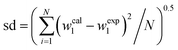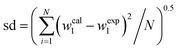DOI:
10.1039/D4RA05917D
(Paper)
RSC Adv., 2024,
14, 34253-34260
Application of deep eutectic solvent-based aqueous two phase systems for extraction of analgesic drugs
Received
15th August 2024
, Accepted 4th October 2024
First published on 28th October 2024
Abstract
The ability of biphasic-aqueous systems to efficiently and simultaneously purify active pharmaceutical compounds has led to extensive study of these systems. As a new environmentally friendly separation technology, deep eutectic solvent (DES)-based aqueous two-phase systems (ATPSs) are extensively applied for the extraction and separation of various bioactive compounds. In this study, two DES-based ATPSs consisting of choline chloride/fructose and choline chloride/glucose as DESs with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and tripotassium phosphate (K3PO4) were prepared. The measured binodal data correlated with Merchuk and Zafarani-Moattar et al. equations. Moreover, the ATPSs were employed to investigate the separation of pharmaceuticals. The partition coefficient and the effect of factors such as the concentration of the deep eutectic solvent on drug partitioning were investigated as novel discoveries. Drugs are likely to be removed in the top DES-rich phase, according to the current data. Finally, the compositions of five tie-lines for each ATPS were meticulously determined. Othmer–Tobias, Bancraft, and Setschenow equations were used for correlation of tie-line data.
1 and tripotassium phosphate (K3PO4) were prepared. The measured binodal data correlated with Merchuk and Zafarani-Moattar et al. equations. Moreover, the ATPSs were employed to investigate the separation of pharmaceuticals. The partition coefficient and the effect of factors such as the concentration of the deep eutectic solvent on drug partitioning were investigated as novel discoveries. Drugs are likely to be removed in the top DES-rich phase, according to the current data. Finally, the compositions of five tie-lines for each ATPS were meticulously determined. Othmer–Tobias, Bancraft, and Setschenow equations were used for correlation of tie-line data.
1. Introduction
The biotechnology industry has made great strides in the last thirty years in the production of biological products through the application of traditional techniques, including precipitation, membrane-based, and chromatography technologies, for the separation, recovery, and purification of biomolecules. Cost-effective and biocompatible methods are crucial to ensuring successful scalability and satisfying customer needs for biotechnology products.1 In separation operations, aqueous two phase systems (ATPSs) are widely used.2,3 These systems offer a sustainable and environmentally friendly alternative to conventional organic-water solvent extraction methods. Recent advancements in the field have focused on the use of deep eutectic solvents (DESs) to create ATPSs for a wide range of separation and analytical purposes.4–8
The novelty of DES-based ATPSs lies in their unique composition and tunable properties, which distinguish them from traditional ATPSs. Deep eutectic solvents are formed by mixing two or more components, typically a hydrogen bond donor and acceptor that combine to create a liquid at room temperature with enhanced solubility and selectivity for various compounds.9 These systems are recognized for their biodegradability, low toxicity, and sustainable nature, making them ideal candidates for green extraction methods.10–12 In the context of drug extraction, DES-based ATPSs offer several advantages, including higher efficiency in selectively partitioning bioactive compounds, improving the solubility of poorly water-soluble drugs, and facilitating the recovery of delicate pharmaceutical agents without compromising their stability.13,14 This innovative approach opens new possibilities for the extraction, separation, and purification of therapeutic drugs, presenting a more sustainable and effective alternative to conventional extraction techniques. The adaptability of DES-based ATPSs to a wide range of compounds highlights their potential for broader applications in the pharmaceutical industry, particularly in drug development and formulation. Their adaptability is based on their distinct molecular structure and chemical properties, which means that to develop their applications, it is necessary to comprehend their thermodynamics and phase behavior. Xu and colleagues have played a pioneering role in demonstrating the use of DESs as phase-forming components in ATPSs.5,6,15,16 They extracted proteins using sugar-based DESs. They created DESs for this reason, which are composed of mixes of D-glucose and D-sorbitol as HBD and ChCl as HBA. Their findings demonstrated the high protein extraction capacity of these sugar-based DESs. Subsequently, the utilization of Deep Eutectic Solvent/Aqueous Two-Phase Systems (DES/ATPS) was employed for the extraction of numerous natural active compounds, including organic raw materials,17 amino acids,18 pharmaceuticals,19 and enzymes such as pepsin.20 This system is characterized by a straightforward operational process and has demonstrated excellent performance in the large-scale recovery of biological products.21,22 However, investigations conducted by Coutinho and colleagues23 revealed that the hydrogen bond between the HBA and HBD of DES might break at higher water content in ATPS. Consequently, the initial molar ratios of HBA and HBD cannot be sustained, leading to their distribution in either the upper or lower phase. In such instances, the authentic DES ceases to exist, compromising the fusion-enhancing property of DES. Farias et al.23 proposed that a pseudo-ternary system can be established when both HBA and HBD exhibit high hydrophilicity and poor solubility in the other phase-forming components. This configuration enables the maintenance of the stoichiometry of the initial DES to a certain extent. This pseudo-ternary DES-based ATPS is categorized as a true quaternary system, preserving the initial molar ratios of HBD and HBA. This is crucial for ensuring the stability of DES properties under specific conditions.24,25 According to the results obtained from the sources, it has been determined that DESs have a high ability to form aqueous two-phase systems and separate pharmaceutical substances. Despite the challenges identified, DES-based ATPSs still hold considerable potential for applications in extraction procedures.23,26
Therefore, in this study, K3PO4 and two deep eutectic solvents made of fructose or glucose as the hydrogen bond donor (HBD) and choline chloride as the hydrogen bond acceptor (HBA) with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were created as aqueous two-phase systems. The experiments were carried out under atmospheric pressure and 298.15 K in temperature. For these ATPSs, the binodal and tie-line values were found. Equations from Zafarani-Moattar et al.27 and Merchuk28 were applied to fit the binodal data. Additionally, tie-lines were established for the ATPSs made up of water, K3PO4, and DESs. Tie-line compositions were modeled using the Othmer–Tobias and Bancraft equations29,30 and Setschenow-type equation.31 Our research delved into using these ATPSs to separate ibuprofen, acetaminophen, and aspirin. For this purpose, partition coefficients (K) and extraction efficiencies (EE%) of the studied drugs were calculated at various tie-lines to describe how the concentration of DESs and the characteristics of the drugs influenced their separation.
1 were created as aqueous two-phase systems. The experiments were carried out under atmospheric pressure and 298.15 K in temperature. For these ATPSs, the binodal and tie-line values were found. Equations from Zafarani-Moattar et al.27 and Merchuk28 were applied to fit the binodal data. Additionally, tie-lines were established for the ATPSs made up of water, K3PO4, and DESs. Tie-line compositions were modeled using the Othmer–Tobias and Bancraft equations29,30 and Setschenow-type equation.31 Our research delved into using these ATPSs to separate ibuprofen, acetaminophen, and aspirin. For this purpose, partition coefficients (K) and extraction efficiencies (EE%) of the studied drugs were calculated at various tie-lines to describe how the concentration of DESs and the characteristics of the drugs influenced their separation.
2. Experimental measurements
2.1. Chemicals
The specific information about the utilized chemicals, including details on purity, CAS number, and origin, is presented in Table 1. To prepare the solutions, double-distilled deionized water with a conductivity of 0.055 μS cm−1 was employed, and all chemicals were utilized without undergoing additional purification processes. The determination of water contents in the chemicals was conducted using the Karl-Fischer method.
Table 1 Information on used chemicalsa,b
| Chemicals |
Source |
CAS no. |
Mass percent (purity) |
| The suppliers provided the purities of the used components. Water contents were determined using the Karl-Fischer method. |
| Ibuprofen |
Zahravi (Iran) |
— |
≥990 |
| Acetaminophen |
Zahravi (Iran) |
— |
≥990 |
| Aspirin |
Zahravi (Iran) |
— |
≥990 |
| Choline chloride |
Daejung |
67-48-1 |
>0.990 |
| Fructose |
Merck |
57-48-7 |
>0.995 |
| Glucose |
Merck |
50-99-7 |
>0.995 |
| Tri-potassium phosphate (K3PO4) |
Merck |
7778-53-2 |
>0.980 |
2.2. Preparation of DES
In this study, two forms of DES were created in molar ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using fructose or glucose as the hydrogen bond donor (HBD) and choline chloride as the hydrogen bond acceptor (HBA). The process described in earlier investigations22,32 was followed in the fabrication of DES. First, sugars and choline chloride were mixed together in a 50 mL round-bottom flask. Next, using a hot plate stirrer, the flask was submerged in a paraffin oil bath that was heated. At 353.15 K, the mixture was agitated for two hours to produce a colorless, uniform liquid. A thermometer with an accuracy of ±0.01 K was applied to measure the DES temperature continuously. All samples were then properly preserved in airtight vials in a temperature-controlled environment. All the materials were dried before making the DES. Karl-Fischer analysis (Metrohm 751 GPD) was used to obtain the prepared DES water content, and the results showed a 0.0008 mass fraction of water content.
1 using fructose or glucose as the hydrogen bond donor (HBD) and choline chloride as the hydrogen bond acceptor (HBA). The process described in earlier investigations22,32 was followed in the fabrication of DES. First, sugars and choline chloride were mixed together in a 50 mL round-bottom flask. Next, using a hot plate stirrer, the flask was submerged in a paraffin oil bath that was heated. At 353.15 K, the mixture was agitated for two hours to produce a colorless, uniform liquid. A thermometer with an accuracy of ±0.01 K was applied to measure the DES temperature continuously. All samples were then properly preserved in airtight vials in a temperature-controlled environment. All the materials were dried before making the DES. Karl-Fischer analysis (Metrohm 751 GPD) was used to obtain the prepared DES water content, and the results showed a 0.0008 mass fraction of water content.
2.3. Methods
2.3.1. Preparation of binodal curves and tie-lines. The cloud point titration technique was applied to determine the binodal curves, adhering to accepted procedures that have been previously reported in investigations.26,33,34 The temperature and pressure used for the trials were 298.15 K and 85 kPa, respectively. In this method, amounts of DES solution were added dropwise to a K3PO4 solution gradually until a hazy solution appeared, signifying the presence of a biphasic zone. Water was then added drop by drop until a zone that was clear and monophasic was observed. The binodal curves were found using 60% (w/w) K3PO4 and 60% (w/w) DES. Stirring was done constantly during the experiment. Using an analytical balance (Shimadzu, 321-34553, Shimadzu Co., Japan) with a precision of ±10−7 kg, the mass fraction of the components was determined. It was found that there was a maximum uncertainty of 0.002 in the mass fraction determination of both salt and DES.For each ATPS, a total of five distinct solutions consisting of {K3PO4 + DES (ChCl/fructose or ChCl/glucose with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) + water} were created to identify the tie-lines. Using a mixture of DES, K3PO4, and water, a precise gravimetric preparation of the biphasic zone was performed for each tie-line, with an accuracy of ±10−7 kg. These solutions were vigorously stirred for 30 minutes; then, they were centrifuged and heated to 298 K in a water bath to achieve equilibrium. Then, analytical techniques were used to identify the compositions of each phase. After separation of the two phases, a flame photometer (JENVEY model PFP7, England) was used to determine the concentrations of potassium phosphate (K3PO4) in the top and bottom phases.
1 molar ratio) + water} were created to identify the tie-lines. Using a mixture of DES, K3PO4, and water, a precise gravimetric preparation of the biphasic zone was performed for each tie-line, with an accuracy of ±10−7 kg. These solutions were vigorously stirred for 30 minutes; then, they were centrifuged and heated to 298 K in a water bath to achieve equilibrium. Then, analytical techniques were used to identify the compositions of each phase. After separation of the two phases, a flame photometer (JENVEY model PFP7, England) was used to determine the concentrations of potassium phosphate (K3PO4) in the top and bottom phases.
The concentration of sugars was obtained using the phenol–sulfuric acid method.35,36 In this process, a test tube containing 1 mL of a 5% aqueous phenol solution was combined with a 2 mL aliquot of the carbohydrate solution. After that, 5 milliliters of sulfuric acid were quickly added to the mixture. To produce color, the sample tubes were immersed in a water bath at 298.15 K for 20 minutes after standing for 10 minutes and vortexing for 30 seconds. Light absorption was measured using a spectrophotometer (model: SPECORD 40-Series Analytik Jena AG-Germany).
The identical steps as previously described were used to prepare the reference solutions, with the exception that the 2 mL aliquot of sugar solution was substituted with double-distilled deionized water.
The refractive index method37 was used to calculate the concentration of choline chloride in both phases. A refractometer (ATAGO DR-A1, Japan) was used to test the refractive index of the solutions with 0.0001 precision. An estimated 0.0002 was the related uncertainty of the refractive index measurement.
The refractive index technique37 states that there is a relationship between the mass fractions of the relevant components and the refractive index of the solution, nD, for diluted aqueous solutions containing a choline chloride, sugar and salt for each phase of ATP. The relation between nD and mass fractions of salt, ws, choline chloride, wc and sugars, wsu, takes the following form:
| | |
nD = n0 + asws + acwc + asuwsu,
| (1) |
where
n0 represents the refractive index of pure water, determined to be 1.3325 at a temperature (
T) of 298.15 K. The constants
as,
ac and
asu, associated with salt, ChCl, and sugars, respectively, were derived from calibration plots of the refractive index corresponding to diluted solutions within the range of mass fraction, as presented in
Table 2. The method yielded an uncertainty of approximately 0.008 for the mass fraction determination of each component of the ATPs.
Table 2 The parameters of eqn (1), am, for ATPs containing the{ChCl (c)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sugars (su) + K3PO4 (s) + water (w)} system
sugars (su) + K3PO4 (s) + water (w)} system
| Material |
Constant |
Value |
C range (w/w) |
R2a |
| For the mass fraction range (C range) of each material, R2 denotes the corresponding correlation coefficient value of the linear calibration plot of the refractive index against the mass fraction of ChCl, salt, and sugars. |
| ChCl |
ac |
0.1452 |
0–0.08 |
0.9975 |
| K3PO4 |
as |
0.1308 |
0–0.10 |
0.9992 |
| Fructose |
asu |
0.1473 |
0–1.20 |
0.9973 |
| Glucose |
asu |
0.1474 |
0–1.20 |
0.9978 |
2.3.2. Separation of drugs. According to the previously determined phase diagrams of {DES (ChCl/fructose or ChCl/glucose) + K3PO4 + H2O} systems, five mixture compositions (presented in Table 3) were chosen to assess the efficacy of the investigated ATPs with respect to ibuprofen, acetaminophen, and aspirin partitioning and extraction efficiency. The general makeup of these combinations was intended to resemble that of the ones that were utilized to create the tie-lines. After vigorously swirling for half an hour, the prepared combinations were placed in a water bath and heated to the appropriate temperature. Following the observation of phase separation, 1 mL of each phase was carefully separated, combined, and supplemented by 0.002 mass fractions of ibuprofen, acetaminophen, and aspirin. To guarantee total phase separation and equilibrium, the samples were then centrifuged for 10 minutes at 2000 rpm and then placed in a water bath for 24 hours. UV spectroscopy (model: SPECORD 40-Series Analytik Jena AG-Germany) was used to measure the amounts of ibuprofen, acetaminophen, and aspirin in both phases following the establishment of equilibrium. The samples were suitably diluted and tested beside blanks that had the same phase composition without drug to reduce interference from the components present in the phases.38 In the UV spectrophotometric method for measuring drug concentration, the solvent's effect on UV absorption is first eliminated. This is typically done by using a blank cell containing only the solvent. After adjusting and zeroing the device against the blank, the sample containing the drug is placed into the UV cell, and its light absorption at a specific wavelength is measured.
Table 3 Mass fractions (wt%) of experimental binodal data for {DES (wDES) + K3PO4 (ws) + H2O} systems at 298.15 Ka
| wDES ChCl/fructose |
ws |
wDES ChCl/glucose |
ws |
| Standard uncertainties (u) for mass fraction, pressure, and temperature are u(wi) = 0.005; u(p) = 0.5 kPa; and u(T) = 0.05 K, respectively (0.68 level of confidence). |
| 41.56 |
26.70 |
43.12 |
24.64 |
| 41.10 |
26.91 |
42.56 |
24.99 |
| 40.39 |
27.32 |
42.06 |
25.14 |
| 39.76 |
27.68 |
41.36 |
25.58 |
| 38.8 |
28.15 |
38.98 |
26.70 |
| 37.91 |
28.58 |
37.18 |
27.67 |
| 36.81 |
29.06 |
35.57 |
28.48 |
| 35.68 |
29.65 |
33.79 |
29.39 |
| 34.45 |
30.3 |
32.13 |
30.42 |
| 33.19 |
30.99 |
30.5 |
31.35 |
| 31.92 |
31.58 |
28.89 |
32.33 |
| 30.89 |
32.26 |
27.48 |
33.27 |
| 29.7 |
32.98 |
25.97 |
34.34 |
| 28.49 |
33.57 |
24.61 |
35.24 |
| 27.42 |
34.24 |
23.31 |
36.04 |
| 26.12 |
34.87 |
22.08 |
36.89 |
Eqn (2) and (3) were used to determine the extraction efficiency (EE%) and partitioning coefficient (K), respectively. The results are as follows:
| |
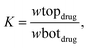 | (2) |
| |
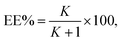 | (3) |
where
wbot
drug and
wtop
drug are the mass fractions of the drug in the bottom and top phases, respectively.
3. Results and discussion
In this study, K3PO4 and two deep eutectic solvents made of fructose or glucose as the hydrogen bond donor (HBD) and choline chloride as the hydrogen bond acceptor (HBA) with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were created as aqueous two-phase systems.
1 were created as aqueous two-phase systems.
3.1. Phase diagram
The binodal curves of obtained ATPs containing K3PO4, DES (with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of ChCl/fructose and ChCl/glucose), and water at 298.15 K are plotted in Fig. 1, and the experimental mass fraction data are listed in Table 3.
1 molar ratio of ChCl/fructose and ChCl/glucose), and water at 298.15 K are plotted in Fig. 1, and the experimental mass fraction data are listed in Table 3.
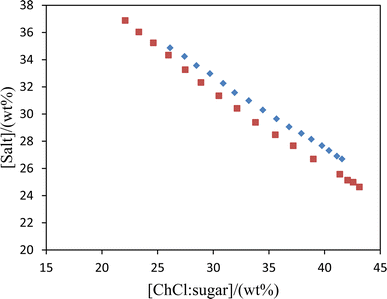 |
| | Fig. 1 Phase diagram for the systems {DES (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) + K3PO4 + water} at 298 K: ChlCl/fructose (♦); ChCl/glucose (■). 1 molar ratio) + K3PO4 + water} at 298 K: ChlCl/fructose (♦); ChCl/glucose (■). | |
The binodal data presented in Table 3 were fitted to the equations presented by Merchuk28 (eqn (4)) and Zafarani-Moattar et al.27 (eqn (5)) using a nonlinear least-square regression method:
| |
ws = a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(bwDES0.5 − cwDES3), exp(bwDES0.5 − cwDES3),
| (4) |
| |
ws = α + β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(wDES) + γwDES, ln(wDES) + γwDES,
| (5) |
where
ws and
wDES are mass fractions of salt and DES, respectively. The adjustable parameters of
eqn (4) and
(5) {(
a,
b, and
c) and (
α,
β and
γ)} together with the corresponding standard deviation, sd, are presented in
Table 4. The obtained sd values indicate the good performance of both equations in the regeneration of the binodal data.
Table 4 Parameters of eqn (4) and (5) for {K3PO4 + ChCl/sugars + H2O} systems at 298.15 K
| DES |
Merchuk (eqn (4)) |
| a |
b |
c |
100sda |
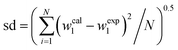 , where w1 and N represent the mass fraction of salt and the number of binodal data, respectively. , where w1 and N represent the mass fraction of salt and the number of binodal data, respectively. |
| ChCl/fructose |
1.7885 |
−2.3702 |
12.2545 |
0.09 |
| ChCl/glucose |
2.7008 |
−3.4492 |
8.0556 |
0.09 |
| DES |
Zafarani-Moattar et al. (eqn (5)) |
| α |
β |
γ |
100sd |
| ChCl/fructose |
0.0670 |
−0.3889 |
−0.6151 |
0.09 |
| ChCl/glucose |
−0.4069 |
−0.5707 |
0.1608 |
0.08 |
3.2. Analysis of tie-lines
Fig. 2 shows the tie-lines and binodal curves for the ATPs made of K3PO4 and NADES (ChCl/glucose or ChCl/fructose) at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Table 5 provides an overview of the experimental tie-lines and the tie-line lengths (TLL) for the studied systems. The top phase is constantly enriched in DES with varying tie-line lengths according to the data, which follow a consistent pattern. Higher K3PO4 concentrations than DES made up the majority of the bottom phase, suggesting a salt-rich phase.
1. Table 5 provides an overview of the experimental tie-lines and the tie-line lengths (TLL) for the studied systems. The top phase is constantly enriched in DES with varying tie-line lengths according to the data, which follow a consistent pattern. Higher K3PO4 concentrations than DES made up the majority of the bottom phase, suggesting a salt-rich phase.
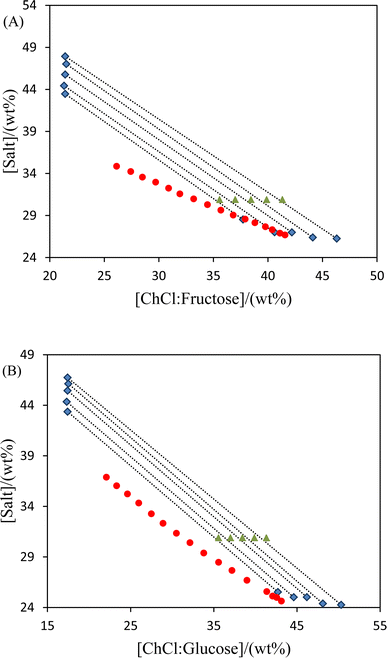 |
| | Fig. 2 Phase diagram and tie-line data for the systems {DES (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) + K3PO4 + water} at 298 K: (A) ChCl/fructose; (B) ChCl/glucose; binodal curve (●), overall composition of tie-line (▲), and phase composition (■). 1 molar ratio) + K3PO4 + water} at 298 K: (A) ChCl/fructose; (B) ChCl/glucose; binodal curve (●), overall composition of tie-line (▲), and phase composition (■). | |
Table 5 Tie-line experimental data at 298.15 K for the {ChCl (HBA) + sugars (HBD) + K3PO4 + H2O} systema
HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD HBD |
Overall composition/wt% |
ChCl-rich phase/wt% |
Salt-rich phase/wt% |
Molar ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
[HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD] HBD] |
[K3PO4] |
[HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD] HBD] |
[K3PO4] |
[HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD] HBD] |
[K3PO4] |
TLL |
| Standard uncertainties (u) for mass fraction, pressure, and temperature are u(wi) = 0.005; u(p) = 0.5 kPa; and u(T) = 0.05 K, respectively (0.68 level of confidence). |
| ChCl/fructose |
35.56 |
30.90 |
43.47 |
28.53 |
21.41 |
37.71 |
23.89 |
| ChCl/glucose |
42.71 |
25.53 |
17.41 |
43.37 |
30.96 |
| ChCl/fructose |
37.00 |
30.90 |
44.44 |
27.02 |
21.31 |
40.61 |
26.82 |
| ChCl/glucose |
44.61 |
25.02 |
17.31 |
44.35 |
33.45 |
| ChCl/fructose |
38.43 |
30.90 |
45.78 |
27.00 |
21.41 |
42.19 |
28.72 |
| ChCl/glucose |
46.19 |
25.03 |
17.41 |
45.45 |
35.29 |
| ChCl/fructose |
39.91 |
30.90 |
47.03 |
26.39 |
21.53 |
44.11 |
31.05 |
| ChCl/glucose |
48.11 |
24.39 |
17.51 |
46.13 |
37.54 |
| ChCl/fructose |
41.35 |
30.90 |
47.94 |
26.26 |
21.41 |
46.31 |
33.25 |
| ChCl/glucose |
50.31 |
24.26 |
17.41 |
46.74 |
39.85 |
3.2.1. Othmer–Tobias and Bancraft equations. The reliability of the obtained tie-lines was ascertained by the correlation of the Othmer–Tobias (eqn (6))29 and Bancroft (eqn (7)) equations:30| |
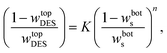 | (6) |
| |
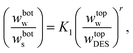 | (7) |
where K, n, K1 and r are fitted parameters. The parameters fitted by Othmer–Tobias and the model along with the associated (R2) and standard deviations are listed in Table 6.
Table 6 Values of parameters of Othmer–Tobias and Bancroft for {K3PO4 + ChCl/sugars + H2O} at T = 298.15 K
| Othmer–Tabias and Bancroft equations (eqn (6) and (7)) |
| |
K |
n |
K1 |
r |
| ChCl/glucose |
2.727 |
2.037 |
0.636 |
0.545 |
| ChCl/fructose |
2.792 |
2.068 |
0.649 |
0.553 |
The presence of a linear relationship between the two sides of the above equations indicates the acceptability of the obtained results. In the present work, we investigate this issue to clarify the results and witness the linearity of the relationship between the two sides and the acceptability and consistency of the obtained results. Using the deviations (Dev.) achieved, we conclude that eqn (6) and (7) can be applied to correlate the tie-lines of the mentioned systems.
3.2.2. Setschenow-type equation. To correlate tie-line data and investigate the salting-out ability in our studied ATPS systems composed of K3PO4, DES (obtained at one molar ratio of ChCl/fructose and ChCl/glucose (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)), and the water Setschenow equation31 at T = 298.15 K are used, which is a simple two-parameter relation proposed by Hey and coworkers:31
1)), and the water Setschenow equation31 at T = 298.15 K are used, which is a simple two-parameter relation proposed by Hey and coworkers:31| |
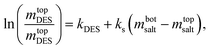 | (8) |
where ks is the salting-out coefficient, kNADES is a constant and mNADES and msalt are the molality of DES and K3PO4, respectively. To evaluate the reliability of eqn (8), in describing the soluting-out ability of DESs in these biocompatible ATPSs containing K3PO4 by determining the soluting-out coefficient, ks, from fitting experimental tie-lines the following objective function (eqn (9)) was used at studied temperature. The values of kNADES and ks are illustrated in Table 7 along with the correlation coefficient, R2, and sd values at different temperatures.| |
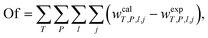 | (9) |
where wT,P,l,j represents the mass percent of the component j in the phase (DES, K3PO4 and water) for lth tie-line at working temperature. The superscripts “cal” and “exp” indicate the calculated and experimental values, respectively. The corresponding correlation coefficient values, R2, together with the tie-line experimental data, values of the fitted parameters (ks, kNADES) and deviations are listed in Table 7. This table shows that the ks values for the two studied systems are very similar. This value for the system countering ChCl/glucose is slightly higher than that of another system, which shows that the soluting-out effect of the DES composed of ChCl/glucose is a little more than that of the DES composed of ChCl/fructose.
Table 7 Values of parameters Setschenow-type, (kNADES, ks), for {K3PO4 + ChCl/sugars + H2O} at T = 298.15 Ka
| Setschenow type equation (eqn (8)) |
| System |
kNADES/kg K mol−1 |
ks/kg K mol−1 |
Dev. |
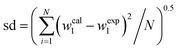 , where N and w1 denote the mass percent of salt and the number of binodal data, respectively. , where N and w1 denote the mass percent of salt and the number of binodal data, respectively. |
| ChCl/glucose |
1.7431 |
0.1181 |
0.03 |
| ChCl/fructose |
1.7341 |
0.1119 |
0.04 |
3.3. Partitioning behavior of drugs in the prepared ATPs
Table 8 shows the values of the extraction efficiency (EE%) and partition coefficients (K), which are determined using eqn (2) and (3) for the chosen drugs. As can be observed, we used five combinations with different weight percentages of DES (35.56, 37.00, 38.43, 39.91, and 41.35 wt%) and a constant concentration of salt (30.90 wt%) to investigate how changing DES concentration influences drug separation. The partition coefficients for ibuprofen, acetaminophen, and aspirin are shown in Fig. 3, and they increase as DES concentrations increase. Operating points are in a wider biphasic zone (longer tie-line) as the DES concentration increases. Additionally, these drugs are partitioned into the DES-rich phase, as shown in Fig. 4. More hydrogen bonds between DES molecules and drug molecules develop when the weight fraction of the DES is larger. Higher partition coefficients and greater extraction efficiency are the results of this improved interaction, which encourages greater drug partitioning into the DES phase. For both DESs, the observed trend due to the similar structure of sugars is very close and very slightly different as follows: K (ChCl/glucose) > K (ChCl/fructose).
Table 8 Partition coefficients (K) and extraction efficiency (EE%) for the {DES [ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sugar] + K3PO4 + H2O} system at 298.15 Ka
sugar] + K3PO4 + H2O} system at 298.15 Ka
Molar ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Overall composition/wt% |
K |
EE% |
| [DES] |
[K3PO4] |
| The partitioning coefficient, temperature, and pressure standard uncertainties (σ) are as follows: u(K) = 0.15, u(T) = 0.05 K and u(p) = 0.5 kPa, respectively. |
| Ibuprofen |
| ChCl/fructose |
41.35 |
30.90 |
2.50 |
71.43 |
| 39.91 |
30.90 |
2.33 |
69.97 |
| 38.43 |
30.90 |
2.18 |
68.55 |
| 37.00 |
30.90 |
1.99 |
66.56 |
| 35.56 |
30.90 |
1.72 |
63.24 |
| ChCl/glucose |
41.35 |
30.90 |
2.62 |
72.38 |
| 39.91 |
30.90 |
2.45 |
71.01 |
| 38.43 |
30.90 |
2.30 |
69.70 |
| 37.00 |
30.90 |
2.11 |
67.85 |
| 35.56 |
30.90 |
1.84 |
64.79 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Acetaminophen |
| ChCl/fructose |
41.35 |
30.90 |
3.51 |
77.83 |
| 39.91 |
30.90 |
3.34 |
76.96 |
| 38.43 |
30.90 |
3.19 |
76.13 |
| 37.00 |
30.90 |
3.01 |
75.06 |
| 35.56 |
30.90 |
2.73 |
73.19 |
| ChCl/glucose |
41.35 |
30.90 |
3.62 |
78.35 |
| 39.91 |
30.90 |
3.45 |
77.53 |
| 38.43 |
30.90 |
3.30 |
76.74 |
| 37.00 |
30.90 |
3.12 |
75.73 |
| 35.56 |
30.90 |
2.84 |
73.96 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Aspirin |
| ChCl/fructose |
41.35 |
30.90 |
5.69 |
85.05 |
| 39.91 |
30.90 |
5.52 |
84.66 |
| 38.43 |
30.90 |
5.37 |
84.30 |
| 37.00 |
30.90 |
5.19 |
83.84 |
| 35.56 |
30.90 |
4.91 |
83.08 |
| ChCl/glucose |
41.35 |
30.90 |
5.87 |
85.44 |
| 39.91 |
30.90 |
5.70 |
85.07 |
| 38.43 |
30.90 |
5.55 |
84.73 |
| 37.00 |
30.90 |
5.37 |
84.30 |
| 35.56 |
30.90 |
5.09 |
83.58 |
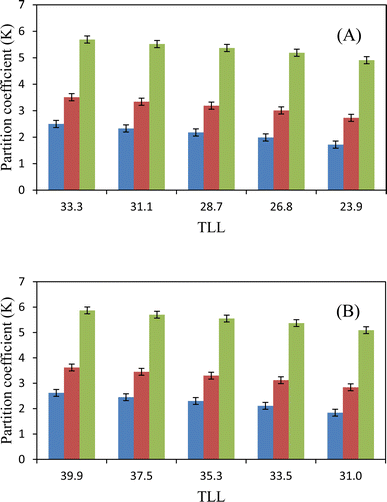 |
| | Fig. 3 Partition coefficient, K, as a function of the TLL in ATPSs composed of {DES (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios) + K3PO4 + H2O}: (A) ChCl/fructose; (B) ChCl/glucose. Ibuprofen 1 molar ratios) + K3PO4 + H2O}: (A) ChCl/fructose; (B) ChCl/glucose. Ibuprofen  acetaminophen acetaminophen  aspirin aspirin  . . | |
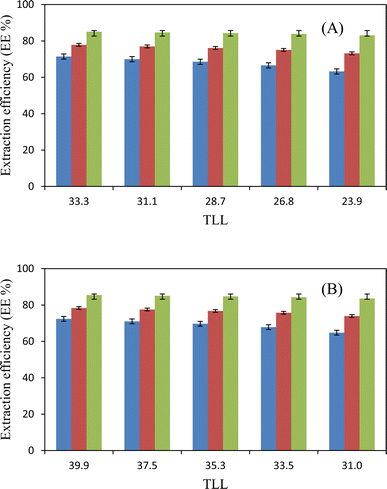 |
| | Fig. 4 Extraction efficiency, EE%, as a function of the TLL in ATPSs composed of {DES (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios) + K3PO4 + H2O}: (A) ChCl/fructose; (B) ChCl/glucose. Ibuprofen 1 molar ratios) + K3PO4 + H2O}: (A) ChCl/fructose; (B) ChCl/glucose. Ibuprofen  acetaminophen acetaminophen  aspirin aspirin  . . | |
3.3.1. Effect of drug structure. The chemical structures and polarity of the medications can be used to explain the observed trend of the partition coefficients (K) and extraction efficiency (EE%) values for ibuprofen, acetaminophen, and aspirin, as shown in Table 8. The non-polar nature of the studied medicines results in advantageous interactions with the hydrophobic components in the top phase. Consequently, there is less medication partitioning in the salt-rich phase. Interestingly, the ibuprofen, acetaminophen, and aspirin structures have non-polar functional groups that increase their affinity for the DES-rich top phase. Table 8 shows that a considerable proportion of pharmaceuticals are mostly partitioned into the DES top phase based on the high values of EE% found.Moreover, the K value trend is consistent with ibuprofen, acetaminophen, and aspirin hydrophobicity, as indicated by the logarithm of the octanol/water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow). An increase in log
Kow). An increase in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow indicates an increase in the hydrophobicity of a solute, which reduces its tendency to bind with water molecules.39 The log
Kow indicates an increase in the hydrophobicity of a solute, which reduces its tendency to bind with water molecules.39 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values for ibuprofen, acetaminophen, and aspirin are 3.97,3 2.34,40 and 1.40,41 respectively. This work emphasizes the importance of drug hydrophobicity as a helpful factor in forecasting drug partitioning between the two phases of an aqueous two-phase system (ATPs), as in a previous study.26
Kow values for ibuprofen, acetaminophen, and aspirin are 3.97,3 2.34,40 and 1.40,41 respectively. This work emphasizes the importance of drug hydrophobicity as a helpful factor in forecasting drug partitioning between the two phases of an aqueous two-phase system (ATPs), as in a previous study.26
3.3.2. Effect of pH on the partitioning of drugs. Potassium phosphate can affect the pH of water when dissolved. K3PO3 is a salt of phosphorous acid (H3PO3), but in its dissociated form, potassium phosphate acts more like a base than an acid. When it dissolves in water, it dissociates into potassium (K+) ions and phosphate ions (PO33−). Phosphate ions (PO33−) can react with water molecules to form hydroxide ions (OH−) through hydrolysis. This reaction increases the concentration of OH− in the solution, making the water more basic. As a result of the increased OH− concentration, the pH of the water increases. This means that K3PO3 increases the pH of water, making it more alkaline.42,43 As can be understood from Table 8, all three drugs have acidic groups and are slightly soluble in the salt-rich phase.
4. Conclusion
In this work, two deep eutectic solvents were synthesized and employed in conjunction with K3PO4 to create ATPSs. These solvents included ChCl as HBA and fructose or glucose as HBD at a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Two correlation equations, Merchuk and Zafarani-Moattar, were satisfactorily used to represent the experimental binodal data. The compositions of the five tie-lines for each ATPS were carefully determined. Three Othmer–Tobias, Bancraft, and Setschenow equations were used for the correlation of the tie-line data. With precision in the standard deviation values obtained from each model, we can observe that the performance of the models used in fitting the tie-line data is good.
1. Two correlation equations, Merchuk and Zafarani-Moattar, were satisfactorily used to represent the experimental binodal data. The compositions of the five tie-lines for each ATPS were carefully determined. Three Othmer–Tobias, Bancraft, and Setschenow equations were used for the correlation of the tie-line data. With precision in the standard deviation values obtained from each model, we can observe that the performance of the models used in fitting the tie-line data is good.
In addition, the capability of the studied systems in drug separation was used to partition ibuprofen, acetaminophen, and aspirin. The results showed that higher concentrations of DESs had a positive effect on the partition coefficient for the studied pharmaceuticals, and they had a tendency to move to the top DES-rich phase. This phenomenon is primarily influenced by the hydrophobic nature and structural characteristics of the drug.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- W. N. Phong, P. L. Show, Y. H. Chow and T. C. Ling, J. Biosci. Bioeng., 2018, 126, 273–281 CrossRef CAS PubMed.
- P.-Å. Albertsson, Nature, 1958, 182, 709–711 CrossRef CAS PubMed.
- F. Ghaffari, M. Khorsandi, H. Shekaari and M. T. Zafarani-Moattar, Sci. Rep., 2022, 12, 13848 CrossRef CAS PubMed.
- Y. Song, R. P. Chandra, X. Zhang and J. N. Saddler, Carbohydr. Polym., 2020, 250, 116956 CrossRef CAS PubMed.
- K. Xu, Y. Wang, Y. Huang, N. Li and Q. Wen, Anal. Chim. Acta, 2015, 864, 9–20 CrossRef CAS PubMed.
- Q. Zeng, Y. Wang, Y. Huang, X. Ding, J. Chen and K. Xu, Analyst, 2014, 139, 2565–2573 RSC.
- F. O. Farias, H. Passos, Á. S. Lima, M. R. Mafra and J. A. P. Coutinho, ACS Sustainable Chem. Eng., 2017, 5, 9402–9411 CrossRef CAS.
- D. Zhang, L. Luo, M. Jin, M. Zhao, J. Niu, S. Deng and X. Long, Biochem. Eng. J., 2022, 188, 108676 CrossRef CAS.
- F. S. Buarque, G. V. Gautério, M. A. Z. Coelho, A. C. Lemes and B. D. Ribeiro, Processes, 2023, 11, 31 CrossRef CAS.
-
(a) Y. H. Choi, J. van Spronsen, Y. Dai, M. Verberne, F. Hollmann, I. W. C. E. Arends, G.-J. Witkamp and R. Verpoorte, Plant Physiol., 2011, 156, 1701–1705 CrossRef CAS PubMed;
(b) M. J. Khinteel and B. F. Alfarhani, Chem. Rev. Lett., 2024, 7 DOI:10.22034/crl.2024.471918.1394;
(c) L. Baramakeh, J. Chem. Lett., 2022, 3, 81–85 Search PubMed;
(d) C. J. Chime, A. S. Ogbuagu and O. B. Ifeagwu, Chem. Res. Technol., 2024, 1, 128–137 Search PubMed;
(e) A. Drakhshan, S. Rezaeian and H. R. Pourianfar, Chem. Rev. Lett., 2024, 7, 454–465 CAS;
(f) R. T. Iorhemen, S. N. Adawara and G. A. Gulanda, J. Chem. Lett., 2021, 2, 89–95 Search PubMed;
(g) V. Agnihotri, A. Kumar and K. Wasewar, Chem. Rev. Lett., 2023, 6, 256–261 CAS.
- M. Marchel, J. Niewisiewicz, A. S. Coroadinha and I. M. Marrucho, Sep. Purif. Technol., 2020, 252, 117480 CrossRef CAS.
- V. Selvanathan, A. D. Azzahari, A. A. A. Halim and R. Yahya, Carbohydr. Polym., 2017, 167, 210–218 CrossRef CAS PubMed.
- M. Feyzi and S. Shahriari, Chem. Pap., 2024, 78, 2955–2966 CrossRef CAS.
- F. Soltanmohammadi, A. Jouyban and A. Shayanfar, Chem. Pap., 2021, 75, 439–453 CrossRef CAS.
- N. Li, Y. Wang, K. Xu, Y. Huang, Q. Wen and X. Ding, Talanta, 2016, 152, 23–32 CrossRef CAS PubMed.
- H. Zhang, Y. Wang, K. Xu, N. Li, Q. Wen, Q. Yang and Y. Zhou, Anal. Methods, 2016, 8, 8196–8207 RSC.
- F. Ghazizadeh Ahsaie and G. Pazuki, J. Mol. Liq., 2021, 339, 117152 CrossRef CAS.
- P. Xu, Y. Wang, J. Chen, X. Wei, W. Xu, R. Ni, J. Meng and Y. Zhou, Talanta, 2019, 202, 1–10 CrossRef CAS PubMed.
- H. Shaker Shiran, M. Baghbanbashi, F. Ghazizadeh Ahsaie and G. Pazuki, J. Mol. Liq., 2020, 303, 112629 CrossRef CAS.
- H. Zhang, Y. Wang, Y. Zhou, K. Xu, N. Li, Q. Wen and Q. Yang, Talanta, 2017, 170, 266–274 CrossRef CAS PubMed.
- S. A. Jafari and M. H. Entezari, J. Mol. Liq., 2020, 312, 113331 CrossRef CAS.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- F. O. Farias, J. F. B. Pereira, J. A. P. Coutinho, L. Igarashi-Mafra and M. R. Mafra, Fluid Phase Equilib., 2020, 503, 112319 CrossRef CAS.
- M. T. Zafarani-Moattar, H. Shekaari and F. Ghaffari, J. Chem. Eng. Data, 2019, 64, 4754–4762 CrossRef CAS.
- M. T. Zafarani-Moattar, H. Shekaari and F. Ghaffari, J. Mol. Liq., 2020, 311, 113347 CrossRef CAS.
- F. Ghaffari, M. T. Zafarani-Moattar and H. Shekaari, Fluid Phase Equilib., 2022, 555, 113348 CrossRef CAS.
- M. T. Zafarani-Moattar and E. Nemati-Kande, Calphad, 2010, 34, 478–486 CrossRef CAS.
- J. C. Merchuk, B. A. Andrews and J. A. Asenjo, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 711, 285–293 CrossRef CAS PubMed.
- D. Othmer and P. Tobias, Liquid-Liquid Extraction Data – The Line Correlation, https://pubs.acs.org/doi/pdf/10.1021/ie50390a600, accessed February 11, 2024 Search PubMed.
- P. González-Tello, F. Camacho, G. Blázquez and F. J. Alarcón, J. Chem. Eng. Data, 1996, 41, 1333–1336 CrossRef.
- M. J. Hey, D. P. Jackson and H. Yan, Polymer, 2005, 46, 2567–2572 CrossRef CAS.
- A. Hayyan, F. S. Mjalli, I. M. AlNashef, T. Al-Wahaibi, Y. M. Al-Wahaibi and M. A. Hashim, Thermochim. Acta, 2012, 541, 70–75 CrossRef CAS.
- H. Passos, D. J. P. Tavares, A. M. Ferreira, M. G. Freire and J. A. P. Coutinho, ACS Sustainable Chem. Eng., 2016, 4, 2881–2886 CrossRef CAS.
- M. T. Zafarani-Moattar, H. Shekaari and E. Pourbagherian, Fluid Phase Equilib., 2020, 514, 112536 CrossRef CAS.
- A. A. Albalasmeh, A. A. Berhe and T. A. Ghezzehei, Carbohydr. Polym., 2013, 97, 253–261 CrossRef CAS PubMed.
- M. DuBois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Colorimetric Method for Determination of Sugars and Related Substances, https://pubs.acs.org/doi/pdf/10.1021/ac60111a017, accessed November 18, 2023 Search PubMed.
- E. L. Cheluget, S. Marx, M. E. Weber and J. H. Vera, J. Solution Chem., 1994, 23, 275–305 CrossRef CAS.
- G. M. D. Ferreira, G. M. D. Ferreira, A. O. Maldaner, L. H. Mendes da Silva and M. C. Hespanhol, Fluid Phase Equilib., 2020, 506, 112367 CrossRef CAS.
- M. Haham, S. Ish-Shalom, M. Nodelman, I. Duek, E. Segal, M. Kustanovich and Y. D. Livney, Food Funct., 2012, 3, 737–744 RSC.
- F. Ghaffari, M. Taghi Zafarani-Moattar and H. Shekaari, RSC Adv., 2024, 14, 12349–12359 RSC.
- J. B. Dressman, A. Nair, B. Abrahamsson, D. M. Barends, D. W. Groot, S. Kopp, P. Langguth, J. E. Polli, V. P. Shah and M. Zimmer, J. Pharm. Sci., 2012, 101, 2653–2667 CrossRef CAS PubMed.
- V. Najdanovic-Visak, J. N. C. Lopes, Z. P. Visak, J. Trindade and L. P. N. Rebelo, Int. J. Mol. Sci., 2007, 8, 736–748 CrossRef CAS.
- Y. K. Yau, C. W. Ooi, E.-P. Ng, J. C.-W. Lan, T. C. Ling and P. L. Show, Bioresources and Bioprocessing, 2015, 2, 1–13 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab and
Rovnag Rzayevb
*ab and
Rovnag Rzayevb
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and tripotassium phosphate (K3PO4) were prepared. The measured binodal data correlated with Merchuk and Zafarani-Moattar et al. equations. Moreover, the ATPSs were employed to investigate the separation of pharmaceuticals. The partition coefficient and the effect of factors such as the concentration of the deep eutectic solvent on drug partitioning were investigated as novel discoveries. Drugs are likely to be removed in the top DES-rich phase, according to the current data. Finally, the compositions of five tie-lines for each ATPS were meticulously determined. Othmer–Tobias, Bancraft, and Setschenow equations were used for correlation of tie-line data.
1 and tripotassium phosphate (K3PO4) were prepared. The measured binodal data correlated with Merchuk and Zafarani-Moattar et al. equations. Moreover, the ATPSs were employed to investigate the separation of pharmaceuticals. The partition coefficient and the effect of factors such as the concentration of the deep eutectic solvent on drug partitioning were investigated as novel discoveries. Drugs are likely to be removed in the top DES-rich phase, according to the current data. Finally, the compositions of five tie-lines for each ATPS were meticulously determined. Othmer–Tobias, Bancraft, and Setschenow equations were used for correlation of tie-line data.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were created as aqueous two-phase systems. The experiments were carried out under atmospheric pressure and 298.15 K in temperature. For these ATPSs, the binodal and tie-line values were found. Equations from Zafarani-Moattar et al.27 and Merchuk28 were applied to fit the binodal data. Additionally, tie-lines were established for the ATPSs made up of water, K3PO4, and DESs. Tie-line compositions were modeled using the Othmer–Tobias and Bancraft equations29,30 and Setschenow-type equation.31 Our research delved into using these ATPSs to separate ibuprofen, acetaminophen, and aspirin. For this purpose, partition coefficients (K) and extraction efficiencies (EE%) of the studied drugs were calculated at various tie-lines to describe how the concentration of DESs and the characteristics of the drugs influenced their separation.
1 were created as aqueous two-phase systems. The experiments were carried out under atmospheric pressure and 298.15 K in temperature. For these ATPSs, the binodal and tie-line values were found. Equations from Zafarani-Moattar et al.27 and Merchuk28 were applied to fit the binodal data. Additionally, tie-lines were established for the ATPSs made up of water, K3PO4, and DESs. Tie-line compositions were modeled using the Othmer–Tobias and Bancraft equations29,30 and Setschenow-type equation.31 Our research delved into using these ATPSs to separate ibuprofen, acetaminophen, and aspirin. For this purpose, partition coefficients (K) and extraction efficiencies (EE%) of the studied drugs were calculated at various tie-lines to describe how the concentration of DESs and the characteristics of the drugs influenced their separation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using fructose or glucose as the hydrogen bond donor (HBD) and choline chloride as the hydrogen bond acceptor (HBA). The process described in earlier investigations22,32 was followed in the fabrication of DES. First, sugars and choline chloride were mixed together in a 50 mL round-bottom flask. Next, using a hot plate stirrer, the flask was submerged in a paraffin oil bath that was heated. At 353.15 K, the mixture was agitated for two hours to produce a colorless, uniform liquid. A thermometer with an accuracy of ±0.01 K was applied to measure the DES temperature continuously. All samples were then properly preserved in airtight vials in a temperature-controlled environment. All the materials were dried before making the DES. Karl-Fischer analysis (Metrohm 751 GPD) was used to obtain the prepared DES water content, and the results showed a 0.0008 mass fraction of water content.
1 using fructose or glucose as the hydrogen bond donor (HBD) and choline chloride as the hydrogen bond acceptor (HBA). The process described in earlier investigations22,32 was followed in the fabrication of DES. First, sugars and choline chloride were mixed together in a 50 mL round-bottom flask. Next, using a hot plate stirrer, the flask was submerged in a paraffin oil bath that was heated. At 353.15 K, the mixture was agitated for two hours to produce a colorless, uniform liquid. A thermometer with an accuracy of ±0.01 K was applied to measure the DES temperature continuously. All samples were then properly preserved in airtight vials in a temperature-controlled environment. All the materials were dried before making the DES. Karl-Fischer analysis (Metrohm 751 GPD) was used to obtain the prepared DES water content, and the results showed a 0.0008 mass fraction of water content.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) + water} were created to identify the tie-lines. Using a mixture of DES, K3PO4, and water, a precise gravimetric preparation of the biphasic zone was performed for each tie-line, with an accuracy of ±10−7 kg. These solutions were vigorously stirred for 30 minutes; then, they were centrifuged and heated to 298 K in a water bath to achieve equilibrium. Then, analytical techniques were used to identify the compositions of each phase. After separation of the two phases, a flame photometer (JENVEY model PFP7, England) was used to determine the concentrations of potassium phosphate (K3PO4) in the top and bottom phases.
1 molar ratio) + water} were created to identify the tie-lines. Using a mixture of DES, K3PO4, and water, a precise gravimetric preparation of the biphasic zone was performed for each tie-line, with an accuracy of ±10−7 kg. These solutions were vigorously stirred for 30 minutes; then, they were centrifuged and heated to 298 K in a water bath to achieve equilibrium. Then, analytical techniques were used to identify the compositions of each phase. After separation of the two phases, a flame photometer (JENVEY model PFP7, England) was used to determine the concentrations of potassium phosphate (K3PO4) in the top and bottom phases.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sugars (su) + K3PO4 (s) + water (w)} system
sugars (su) + K3PO4 (s) + water (w)} system
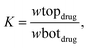
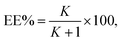
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were created as aqueous two-phase systems.
1 were created as aqueous two-phase systems.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of ChCl/fructose and ChCl/glucose), and water at 298.15 K are plotted in Fig. 1, and the experimental mass fraction data are listed in Table 3.
1 molar ratio of ChCl/fructose and ChCl/glucose), and water at 298.15 K are plotted in Fig. 1, and the experimental mass fraction data are listed in Table 3.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) + K3PO4 + water} at 298 K: ChlCl/fructose (♦); ChCl/glucose (■).
1 molar ratio) + K3PO4 + water} at 298 K: ChlCl/fructose (♦); ChCl/glucose (■).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(bwDES0.5 − cwDES3),
exp(bwDES0.5 − cwDES3),
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(wDES) + γwDES,
ln(wDES) + γwDES,
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Table 5 provides an overview of the experimental tie-lines and the tie-line lengths (TLL) for the studied systems. The top phase is constantly enriched in DES with varying tie-line lengths according to the data, which follow a consistent pattern. Higher K3PO4 concentrations than DES made up the majority of the bottom phase, suggesting a salt-rich phase.
1. Table 5 provides an overview of the experimental tie-lines and the tie-line lengths (TLL) for the studied systems. The top phase is constantly enriched in DES with varying tie-line lengths according to the data, which follow a consistent pattern. Higher K3PO4 concentrations than DES made up the majority of the bottom phase, suggesting a salt-rich phase.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD
HBD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD]
HBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD]
HBD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD]
HBD]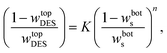
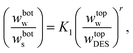
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)), and the water Setschenow equation31 at T = 298.15 K are used, which is a simple two-parameter relation proposed by Hey and coworkers:31
1)), and the water Setschenow equation31 at T = 298.15 K are used, which is a simple two-parameter relation proposed by Hey and coworkers:31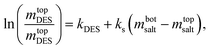
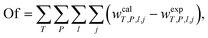
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sugar] + K3PO4 + H2O} system at 298.15 Ka
sugar] + K3PO4 + H2O} system at 298.15 Ka
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios) + K3PO4 + H2O}: (A) ChCl/fructose; (B) ChCl/glucose. Ibuprofen
1 molar ratios) + K3PO4 + H2O}: (A) ChCl/fructose; (B) ChCl/glucose. Ibuprofen  acetaminophen
acetaminophen  aspirin
aspirin  .
.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios) + K3PO4 + H2O}: (A) ChCl/fructose; (B) ChCl/glucose. Ibuprofen
1 molar ratios) + K3PO4 + H2O}: (A) ChCl/fructose; (B) ChCl/glucose. Ibuprofen  acetaminophen
acetaminophen  aspirin
aspirin  .
.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow). An increase in log
Kow). An increase in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow indicates an increase in the hydrophobicity of a solute, which reduces its tendency to bind with water molecules.39 The log
Kow indicates an increase in the hydrophobicity of a solute, which reduces its tendency to bind with water molecules.39 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values for ibuprofen, acetaminophen, and aspirin are 3.97,3 2.34,40 and 1.40,41 respectively. This work emphasizes the importance of drug hydrophobicity as a helpful factor in forecasting drug partitioning between the two phases of an aqueous two-phase system (ATPs), as in a previous study.26
Kow values for ibuprofen, acetaminophen, and aspirin are 3.97,3 2.34,40 and 1.40,41 respectively. This work emphasizes the importance of drug hydrophobicity as a helpful factor in forecasting drug partitioning between the two phases of an aqueous two-phase system (ATPs), as in a previous study.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Two correlation equations, Merchuk and Zafarani-Moattar, were satisfactorily used to represent the experimental binodal data. The compositions of the five tie-lines for each ATPS were carefully determined. Three Othmer–Tobias, Bancraft, and Setschenow equations were used for the correlation of the tie-line data. With precision in the standard deviation values obtained from each model, we can observe that the performance of the models used in fitting the tie-line data is good.
1. Two correlation equations, Merchuk and Zafarani-Moattar, were satisfactorily used to represent the experimental binodal data. The compositions of the five tie-lines for each ATPS were carefully determined. Three Othmer–Tobias, Bancraft, and Setschenow equations were used for the correlation of the tie-line data. With precision in the standard deviation values obtained from each model, we can observe that the performance of the models used in fitting the tie-line data is good.