Xi'an, 710127, China. E-mail: junjieou@nwu.edu.cn; junjieou@dicp.ac.cn; Fax: +86-29-81535026; Tel: +86-29-81535026
cCAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China
First published on 24th October 2024
Abstract
Covalent organic frameworks (COFs) possess an excellent ability for absorbing heavy metals, but their uneven particle size, difficult separation, and poor dispersion limit their wide application in the treatment of heavy metal pollution. In this paper, a monodisperse poly(4-allyloxybenzaldehyde-co-divinylbenzene) microsphere (denoted as PAD) was prepared with 4-allyloxybenzaldehyde as a functional monomer and divinylbenzene (DVB) as a crosslinker by one-step seed swelling polymerization. Subsequently, oxalyldihydrazide (ODH) and 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde (Tp) were chosen as the precursors for coating the COF layer onto the surface of PAD through a one-pot method. The resulting monodisperse particles (diameter = 6.3 μm) with a core–shell structure were assigned as PAD@COF and possessed excellent dispersibility in water along with a high specific surface area of 163.8 m2 g−1. In isothermal and dynamic adsorption experiments, the maximum adsorption capacity of Cu2+ reached 270.9 mg g−1, with the adsorption amount reaching 93 mg g−1 after only 10 min. The Langmuir isothermal adsorption model and pseudo-second-order kinetic model were consistent with the adsorption process, indicating that the adsorption of Cu2+ on PAD@COF occurred as a monolayer and that the adsorption process was controlled by chemical processes.
1 Introduction
Covalent organic frameworks (COFs), as porous materials constructed by the covalent bonds of organic units, were originally discovered in 2005.1 Due to their high specific surface area, controllable pore structure, and surface functionalization,2 COFs exhibit a wide range of application prospects in many fields such as gas adsorption and separation,3 energy storage,4 catalysis,5 desalination,6 sensing,7 detection,8 photoelectric applications9 and environmental purification.10 The highly interconnected structure of COFs provides a robust foundation for the development of adsorption materials with exceptional selectivity and superior adsorption properties. In recent years, COFs have been widely utilized in water remediation,11 organic pollutant adsorption,12 electrocatalysis,13 photocatalysis and other fields,14,15 and their synthesis has also been investigated.16,17 However, most current COF materials involve two-dimensional structures18 and lack regular morphology,19 making the molding problem of COF materials a significant challenge.The size and shape of COF components cannot be controlled under solvothermal conditions, often resulting in mixed shapes and grain sizes that are difficult to separate.20 A few microspheres or particles with controllable morphology were synthesized as the core, and then the COF layer was integrated onto the surfaces of these microspheres, thus improving the controllable morphology of COFs. For example, Gao et al. took Fe3O4 as the core and then made surface modifications for the selective enrichment of hydrophobic peptides.21 Xu and co-workers reported a core–shell composite of COF material grown on the surface of aminopropyl-functionalized SiO2 microspheres for enantiomeric separation by high-performance liquid chromatography.22 Additionally, the direct preparation method of spherical COF materials was developed. Ma and colleagues selected 2,5-divinylterephthalaldehyde and 1,3,5-tris(4-aminophenyl)benzene as precursors dissolved in acetonitrile and then added acetic acid at room temperature to synthesize a micron-scale spherical COF for the selective enrichment of hydrophobic peptides.23 Recently, Zhu et al. developed a polymerization-induced phase separation method for the controlled synthesis of imine-linked polymers, which was then crystallized to produce monolithic COF, expanding a new avenue for shape-controlled COF synthesis.24 More recently, Xu et al. successfully prepared surface-functionalized COF microspheres by the in situ linker exchange and applied them as stationary phase for chiral chromatography to achieve effective enantioseparation.25 However, these synthetic approaches of spherical or monolithic COF have not been widely applied, and the difficulty of synthesis should not be underestimated. At present, the organization of three-dimensional COF synthesis often involves secondary phases (polymers, silica, etc.) to provide templates or supports to stabilize the structure.26 In addition, because of the covalent mechanism of the synthesis process, many core–shell structured materials are irreversible once the reaction is carried out, and the materials lack reusable characteristics and are environmentally hazardous; thus, the recycling of materials is also a major focus of our attention. It is well known that the imide bond is a covalent bond formed by the condensation reaction of the aldehyde group and amino group, which contains certain reversibility.27 Under mild conditions, the imine bond can be hydrolyzed, resulting in the decomposition of the product into aldehyde and amino groups. This reversibility allows imine bonds to play an important role in some biochemical reactions and organic synthesis, facilitating the degradation or breakdown of materials into their original functional groups.
Herein, we selected 4-allyloxybenzaldehyde, a functional monomer containing an aldehyde group, to prepare micron-scale monodisperse microspheres by one-step seeded swelling polymerization. Subsequently, 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde (Tp) and oxalyldihydrazide (ODH) were selected to generate porous COF, which was grafted onto the surface of PAD through a one-pot method and named as PAD@COF. The novel composite PAD@COF not only improves the preparation efficiency of COF materials but also provides a new possibility for its application in adsorption separation and other aspects.
2 Experimental section
2.1 Reagents and materials
4-Allyloxybenzaldehyde (97%) was obtained from Alfa Aesar (Shanghai, China). Dibutyl phthalate (DBP), Tp and acetic acid were from Innochem (Beijing, China). Azodiisobutyronitrile (AIBN), styrene (St), divinylbenzene (DVB), polyvinyl alcohol (PVA), sodium dodecyl sulfate (SDS), ODH and n-butanol were purchased from Aladdin (Shanghai, China). Toluene and anhydrous ethanol were acquired from Beilian Fine Chemicals Development (Tianjin, China). Tetrahydrofuran (THF) was supplied by Jinshan Chemical Reagent (Chengdu, China). Other chemical reagents were of analytical grade.2.2 Synthesis of monodisperse poly(4-allyloxybenzaldehyde-co-divinylbenzene) microspheres
According to a previous report,28 polystyrene seeds were synthesized via dispersion polymerization. Monodisperse poly(4-allyloxybenzaldehyde-co-divinylbenzene) microspheres were synthesized by one-step seeded swelling polymerization using 4-allyloxybenzaldehyde as a functional monomer and DVB as the crosslinking agent. Typically, a certain volume of polystyrene seeds was placed in a round-bottomed flask for swelling at room temperature, followed by a certain volume of the water phase (SDS and PVA). After that, the oil phase was configured with a certain volume of 4-allyloxybenzaldehyde, DVB, toluene, DBP, and cyclohexanol, and AIBN was added and sonicated until it dissolved completely. The water and oil phases were mixed uniformly through ultrasonication until emulsification was completed and there were no oil droplets on the surface. The resultant solution, after cell disruption, was poured into a flask, sealed under nitrogen protection, and swollen at 25 °C for 24 h at a mechanical speed of 100 rpm, and then reacted at 70 °C for 24 h. After washing, the monodisperse poly(4-allyloxybenzaldehyde-co-divinylbenzene) microspheres were acquired and denoted as PAD.2.3 Surface modification of poly(4-allyloxybenzaldehyde-co-divinylbenzene) microspheres with the COF layer
Based on our previous result,29 ODH and Tp were selected as surface-modified monomers for monodisperse polymer microspheres. The monomer material with amine and aldehyde groups was grafted onto the surface of PAD. In brief, 36 mg ODH was added into 10 mL water and dispersed evenly by ultrasonication. Then, 42 mg Tp was weighed and placed in a mixture of 2.4 mL toluene, n-butanol, and glacial acetic acid (v/v/v = 5/5/2) and ultrasonically dispersed. Then, 135 mg monodisperse PAD was placed in 5 mL water for ultrasonic dispersion, and the condensation reflux reaction was carried out at 120 °C by magnetic agitation for 24 h. After the precipitate was centrifuged with THF and anhydrous ethanol two times, the washed solid precipitate was freeze-dried for 12 h, and the dried powder products were collected and weighed for preservation. The dried product was denoted as PAD@COF.For comparison, an ampoule bottle of dimensions 10 mm (o.d.) × 5 mm (i.d.) was filled with ODH (36 mg), Tp (42 mg), 0.5 mL of anhydrous dioxane and 0.5 mL of glacial acetic acid. After ultrasonic dispersion for 30 min and sealing with a flame, the reactants were heated at 120 °C for 48 h. The washed and dried product was denoted as COF.
2.4 Evaluation of reusability
The cyclic stability of the material was assessed through adsorption–desorption experiments by ultrasonically washing the adsorbed material with 0.1 mol L−1 HCl, drying the precipitate, and reusing it for copper ion adsorption under the same conditions for 10 cycles.2.5 Determination of PAD@COF in real samples
The adsorption capability of PAD@COF for Cu2+ in actual samples was measured. Lake water (Ming Lake, Yinchuan, China) was chosen as the sample. After centrifugal, filtration and the addition of Cu2+, a water sample containing Cu2+ (3.5 mg L−1) was obtained. Then, 8 mg of PAD@COF were mixed with 100 mL of this water sample. After shaking at room temperature for 1 h, the supernatant was collected to measure the concentration of Cu2+ and calculate the adsorption amount.3 Results and discussion
3.1 Design and synthesis of monodisperse aldehyde-functionalized microspheres (PAD)
A monomer of 4-allylbenzaldehyde containing an aldehyde group was selected to synthesize monodisperse microspheres by a one-step seed swelling polymerization method. Given the reversible imine bonds, the synthesized microspheres containing the aldehyde group were expected to integrate with the COF-containing terminal amine group to synthesize core–shell COF microspheres, as shown in Fig. 1. Due to the reversible condensation reaction between the amino group and the aldehyde group, the imine bond can be hydrolyzed for suitable degradation so that the product can be decomposed into the aldehyde group and the amino group again. Therefore, the resulting microspheres are green and environment friendly and can be reused.In this case, polystyrene seeds were successfully prepared via dispersion polymerization. The polystyrene seed exhibited outstanding monodispersity and smooth surface characteristics, and the average diameter of seed particle size was about 3 μm (Fig. S1†). To begin with, the oil phase consisted of 4-allyloxybenzaldehyde, DVB, and porogenic solvents, while the aqueous solution contained 5% PVA and 0.2% SDS. After the swelling and polymerization reaction, the resulting PAD resins with different morphologies were successfully prepared. Eventually, the mass ratio of the seed to the total monomers, the molar ratio of the monomer to the crosslinker, different binary porogenic systems (DBP-toluene system, toluene–cyclohexanol system, and DBP–cyclohexanol system) and the ratio of the oil–water phase on the resin morphology were carefully investigated, and the specific synthetic parameters are listed in Table 1.
| Materials | Seed/total monomers (mg/mg) | Monomer/cross-linking agent (n/n) | Porogenic agent system (v/v) | Oil/water (v/v) | Size (μm) | Productivity (%) |
|---|---|---|---|---|---|---|
a In these cases, the volume ratio of binary porogenic agents (DBP/toluene, toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol, DBP cyclohexanol, DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol) was 1 cyclohexanol) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. “—” indicates uneven size. For PAD-12, five times the amount was added compared to PAD-9, and similarly, for PAD-16 compared to PAD-15. 1. “—” indicates uneven size. For PAD-12, five times the amount was added compared to PAD-9, and similarly, for PAD-16 compared to PAD-15. |
||||||
| PAD-1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
2.4 | 7.0 |
| PAD-2 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
6.9 | 6.6 |
| PAD-3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
6.1 | 30.7 |
| PAD-4 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
2.6 | 55.1 |
| PAD-5 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
7.2 | 26.5 |
| PAD-6 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
5.5 | 42.8 |
| PAD-7 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
6.8 | 37.5 |
| PAD-8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene toluene |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
— | 41.9 |
| PAD-9 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
5.9 | 19.9 |
| PAD-10 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
5.9 | 14.9 |
| PAD-11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
5.4 | 18.9 |
| PAD-12 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
7.1 | 33.2 |
| PAD-13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
6.6 | 35.3 |
| PAD-14 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
— | 9.5 |
| PAD-15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
5.1 | 21.4 |
| PAD-16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
6.2 | 39.5 |
| PAD-17 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
6.4 | 39.2 |
| PAD-18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol cyclohexanol |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
6.3 | 35.6 |
When the monomer/crosslinker (n/n) was almost 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the amount of polystyrene seed was 233 mg, and the oil–water volume ratio was maintained at 3
1, the amount of polystyrene seed was 233 mg, and the oil–water volume ratio was maintained at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. As presented in Fig. 2a, PAD-1 with a broken spherical shape was generated, only a few retained the spherical structures with a particle size of 2.4 μm, and the final yield was only 7.0%. The mass ratio of the seed/total monomers remained 3
25. As presented in Fig. 2a, PAD-1 with a broken spherical shape was generated, only a few retained the spherical structures with a particle size of 2.4 μm, and the final yield was only 7.0%. The mass ratio of the seed/total monomers remained 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, PAD-2 possessed a spherical shape but a few small particles adhered on its surface and gave uneven morphology, the particle size of the sphere was 6.9 μm (Fig. S2a†), and the yield was only 6.6%. It was inferred that the seeds could not fully absorb the oil phase due to the reduction of the seed amount, and the self-polymerization of the excess crosslinking agent and monomer would produce more small particles. The following experiments were attempted to be improved by changing the proportion of the functional monomer and crosslinker. When the amount of functional monomer to crosslinker was 7
10, PAD-2 possessed a spherical shape but a few small particles adhered on its surface and gave uneven morphology, the particle size of the sphere was 6.9 μm (Fig. S2a†), and the yield was only 6.6%. It was inferred that the seeds could not fully absorb the oil phase due to the reduction of the seed amount, and the self-polymerization of the excess crosslinking agent and monomer would produce more small particles. The following experiments were attempted to be improved by changing the proportion of the functional monomer and crosslinker. When the amount of functional monomer to crosslinker was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the surface adhesion of PAD-3 was reduced but the shape was not uniform, as depicted in Fig. S2b.† The self-polymerization of the functional monomer and crosslinker occurred around the product; nevertheless, the yield increased significantly to 30.7%. When monomer/crosslinker (n/n) was almost 1
5, the surface adhesion of PAD-3 was reduced but the shape was not uniform, as depicted in Fig. S2b.† The self-polymerization of the functional monomer and crosslinker occurred around the product; nevertheless, the yield increased significantly to 30.7%. When monomer/crosslinker (n/n) was almost 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the resulting PAD-4 was uniformly spherical. However, compared to the initial polystyrene seeds (Fig. S1†), the microsphere size was only 2.6 μm (Fig. S2c†), indicating that the seeds did not expand successfully. The oil phase cannot be completely absorbed due to the lack of the water phase, and most of the resins are self-polymerized precursors. As a result, the oil–water volume ratio was changed to 3
1, the resulting PAD-4 was uniformly spherical. However, compared to the initial polystyrene seeds (Fig. S1†), the microsphere size was only 2.6 μm (Fig. S2c†), indicating that the seeds did not expand successfully. The oil phase cannot be completely absorbed due to the lack of the water phase, and most of the resins are self-polymerized precursors. As a result, the oil–water volume ratio was changed to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and the monomer/crosslinker (n/n) was almost 1
50 and the monomer/crosslinker (n/n) was almost 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As depicted in Fig. S2d,† the prepared PAD-5 resin exhibited a uniform spherical structure, but the particle size of the spherical resin reached 7.2 μm, and the final yield was 26.5%. Subsequently, the amount of polystyrene seed was doubled (PAD-6), the size of the acquired microspheres was reduced to 5.5 μm (Fig. 2b), the product adhesion slowed down, but the surface of the microspheres became rougher, whose yield reached 42.8%. By changing the total amount of monomers (4-allyloxybenzaldehyde and DVB), the acquired PAD-7 (Fig. S2e†) and PAD-8 (Fig. S2f†) were not satisfactory. In short, these results demonstrated that the DBP–toluene system is not suitable for the fabrication of the PAD spheres.
1. As depicted in Fig. S2d,† the prepared PAD-5 resin exhibited a uniform spherical structure, but the particle size of the spherical resin reached 7.2 μm, and the final yield was 26.5%. Subsequently, the amount of polystyrene seed was doubled (PAD-6), the size of the acquired microspheres was reduced to 5.5 μm (Fig. 2b), the product adhesion slowed down, but the surface of the microspheres became rougher, whose yield reached 42.8%. By changing the total amount of monomers (4-allyloxybenzaldehyde and DVB), the acquired PAD-7 (Fig. S2e†) and PAD-8 (Fig. S2f†) were not satisfactory. In short, these results demonstrated that the DBP–toluene system is not suitable for the fabrication of the PAD spheres.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and the morphology of the product was controlled by changing the dosage of polystyrene seeds or the monomer/crosslinker (n/n). PAD-9 was obtained with the monomer/crosslinker (n/n) ratio of almost 1
50, and the morphology of the product was controlled by changing the dosage of polystyrene seeds or the monomer/crosslinker (n/n). PAD-9 was obtained with the monomer/crosslinker (n/n) ratio of almost 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, when the volume ratio of cyclohexanol/toluene was 1
1, when the volume ratio of cyclohexanol/toluene was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the dosage of polystyrene seed was 155 mg. As shown in Fig. S3a,† the prepared spherical resin exhibited good monodispersity, particle size of 5.9 μm and yield of 19.9%, but the surface was rough. Adjusting the mass ratio of seed/total monomers at 3
1 and the dosage of polystyrene seed was 155 mg. As shown in Fig. S3a,† the prepared spherical resin exhibited good monodispersity, particle size of 5.9 μm and yield of 19.9%, but the surface was rough. Adjusting the mass ratio of seed/total monomers at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (PAD-10), the resin possessed excellent monodispersity, and its size was 5.9 μm (Fig. 2c), but the yield further decreased to 14.9%. When the mass ratio of seed/total monomers was 1
5 (PAD-10), the resin possessed excellent monodispersity, and its size was 5.9 μm (Fig. 2c), but the yield further decreased to 14.9%. When the mass ratio of seed/total monomers was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (PAD-11), the particle size of the sphere was 5.4 μm but it was broken, and the adhesion was serious (Fig. 2d), resulting in a low yield of 18.9%. It was hypothesized that the limited amount of seeds could not adequately absorb the monomers, leading to the formation of small particles that adhered to the surface of PAD. Therefore, the polystyrene seeds were increased by 1.5 times (PAD-10), which was the best choice. Based on PAD-9, the monomer, crosslinker, initiator, aqueous phase and polystyrene seeds were expanded by five times in equal proportions. Although the product (PAD-12) displayed spherical morphology and particle size of 7.1 μm (Fig. S3b†), the adhesion was more serious. It was presumed that the change in the reaction conditions, mass transfer problem and the difference of solvent selection in the scale-up experiment may lead to significant differences in the morphology of the microspheres. As depicted in Fig. S3c,† when the amount of the functional monomer to the crosslinker was 1
2 (PAD-11), the particle size of the sphere was 5.4 μm but it was broken, and the adhesion was serious (Fig. 2d), resulting in a low yield of 18.9%. It was hypothesized that the limited amount of seeds could not adequately absorb the monomers, leading to the formation of small particles that adhered to the surface of PAD. Therefore, the polystyrene seeds were increased by 1.5 times (PAD-10), which was the best choice. Based on PAD-9, the monomer, crosslinker, initiator, aqueous phase and polystyrene seeds were expanded by five times in equal proportions. Although the product (PAD-12) displayed spherical morphology and particle size of 7.1 μm (Fig. S3b†), the adhesion was more serious. It was presumed that the change in the reaction conditions, mass transfer problem and the difference of solvent selection in the scale-up experiment may lead to significant differences in the morphology of the microspheres. As depicted in Fig. S3c,† when the amount of the functional monomer to the crosslinker was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the mass ratio of seed/total monomers remained 1
1, the mass ratio of seed/total monomers remained 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and PAD-13 exhibited a spherical shape but was not uniform. Subsequently, when the mass ratio of the seed/total monomers remained at 2
3, and PAD-13 exhibited a spherical shape but was not uniform. Subsequently, when the mass ratio of the seed/total monomers remained at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the resulting PAD-14 contained a few spheres and most of the irregular particles (Fig. S3d†), whose yield was only 9.5%. Compared with the DBP–toluene system, the morphology of the products fabricated with the toluene–cyclohexanol system was improved but the adhesion of small particles still existed.
5, the resulting PAD-14 contained a few spheres and most of the irregular particles (Fig. S3d†), whose yield was only 9.5%. Compared with the DBP–toluene system, the morphology of the products fabricated with the toluene–cyclohexanol system was improved but the adhesion of small particles still existed.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 using DBP and cyclohexanol as porogenic agents, the prepared resin (PAD-15) possessed uniform morphology and the particle size was 5.1 μm (Fig. S4a†), but a few small particles adhered on its surface, and the yield was 21.4%. Similarly, when the amounts of the monomer, crosslinker, initiator, aqueous phase and polystyrene seeds were expanded by five times in equal proportions (PAD-16), the monodisperse microspheres were acquired, the particle size of the microspheres reached 6.2 μm (Fig. S4b†), and the yield was significantly increased to 39.5%. Although the homogeneity and monodisperse of the spheres were significantly improved by the above experimental improvement, the surface of the resin ball was still rough. When the monomer/crosslinker (n/n) was 3
50 using DBP and cyclohexanol as porogenic agents, the prepared resin (PAD-15) possessed uniform morphology and the particle size was 5.1 μm (Fig. S4a†), but a few small particles adhered on its surface, and the yield was 21.4%. Similarly, when the amounts of the monomer, crosslinker, initiator, aqueous phase and polystyrene seeds were expanded by five times in equal proportions (PAD-16), the monodisperse microspheres were acquired, the particle size of the microspheres reached 6.2 μm (Fig. S4b†), and the yield was significantly increased to 39.5%. Although the homogeneity and monodisperse of the spheres were significantly improved by the above experimental improvement, the surface of the resin ball was still rough. When the monomer/crosslinker (n/n) was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (PAD-17), the surface roughness of the microspheres was reduced, the particle size was 6.4 μm (Fig. 2e), and the yield reached 39.2%. When the monomer/crosslinker (n/n) was 2
2 (PAD-17), the surface roughness of the microspheres was reduced, the particle size was 6.4 μm (Fig. 2e), and the yield reached 39.2%. When the monomer/crosslinker (n/n) was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (PAD-18), the surface roughness of the prepared microspheres changed significantly, the particle size of the microspheres was 6.3 μm (Fig. 2f), and the yield was 35.6%. Ultimately, the product PAD-18 was selected for following modification.
1 (PAD-18), the surface roughness of the prepared microspheres changed significantly, the particle size of the microspheres was 6.3 μm (Fig. 2f), and the yield was 35.6%. Ultimately, the product PAD-18 was selected for following modification.3.2 Characterization of materials
As the PAD contains aldehyde groups, it can react with the amino groups of ODH to produce hydrazone bonds. As a result, ODH and Tp were mixed with PAD to coat a layer of two-dimensional COF via a “one-pot” method. Fig. 2g depicts the morphology of powdered COF, while Fig. 2h presents the SEM image of PAD@COF. Compared with the pristine PAD microspheres, the color is remarkably changed from white to yellow. Additionally, a layer of material appears on the surface of the PAD microsphere, which also proved the successful fabrication of PAD@COF.The FT-IR spectra of PAD, COF and PAD@COF are depicted in Fig. 3a. The peak at 1260 cm−1 in the PAD spectrum was the stretching vibration peak of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–C, and the stretching vibration peak of C
C–O–C, and the stretching vibration peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was proved at 1731 cm−1. In the COF spectrum, the peak at 1681 cm−1 is related to the C
O was proved at 1731 cm−1. In the COF spectrum, the peak at 1681 cm−1 is related to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, the peak at 1623 cm−1 corresponded to the –C–N stretching vibration, and the vibration peak at 3235 and 3419 cm−1 is considered to be the symmetric and asymmetric stretching vibration mode of –NH2 groups in ODH. It is worth noting that in the PAD@COF spectrum, the C
O stretching vibration, the peak at 1623 cm−1 corresponded to the –C–N stretching vibration, and the vibration peak at 3235 and 3419 cm−1 is considered to be the symmetric and asymmetric stretching vibration mode of –NH2 groups in ODH. It is worth noting that in the PAD@COF spectrum, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak at 1683 cm−1 corresponded to the stretching vibration peaks of PAD and COF, and the symmetric and asymmetric stretching vibration modes of –NH2 groups at 3236 and 3417 cm−1 corresponded to the N–H stretching vibration of ODH in the COF monomer. The appearance of these three characteristic peaks confirmed the successful synthesis of PAD@COF.
O stretching vibration peak at 1683 cm−1 corresponded to the stretching vibration peaks of PAD and COF, and the symmetric and asymmetric stretching vibration modes of –NH2 groups at 3236 and 3417 cm−1 corresponded to the N–H stretching vibration of ODH in the COF monomer. The appearance of these three characteristic peaks confirmed the successful synthesis of PAD@COF.
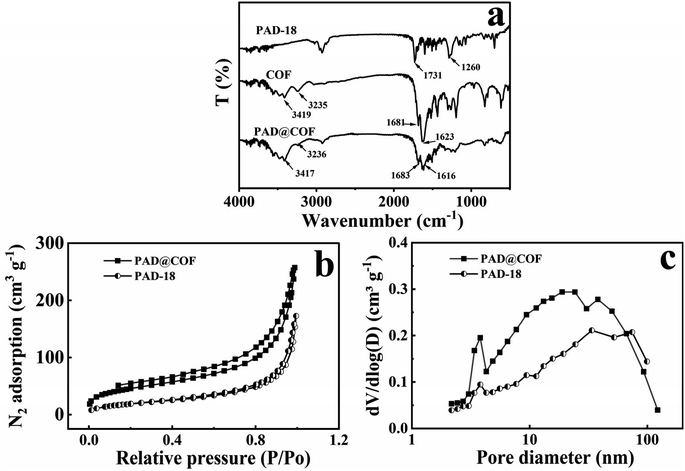 | ||
| Fig. 3 (a) FT-IR spectra of PAD-18, COF and PAD@COF, (b) nitrogen adsorption isotherms and (c) pore diameter distribution of PAD-18 and PAD@COF. | ||
The elemental composition of PAD and PAD@COF was determined by XPS. As presented in Fig. S5a,† the existence of carbon, oxygen and nitrogen elements was detected in PAD@COF, while nitrogen element was not observed. Additionally, the elemental analysis of PAD demonstrated that the composition of PAD is primarily comprised of 84.99% carbon and 15.01% oxygen (Table S1†), indicating the absence of nitrogen. PAD@COF mainly contains carbon, oxygen and nitrogen elements with contents of 75.64%, 20.72% and 3.65%, respectively. Among them, nitrogen was mainly derived from ODH. For PAD@COF, it can be observed from Fig. S5b–d† that the C 1s profile consisted of four fitting peaks, where C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–N, C–O, and C–C were centered at 288.2, 285.8, 285.5 and 284.3 eV,30 respectively, while the O 1s also consisted of three fitting peaks, viz., O
O, C–N, C–O, and C–C were centered at 288.2, 285.8, 285.5 and 284.3 eV,30 respectively, while the O 1s also consisted of three fitting peaks, viz., O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, O–H and O–C, centered on 531.3, 532.8 and 532.5 eV, respectively.31 The N 1s profile of the PAD@COF consisted of two fitting peaks, including –NH2 and –N centered on the binding energy of 398.8 and 399.7 eV, respectively.32 Chemical shifts of C
C, O–H and O–C, centered on 531.3, 532.8 and 532.5 eV, respectively.31 The N 1s profile of the PAD@COF consisted of two fitting peaks, including –NH2 and –N centered on the binding energy of 398.8 and 399.7 eV, respectively.32 Chemical shifts of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and O
O, C–O, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C were observed in the spectrum of PAD@COF compared to PAD, as well as new C–N peaks and N 1s peaks, indicating that the COF was successfully coated onto PAD.
C were observed in the spectrum of PAD@COF compared to PAD, as well as new C–N peaks and N 1s peaks, indicating that the COF was successfully coated onto PAD.
The pore structure and specific surface area pore structure of PAD and PAD@COF were analyzed, and the results are listed in Table 2. The specific surface area of PAD-6 synthesized in the DBP-toluene system was measured to be 10.7 m2 g−1, while that of PAD-11 synthesized in the toluene–cyclohexanol system was measured to be 12.9 m2 g−1. PAD-15, PAD-17, and PAD-18 were synthesized using DBP–cyclohexanol as the porogenic agent, resulting in specific surface areas of 26.7, 60.9, and 71.8 m2 g−1, respectively. According to our previous report,29 the specific surface area of COF was 835 m2 g−1. PAD-18 was coated with COF to obtain PAD@COF with a specific surface area of 163.8 m2 g−1. The BET curves of all the samples in Fig. 3b show a IV-type isotherm pattern based on IUPAC, indicating the existence of mesopores.33–35 The rapid uptake of nitrogen from 0.8–1.0 was observed, indicating that PAD@COF possessed a macroporous structure. All these indicated that the material had a porous structure. The pore volume and specific surface area of PAD@COF increased by 35.0% and 56.2%, respectively, compared with PAD-18. It was due to the increase in the adsorption pores on the surface of the microspheres after the modification of the COF layer, which also indicated that the COF layer was successfully grafted to the surface of PAD. With the increase in relative pressure, the slope of the curve decreased, and the adsorption from single layer to multi-layer gradually changed.36 The pore size distribution depicted in Fig. 3c also confirmed the presence of mesopores and macropores within the range of 10–60 nm in the PAD@COF material, thereby providing adsorption sites for heavy metals. Furthermore, the existence of these large macropores was conducive to enhancing the mass transfer rate.
| Materials | Specific surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| PAD-6 | 10.7 | 0.02 | 8.9 |
| PAD-11 | 12.9 | 0.03 | 7.7 |
| PAD-15 | 26.7 | 0.19 | 29.4 |
| PAD-17 | 60.9 | 0.23 | 14.9 |
| PAD-18 | 71.8 | 0.26 | 14.5 |
| COF | 835 | 0.89 | 15.5 |
| PAD@COF | 163.8 | 0.40 | 9.7 |
3.3 Adsorption ability
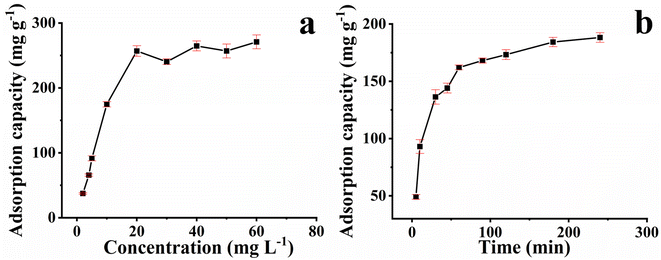
As presented in Fig. 4a, the adsorption performance of PAD@COF can be investigated through isothermal adsorption experiments. When the concentration of Cu2+ was less than 20 mg L−1, with the increase in Cu2+ concentration, the adsorption of Cu2+ by PAD@COF rapidly increased. When the concentration of Cu2+ was about 40 mg L−1, the adsorption equilibrium was reached, and the maximum adsorption amount of Cu2+ was 270.9 mg L−1. The equilibrium adsorption isotherm was fitted through the experimental data, as shown in Table S2 and Fig. S6a–d.† It could be observed that the Langmuir model (RL = 0.999) possessed a higher degree of fitting and correlation coefficient.The experimental results demonstrated that the Langmuir model (RL = 0.999) featured a higher degree of fitting and higher correlation coefficient than that of the Freundlich model (RF = 0.932). The adsorption was chiefly carried out on the surface of PAD@COF. During the isothermal adsorption experiment, an increase in Cu2+ concentration resulted in a corresponding increase in Qe. This phenomenon can be attributed to the disparity in Cu2+ concentration, which consequently led to enhanced mass transfer of Cu2+ onto the adsorbent surface.37
The kinetic adsorption properties of PAD@COF for Cu2+ were investigated at 30 mg L−1 concentration and different adsorption times. As shown in Fig. 4b, the kinetic adsorption results showed that the adsorption capacity increased rapidly within the initial 50 min and then gradually slowed down, reaching the adsorption equilibrium at about 180 min. The faster first stage could be explained by the fact that abundant binding sites enhance the contact between PAD@COF and Cu2+, and the adsorption reaction mainly occurs on the surface of the adsorbed material. When the adsorption sites on PAD@COF tend to be saturated, the effective adsorption sites are occupied by Cu2+, resulting in a slowing down of the increase in the adsorption capacity until equilibrium. The linear regression equation was adopted to fit the adsorption data, as shown in Fig. S6e and f.† The relevant kinetic parameters determined by the pseudo-first-order and pseudo-second-order models are listed in Table S3.† The correlation coefficient (R2 = 0.999) of the pseudo-second-order model was higher than that of the pseudo-first-order (R1 = 0.982). It could be concluded that the experimental data fit the pseudo-second-order model well, which indicated that chemical adsorption mainly occurred on its surface.
The comparison of this kind of microsphere with other materials on the adsorption of Cu2+ is exhibited in Table 3. Li and his group prepared a magnetic cellulose nanofiber hydrogel to remove Cu2+ from wastewater using cellulose nanofiber materials prepared from agricultural waste soybean residue, poly(vinyl alcohol) with excellent biocompatibility, hydrophilic diatomite, and nano-Fe3O4. The adsorption equilibrium of magnetic hydrogel reached 720 min and the maximum adsorption capacity was 100 mg g−1.38 Bai et al. synthesized spherical Cu2+-imprinted polymer using methacrylic acid as the monomer and poly(glycidyl methacrylate-co-polyethylene glycol dimethacrylate) as a matrix through atom-transfer radical polymerization. The adsorption equilibrium of the product was reached within 30 min and the maximum adsorption capacity reached 85.6 mg g−1.39 Lu and co-workers adopted surface ion-imprinting technology to selectively adsorb and detect Cu2+ in lake water using mesoporous silica-modified rice husk as the carrier and organosilane as the ion-acceptor, and the maximum adsorption capacity was 87.8 mg g−1.40 Zou et al. selected cicada shells as raw materials and prepared a porous nitrogen-doped activated biochar by chemical activation treatment, which exhibited excellent electrochemical properties and was applied to manufacture Cu2+ sensors, and the maximum adsorption capacity of Cu2+ was 110 mg g−1.41 Xu et al. collected sludge from a paper mill to prepare sludge-chitosan nanocomposite beads with a maximum adsorption capacity of 115 mg g−1 for Cu2+.42 There are also studies using 2,4,6-triformylphloroglucinol and tri(4-aminophenyl)amine as raw materials to successfully synthesize two-dimensional imine-based COF material,43 which manifested a large specific surface area (686 m2 g−1) and also exhibited high sensitivity in the fluorescence test. At pH = 6, the adsorption capacity of Cu2+ reached 229 mg g−1, which can be utilized as an adsorbent and fluorescence probe for Cu2+ removal and detection. Our group previously reported a COF synthetic strategy using flexible alkyl amine as the building unit and intramolecular hydrogen bonding as the network connection.29 By exploring ODH and Tp, a type of COF material was acquired and its specific surface area was as high as 835 m2 g−1, exhibiting a superior adsorption capacity of 324 mg g−1.29 Herein, although the maximum adsorption capacity of PAD@COF was marginally lower than that of the hydrazone-linked COF, it exhibited superior adsorption performance compared to other six kinds of Cu2+ adsorption materials. Furthermore, PAD@COF demonstrated a shorter equilibrium adsorption time in comparison to the magnetic hydrogel, activated carbon and imine-based COF materials.
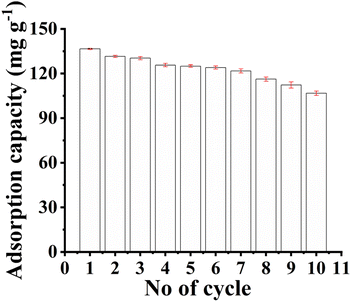
The reusability of the adsorbent is a crucial factor in practical applications. Multiple cycles of adsorption and desorption can be utilized to evaluate the adsorption performance of the adsorbent and reduce the adsorption cost. The reusability of PAD@COF was investigated after 10 cycles of adsorption and desorption experiments. The amounts of adsorption for each cycle were 136.30, 131.45, 130.06, 125.90, 124.51, 123.82, 121.74, 116.19, 112.73, and 107.18 mg g−1, respectively. As shown in Fig. 5, the adsorption capacity of PAD@COF slightly decreased, but the adsorption efficiency remained over 78.6% after the 10 cycles. It was evident that the washing process has minimal impact on the porous structure and chemical properties of PAD@COF. Therefore, PAD@COF possessed good cyclic stability and regeneration properties.
The adsorption ability of PAD@COF in real environments was assessed. The adsorption amount of PAD@COF for Cu2+ was 43.75 mg g−1 within 1 h, and the LOD did not change significantly in the lake water sample. This indicated that PAD@COF enabled the rapid adsorption of Cu2+.
3.4 Degradation and recovery
PAD@COF was added into different concentrations of acid solution. As shown in Fig. S7,† PAD@COF appears similar to the COF as the coating was gradually hydrolyzed with the increase in the concentration, achieving recovery and reuse of the aldehyde-containing microspheres.4 Conclusion
In this work, monodisperse resin microspheres (PAD) with a great quantity of aldehyde groups on the surface were synthesized via seed swelling polymerization, and the effect of different porogenic systems on the morphology of the resin microspheres was mainly investigated. Subsequently, PAD was utilized as the matrix, and COF was grafted onto the surface of the microspheres by a simple one-pot method (PAD@COF). The obtained PAD@COF exhibited controllable particle size and potential for the rapid removal of Cu2+ in water. Furthermore, due to the presence of aldehyde groups of the PAD microspheres, it could be reused by a reversible condensation reaction, which presented the extensive integration of other COFs. Additionally, the microspheres are apt to be modified through the Ugi reaction with aldehyde groups. It is optimistically expected that this kind of aldehyde-containing monodisperse microspheres can be utilized as chromatographic stationary phases in the future.Data availability
The data are available from the corresponding author on reasonable request.Author contributions
Xiaoqiong Wang: conceptualization, data curation, methodology, investigation, software, visualization, and writing – original draft. Qingyan Bai: sample preparation, methodology, and software. Mingjia Yan: validation, and visualization. Yashuai Zhao: investigation and data curation. Shujuan Ma: validation and resources. Chunmiao Bo: supervision. Junjie Ou: project administration, funding acquisition and writing – review & editing.Conflicts of interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.Acknowledgements
Financial support is gratefully acknowledged from the Key research and development program of Ningxia (No. 2022BFE02002), the National Natural Sciences Foundation of Ningxia (No. 2021AAC02017) and the CAS-Weigao Research & Development Program ([2017]-009) to J. Ou.Notes and references
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- Z. Xiao, X. Nie, Y. Li, Y. Nie, L. Lu and X. Tian, ACS Appl. Mater. Interfaces, 2023, 15, 9524–9532 CrossRef CAS PubMed.
- D. Xu, Y. Jin, C. Li, Y. Fan, S. Kawi, X. Meng, J. Song and N. Yang, J. Membr. Sci., 2024, 700, 122678 CrossRef CAS.
- L. Zhong, C. Wang, J. He, Z. lin, X. Yang, R. Li, S. Zhan, L. Zhao, D. Wu, H. Chen, Z. Tang, C. Zhi and H. Lv, Adv. Mater., 2024, 2314050 CrossRef CAS PubMed.
- L. Li, X. Lv, Y. Xue, H. Shao, G. Zheng and Q. Han, Angew. Chem., Int. Ed., 2024, 63, e202320218 CrossRef CAS PubMed.
- F. Kong, L. Yue, Z. Yang, G. Sun and J. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 21379–21389 CrossRef CAS PubMed.
- Y. Liu, P. Li, R. Cui, C. Qin, L. Wu, X. Zhang, B. Li, J. Ping, Y. Wang, J. Pan, Y. Ying, D. Li, D. Shi and L. Xu, Trends Anal. Chem., 2024, 174, 117678 CrossRef CAS.
- K. Hu, Y. Wang, G. Wang, Y. Wu and Q. He, Food Chem., 2023, 429, 136801 CrossRef CAS PubMed.
- X. Zhong, Q. Ling, P. Kuang and B. Hu, Chem. Eng. J., 2024, 483, 149339 CrossRef CAS.
- S. A, H. R and A. G, npj Clean Water, 2024, 7, 31 CrossRef.
- R. Sen Gupta, S. Islam, A. Malakar, T. Das and S. Bose, J. Mater. Chem. A, 2024, 12, 19094–19108 RSC.
- J. Wang, A. Hassan, A. Elewa and A. El-Mahdy, J. Mater. Chem. A, 2024, 12, 14005–14021 RSC.
- H. Dong, M. Lu, Y. Wang, H. Tang, D. Wu, X. Sun and F. Zhang, Appl. Catal., B, 2022, 303, 120897 CrossRef CAS.
- C. Li, M. Gao, X. Sun, H. Tang, H. Dong and F. Zhang, Appl. Catal., B, 2020, 266, 118586 CrossRef CAS.
- H. Dong, X. Meng, X. Zhang, H. Tang, J. Liu, J. Wang, J. Wei, F. Zhang, L. Bai and X. Sun, Chem. Eng. J., 2020, 379, 122342 CrossRef CAS.
- K. Miller, J. Gayle, S. Roy, M. Abdellah, R. Hardian, L. Cseri, P. Demingos, H. Nadella, F. Lee, M. Tripathi, S. Gupta, G. Guo, S. Bhattacharyya, X. Wang, A. Dalton, A. Garg, C. Singh, R. Vajtai, G. Szekely and P. Ajayan, Small, 2024, 20, 2401269 CrossRef CAS PubMed.
- S. Kumar, G. Ignacz and G. Szekely, Green Chem., 2021, 23, 8932–8939 RSC.
- M. Xie, K. Quan, H. Li, B. Liu, J. Chen, Y. Yu, J. Wang and H. Qiu, Chem. Commun., 2023, 59, 314–317 RSC.
- H. Long, Y. Deng, Y. Zhang, S. Tang and W. Chen, Microchem. J., 2024, 197, 109900 CrossRef CAS.
- J. Tan, S. Namuangruk, W. Kong, N. Kungwan, J. Guo and C. Wang, Angew. Chem., Int. Ed., 2016, 55, 13979–13984 CrossRef CAS PubMed.
- C. Gao, G. Lin, Z. Lei, Q. Zheng, J. Lin and Z. Lin, J. Mater. Chem. B, 2017, 5, 7496–7503 RSC.
- N. Xu, P. Guo, J. Chen, J. Zhang, B. Wang, S. Xie and L. Yuan, Talanta, 2021, 235, 122754 CrossRef CAS PubMed.
- W. Ma, Q. Zheng, Y. He, G. Li, W. Guo, Z. Lin and L. Zhang, J. Am. Chem. Soc., 2019, 141, 18271–18277 CrossRef CAS PubMed.
- Y. Su, M. Qin, J. Kong, Q. Zhai, D. Yuan, Z. Liu and Y. Fang, Adv. Funct. Mater., 2024, 2400433 CrossRef CAS.
- J. Xu, G. Feng, D. Ao, X. Li, M. Li, S. Lei and Y. Wang, Adv. Mater., 2024, 2406256, DOI:10.1002/adma.202406256.
- H. S. Sasmal, A. Kumar Mahato, P. Majumder and R. Banerjee, J. Am. Chem. Soc., 2022, 144, 11482–11498 CrossRef CAS PubMed.
- Q. Zhu, G. Zhang, L. Zhang, S. Wang, J. Fu, Y. Wang, L. Ma, L. He and G. Tao, J. Am. Chem. Soc., 2023, 145, 6177–6183 CrossRef CAS PubMed.
- J. Hao, F. Wang, X. Dai, B. Gong and Y. Wei, Talanta, 2011, 85, 482–487 CrossRef CAS PubMed.
- Y. Li, C. Wang, S. Ma, H. Zhang, J. Ou, Y. Wei and M. Ye, ACS Appl. Mater. Interfaces, 2019, 11, 11706–11714 CrossRef CAS PubMed.
- P. Wang, L. Yin, J. Wang, C. Xu, Y. Liang, W. Yao, X. Wang, S. Yu, J. Chen, Y. Sun and X. Wang, Chem. Eng. J., 2017, 326, 863–874 CrossRef CAS.
- S. Wan, W. Ding, Y. Wang, J. Wu, Y. Gu and F. He, Chem. Eng. J., 2018, 350, 1135–1143 CrossRef CAS.
- K. Zhang, B. Chen, J. Mao, L. Zhu and B. Xing, Environ. Pollut., 2018, 240, 342–352 CrossRef CAS PubMed.
- N. Gan, Q. Sun, L. Zhao, S. Zhang, Z. Suo, X. Wang and H. Li, J. Mater. Chem. B, 2021, 9, 5628–5635 RSC.
- Y. Dou, N. Liu, X. Zhang, W. Jiang, X. Jiang and L. Yu, Chem. Eng. J., 2023, 463, 142398 CrossRef CAS.
- A. V. Narendra Kumar and W. S. Shin, Chem. Eng. J., 2023, 465, 142922 CrossRef CAS.
- X. Xie, H. Gao, X. Luo, T. Su, Y. Zhang and Z. Qin, J. Environ. Chem. Eng., 2019, 7, 103183 CrossRef.
- J. Dong, Y. Du, R. Duyu, Y. Shang, S. Zhang and R. Han, Bioresour. Technol. Rep., 2019, 6, 96–102 CrossRef.
- P. Li, H. Liu, M. Zhou, H. Lei, B. Jian, R. Liu, X. Li, Y. Wang and B. Zhou, Ind. Crops Prod., 2022, 186, 115257 CrossRef CAS.
- Q. Bai, C. Huang, S. Ma, B. Gong and J. Ou, Sep. Purif. Technol., 2023, 315, 123666 CrossRef CAS.
- X. Lv, H. Hu, L. Yao, L. Deng, X. Liu, L. Yu and H. He, Spectrochim. Acta, Part A, 2023, 298, 122723 CrossRef CAS PubMed.
- J. Zou, J. Liu, Q. Yu, Y. Gao, S. Chen, X. Huang, D. Hu, S. Liu and L. Lu, Molecules, 2022, 27, 4516 CrossRef CAS PubMed.
- K. Xu, L. Li, Z. Huang, Z. Tian and H. Li, Sci. Total Environ., 2022, 846, 157399 CrossRef CAS PubMed.
- Y. Zhao, Y. Li, W. Chen and X. Jin, Inorg. Chem., 2024, 63, 1879–1887 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra05820h |
| This journal is © The Royal Society of Chemistry 2024 |