Open Access Article
Open Access ArticleAscorbic acid mediated fluorescence emission of MnO2 modified upconversion nanoparticles for anti-counterfeiting
Yongling Zhang *ab,
Xiang Liub,
Yuhai Wangb,
Mingxing Songb and
Zhengkun Qin
*ab,
Xiang Liub,
Yuhai Wangb,
Mingxing Songb and
Zhengkun Qin *b
*b
aSchool of Chemistry and Pharmaceutical Engineering, Jilin Institute of Chemical Technology, Jilin 132022, China. E-mail: yong1ling@163.com
bCollege of Information & Technology, Jilin Normal University, Siping 136000, China. E-mail: qin_zhengkun@126.com
First published on 21st October 2024
Abstract
Anti-counterfeiting ink can prevent important documents from being forged or tampered with. We reported a strategy to improve upconversion luminescence intensity of NaYF4:18%Yb3+,0.5%Tm3+ core nanoparticles (NPs) by coating the NaYF4 shell. We synthesized NaYF4:18%Yb3+,0.5%Tm3+ core NPs and NaYF4:18%Yb3+,0.5%Tm3+/NaYF4 core–shell NPs by high temperature thermal decomposition method. In comparison with the core NPs, the upconversion luminescence intensity of the core–shell NPs was enhanced by 2.3 times in the wavelength range of 445 nm to 495 nm. We designed composite nanomaterials based on NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs and MnO2, and synthesized NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs by physical doping method. Here, MnO2 acts as a quencher to quench the upconversion fluorescence of Tm3+ ions of the core–shell NPs. Afterwards, we used the prepared product for document anti-counterfeiting. And then reducing agent (AA) can destroy the structure of MnO2 to restore the upconversion luminescence of Tm3+ ions. We use NaYF4:18%Yb3+,0.5%Tm3+/NaYF4@MnO2 composite NPs as anti-counterfeiting ink to write the letter “L”. Under ambient conditions or the irradiation of 980 nm continuous light, “L” does not emit light. After AA is evenly applied on the letter “L”, “L” can emit blue fluorescence under the irradiation of 980 nm continuous light. These results showed that NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs can be used in important document anti-counterfeiting tasks to enhance information security.
1. Introduction
Important documents may be forged and tampered with by malicious individuals during the transmission process. For example, key statements and signatures in commercial documents are forged and tampered with by malicious individuals, leading to legal disputes.1–3 Therefore, it is very necessary to create a safe, effective and easy to popularize anti-counterfeiting technique. Among many anti-counterfeiting techniques, luminescent materials are undoubtedly the best choice because of their unique physico-chemical and optical properties, which can make complex patterns printed on different substrates.4–7 Rare-earth upconversion luminescent materials are often chosen; they can enable conversion from near-infrared light to visible light due to the anti-stokes effect where two or more low-energy photons are sequentially absorbed, and then higher-energy photons are emitted.8–11 Rare-earth upconversion luminescent materials have the characteristics of strong photostability, long fluorescence lifetime, simple synthesis method and low toxicity and stable chemical properties, which allow us to effectively use them in anti-counterfeiting work.12–15 Over the past few decades, anti-counterfeiting technology based on rare-earth upconversion luminescent materials has been researched and applied by researchers in different degrees.16–19 Mengxiao Li's team20 proposed to coat a layer of novel fluorescent carbon quantum dots (CDs) on the surface of the synthesized NaYF4:RE3+ nanomaterials and mixed them with poly (acrylic acid) (PAA) aqueous solution as an anti-counterfeiting ink. They used the anti-counterfeiting ink to print out QR codes, clover and other patterns. These patterns can't be seen under visible light irradiation. But blue fluorescence patterns can be observed under 365 nm UV light irradiation, and green fluorescence patterns can be observed under 980 nm continuous pump light irradiation, so the material can be used in anti-counterfeiting technology. Wu Wei et al.21 choose traditional screen printing technology to deposit water-soluble NaYF4:Yb3+,Tm3+/Er3+/Eu3+ upconversion NPs on paper and polyethylene terephthalate(PET) and made different patterns. There was no pattern on paper. There were white patterns on PET. Under 980 nm continuous pump light irradiation, the blue, yellow-green and green fluorescence corresponding to NaYF4:Yb3+,Tm3+/Er3+/Eu3+ upconversion NPs can be observed on paper and PET, respectively. Huang Ling et al.22 prepared NaYF4:2%Er3+,0.5%Tm3+/NaYF4 core–shell NPs by high temperature thermal decomposition method, and made them as anti-counterfeiting ink to write the letters “I, A, M”. Under 980 nm continuous pump light irradiation and the repetition rate was constant at 100 Hz, the letters “I, A, M” emitted green upconversion fluorescence when the pulse time was 100 us; the letters “I, A, M” emitted yellow upconversion fluorescence when the pulse time was 500 μs; the letters “I, A, M” emitted red upconversion fluorescence when the pulse time was 6000 us. But these anti-counterfeiting technologies are single and easy to crack, so we have been studying a more precise anti-counterfeiting technology, so that the anti-counterfeiting security is stronger.In this article, we proposed to wrap a NaYF4 passive shell outside NaYF4:18%Yb3+,0.5%Tm3+ core NPs to improve the luminescence intensity of NaYF4:18%Yb3+,0.5%Tm3+/NaYF4 core–shell NPs. The upconversion luminescence intensity (∼477 nm) of the NPs was enhanced by 2.3 times after coating a NaYF4 passive shell, due to the surface luminescence quenching effect was suppressed by wrapping the passive shell. Then we physically mixed high-luminosity core–shell NPs with MnO2 nanosheets to form NaYF4:18%Yb3+,0.5%Tm3+/NaYF4@MnO2 composite NPs. We found resonant energy transfer between MnO2 and Tm3+ ions can quench the upconversion luminescence of core–shell NPs. We also found that AA can disrupt the structure of MnO2 and reduce it to Mn2+ ions, and that Mn2+ ions can't occur energy transfer with Tm3+ ions, thus restoring the upconversion luminescence of the core–shell NPs. Finally, we conducted the experiments about composite NPs on anti-counterfeiting research and concluded that they can further enhance the security of anti-counterfeiting.
2. Experimental
2.1 Chemicals
Yttrium acetate hexahydrate(Y(CH3COO)3·6H2O), Ytterbium acetate hexahydrate(Yb(CH3COO)3·6H2O) and Thulium acetate hexahydrate(Tm(CH3COO)3·6H2O) were purchased from Jining Tianyi New Materials Co., Ltd, Shandong, China. Oleic acid (OA), 1-octadecene (ODE) and ammonium fluoride (NH4F) were purchased from Alfa Aesar Co., Ltd, Shanghai, China. Sodium hydroxide was purchased from Tianjin Guangfu Techology Development Co., Ltd, China. Potassium hypermanganate (KMnO4) was purchased from Tianjin Fuchen Reagent Co., Ltd, China. Methanol (CH3OH) and ethanol (CH3CH2OH) were purchased from Sinopharm Chemical Reagents Co., Ltd, China. Cyclohexane (C6H12) was purchased from Chengdu Cologne Chemical Co., Ltd, China.2.2 Synthetic procedures
2.3 Characterization
The crystal phase of the samples were measured by Model Rigaku Ru-200b X-ray powder analyzer produced by Rigaku Corporation Japan (λ = 1.5406 Å, scanning range from 10° to 70°). Morphological of products were measured by a High Resolution Transmission Electron Microscope (JEM-F200). Up-conversion fluorescence spectra of these products were measured by a Hitachi F-4500 fluorescence spectrometer. Anti-counterfeiting application photos were taken by Nikon D3200.3. Results and discussion
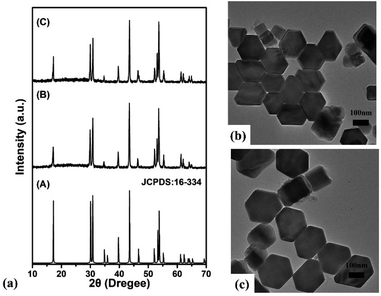
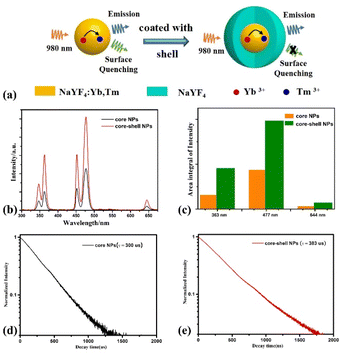
NaYF4:18%Yb3+,0.5%Tm3+ core NPs was prepared by high-temperature thermal decomposition method. And further NaYF4:18%Yb3+,0.5%Tm3+/NaYF4 core–shell NPs was prepared by a coating NaYF4 shell method. The XRD standard card of β-phase NaYF4 (JCPDS: 16-334) is shown in Fig. 1(a). The crystal phase of the samples was tested by using X-ray diffraction (XRD), as shown in Fig. 1(b) and (c). We can see that all diffraction peaks of the prepared samples can match well with the NaYF4 standard PDF (JCPDS: 16-334) without any other impurity peaks, exhibiting a β-phase structure, confirming that the coating of a NaYF4 passive shell has no effect on crystal phase of the sample. Transmission Electron Microscope (TEM) images of NaYF4:18%Yb3+,0.5%Tm3+ core NPs and NaYF4:18%Yb3+,0.5%Tm3+/NaYF4 core–shell NP. The TEM image of as-prepared NaYF4:Yb3+,Tm3+ core NPs and as-prepared NaYF4: Yb3+,Tm3+/NaYF4 core–shell NPs are presented in Fig. 1(b) and (c). As can be seen from Fig. 1(b), that the shapes of NaYF4:Yb3+,Tm3+ core NPs is hexagonal and have uniform morphology. The average size of the as-prepared core NPs is about 140 nm. From Fig. 1(c), it can be seen that the NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs is also hexagonal and the average size of the as-prepared core–shell NPs is about 190 nm. The above results indicate that after coating NaYF4 shell, the morphology of the as-prepared NPs did not change, and the average size of the as-prepared core–shell NPs increased, with the NaYF4 shell thickness of about 50 nm.Fig. 2(a) is schematic structure diagram of NaYF4:Yb3+,Tm3+ core NPs and NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs. To explore the effect of NaYF4 shell on the luminescence properties of the as-prepared sample, we tested the upconversion fluorescence spectra of NaYF4:18%Yb3+,0.5%Tm3+ core NPs and NaYF4:18%Yb3+,0.5%Tm3+/NaYF4 core–shell NPs under 980 nm continuous light excitation. The test results are shown in the figure Fig. 2(b). From the upconversion emission spectra, there are five emission peaks can be observed at 345 nm, 363 nm, 451 nm, 477 nm and 644 nm, which correspond to the radiative transitions of Tm3+ ions: 1I6 → 3F4, 1D2 → 3H6, 1D2 → 3F4, 1G4 → 3H6 and 3F3 → 3H6. From Fig. 2(b), we can also see that the upconversion luminescence intensity of NaYF4 core NPs is relatively weak. This is because the core NPs have a larger specific surface area and more surface defects, which will lead to the occurrence of surface luminescence quenching effect. After coating with the NaYF4 shell, the upconversion emission intensity of the NPs was significantly enhanced under the excitation of 980 nm continuous light, as shown in Fig. 2(b). Fig. 2(c) shows the ratio of the luminescence intensity of the upconversion emission peaks of the core NPs and the core–shell NPs under 980 nm continuous pump light excitation. After coating a NaYF4 shell on the core NP, its upconversion luminescence intensity (∼477 nm) is 2.3 times that of the core NPs, due to the surface passivation,27 as shown in Fig. 2(c). The above results indicate that coating a NaYF4 shell on the core NPs can effectively enhance its upconversion luminescence intensity. Therefore, a high brightness core–shell NP with a size of approximately 190 nm was prepared using core–shell coating method and used for subsequent research. In addition, we also tested the fluorescence attenuation curve the fluorescence decay curve of 1G4 energy level of Tm3+ of NaYF4:Yb3+,Tm3+ core NPs and NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs, as shown in Fig. 2(d) and (e). After being coated with a NaYF4 shell, lifetimes of 1G4 energy level of Tm3+ of the NaYF4:Yb3+,Tm3+ core NPs increased from 300 μs to 383 μs. This is because the shell coating can effectively reduce the surface defects of the NPs. The lifetime variation trend and fluorescence spectral intensity variation trend of the core NPs and the core–shell NPs are consistent, as shown in Fig. 2(b).
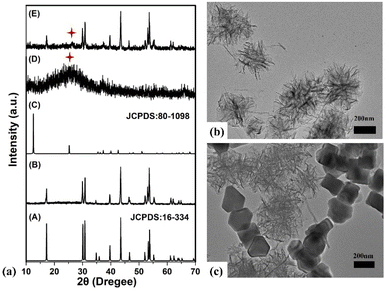
NaYF4:18%Yb3+,0.5%Tm3+/NaYF4 core–shell NPs was prepared by high-temperature thermal decomposition method. MnO2 nanosheets was synthesized by hydrothermal method, and NaYF4:18%Yb3+,0.5%Tm3+/NaYF4@MnO2 composite NPs was synthesized by physical mixing method. We measured the XRD of these samples. The XRD standard card of β-phase NaYF4 (JCPDS: 16-334), NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs, the XRD standard card of δ-phase MnO2 (JCPDS: 80-1098), MnO2 nanosheets and NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs was displayed in Fig. 3(a). From Fig. 3(a), it can be observed that the diffraction peaks of as-prepared core–shell NPs is sufficient to match that of β-phase NaYF4, and the diffraction peaks of as-prepared MnO2 nanosheets can be matched well with pure δ-phase MnO2. We can observe the crystal phase of as-prepared the core–shell NPs is β-phase NaYF4, and the crystal phase of MnO2 nanosheets is δ-phase MnO2. The composite NPs have all the diffraction peaks of the δ-phase MnO2 and the β-phase NaYF4, which indicates that we have successfully prepared NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs. Fig. 2(b) is the TEM image of MnO2 nanosheets, whose shape is in a flower-like nanosheet. The prepared δ-phase MnO2 nanosheets have a birnessite framework and layered structure, whose layered thickness is about ∼35 nm. Fig. 2(c) is the TEM image of NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs. It can be observed that the MnO2 nanosheets are evenly mixed with NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs to form composite NPs.
To investigate the effect of MnO2 nanosheets on the luminescence properties of NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs, we tested the upconverted luminescence spectra of NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs and NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs under 980 nm continuous pump light excitation. A in Fig. 4(a) is the upconversion luminescence spectra of NaYF4:Yb3+,Tm3+/NaYF4/NaYF4 core–shell NPs. Under 980 nm continuous pump light excitation, we can see NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs have five distinct emission peaks that are located at 345 nm (1I6 → 3F4), 363 nm (1D2 → 3H6), 451 nm (1D2 → 3F4), 477 nm (1G4 → 3H6) and 644 nm (3F3 → 3H6). B in Fig. 4(a) is the upconversion luminescence spectra of NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs. It can be seen NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs have hardly emission peaks. To facilitate the observation of the emission peaks of the composite NPs, B in Fig. 4(a) was multiplied by 40 times, resulting in C in Fig. 4(a).28 Under 980 nm continuous pump light excitation, we can see the composite NPs have two emission peaks that are located at 477 nm (1G4 → 3H6) and 644 nm (3F3 → 3H6). The above phenomenon is due to the resonance energy transfer between Tm3+ ions and MnO2 nanosheets,28 which can quench the upconversion luminescence of the core–shell NPs. The mechanism diagram of the energy transfer between Tm3+ ions and MnO2 nanosheets is shown in Fig. 4(b). Luminescence process of Yb3+-Tm3+ co-doped system mainly consists of excited state absorption (ESA), energy transfer upconversion (ETU) and cross-relaxation (CR). For the up-conversion luminescence of the core–shell NPs, Yb3+ ions as sensitizers have a large absorption cross section at 980 nm. Under 980 nm continuous pump excitation, Yb3+ ions absorb energy to continuously transfer electrons from 7F7/2 ground state level to 7F5/2 excited state level, and then transfer energy to adjacent Tm3+ ions, so that the electrons can be populated to the excited state levels of Tm3+ ions, such as 3H5 level, 3F2 level, 3F3 level and 1G4 level. The energy transfer process is the following: 2F5/2 (Yb3+) + 3F4 (Tm3+) → 2F7/2 (Yb3+) + 3H5 (Tm3+), 2F5/2 (Yb3+) + 3H4 (Tm3+) → 2F7/2 (Yb3+) + 3F2,3 (Tm3+), 2F5/2(Yb3+) + 3H6 (Tm3+) → 2F7/2 (Yb3+) + 1G4 (Tm3+). Due to the large energy mismatch (∼3500 cm−1) between Yb3+ ions and Tm3+ ions, the ions cannot be directly placed on the 1D2 level of Tm3+ ions through energy transmission, so we need CR (2F2,3 (Tm3+) + 3H4 (Tm3+) → 1D2 (Tm3+) + 3H6 (Tm3+)) process. Because of the efficient generation of CR process, more and more Tm3+ ions are located at the 1D2 level, resulting in an effective energy transfer process of 2F5/2 (Yb3+) + 1I6 (Tm3+) → 2F7/2 (Yb3+) + 3H4 (Tm3+). Finally, Tm3+ ions are successfully positioned on 3H5 level, 3F2,3 level, 1G4 level, 1D2 level and 1I6 level, and upconversion of luminescence occurs through the radiation transition.
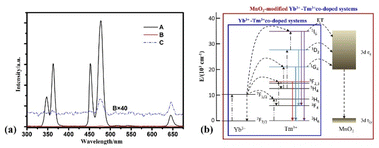 | ||
| Fig. 4 (a) Upconversion spectra of (A) NaYF4:18%Yb3+,0.5%Tm3+/NaYF4 core–shell NPs and (B) NaYF4:18%Yb3+,0.5%Tm3+/NaYF4@MnO2 composite NPs under 980 nm continuous pump light excitation, (C) is 40 times the product of (B). (b) The schematic diagram of the energy transfer process of Yb3+-Tm3+ co-doped system and MnO2-modified Yb3+-Tm3+ codoped system under 980 nm continuous pump light excitation.28 | ||
There is a resonate energy transfer occurring between the core–shell NPs and MnO2 nanosheets.28 The main levels (3H5, 3F2,3, 1G4, 1D2 and 1I6) of Tm3+ ions don't produce a radiative transition, but transfer energy to the 3d eg level of the adjacent MnO2 nanosheets. Subsequently, the energy at the 3d eg level of the MnO2 nanosheets jumps by radiation transition to the 3d t2g level of the MnO2 nanosheets, thus quenching the upconversion luminescence of the core–shell NPs.
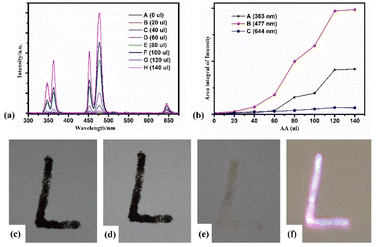
In previous studies, we found that MnO2 nanosheets can quench the upconversion luminescence of NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs. Next, we prepared an aqueous solution of 1 mL NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs containing 0.008 mmol NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs and 0.012 mmol MnO2 nanosheets. To observe the changes in the upconversion luminescence properties of AA to composite NPs, we tested the upconversion luminescence spectra of NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs solutions after adding different amounts of AA under 980 nm continuous pump light excitation, as shown in Fig. 5(a). Fig. 5(b) showed the intensity ratio of the emission peaks of the composite particle solutions after adding different amounts of AA at 363 nm, 477 nm, and 644 nm. Firstly, we made up an aqueous AA solution of 0.1 mmol mL−1, and dropped it into a 1 mL of NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs solutions, droplet amount were 0 μl, 20 μl, 40 μl, 60 μl, 80 μl, 100 μl, 120 μl and 140 μl. We see that under 980 nm continuous pump light excitation, when the droplet amount of AA aqueous solution is 0 μl (No drip of the aqueous AA solution was added), the emission peak of composite NPs is almost invisible, due to the resonance energy transfer of MnO2 and Tm3+ ions, quenching the upconversion of Tm3+ ions. When we gradually added AA aqueous solution of 0.1 mmol mL−1 to the composite nanoparticle solution, we found that the upconversion luminescence of NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs was gradually restored with the increasing amount of AA droplets. When the amount of AA droplets was from 20 μl to 120 μl, the upconversion luminescence intensity of Tm3+ ions gradually increased with the amount of aqueous AA solution. The upconversion luminescence intensity of Tm3+ ions is maximum when the AA droplet volume is 120 μl. When the AA droplet amount is 140 μl, the upconversion luminescence intensity of Tm3+ ions was almost unchanged compared with the AA drop amount of 120 μl (trend diagram of changes of luminescence intensity is shown in Fig. 5(b)). From the above results, it is known that when the droplet amount of AA solution is 120 μl, it completely reacts with MnO2 in the composite particles, that is, 0.012 mmol AA just completely reacts with 0.012 mmol MnO2. This is because MnO2 can be effectively deoxidize to Mn2+ ions by AA, meanwhile AA is oxidized to DAA. The specific reaction process is as follows29
| MnO2 + AA + 8H+ → Mn2+ + DAA + 4H2O | (1) |
Resonance energy transfer can't occur between Mn2+ ions and Tm3+ ions, so when the MnO2 in the composite particles completely reacts with AA, the upconversion luminescence of core–shell NPs is restored. Fig. 5(a) also demonstrated that AA can restore the upconversion fluorescence of NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs, without changing the position of emission peaks of the upconversion luminescence of the core–shell NPs.
We have previously proved that MnO2 can quench the upconversion luminescence of NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs and AA can deoxidize MnO2 to Mn2+ ions to restore the upconversion luminescence of NaYF4:Yb3+,Tm3+/NaYF4 core–shell NPs. Next, we used NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs as anti-counterfeiting materials to conduct experiments on anti-counterfeiting research. We used the prepared NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs aqueous solution as anti-counterfeiting materials to write the letter “L” on the paper. Under natural light environment, we can see the black letter “L” on the paper, the photograph was shown in Fig. 5(c). There was no change about letter “L” under 980 nm continuous pump light irradiation, the photograph was shown in Fig. 5(d). After smearing AA aqueous solution, the black of letter “L” disappeared, the photograph was shown in Fig. 5(e). At this point, letter “L” emitted bright blue-purple fluorescence under 980 nm continuous pump light irradiation, the photograph was shown in Fig. 5(f). Therefore, the NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs can be applied in the field of fluorescence anti-counterfeiting, and composite NPs need to deoxidize by specific reducing agents to display fluorescence under continuous pump light irradiation, which is more safe than ordinary fluorescence anti-counterfeiting.
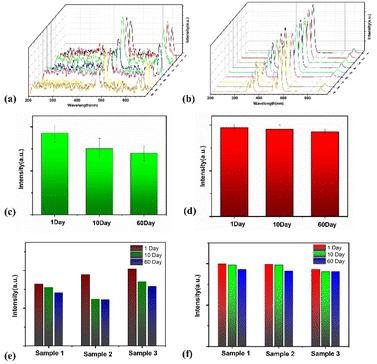
To test the stability of NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs, three identical samples of NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs were synthesized. On the first day, the fluorescence spectra of the three samples were tested under 980 nm continuous light excitation, as shown in Fig. 6(a) at 1, 2, and 3. On the 10th day, the fluorescence spectra of the three samples were obtained under 98 0 nm continuous light excitation, as shown in Fig. 6(a) at 4, 5, and 6. On the 60th day, the fluorescence spectra of the three samples were measured under 980 nm continuous light excitation, as shown in Fig. 6(a) at 7, 8, and 9. Then, these three samples were reduced by AA and the fluorescence properties of the three samples after adding AA were tested. On the first day, the fluorescence spectra of the three samples after adding AA were tested under 980 nm continuous light excitation, as shown in 1, 2, and 3 of Fig. 6(b). On the 10th day, the fluorescence spectra of the three samples after adding AA were obtained under 980 nm continuous light excitation, as shown in 4, 5, and 6 of Fig. 6(b). On the 60th day, the fluorescence spectra of the three samples after adding AA were measured under 980 nm continuous light excitation, as shown in 7, 8, and 9 of Fig. 6(b). Fig. 6(c) show comparison of the luminescence intensity of NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs at 477.6 nm under continuous pump light excitation at 980 nm. The results indicate that NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs exhibit reliable fluorescence characteristics and good experimental reproducibility, under the excitation of 980 nm continuous light. Fig. 6(d) show comparison of the luminescence intensity of NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs after adding AA at 477.6 nm under continuous pump light excitation at 980 nm. From Fig. 6(d), it can be seen that NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs after adding AA exhibit reliable fluorescence characteristics and good experimental reproducibility, under the excitation of 980 nm continuous light. Fig. 6(e) show under 980 nm continuous pump light excitation, the luminescence intensity of the three NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs samples at 477.6 nm was measured on the 1st, 10th, and 60th day, respectively. From Fig. 6(e), it can be seen that NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs has good stability. Fig. 6(f) show under 980 nm continuous pump light excitation, the luminescence intensity of the three NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs samples after adding AA at 477.6 nm was measured on the 1st, 10th, and 60th day, respectively. Fig. 6(f) shows that NaYF4:Yb3+,Tm3+/NaYF4@MnO2 composite NPs s after adding AA has also good stability.”
4. Conclusion
In summary, we presented a method to improve the upconversion luminescence intensity of NaYF4:18%Yb3+,0.5%Tm3+ core NPs, that is, a NaYF4 passive shell was wrapped outside the core NPs. Compared with NaYF4:18%Yb3+,0.5%Tm3+ core NPs, the upconversion luminescence intensity (∼477 nm) of NaYF4:18%Yb3+,0.5%Tm3+/NaYF4 core–shell NPs was increased by 2.3 times. We also reported a novel design based on composite particles of physical mixing of rare-earth upconversion NPs and MnO2 nanosheets, which can further enhance the security of fluorescence anti-counterfeiting. In this design, MnO2 nanosheets can be used as a quencher for the upconversion luminescence of rare earth ions. The AA can destroy the structure of MnO2 and reduce it to Mn2+ ions, which can't conduct resonant energy transfer with rare earth ions, so the upconversion luminescence of rare earth ions restore. Rare earth materials after using a specific reducing agent can display fluorescence under pump light to make fluorescence security more encryption.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Author contributions
Y. Z. and X. L. conceived and designed the experiments; Y. Z. and X. L. performed the experiments; Y. Z., Y. W. M. S. and Z. Q. analyzed the data and contributed reagents/materials/analysis tools. Y. Z. and X. L. wrote the paper. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Jilin Provincial Natural Science Foundation No. YDZJ202201ZYTS425. Jilin Institute of Chemical Technology Doctoral Initiation Fund Project, No. 2023011. TEM data was obtained using equipment maintained by Jilin Insititute of Chemical Technology Center of Characterization and Analysis.References
- W. J. Yao, Q. Y. Tian and W. Wu, Adv. Opt. Mater., 2018, 7, 1801171 CrossRef CAS; A. Abdollahi, H. Roghani-Mamaqani, B. Razavi and M. Salami-Kalajahi, ACS Nano, 2020, 14, 14417–14492 CrossRef PubMed.
- L. Yan, H. Zheng, Q. Zhang, B. Zhou, L. Yan, J. Huang, Z. An, Q. Zhang and B. Zhou, Nano Lett., 2022, 22, 7042–7048 CrossRef CAS.
- Y. Wu, X. Chen and W. Wu, Small, 2023, 19, 2206709 CrossRef CAS.
- J. M. Meruga, W. M. Cross, P. S. May, Q. Luu, G. A. Crawford and J. J. Kellar, Nanotechnology, 2012, 23, 395201 CrossRef.
- P. Kumar, J. Dwivedi and B. K. Gupta, J. Mater. Chem. C, 2014, 2, 10468–10475 RSC.
- K. Park, M. Park, H. S. Jang, J. H. Park, J. Kim, Y. Cho, I. K. Han, D. J. Byun and H. Ko, Adv. Funct. Mater., 2018, 28, 1800369 CrossRef.
- T. Y. Sun, B. Z. Xu, B. Chen, X. Chen, M. Y. Li, P. Shi and F. Wang, Nanoscale, 2017, 9, 2701–2705 RSC.
- L. G. Zhang, S. L. Zhao, Z. Q. Liang, J. J. Zhang, W. Zhu, P. Liu and H. K. Sun, J. Alloys Compd., 2017, 699, 1–6 CrossRef CAS.
- L. Anbharasi, M. Gunaseelan, V. N. K. B. Adusumalli, S. Yamini, V. Mahalingam and J. Senthilselvan, AIP Conf. Proc., 2019, 2115, 030179 CrossRef.
- F. Auzel, Chem. Rev., 2004, 104, 139–173 CrossRef CAS.
- M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano Lett., 2008, 8, 3834–3838 CrossRef CAS PubMed.
- B. Marco, C. Luís and X. G. Liu, Phys. Today, 2015, 68, 38–44 Search PubMed.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CrossRef CAS PubMed.
- X. Chen, D. F. Peng, Q. Ju and F. Wang, Chem. Soc. Rev., 2015, 44, 1318–1330 RSC.
- B. Zhou, B. Y. Shi, D. Y. Jin and X. G. Liu, Nat. Nanotechnol., 2015, 10, 924–936 CrossRef CAS PubMed.
- L. Lei, D. Q. Chen, C. Li, F. Huang, J. J. Zhang and S. Q. Xu, J. Mater. Chem. C, 2018, 6, 5427–5433 RSC.
- Q. Q. Ma, J. Wang, Z. H. Li, D. Wang, X. X. Hu, Y. S. Xu and Q. Yuan, Inorg. Chem. Front., 2017, 4, 1166–1172 RSC.
- Y. L. Liu, K. L. Ai and L. H. Lu, Nanoscale, 2011, 3, 4804–4810 RSC.
- M. L. You, J. J. Zhong, Y. Hong, Z. F. Duan, M. Lin and F. Xu, Nanoscale, 2015, 7, 4423–4431 RSC.
- M. X. Li, W. J. Yao, J. Liu, Q. Y. Tian, L. Liu, J. Ding, Q. W. Xue, Q. Lu and W. Wu, J. Mater. Chem. C, 2017, 5, 6512–6520 RSC.
- W. J. Yao, Q. Y. Tian, J. Liu, Z. H. Wu, S. Y. Cui, J. Ding, Z. G. Dai and W. Wu, J. Mater. Chem. C, 2016, 4, 6327–6335 RSC.
- Y. D. Han, H. Y. Li, Y. B. Wang, Y. Pan, L. Huang, F. Song and W. Huang, Sci. Rep., 2017, 7, 1320 CrossRef PubMed.
- Y. L. Zhang, P. Lv, X. Liu, H. Y. Chi, G. Q. Xi and Z. K. Qin, Nano, 2020, 15, 2050023–2050031 CrossRef CAS.
- Y. L. Zhang, X. H. Liu, Y. B. Lang, Z. Yuan, D. Zhao, G. S. Qin and W. P. Qin, J. Mater. Chem. C, 2015, 3, 2045–2053 RSC.
- Y. L. Zhang, F. Wang, Y. B Lang, J. Yin, M. L. Zhang, X. H. Liu, D. M. Zhang, D. Zhao, G. S. Qin and W. P. Qin, J. Mater. Chem., 2015, 3, 9827–9832 RSC.
- Y. Zhang, S. J. Deng, Y. H. Li, B. Liu, G. X. Pan, Q. Liu, X. L. Wang, X. H. Xia and J. P. Tu, Energy Stor. Mater., 2020, 29, 52–59 Search PubMed.
- D. Q. Chen and P. Huang, Dalton Trans., 2014, 43, 11299–11304 RSC.
- R. R. Deng, X. J. Xie, M. Vendrell, Y. T. Chang and X. G. Liu, J. Am. Chem. Soc., 2011, 133, 20168–20171 CrossRef CAS.
- Q. Yang, X. Y. Wang, H. L. Peng, M. Arabi, J. H. Li, H. Xiong, J. Choo and L. G. Chen, Sens. Actuators, B, 2019, 302, 127176 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |