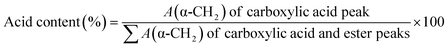Open Access Article
Open Access ArticleMicrowave-driven oleic acid esterification over chlorosulfonic acid-treated hydroxyapatite: synergism for intensified biodiesel production
Boutaina Rezki *a,
Younes Essamlalib,
Othmane Amadineb,
Said Sairb,
Mina Aadila and
Mohamed Zahouilyab
*a,
Younes Essamlalib,
Othmane Amadineb,
Said Sairb,
Mina Aadila and
Mohamed Zahouilyab
aFaculty of Sciences and Technology, Laboratory of Materials, Catalysis and Natural Resources Valorization, Hassan II University of Casablanca, 20650, Mohammedia, Casablanca-Settat, Morocco. E-mail: boutainachimie@gmail.com; boutaina.rezki@etu.fstm.ac.ma
bMoroccan Foundation for Advance Science Innovation and Research, VARENA Center, Mohammed VI Polytechnic University, Ben Guerir, Morocco
First published on 21st October 2024
Abstract
Treating hydroxyapatite (HAP) with sulfonic acid without structural destruction remains challenging owing to the sensitivity of HAP to acidic pH. In this work, natural derived HAP was prepared using natural phosphate via a dissolution/precipitation process. Notwithstanding the challenge, the prepared HAP was treated with three concentrations of chlorosulfonic acid in dichloromethane to prepare HAP-S1, HAP-S2 and HAP-S3 depending on the acid content under carefully controlled conditions. The treatment of HAP with the lowest acid concentration led to the preservation of the apatite framework with surface modification of sulfonic acid groups. As the acid concentration increased, HAP, CaHPO4 and CaSO4 were obtained. A further increase in the acid concentration led to the formation of CaSO4 with the coexistence of Ca(H2PO4)2·H2O. Subsequently, the catalytic activity of HAP-S1 was evaluated in oleic acid esterification under microwave irradiation, resulting in a yield of up to 87% under optimized conditions of 10 wt% of catalyst to oleic acid weight, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol to oleic acid molar ratio, and 40 min reaction duration under microwave irradiation at 150 °C. The activity of HAP-S1 was attributed to the active S
1 methanol to oleic acid molar ratio, and 40 min reaction duration under microwave irradiation at 150 °C. The activity of HAP-S1 was attributed to the active S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and S–O–H functional groups, and a possible mechanism of acid-catalyzed esterification is proposed. The catalyst was also tested in the transesterification reaction of rapeseed oil, achieving a conversion of 40.2% after 60 min reaction duration under microwave irradiation. Furthermore, the catalyst was evaluated in a two-step esterification reaction to investigate its activity towards acidified feedstocks. Results showed that after two successive runs, acidity was reduced by 79.6% with a total FAME yield of 40.13%. The obtained results indicate that this catalyst, being an acid catalyst, is more suitable for direct esterification.
O and S–O–H functional groups, and a possible mechanism of acid-catalyzed esterification is proposed. The catalyst was also tested in the transesterification reaction of rapeseed oil, achieving a conversion of 40.2% after 60 min reaction duration under microwave irradiation. Furthermore, the catalyst was evaluated in a two-step esterification reaction to investigate its activity towards acidified feedstocks. Results showed that after two successive runs, acidity was reduced by 79.6% with a total FAME yield of 40.13%. The obtained results indicate that this catalyst, being an acid catalyst, is more suitable for direct esterification.
1. Introduction
Acid catalysis is one of the most important areas of catalysis science. Typically, Lewis or Brønsted acid catalysts are responsible for the processing of important transformations such as Fischer esterification.1 In particular, the esterification reaction of fatty acids such as oleic acids (OA) and palm fatty acid distillate (PFAD) with methanol has attracted significant interest because the obtained monoalkyl esters have similar physicochemical properties to those of petrodiesel.2,3 Monoalkyl esters, namely, biodiesel, can be blended with conventional diesel and used in engines without further modification.4,5 Generally, the esterification of fatty acids is carried out using homogeneous acid catalysts such as H2SO4, H3PO4, HCl, and p-toluenesulfonic acid, where the proton (H+) is the acid moiety, according to the Brønsted–Lowry acid–base theory.6 However, although homogeneous catalysts provide high ester yields under mild conditions, they are corrosive to reactors, and further neutralization steps are required to purify the final product. Therefore, to facilitate the separation and regeneration steps, many solid catalysts have been investigated in esterification such as sulfated metal oxides,7 sulfonated mixed metal oxides,8 sulfonic acid-functionalized mesoporous carbon materials or silicas,9 heteropolyacids,10 polyoxometalates,11 TiO2/NP12 and acid-derived apatite catalysts.13,14 It should be noted that biodiesel can be obtained by adopting different processes depending on the acidity of feedstocks and the type of catalysts.15 In general, biodiesel can be produced via direct base-catalyzed transesterification of oils.16,17 Moreover, it is obtained from highly acidic feedstocks by a simultaneous esterification/transesterification process using bifunctional or strong acid catalysts.18 It is also produced by a two-step esterification and transesterification process by reducing the acidity of the initial feedstock via esterification to make it suitable for the transesterification reaction.19Hydroxyapatite (HAP) is an attractive inorganic compound due to its flexible framework, which can accept many partial cationic and anionic substitutions, and also due to its excellent surface activity related to its possible functionalization.20,21 In this context, the surface modification of HAP has received significant interest in acid-catalyzed transformations.22–24 For example, supported synthetic apatite with sulfonic acid groups (HAP-SO3H) provided new strong catalytic acid sites for condensation and cyclization between amidoxime and aldehyde, as reported by El Mansouri et al.22 In addition, magnetic sulfonated hydroxyapatite-encapsulated-γ-Fe2O3 has been widely used in many acid-catalyzed reactions, for example, it was found to exhibit a good performance in the N-formylation of amines, as detailed by Ma'mani et al.25–28 Furthermore, HAP-AEPH2-SO3H was found to be an efficient catalyst for the esterification of carboxylic acids with alcohols, as reported by Siavashi et al.29 Although many solid catalysts have been employed for biodiesel production, apatite-based catalysts have recently attracted attention due to their effectiveness in promoting the synthesis of biodiesel from diverse feedstocks.21 Herein, it is essential to highlight that the utilization of apatite-derived catalysts aligns with the sustainable catalysis principles. This relies on utilizing materials with high natural abundance to produce high-value chemicals in an environmentally sustainable approach. In this context and motivated by the abundance of phosphate mineral in Morocco, the main objective of the present study is the preparation of an acidic catalyst based on natural hydroxyapatite modified with sulfonic acid and its application in biodiesel production through the oleic acid esterification reaction as a model reaction.
Alternatively, thermo-catalytic conversions occur in heated reactors using various heating modes (conventional oil-bath reflux heating, electrical heating, and microwave-assisted heating). The use of microwave irradiation for the synthesis of biodiesel significantly reduces the time needed for the optimal conversion and produces a higher yield compared to conventional methods.30 Due to its unique thermal effects, when applying microwave frequencies, microwaves directly heat the internal sample via dielectric heating. The polar molecules in reaction medium align with the electromagnetic field, generating heat through molecular rotation, friction, and collision.31 In contrast, conventional heating, which transfers heat through conduction and convection, may lead to uneven temperature and thermal gradients.32
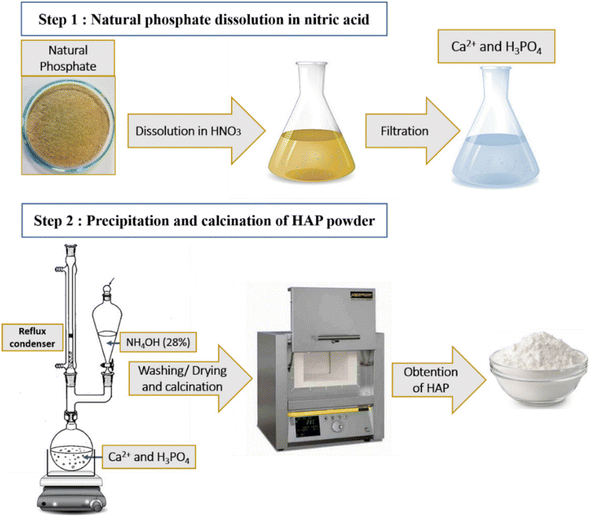
Herein, in the presented research, natural hydroxyapatite was obtained by the dissolution–precipitation process of natural phosphate, and then the obtained liquid phase was precipitated using a highly basic solution. Subsequently, the obtained hydroxyapatite was subjected to a series of treatments with chlorosulfonic acid (ClSO3H) in dichloromethane (DCM). To the best of our knowledge, this is the first report studying the effect of the concentration sulfonic acid in DCM on the structure of natural hydroxyapatite by various characterization techniques. Then, the sulfonated hydroxyapatite (HAP-S1) was utilized in oleic acid esterification with methanol under microwave irradiation. Other tests, such as the transesterification of rapeseed oil and the two-step esterification–transesterification process, were carried out to investigate the activity and selectivity of the catalyst towards the synthesis of biodiesel from different feedstocks. Herein, in this work, and despite the challenges of the deactivation and short-term stability of the catalysts, the acid-based-apatite catalysts are shown to be a promising alternative to other catalysts due to their abundance, easy accessibility and cost-effectiveness. Thus, further research should be conducted to propose better modifications of apatite to realize long-term stability.
2. Material preparation and characterization
2.1. Materials and reagents
Natural phosphorus rock was obtained from Moroccan sedimentary phosphate deposits at mines in the Khouribga region. Oleic acid (technical grade, 90%) was provided by Alfa Aesar. Rapeseed oil was purchased from a local company in the city of Mohammedia, Morocco. Ammonium hydroxide (NH4OH) (28–30%), nitric acid (HNO3) (65–67%), phenolphthalein (C20H14O4) and potassium hydroxide (KOH) were purchased from Aldrich Company. Methanol (CH3OH), ethanol (C2H5OH), diethyl ether (C2H5)2O, dichloromethane (CH2Cl2) and chlorosulfonic acid (ClSO3H) were all analytical grade and used as received without further purification.2.2. Materials preparation
2.3. Material characterization
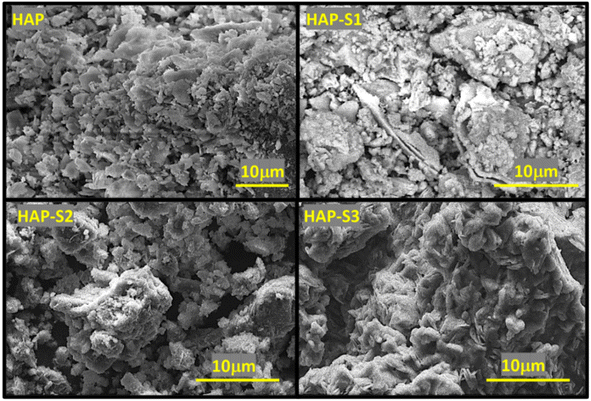
The X-ray diffraction (XRD) data of the prepared materials were collected at room temperature using a D2 PHASER diffractometer, with the Bragg–Brentano geometry, using CuKa radiation (λ = 1.5406 Å) at 30 kV and 10 mA. The patterns were scanned in steps of 0.02° (2θ). The Fourier transform infrared (FT-IR) spectra of the samples were obtained using a Bruker Vector 22 spectrometer in the range of 4000–500 cm−1 at a resolution of 16 cm−1. Scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX) and elemental mapping analysis were performed using a Tecnai G2 microscope at 120 kV. Solid-state 31P nuclear magnetic resonance experiments were performed on a Bruker Advance 600 MHz spectrometer, where powder samples were packed into 4 mm zirconia magic-angle spinning (MAS) rotors and spun at a rotation frequency of 10 kHz. The 31P CP MAS spectra were obtained using the standard cross-polarisation pulse technique followed by 1H high-power decoupling.2.4. Reaction procedure
The feedstocks in this study, namely, oleic acid and refined rapeseed oil, were utilized without any pretreatment or preheating.
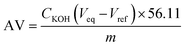 | (1) |
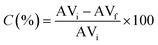 | (2) |
In addition, the oleic acid conversion (wt%) under the optimal conditions was also performed using 1H-NMR adopting the following equations:
| Acid conversion (%) = [100 − acid content (%)] |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol to oleic acid molar ratio, temperature of 150 °C) for both 40 min and 60 min. At the end of the reaction, the catalyst was removed by centrifugation. Subsequently, the liquid mixture was subjected to rotary evaporation to remove the excess methanol. The separated ester phase was analysed by nuclear magnetic resonance (1H-NMR, Bruker Avance 600 MHz) using deuterated chloroform (CDCl3) as the solvent and tetramethylsilane (TMS) as the internal standard. The oil conversion was calculated using the method reported by Liza et al.34
1 methanol to oleic acid molar ratio, temperature of 150 °C) for both 40 min and 60 min. At the end of the reaction, the catalyst was removed by centrifugation. Subsequently, the liquid mixture was subjected to rotary evaporation to remove the excess methanol. The separated ester phase was analysed by nuclear magnetic resonance (1H-NMR, Bruker Avance 600 MHz) using deuterated chloroform (CDCl3) as the solvent and tetramethylsilane (TMS) as the internal standard. The oil conversion was calculated using the method reported by Liza et al.342.5. Catalyst characterization
| Material | Lattice parameters | Volume (Å3) | ||
|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | ||
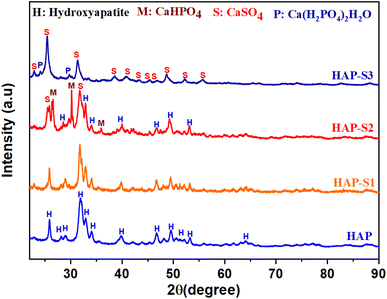
| HAP | 9.42779 | 9.42779 | 6.8975 | 530.57 |
| HAP-S1 | 9.41393 | 9.41393 | 6.88259 | 528.23 |
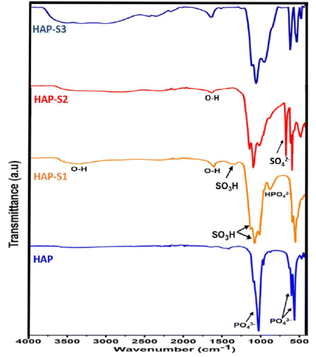
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the sulfonic group (SO3H) at 1350–1342 cm−1, confirming that the SO3H groups were anchored to HAP framework.38 Furthermore, a new band appeared at 3600–3036 cm−1, which was attributed to the OH groups that appeared after sulfonation.38 An additional tiny peak at 888 cm−1 attributed to the presence of HPO42− ions appeared in the spectrum of HAP-S1, which can be explained by the possible partial transformation of the surface PO43− groups into HPO42−, as detailed by Dorozhkin.36,39 A further increase in the acid concentration treatment led to the appearance of the symmetric and asymmetric stretching vibrations of SO42− at 670 and 482 cm−1 in the spectrum of HAP-S2, respectively.40 In addition, the intensity of the peaks in the 1000–1130 cm−1 increased due to the coexistence of SO42− bands, which overlapped with the vibrational modes of the PO43− groups, as reported by Oulguidoum et al.41 In the case of HAP-S3, the characteristic peaks at 670 and 482 cm−1 are related to the presence of the SO42− groups. The other characteristic peaks of the monocalcium phosphate monohydrate (MCPM) groups may overlap with the characteristic CaSO4 groups. For example, the fundamental vibrational bending and stretching modes of O–P–O, P–O, in-plane P–O–H and out-of-plane P–O–H can be observed in the region of 450–600, 900–1100, 1200–1250, and 900–800 cm−1, respectively.42 Furthermore, the vibrational modes of the H2O molecules appear in the range of 3200 to 3500 cm−1 and at 1646 cm−1, which are related to the bending, symmetric, and asymmetric stretching of the H–O–H bonds, respectively. In addition, the O–H stretching mode of H2PO4− appeared as three A, B, and C bands at about 3100–3200 cm−1 (A band), 2300–2400 cm−1 (B band), and 1600–1800 cm−1 (C band).43
O of the sulfonic group (SO3H) at 1350–1342 cm−1, confirming that the SO3H groups were anchored to HAP framework.38 Furthermore, a new band appeared at 3600–3036 cm−1, which was attributed to the OH groups that appeared after sulfonation.38 An additional tiny peak at 888 cm−1 attributed to the presence of HPO42− ions appeared in the spectrum of HAP-S1, which can be explained by the possible partial transformation of the surface PO43− groups into HPO42−, as detailed by Dorozhkin.36,39 A further increase in the acid concentration treatment led to the appearance of the symmetric and asymmetric stretching vibrations of SO42− at 670 and 482 cm−1 in the spectrum of HAP-S2, respectively.40 In addition, the intensity of the peaks in the 1000–1130 cm−1 increased due to the coexistence of SO42− bands, which overlapped with the vibrational modes of the PO43− groups, as reported by Oulguidoum et al.41 In the case of HAP-S3, the characteristic peaks at 670 and 482 cm−1 are related to the presence of the SO42− groups. The other characteristic peaks of the monocalcium phosphate monohydrate (MCPM) groups may overlap with the characteristic CaSO4 groups. For example, the fundamental vibrational bending and stretching modes of O–P–O, P–O, in-plane P–O–H and out-of-plane P–O–H can be observed in the region of 450–600, 900–1100, 1200–1250, and 900–800 cm−1, respectively.42 Furthermore, the vibrational modes of the H2O molecules appear in the range of 3200 to 3500 cm−1 and at 1646 cm−1, which are related to the bending, symmetric, and asymmetric stretching of the H–O–H bonds, respectively. In addition, the O–H stretching mode of H2PO4− appeared as three A, B, and C bands at about 3100–3200 cm−1 (A band), 2300–2400 cm−1 (B band), and 1600–1800 cm−1 (C band).43
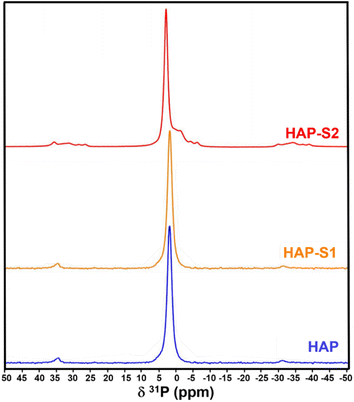
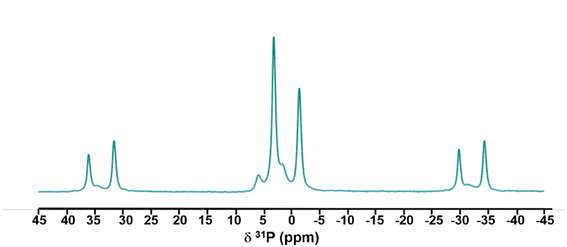
CP 31P-NMR was performed on the HAP-S3 sample, as shown in Fig. 5, revealing the presence of several signals. The interpretation of the spectrum of HAP-S3 requires sufficient caution due to the overlapping of these signals. Considering the complexity of these results and the high sensitivity of this technique to the presence of P in the sample, the intense peak at 3.1 ppm can be attributed to the possible presence of orthophosphate group residues of Ca10(PO4)6(OH)2.46 The overlap of a small peak with the previous HAP-associated signal at −1.46 ppm followed by an intense signal at 1.50 and a non-symmetric signal at 5.77 ppm may be attributed to the presence of Ca(H2PO4)2·H2O and the possible existence of acidic phosphate residues such as CaHPO4, CaHPO4·2H2O, and Ca(H2PO4), which could be precipitated during the complex dissolution–precipitation process of the apatite in highly acidic medium.47,48 Furthermore, the possible substitution of orthophosphate in the calcium sulfate crystals can also be a possible reason, which may explain the multiple signals detected.
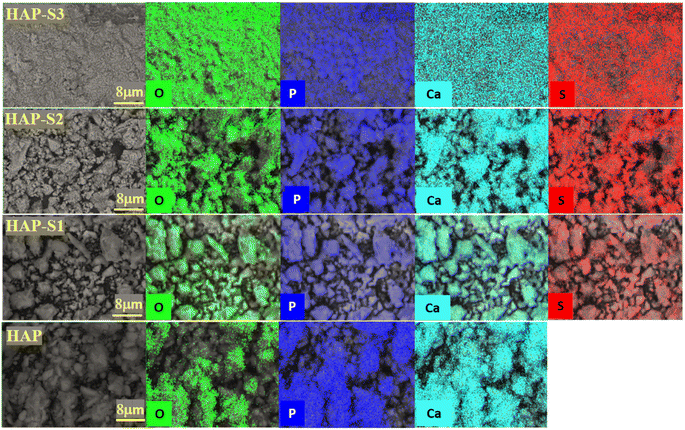
The surface elemental analysis of the prepared samples by energy dispersive spectroscopy is presented in Table 2. According to the results, the materials are mainly composed of Ca, P and O for all the samples, besides the presence of sulfur for HAP-S1, HAP-S2 and HAP-S3. In fact, the Ca and P contents decreased slightly after the treatment of apatite in acid medium. However, the sulfur content increased with an increase in the sulfonic acid concentration.
| Material | Element content (wt%) | ||||
|---|---|---|---|---|---|
| Ca | P | O | S | C | |
| HAP | 42.71 | 18.4 | 35.36 | — | 3.53 |
| HAP-S1 | 40.84 | 16.27 | 34.19 | 6.47 | 2.23 |
| HAP-S2 | 36.62 | 14.61 | 41.97 | 11.02 | 1.21 |
| HAP-S3 | 27.86 | 7.20 | 50.57 | 14.37 | — |
3. Catalytic investigation in esterification, transesterification and two-step process
3.1. Oleic acid esterification investigation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
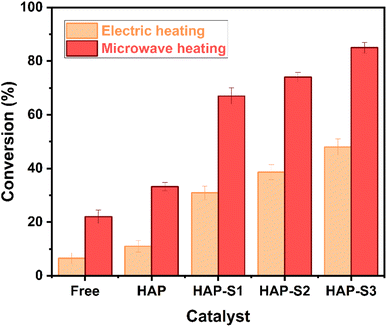
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and catalyst amount of 7 wt% at 150 °C for 60 min as control experiments (Fig. 8). The first experiment was conducted without any catalyst, resulting in the production of 6.5% OAME. Under similar conditions, in the presence of HAP, HAP-S1, HAP-S2 and HAP-S3, yields of 11%, 30.9%, 38.6%, and 48%, respectively, were obtained. This enhancement in the obtained yield is due to the presence of active sites in the surface of the utilized materials. We observed that higher concentrations of sulfonic acid-treated HAP resulted in an increase in the acidity of the catalyst, which enhanced its catalytic activity by providing more active sites for the reaction. Nevertheless, we believe that better results can be obtained by conducting these reactions for longer durations. In fact, the majority of the reviewed works that carried out the esterification of OA in batch reactors required more rigorous reaction conditions to obtain high biodiesel yields, high molar ratios of alcohol-to-oil, and also presented longer reaction times than that used in the present tests.12,49 Alternatively, performing the same experiments under MW irradiation (Fig. 8) resulted in higher yields compared to that obtained by the heated stainless-steel batch reactor, confirming the beneficial effect of MW. In particular, an oleic acid conversion of 22% was obtained with the free catalytic system, whereas 33.2%, 67%, 74% and 85% was obtained using HAP, HAP-S1, HAP-S2 and HAP-S3, respectively. In fact, in electrically assisted heating, the heat energy is transferred from the surface to the reactor by convection and conduction. Therefore, a large amount of heat and long reaction time are required to achieve the satisfactory conversion of fatty acids to FAME. In contrast, microwave irradiation can deliver the energy directly to the reactants which enhances the heat transfer due to the interaction of the dipole moment of the polar methanol with the incident radiation. Actually, the role of microwave irradiation is pivotal in accelerating the reaction rates. The ability of microwaves to provide uniform heating and enhance the mass transfer leads to faster reaction kinetics compared to conventional heating methods. The interaction between radiation and dipole moment of methanol results in the rotation, friction and collision of molecules, resulting in instantaneous localized superheating, where high energy is delivered to the reagents at a very fast rate. Here, the reaction mixture was rapidly heated when exposed to MW irradiation and formed hot spots, known as the “microwave thermal effect”, which is responsible for the increased reaction efficiency.50
1 and catalyst amount of 7 wt% at 150 °C for 60 min as control experiments (Fig. 8). The first experiment was conducted without any catalyst, resulting in the production of 6.5% OAME. Under similar conditions, in the presence of HAP, HAP-S1, HAP-S2 and HAP-S3, yields of 11%, 30.9%, 38.6%, and 48%, respectively, were obtained. This enhancement in the obtained yield is due to the presence of active sites in the surface of the utilized materials. We observed that higher concentrations of sulfonic acid-treated HAP resulted in an increase in the acidity of the catalyst, which enhanced its catalytic activity by providing more active sites for the reaction. Nevertheless, we believe that better results can be obtained by conducting these reactions for longer durations. In fact, the majority of the reviewed works that carried out the esterification of OA in batch reactors required more rigorous reaction conditions to obtain high biodiesel yields, high molar ratios of alcohol-to-oil, and also presented longer reaction times than that used in the present tests.12,49 Alternatively, performing the same experiments under MW irradiation (Fig. 8) resulted in higher yields compared to that obtained by the heated stainless-steel batch reactor, confirming the beneficial effect of MW. In particular, an oleic acid conversion of 22% was obtained with the free catalytic system, whereas 33.2%, 67%, 74% and 85% was obtained using HAP, HAP-S1, HAP-S2 and HAP-S3, respectively. In fact, in electrically assisted heating, the heat energy is transferred from the surface to the reactor by convection and conduction. Therefore, a large amount of heat and long reaction time are required to achieve the satisfactory conversion of fatty acids to FAME. In contrast, microwave irradiation can deliver the energy directly to the reactants which enhances the heat transfer due to the interaction of the dipole moment of the polar methanol with the incident radiation. Actually, the role of microwave irradiation is pivotal in accelerating the reaction rates. The ability of microwaves to provide uniform heating and enhance the mass transfer leads to faster reaction kinetics compared to conventional heating methods. The interaction between radiation and dipole moment of methanol results in the rotation, friction and collision of molecules, resulting in instantaneous localized superheating, where high energy is delivered to the reagents at a very fast rate. Here, the reaction mixture was rapidly heated when exposed to MW irradiation and formed hot spots, known as the “microwave thermal effect”, which is responsible for the increased reaction efficiency.50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
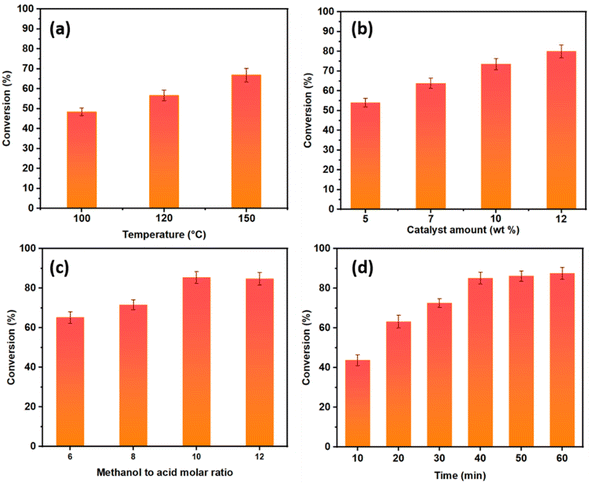
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reaction time of 60 min). The oleic acid conversion was found to increase with an increase in temperature due to the higher molecular collisions between the reagents as a result of the improved mass transfer, which increased the reaction rate.51 To investigate the effect of the catalyst amount on the esterification conversion, different amounts of catalyst ranging from 5 to 12 wt% were used, while keeping the other experimental conditions constant. Based on the results obtained (Fig. 9b), the conversion increased as the catalyst amount increased from 5 to 10 wt%. Therefore, the catalyst amount of 10 wt% was selected as the optimum value given that further increasing the catalyst amount to 12 wt% resulted in an OA conversion increase of only 6.45% with a final total value of 80%. In general, increasing the catalyst amount increased the number of available catalytic acid active sites responsible for promoting the esterification process. The effect of the molar ratio of methanol to acid was also studied. In fact, the stoichiometric ratio of methanol to oleic acid for esterification is defined as one. However, to drive the reaction equilibrium towards ester production, an excess amount of methanol is recommended.12 As shown in Fig. 9c, the yield of methyl oleates increased from 65.1% to 85.47% by increasing the molar ratio from 6
1 and reaction time of 60 min). The oleic acid conversion was found to increase with an increase in temperature due to the higher molecular collisions between the reagents as a result of the improved mass transfer, which increased the reaction rate.51 To investigate the effect of the catalyst amount on the esterification conversion, different amounts of catalyst ranging from 5 to 12 wt% were used, while keeping the other experimental conditions constant. Based on the results obtained (Fig. 9b), the conversion increased as the catalyst amount increased from 5 to 10 wt%. Therefore, the catalyst amount of 10 wt% was selected as the optimum value given that further increasing the catalyst amount to 12 wt% resulted in an OA conversion increase of only 6.45% with a final total value of 80%. In general, increasing the catalyst amount increased the number of available catalytic acid active sites responsible for promoting the esterification process. The effect of the molar ratio of methanol to acid was also studied. In fact, the stoichiometric ratio of methanol to oleic acid for esterification is defined as one. However, to drive the reaction equilibrium towards ester production, an excess amount of methanol is recommended.12 As shown in Fig. 9c, the yield of methyl oleates increased from 65.1% to 85.47% by increasing the molar ratio from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while keeping the other parameters constant. However, the conversion remained constant with a further increase in the molar ratio to 12
1, while keeping the other parameters constant. However, the conversion remained constant with a further increase in the molar ratio to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reaction duration was varied from 10 to 60 min in the presence of 7 wt% of HAP-S1 catalyst and the optimal methanol to acid molar ratio of 10
1. The reaction duration was varied from 10 to 60 min in the presence of 7 wt% of HAP-S1 catalyst and the optimal methanol to acid molar ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As shown in Fig. 9d, the conversion increased with the prolongation of the reaction time. After 10 min of reaction under MW irradiation, a yield of 42.75% was obtained. Increasing the reaction time to 40 min resulted in a methyl oleate yield of 85%. Thus, this duration was determined to be the optimal reaction time. Therefore, it should be noted that in general, the catalytic performances are also related to the strength and density of the catalytic sites.32,52
1. As shown in Fig. 9d, the conversion increased with the prolongation of the reaction time. After 10 min of reaction under MW irradiation, a yield of 42.75% was obtained. Increasing the reaction time to 40 min resulted in a methyl oleate yield of 85%. Thus, this duration was determined to be the optimal reaction time. Therefore, it should be noted that in general, the catalytic performances are also related to the strength and density of the catalytic sites.32,52
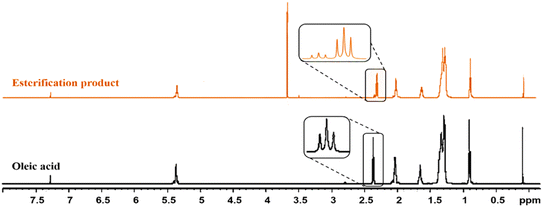
As shown, the typical triplets of the oleic acid methylene group (α-CH2) adjacent to the carbonyl group are present in the oleic acid spectrum at 2.34–2.37 ppm. Under the optimal conditions, the spectrum of methyl oleate revealed the presence of new characteristic peaks, with a triplet appearing at 2.30–2.33 ppm assigned to the (α-CH2) of methyl oleate, and these signals appeared at a chemical shift lower than that of (α-CH2) of the carboxylic group due to their deshielding effect. In fact, the intensities of these triplets are proportional to the concentration of each molecule in the final product. In addition, a new intense singlet appeared at 3.67 ppm, which is attributed to the protons of the methoxy group (R–O–CH3) of the methyl esters.
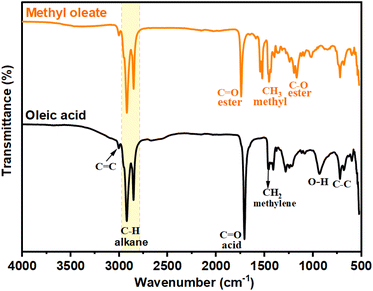
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl groups present in the carboxyl group of oleic acid, and the intense peak at 1739 cm−1 confirms the stretching vibration of C
O carbonyl groups present in the carboxyl group of oleic acid, and the intense peak at 1739 cm−1 confirms the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O present in the esters.
O present in the esters.
3.2. Possible esterification mechanism
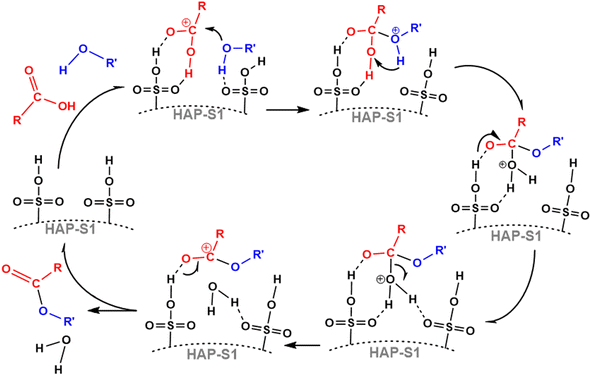
The esterification process is the combination of an organic acid with an alcohol to form an ester. From a mechanistic point of view, the esterification process is a catalytic nucleophilic acyl substitution that occurs based on the nature of electrophilicity of the carbon atom of carbonyl and the nucleophilicity of the alcohol. Based on this information, we propose a reasonable mechanism for the esterification reaction over the HAP-S1 catalyst. In fact, two plausible mechanisms can be proposed according to the literature because there are two different active sites in the active sulfonic group, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and S–O–H, as described by Vafaeezadeh and Fattahi53 and Dou et al.54 Therefore, we hypothesize that the reaction process probably follows a dual-site mechanism, in which two separate SO3H groups are involved in the reaction pathway, as shown in the Scheme 1. Firstly, the interaction of the oleic acid carbonyl with the Brønsted acid site (S–O–H group) of the catalyst surface leads to the formation of a carbocation (electro-deficient carbon of the acid). Similarly, the adsorption of the adjacent Lewis acid site (S
O and S–O–H, as described by Vafaeezadeh and Fattahi53 and Dou et al.54 Therefore, we hypothesize that the reaction process probably follows a dual-site mechanism, in which two separate SO3H groups are involved in the reaction pathway, as shown in the Scheme 1. Firstly, the interaction of the oleic acid carbonyl with the Brønsted acid site (S–O–H group) of the catalyst surface leads to the formation of a carbocation (electro-deficient carbon of the acid). Similarly, the adsorption of the adjacent Lewis acid site (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group) via the hydroxyl oxygen leads to the formation of an h-bonded alcohol molecule, which attacks the protonated carbonyl group to form a tetrahedral intermediate. Subsequently, internal rearrangement, followed by the elimination of a water molecule from the obtained intermediate results in the production of methyl oleates (Scheme 1).
O group) via the hydroxyl oxygen leads to the formation of an h-bonded alcohol molecule, which attacks the protonated carbonyl group to form a tetrahedral intermediate. Subsequently, internal rearrangement, followed by the elimination of a water molecule from the obtained intermediate results in the production of methyl oleates (Scheme 1).
3.3. Transesterification of rapeseed oil
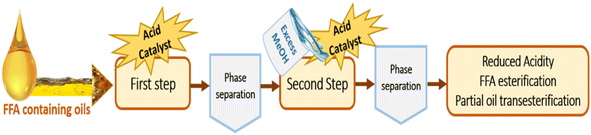
The results obtained for the rapeseed oil transesterification indicate that a conversion of only 17.6% was achieved after 40 min of reaction. Alternatively, for a longer reaction time of 60 min, the conversion reached 40.2%. Thus, based on the obtained results, the acid sulfonated apatite could promote the conversion of vegetable oil. However, the kinetic rate was found to be lower than that of the base-catalyzed process. In general, acid-catalyzed transesterification is not widely applied due to the requirement of catalysts with a high total acid site density,32 high alcohol to oil molar ratio, longer reaction time and additional energy to achieve high yields.56–58 Therefore, the majority of sulfonated acid catalysts are evaluated for the esterification of free fatty acids.3.4. Double-step esterification/transesterification of acidic oil
After the successful application of the selected acid catalyst in the esterification of oleic acid and its potential application in the transesterification reaction, we aimed to study it in the esterification of FFA-containing oils to investigate the ability of the catalyst to perform simultaneous esterification and transesterification reactions. In this context, a mixture of rapeseed oil with 5 wt% oleic acid was prepared and used as a model feedstock for the production of biodiesel from a low-cost feedstock. The initial acid value of the feedstock was found to be 15.26 mg KOH per g. Esterification was carried out under the optimized conditions for 60 min. At the end of the reaction, the catalyst was separated by centrifugation and the excess methanol was evaporated by rotary evaporation. Based on the titration method, the acid value was determined to be 7.5 mg KOH per g. The catalyst could reduce the acidity of the original feedstock by 50.85%. The evaporated ester phase containing the remaining FFA, untransesterified triglycerides, diglycerides and monoglyceride was introduced in a second MW-driven reaction, in which 10 wt% of fresh catalyst was added in addition to an amount of methanol equal to that introduced in the first step. The final product exhibited an acid value of 3.1 mg KOH per g, and thus the total FFA conversion after the two-step reaction was 79.6% (according to titration results) with a total FAME production of 40.13% (Table 3), as determined by 1H-NMR according to the method reported in the literature.34,59 In fact, the competitive FFA esterification and TG transesterification by the acid catalytic sites and the possible hydrolysis of TG by the generated water may limit the total biodiesel yield.32,60 Also, the reaction mechanisms of the two pathways are different given that the methanolysis of FFAs proceeds via simple esterification, while TGs proceed via transesterification, which involves several consecutive steps.61 A relevant aspect should also be noted, where the achieving high yields by simultaneous esterification/transesterification is related to the strength of the catalytic sites and their high surface density, in addition to the further optimization of the reaction parameters, which can play an important role increasing the conversion.52| Feedstock's initial acid value (mg KOH per g) | First step | Second step | |
|---|---|---|---|
| First reaction acid value (mg KOH per g) | Second reaction acid value (mg KOH per g) | Total FAME yield (%) | |
| 15.26 | 7.5 | 3.11 | 40.13 |
Based on these results, it can be concluded that the HAP-S1 catalyst is a potential solid acid catalyst for FFA removal from acidic feedstocks. Consequently, given that the acid value of the final product was less than 4 mg KOH per g after two reaction runs, it is recommended to adopt an alkali-catalyzed transesterification to increase the biodiesel yield and achieve the ester value required by ASTM and EN 14214.19,62–64 A summary of the two-step process adopted is shown in Fig. 12.
3.5. Comparison with reported catalysts for the microwave-assisted esterification of oleic acid under
To allow a proper comparison with the results obtained herein, the data of the relevant works published in the literature dealing with the microwave-assisted esterification of oleic acid for biodiesel are presented in Table 4. The synthesized catalyst showed competitive catalytic activity with respect to other catalytic systems such as sulfated zirconia, Nb2O5, sulfonated silica I-ISS-300, waste flint kaolin, MOF-based catalyst UiO-66-SO3H, ionic liquid [Bmim]HSO4 and homogeneous H2SO4 catalyzed system. Moderate alcohol to acid molar ratios were reported for entries 2 to 6, but high molar ratios were required (for 7 and 8) to achieve the optimal conversion of OA to OAME.| Entry | Catalyst | Time (min) | T (°C) | Catalyst amount (wt%) | Methanol/oleic acid | Methyl oleate yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | HAP-S1 | 40 | 150 | 10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 | This work |
| 2 | Sulfated zirconia | 20 | 200 | 5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
68.7 | 65 |
| 3 | Nb2O5 | 20 | 200 | 5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
68 | 65 |
| 4 | I-ISS-300 | 30 | 120 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 | 66 | |
| 5 | [Bmim]HSO4 | 30 | 120 | 10 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84.7 | 67 |
| 6 | H2SO4 | 30 | 120 | 10 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87.7 | 67 |
| 7 | Wasted flint kaolin | 40 | 115 | — | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96.5 | 68 |
| 8 | UiO-66-SO3H | 60 | 100 | 8 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
98.3 | 69 |
4. Conclusion
In this study, hydroxyapatite-derived natural phosphate was prepared via the dissolution–precipitation of natural Moroccan phosphate in nitric acid. Subsequently, the hydroxyapatite was treated with chlorosulfonic acid in dichloromethane at three acidity levels. Specifically, the HAP-S1 material was investigated in the oleic acid esterification, resulting in an optimal oleic acid methyl ester yield of 87% after 40 min of reaction under microwave irradiation. This confirmed the synergistic influence between microwave irradiation and the prepared catalyst. Also, the catalyst was tested in the transesterification reaction of rapeseed oil, but the final yield was found to be lower than that of the esterification reaction, given that a lower transesterification rate was observed. Furthermore, the catalyst was found to be a potential catalyst for the esterification of FFA-containing feedstocks, given that it could reduce the FFA content of acidified rapeseed oil as a model of a low-quality feedstock after a two-step process. However, the extra purity of the acid used and the meticulous preparation procedure may affect the catalyst preparation procedure and increase the overall cost of catalyst preparation, despite the abundance of the apatite source in Morocco. Thus, further research should be conducted to evaluate the energetic and economic aspects of the developed material for the synthesis of biodiesel.Data availability
Data for this article, including (material characterisation and conversion results) are available from the corresponding author, Dr Boutaina REZKI. All collected data are present in the paper as figures or tables. The data can be requested from the corresponding author.Author contributions
Boutaina Rezki: conceptualization, investigation, data curation, methodology, formal analysis, writing original draft, writing – review & editing. Younes Essamlali: formal analysis, visualization, validation. Othmane Amadine: provided help for biodiesel analysis realization. Said Sair: SEM-mapping analysis for the materials. Mina Aadil: co-supervision. Mohamed Zahouily: project administration, supervision, validation, writing – review & editing and resource.Conflicts of interest
The authors declare that there are no conflicts of interest to declare.Acknowledgements
Authors express their gratitude to the University of Hassan II of Casablanca in Morocco. Authors also thank the Moroccan Foundation for Advanced Science, Innovation and Research (MAScIR) – UM6P for characterization facilities. Dr Boutaina Rezki express special thanks to Dr Oussama Oulhakem, Dr Abdallah Nayad, Dr Az-Eddine El Mansouri and Dr Dalia Allouss for their assistance.References
- R. Vitiello, F. Taddeo, V. Russo, R. Turco, A. Buonerba and A. Grassi, et al., Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts, ChemEngineering, 2021, 5, 1–14 Search PubMed.
- N. Z. Abdul Kapor, G. P. Maniam, M. H. A. Rahim and M. M. Yusoff, Palm fatty acid distillate as a potential source for biodiesel production-a review, J. Cleaner Prod., 2017, 143, 1–9, DOI:10.1016/j.jclepro.2016.12.163.
- H. A. Farag, A. El-maghraby and N. A. Taha, Optimization of factors affecting esterification of mixed oil with high percentage of free fatty acid, Fuel Process. Technol., 2011, 92, 507–510, DOI:10.1016/j.fuproc.2010.11.004.
- P. Saxena, S. Jawale and M. H. Joshipura, A review on prediction of properties of biodiesel and blends of biodiesel, Procedia Eng., 2013, 51, 395–402, DOI:10.1016/j.proeng.2013.01.055.
- R. N. Vilas-Bôas, L. Lucchetti, L. D. Fernandes, M. A. S. da Costa and M. F. Mendes, Preparation and Utilization of Hydroxyapatite-Supported Na and CaO–CeO2 Catalysts for Biodiesel Production Using Vegetable Oil Deodorization Distillate as Raw Material, Catal. Lett., 2023, 153, 2456–2470, DOI:10.1007/s10562-022-04173-2.
- I. Atadashi, M. Aroua, A. A. Aziz and N. M. Sulaiman, The effects of catalysts in biodiesel production: A review, J. Ind. Eng. Chem., 2013, 19, 14–26, DOI:10.1016/j.jiec.2012.07.009.
- P. C. Duarte, F. Carvalho Ballotin, H. Limborço, J. Domingos Ardisson, R. Montero Lago and C. T. Ana Paula de, Heterogeneous acid catalyst based on sulfated iron ore tailings for oleic acid esterification, Appl. Catal., A, 2020, 600, 117624, DOI:10.1016/j.apcata.2020.117624.
- S. Soltani, U. Rashid, I. Arbi Nehdi and S. Ibrahim Al-Rasayes, Esterification of Palm Fatty Acid Distillate Using a Sulfonated Mesoporous CuO-ZnO Mixed Metal Oxide Catalyst, Chem. Eng. Technol., 2017, 1–10, DOI:10.1002/ceat.201700138.
- L. Tumkot, A. T. Quitain, P. Boonnoun, N. Laosiripojana, T. Kida and A. Shotipruk, Synergizing Sulfonated Hydrothermal Carbon and Microwave Irradiation for Intensified Esterification Reaction, ACS Omega, 2020, 5, 23542–23548, DOI:10.1021/acsomega.0c01660.
- S. Zhang, Y. Zu, Y. Fu, M. Luo, D. Zhang and T. Efferth, Rapid microwave-assisted transesterification of yellow horn oil to biodiesel using a heteropolyacid solid catalyst, Bioresour. Technol., 2010, 101, 931–936, DOI:10.1016/j.biortech.2009.08.069.
- N. Narkhede, S. Singh and A. Patel, Recent Progress on Supported Polyoxometalates for Biodiesel Synthesis via Esterification and Transesterification, Green Chem., 2015, 17, 89–107, 10.1039/C4GC01743A.
- Y. Essamlali, M. Larzek, B. Essaid and M. Zahouily, Natural phosphate supported titania as a novel solid acid catalyst for oleic acid esterification, Ind. Eng. Chem. Res., 2017, 56, 5821–5832, DOI:10.1021/acs.iecr.7b00607.
- R. Chakraborty and D. Roychowdhury, Optimization of biological-hydroxyapatite supported iron catalyzed methyl oleate synthesis using response surface methodology, J. Taiwan Inst. Chem. Eng., 2013, 45, 92–100, DOI:10.1016/j.jtice.2013.05.006.
- R. Chakraborty and D. Roychowdhury, Fish bone derived natural hydroxyapatite-supported copper acid catalyst: Taguchi optimization of semibatch oleic acid esterification, Chem. Eng. J., 2013, 215–216, 491–499, DOI:10.1016/j.cej.2012.11.064.
- J. V. Ruatpuia, G. Halder, S. Mohan, B. Gurunathan, H. Li and F. Chai, et al., Microwave-assisted biodiesel production using ZIF-8 MOF-derived nanocatalyst: A process optimization, kinetics, thermodynamics and life cycle cost analysis, Energy Convers. Manage., 2023, 292, 117418, DOI:10.1016/j.enconman.2023.117418.
- V. K. Mishra and R. Goswami, A review of production, properties and advantages of biodiesel, Biofuels, 2017, 9, 273–289, DOI:10.1080/17597269.2017.1336350.
- Q. Chen, A. Wang, W. Quan and W. Gang, Efficient synthesis of biodiesel from Hyoscyamus niger L. seed oil by base catalysis, Fuel Process. Technol., 2023, 241, 107630, DOI:10.1016/j.fuproc.2022.107630.
- S. Elias, A. M. Rabiu, B. I. Okeleye, V. Okudoh and O. Oyekola, Bifunctional Heterogeneous Catalyst for Biodiesel Production from Waste Vegetable Oil, Appl. Sci., 2020, 10, 3153, DOI:10.3390/app10093153.
- B. Rezki, Y. Essamlali, M. Aadil, N. Semlal and M. Zahouily, Biodiesel production from rapeseed oil and low free fatty acid waste cooking oil using a cesium modified natural phosphate catalyst, RSC Adv., 2020, 3, 41065–41077, 10.1039/d0ra07711a.
- I. Cacciotti, Cationic and Anionic Substitutions in Hydroxyapatite, Handbook of Bioceramics and Biocomposites, 2016, pp. 145–211, DOI:10.1007/978-3-319-12460-5.
- B. Rezki, Y. Essamlali, O. Amadine, S. Sair, M. Aadil and C. Len, et al., A comprehensive review on apatite-derived catalysts for sustainable biodiesel production: Classification, features and challenges, J. Environ. Chem. Eng., 2024, 12, 111913, DOI:10.1016/j.jece.2024.111913.
- A. El Mansouri, A. Oubella, M. Maatallah, M. Y. Aititto, M. Zahouily and H. Morjani, et al., Design, synthesis, biological evaluation and molecular docking of new uracil analogs-1,2,4-oxadiazole hybrids as potential anticancer agents, Bioorg. Med. Chem. Lett., 2020, 30, 127438, DOI:10.1016/j.bmcl.2020.127438.
- H. Yook, J. Hwang, W. Yeo, J. Bang, J. Kim and T. Y. Kim, et al., Design Strategies for Hydroxyapatite-Based Materials to Enhance Their Catalytic Performance and Applicability, Adv. Mater., 2022, 35, 1–36, DOI:10.1002/adma.202204938.
- I. El yaakouby, I. Rhrissi, Y. Abouliatim, M. Hlaibi and N. Kamil, Moroccan sardine scales as a novel and renewable source of heterogeneous catalyst for biodiesel production using palm fatty acid distillate, Renewable Energy, 2023, 217, 119223, DOI:10.1016/j.renene.2023.119223.
- L. Ma'mani, M. Sheykhan, A. Heydari, M. Faraji and Y. Yamini, Sulfonic acid supported on hydroxyapatite-encapsulated-γ-Fe2O3 nanocrystallites as a magnetically Brønsted acid for N-formylation of amines, Appl. Catal., A, 2010, 377, 64–69, DOI:10.1016/j.apcata.2010.01.020.
- M. Mamaghani, Z. Taati, M. Rasoulian, J. Yousefizad, N. Toraji and M. Mohsenimehr, et al., [γ-Fe2O3@HAp-SO3H] an efficient nanocatalyst for the synthesis of highly functionalised 2-thioxopyrido[2,3-d]pyrimidines, J. Chem. Res., 2016, 40, 29–34, DOI:10.3184/174751916X14495860660659.
- K. F. Sina, A. Yahyazadeh, N. O. Mahmoodi, K. R. Moghadam, M. Nikpassand and M. Rasa, Ultrasonic Assisted Synthesis of 2, 3-Dihydroquinazolin-4(1H)-ones Involving Three-Component Reaction of Isatoic Anhydride, Amines and Pyrazole Carbaldehydes Catalyzed by [Ɣ-Fe2O3@HAp-SO3H], Lett. Org. Chem., 2020, 17, 24–30, DOI:10.2174/1570178616666190401183953.
- J. Deng, L.-P. Mo, F.-Y. Zhao, L.-L. Hou, L. Yang and Z.-H. Zhang, Sulfonic acid supported on hydroxyapatite-encapsulated -γ-Fe2O3 nanocrystallites as a magnetically separable catalyst for one-pot reductive amination of carbonyl compounds, Green Chem., 2011, 13, 2576–2584, 10.1039/c1gc15470b.
- N. Yousefi and S. Batool, Sulfonated nanohydroxyapatite functionalized with 2-aminoethyl dihydrogen phosphate (HAP@AEPH2-SO3H) as a reusable solid acid for direct esterification of carboxylic acids with alcohols, Res. Chem. Intermed., 2016, 42, 5789–5806, DOI:10.1007/s11164-015-2404-8.
- V. Akubude, K. Jaiyeoba, T. Oyewusi, E. Abbah, J. Oyedokum and V. Okafor, Overview on Different Reactors for Biodiesel Production, 2021, pp. 341–59 Search PubMed.
- S. Nomanbhay and M. Yin Ong, A Review of Microwave-Assisted Reactions for Biodiesel Production, Bioengineering, 2017, 4, 1–21, DOI:10.3390/bioengineering4020057.
- S. Chellappan, K. Aparna, C. Chingakham, V. Sajith and V. Nair, Microwave assisted biodiesel production using a novel Brønsted acid catalyst based on nanomagnetic biocomposite, Fuel, 2019, 246, 268–276, DOI:10.1016/j.fuel.2019.02.104.
- S. El Asri, A. Laghzizil, A. Saoiabi, A. Alaoui, K. El Abassi and R. M'hamdi, et al., A novel process for the fabrication of nanoporous apatites from Moroccan phosphate rock, Colloids Surf., A, 2009, 350, 73–78, DOI:10.1016/j.colsurfa.2009.09.006.
- L. A. Anderson and A. K. Franz, Real-Time Monitoring of Transesterification by 1 H NMR Spectroscopy: Catalyst Comparison and Improved Calculation for Biodiesel Conversion, Energy Fuels, 2012, 26, 6404–6410 Search PubMed.
- K. C. V. Kumar, T. J. Subha, K. Ahila, B. Ravindran, S. Chang and A. H. Mahmoud, et al., Spectral characterization of hydroxyapatite extracted from Black Sumatra and Fighting cock bone samples : A comparative analysis, Saudi J. Biol. Sci., 2020, 28, 840–846, DOI:10.1016/j.sjbs.2020.11.020.
- S. Dorozhkin, Dissolution mechanism of calcium apatites in acids: A review of literature, World J. Methodol., 2012, 2, 1–17, DOI:10.5662/wjm.v2.i1.1.
- S. O. Nur, U. Rashid, M. Sufri Mastuli and Y. Hin Taufiq-Yap, Esterification of palm fatty acid distillate (PFAD) to biodiesel using bi-functional catalyst synthesized from waste angel wing shell (Cyrtopleura costata), Renewable Energy, 2019, 131, 187–196, DOI:10.1016/j.renene.2018.07.031.
- M. Zarghani and B. Akhlaghinia, Sulfonated nanohydroxyapatite functionalized with 2-aminoethyl dihydrogen phosphate (HAP@AEPH2-SO3H) as a new recyclable and eco-friendly catalyst for rapid one-pot synthesis of 4,4′-(aryl methylene)bis(3-methyl-1H-pyrazol-5-ol)s, RSC Adv., 2015, 5, 87769–87780, 10.1039/c5ra16236j.
- Å. Bengtsson and S. Sjöberg, Surface complexation and proton-promoted dissolution in aqueous apatite systems, Pure Appl. Chem., 2009, 81, 1569–1584, DOI:10.1351/PAC-CON-08-10-02.
- R. Jahanshahi and B. Akhlaghinia, Sulfonated Honeycomb Coral (HC-SO3H): a new, green and highly efficient heterogeneous catalyst for the rapid one-pot bis (3-methyl-1H-pyrazol-5-ol)s, Chem. Pap., 2017, 71, 1351–1364, DOI:10.1007/s11696-016-0127-y.
- A. Oulguidoum, H. Bouyarmane, A. Laghzizil, J. Nunzi and A. Saoiabi, Development of sulfonate-functionalized hydroxyapatite nanoparticles for cadmium removal from aqueous solutions, Colloid Interface Sci. Commun., 2019, 30, 100178, DOI:10.1016/j.colcom.2019.100178.
- S. Seesanong, C. Seangarun, B. Boonchom, N. Laohavisuti, K. Chaiseeda and W. Boonmee, Composition and Properties of Triple Superphosphate Obtained from Oyster Shells and Various Concentrations of Phosphoric Acid, ACS Omega, 2021, 6, 22065–22072, DOI:10.1021/acsomega.1c02660.
- B. Boonchom, Parallelogram-like microparticles of calcium dihydrogen phosphate monohydrate (Ca(H2PO4)2·H2O) obtained by a rapid precipitation route in aqueous and acetone media, J. Alloys Compd., 2009, 482, 199–202, DOI:10.1016/j.jallcom.2009.03.157.
- W. P. Rothwell, J. Waugh and J. P. Yesinowskit, High-Resolution Variable-Temperature 31P NMR of Solid Calcium Phosphates, J. Am. Chem. Soc., 1980, 102, 2637–2643 Search PubMed.
- B. Louati, F. Hlel, K. Guidara and M. Gargouri, Analysis of the effects of thermal treatments on CaHPO4 by 31P NMR spectroscopy, J. Alloys Compd., 2005, 394, 13–18, DOI:10.1016/j.jallcom.2004.10.025.
- S. Verma and R. Murugavel, Di-tert-butylphosphate Derived Thermolabile Calcium Organophosphates: Precursors for Ca(H2PO4)2, Ca(HPO4), α-/β-Ca(PO3)2, and Nanocrystalline Ca10(PO4)6(OH)2 Sonam, Inorg. Chem., 2020, 59, 13233–13244, DOI:10.1021/acs.inorgchem.0c01591.
- Y. Wang, M. Tang, A. Yusuf, Y. Wang, X. Zhang and G. Yang, et al., Preparation of Catalyst from Phosphorous Rock Using an Improved Wet Process for Transesterification Reaction, Ind. Eng. Chem. Res., 2021, 60, 8094–8107, DOI:10.1021/acs.iecr.1c01072.
- C. Chen, H. Zheng, S. Tang, T. Zhang and L. Lv, Study on Controlling the Dissolution of Calcium Dihydrogen Phosphate Using Ternary Phase Diagrams to Improve the Production Process of Phosphoric Acid, Ind. Eng. Chem. Res., 2022, 61(43), 15970–15979, DOI:10.1021/acs.iecr.2c03146.
- P. Yin, L. Chen, Z. Wang, R. Qu, X. Liu and Q. Xu, et al., Biodiesel production from esterification of oleic acid over aminophosphonic acid resin D418, Fuel, 2012, 102, 499–505, DOI:10.1016/j.fuel.2012.05.027.
- C. A. R. Melo-Júnior, C. E. R. Albuquerque, M. Fortuny, C. Dariva, S. Egues and A. F. Santos, et al., Use of microwave irradiation in the noncatalytic esterification of C18 fatty acids, Energy Fuels, 2009, 23, 580–585, DOI:10.1021/ef800766x.
- L. Tumkot, A. T. Quitain, P. Boonnoun, N. Laosiripojana, T. Kida and A. Shotipruk, Synergizing sulfonated hydrothermal carbon and microwave irradiation for intensified esterification reaction, ACS Omega, 2020, 5, 23542–23548, DOI:10.1021/acsomega.0c01660.
- S. Chellappan, V. Nair, V. Sajith and K. Aparna, Experimental validation of biochar based green Bronsted acid catalysts for simultaneous esterification and transesterification in biodiesel production, Bioresour. Technol. Rep., 2018, 28–44, DOI:10.1016/j.biteb.2018.04.002.
- M. Vafaeezadeh and A. Fattahi, DFT investigations for “Fischer” esterification mechanism over silica-propyl-SO3H catalyst: is the reaction reversible?, Comput. Theor. Chem., 2015, 1071, 27–32, DOI:10.1016/j.comptc.2015.07.028.
- Y. Dou, H. Zhang, A. Zhou, F. Yang, L. Shu and Y. She, et al., Highly efficient catalytic esterification in a -SO3H functionalized Cr(III)-MOF, Ind. Eng. Chem. Res., 2018, 57, 8388–8395, DOI:10.1021/acs.iecr.8b01239.
- J. M. Fraile, E. García-bordejé and L. Roldán, Deactivation of sulfonated hydrothermal carbons in the presence of alcohols: Evidences for sulfonic esters formation, J. Catal., 2012, 289, 73–79, DOI:10.1016/j.jcat.2012.01.017.
- J. A. Melero, L. F. Bautista, G. Morales, J. Iglesias and D. Briones, Biodiesel Production with Heterogeneous Sulfonic Acid-Functionalized Mesostructured Catalysts, Energy Fuels, 2009, 23, 539–547 Search PubMed.
- V. Mandari and S. K. Devarai, Biodiesel Production Using Homogeneous, Heterogeneous, and Enzyme Catalysts via Transesterification and Esterification Reactions: a Critical Review, BioEnergy Res., 2021, 15, 935–961, DOI:10.1007/s12155-021-10333-w.
- M. Chai, Q. Tu, M. Lu and Y. J. Yang, Esterification pretreatment of free fatty acid in biodiesel production, from laboratory to industry, Fuel Process. Technol., 2014, 15, 106–113 Search PubMed.
- G. Gelbard, O. Br, R. M. Vargas, F. Vielfaure and U. E. Schuchardt, 1H Nuclear Magnetic Resonance Determination of the Yield of the Transesterification of Rapeseed Oil with Methanol, J. Am. Oil Chem. Soc., 1995, 72, 1239–1241 Search PubMed.
- N. Boz, N. Degirmenbasi and D. M. Kalyon, Esterification and Transesterification of Waste Cooking Oil over Amberlyst 15 and Modified Amberlyst 15 Catalysts, Appl. Catal., B, 2014, 165, 723–730, DOI:10.1016/j.apcatb.2014.10.079.
- S. Martinez Luz, R. Romero, R. Natividad and J. González, Optimization of biodiesel production from sunflower oil by transesterification using Na2O/NaX and methanol, Catal. Today, 2014, 220–222, 12–20, DOI:10.1016/j.cattod.2013.08.022.
- R. Sheikh, M. Choi, J. Im and Y. Park, Study on the solid acid catalysts in biodiesel production from high acid value oil, J. Ind. Eng. Chem., 2013, 19, 1413–1419, DOI:10.1016/j.jiec.2013.01.005.
- A. D. Ogunsola, M. O. Durowoju, A. O. Alade, S. O. Jekayinfa and O. Ogunkunle, Modeling and optimization of two-step shea butter oil biodiesel synthesis using snail shells as heterogeneous base, Energy Adv., 2022, 1, 113–128, 10.1039/d1ya00042j.
- S. Niju, F. Raj Russell, C. Anushya and M. Balajii, Optimization of acid catalyzed esterification and mixed metal oxide catalyzed transesterification for biodiesel production from Moringa oleifera oil, Green Process. Synth., 2019, 8, 756–775 Search PubMed.
- C. A. R. Melo Junior, C. E. R. Albuquerque, S. A. C. Juliana, C. Dariva, M. Fortuny and A. F. Santos, et al., Solid-Acid-Catalyzed Esterification of Oleic Acid Assisted by Microwave Heating, Ind. Eng. Chem. Res., 2010, 49, 12135–12139 Search PubMed.
- Z. Hasan, J. W. Yoon and S. H. Jhung, Esterification and acetylation reactions over in situ synthesized mesoporous sulfonated silica, Chem. Eng. J., 2014, 278, 105–112, DOI:10.1016/j.cej.2014.12.025.
- S. Bölük and Ö. Sönmez, Microwave-Assisted Esterification of Oleic Acid using an Ionic Liquid Catalyst, Chem. Eng. Technol., 2020, 43, 1–19, DOI:10.1002/ceat.202000045.
- A. D. O. Nazaré, L. Rafaela da Silva Costa, L. Helena de Oliveira Pires, L. S. d. N. Adriano, R. Angélica and E. F. d. C. Carlos, et al., Microwave-assisted preparation of a new esterification catalyst from wasted flint kaolin, Fuel, 2013, 103, 626–631, DOI:10.1016/j.fuel.2012.07.017.
- G. S. Prasad, K. Ngaosuwan, S. Assabumrungrat, M. Selvaraj, G. Halder and S. Lalthazuala Rokhum, Microwave assisted biodiesel production using sulfonic acid-functionalized metal-organic frameworks UiO-66 as a heterogeneous catalyst, Renewable Energy, 2022, 197, 161–169 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |