Open Access Article
Open Access ArticleLow temperature synthesis of franklinite stabilized cefixime as a multifunctional nanoformulation
Amna Munsafab,
Muhammad Naeem Ahmed *a,
Aroosa Zafarc,
Bilal Akram
*a,
Aroosa Zafarc,
Bilal Akram *bd and
Mahmoud A. A. Ibrahim
*bd and
Mahmoud A. A. Ibrahim ef
ef
aDepartment of Chemistry, The University of Azad Jammu & Kashmir, Muzaffarabad 13100, Pakistan
bDepartment of Chemistry, Women University of Azad Jammu & Kashmir, Bagh 12500, AJ&K, Pakistan
cDepartment of Pharmacy, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad 45320, Pakistan
dDepartment of Chemistry, Tsinghua University, Beijing, China
eComputational Chemistry Laboratory, Chemistry Department, Faculty of Science, Minia University, Minia 61519, Egypt
fSchool of Health Sciences, University of KwaZulu-Natal, Westville Campus, Durban 4000, South Africa
First published on 6th November 2024
Abstract
Cefixime, an antibiotic with low solubility, stability, bioavailability and therapeutic effectiveness, needs to be administered in larger doses for effective treatment. This can lead to higher healthcare costs and increased risk of side effects, negatively affecting public health. Herein, we aim to develop a strategy to overcome the aforementioned limitations by stabilizing it using franklinite nanostructures. Franklinite nanostructures (ZnFe2O4) were synthesized via a green method and subsequently used as a support to stabilize cefixime (Cef). The successful formation of ZnFe2O4 nanostructures and subsequent loading of the drug was confirmed using various microscopic and spectroscopic analyses. Solubility measurements and dissolution tests for the franklinite stabilized cefixime (Cef–ZnFe2O4) indicated increased solubility, enhanced in vitro bioavailability and greater absorption under physiological conditions. Hemolytic assay affirmed the safety and efficacy of drug stabilized by franklinite. Biological assessment of Cef–ZnFe2O4 revealed that it has strong antifungal, antioxidant and kinase inhibition potential as compared to its bare counterpart. These findings emphasize the potential of newly designed Cef–ZnFe2O4 as a promising nanoformulation with enhanced solubility, efficacy, safety and biological activities.
Introduction
Traditional medications, despite their clinical applications, often face limitations in optimizing their therapeutic efficacy while minimizing associated side effects. These limitations include low bioavailability, less solubility and emergence of antimicrobial resistance. These issues demand high drug doses to achieve the desired effect, leading to increased side effects, reduced adherence and rapid excretion from the body.1,2 Cefixime is a broad-spectrum cephalosporin antibiotic characterized by poor solubility and instability under certain conditions. These properties restrict its absorption in the gastrointestinal tract and consequently diminish its therapeutic effectiveness.3,4 Furthermore, these limitations can result in less-than-ideal therapeutic outcomes and contribute to the development of antibiotic resistance.To address these concerns, nanotechnology has opened a path to advanced treatment strategies through nanomedicines.5 The scientific community has been recently showing increasing interest in nanomaterials owing to the combined physicochemical characteristics of their constituent parts.6–8 These nanomaterials hold significant importance across various scientific and technological fields, including optics, electronics, environmental science, aerospace9,10 and medicine, due to their diverse applications, which are influenced by their structures, compositions and stabilities.11,12 Metal oxide-based nanomaterials, owing to their inherent properties, have been reported as promising solutions for addressing a range of ailments, including microbial infections,13 inflammations,14 malignancies15 and liver disorders.16–20 Due to their biocompatibility, chemical stability, ease of separation and cost-effectiveness, they are ideal for biomedical applications.21–23 By incorporating therapeutic agents into nanoscale matrices, nanomaterials offer several advantages, including increased drug stability, solubility, bioavailability and efficacy.24–28
Transition metal ferrites possess versatile characteristics, such as high surface area, high chemical stability, high surface active sites, strong magnetic properties and ease of functionalization. These properties make them key components in a wide range of industrial and technological applications.29 Franklinite, in particular, offers a unique combination of properties. The presence of both Fe and Zn atoms enhances the stability of franklinite nanostructures and imparts bi-functional properties, making them ideal for specialized applications.30,31 In recent years, these nanostructures have gained growing attention for biomedical uses due to their physiological compatibility, biosafety and potential in targeted drug delivery.32 Therefore, the combination of superior stability, magnetic properties, biosafety and potential for functionalization makes franklinite an excellent choice to stabilize cefixime for advanced biomedical applications.
The quest for an environmentally sustainable and revolutionary method to generate metal oxide-based nanocomposites is unceasing. Nanocomposites can be achieved through several methods, including the sol–gel process, pulse laser desorption, mechanical milling, spray pyrolysis, thermal evaporation and microwave-assisted techniques.33 Unfortunately, these methods are highly expensive and involve the extensive use of toxic chemicals requiring a large workforce and posing dangers to the environment. Some hazardous chemicals used in chemical methods can have harmful effects in the medical field.34 However, green synthesis using biowaste promotes a non-toxic, eco-friendly approach to nanomaterial synthesis.35,36 Agricultural wastes contain abundant secondary metabolites that reduce the metal ions through enzymes, amino acids, phenolic compounds and vitamins, which act as natural reducing agents.37 Additionally, this approach also lowers the synthesis costs and energy requirements as compared to physical and chemical methods.38,39
Current research seeks to examine the biological efficacy, solubility and biocompatibility of franklinite stabilized cefixime under various physiological conditions synthesized using Buddleja asiatica leaves. Through the fusion of green chemistry and nanocomposite innovation, our investigation aims to thoroughly evaluate the therapeutic capabilities of the composite. This assessment includes a range of in vitro assays such as antioxidant, hemolytic, antifungal and protein kinase inhibition assays offering a holistic understanding of its pharmacological potential.
Experimental
Materials and methods
The chemicals used in the recent exploration were of analytical grade and were utilized as provided.
Prior to usage, glass wares were washed with a 5% nitric acid solution and rinsed twice with deionized water. All the solutions utilized in this study were prepared in deionized water.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes to separate the cefixime loaded composite, which was then washed double with distilled water and once with DMSO to get rid of the unloaded cefixime. After the drying process, the drug loading efficiency was determined.
000 rpm for 10 minutes to separate the cefixime loaded composite, which was then washed double with distilled water and once with DMSO to get rid of the unloaded cefixime. After the drying process, the drug loading efficiency was determined.In vitro biocompatibility evaluation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant was removed, and 1 ml PBS was added to each Eppendorf tube and centrifuged to wash the blood cells. The supernatant was discarded, and the process was repeated three times. The PBS was added to the Eppendorf tube to make a 5% solution of cells. 150 μl of the sample was added to the Eppendorf tube, then 350 μl of blood cells were added to each Eppendorf tube. The Eppendorf tubes were incubated at 37 °C for 30 min and then centrifuged at 10
000 rpm for 10 min. The supernatant was removed, and 1 ml PBS was added to each Eppendorf tube and centrifuged to wash the blood cells. The supernatant was discarded, and the process was repeated three times. The PBS was added to the Eppendorf tube to make a 5% solution of cells. 150 μl of the sample was added to the Eppendorf tube, then 350 μl of blood cells were added to each Eppendorf tube. The Eppendorf tubes were incubated at 37 °C for 30 min and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. 200 μl of supernatant was added to 96 well plates and absorbance was measured at 530 nm. The % age hemolytic activity was calculated by the formula,
000 rpm for 5 min. 200 μl of supernatant was added to 96 well plates and absorbance was measured at 530 nm. The % age hemolytic activity was calculated by the formula,| % hemolysis = As − An/Ap − An × 100 |
| Free radical scavenging activity = 1 − As/Ac × 100 |
Results and discussion
Synthesis of franklinite (ZnFe2O4) nanoparticles
Franklinite (ZnFe2O4) nanoparticles were obtained using an aqueous extract of B. asiatica leaves as a reducing agent, zinc acetate dihydrate and iron sulphate as precursors at room temperature. The easily obtained ZnFe2O4 nanoparticles were further used as a support to stabilize Cef. Drug-loading efficiency was estimated to be 76%.X-ray diffraction analysis
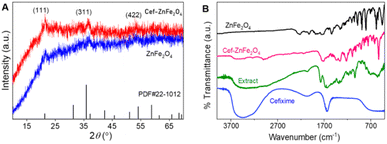
The XRD analysis of the synthesized bare and cefixime functionalized ZnFe2O4 displays a semi-crystalline structure by the presence of three significant peaks shown in Fig. 1A. These peaks at 2θ values of 18.19°, 35.26° and 53.1°, correspond to (111), (311) and (422) reflection planes, respectively. In the synthesized material, the peak at 18.19° was slightly shifted and appeared at 21°. The diffraction pattern displays the franklinite structure of the as-synthesized nanoparticles. Diffraction patterns match the standard JCPDS card no. 22-1012. The X-ray diffraction patterns of both bare and cefixime-functionalized franklinite ZnFe2O4 nanostructures exhibit a higher degree of similarity, particularly in terms of the position of the peaks. A slight difference observed between the two sets of XRD patterns is in the intensity of the peaks. Specifically, the peaks corresponding to the bare franklinite are somewhat more pronounced than those of the drug-loaded nanoparticles. This difference suggests that the inclusion of cefixime into the nanostructures affects the crystallinity, slightly reducing the intensity of diffraction peaks.Fourier transform infrared (FTIR) analysis
A comparison of FTIR spectra of the plant extract, cefixime, bare and cefixime functionalized ZnFe2O4 nanostructures indicated their functional groups and possible interactions, as shown in Fig. 1B. Cefixime shows major peaks at 3500–3000 cm−1 resembling NH or OH stretching vibrations, along with a carbonyl peak at 1700 cm−1. The extract noticeably displays a broad peak around 3500 cm−1 indicative of the OH group, potentially from phenols or alcohols coupled with faint signals at 2000 cm−1 along with small peaks in the range of 1500 cm−1 to 500 cm−1, which are due to phytochemicals. The primary peaks in the region of 600 cm−1 450 cm−1 are indicative of metal–oxygen bonds typically for metal oxides. Cefixime-functionalized ZnFe2O4 nanostructure interestingly has a spectrum that combines characteristics from the two compounds, a faint peak at 3000 cm−1 that resembles both OH or NH groups, a peak at 1700 cm−1 that represents a signal from the extract properties, and peaks at 600–450 cm−1 that resembles metal–oxygen bonds.Cefixime shows a sharp carbonyl peak at 1700 cm−1 but the noticeable non-uniformity of this peak in the FTIR spectrum of Cef–ZnFe2O4 shows strong interactions between the cefixime and franklinite surface. This change is likely caused by the carbonyl groups in cefixime binding tightly to the Zn2+ and Fe3+ ions on the franklinite surface, changing the vibrational characteristics of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. Additionally, the hydrogen bonding between cefixime and franklinite weakens the peak of carbonyl. Similarly, functionalization with nanostructures may induce slight conformational changes in the cefixime molecule, altering the orientation of the carbonyl group and further affecting its vibrational behavior. These combined effects lead to a significant change in the appearance of the peak, highlighting the profound interactions between cefixime and the franklinite nanostructures.
O bond. Additionally, the hydrogen bonding between cefixime and franklinite weakens the peak of carbonyl. Similarly, functionalization with nanostructures may induce slight conformational changes in the cefixime molecule, altering the orientation of the carbonyl group and further affecting its vibrational behavior. These combined effects lead to a significant change in the appearance of the peak, highlighting the profound interactions between cefixime and the franklinite nanostructures.
Energy dispersive X-ray spectroscopy (EDX) analysis
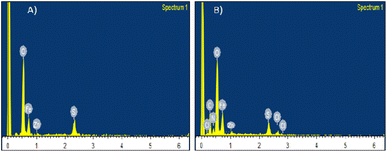
EDX spectra shown in Fig. 2A displays remarkable peaks corresponding to iron, zinc and oxygen compatible with the elemental composition expected for ZnFe2O4. Peak corresponding to zinc is weaker than those for iron, meaning that there is a less percentage of zinc as compared to iron. EDX spectra helped to confirm that zinc is also a part of the material, along with iron. Similarly, EDX spectra (B) of Cef–ZnFe2O4 Nanostructures exhibit notable peaks corresponding to iron, zinc, oxygen, nitrogen, carbon and sulphur consistent with the expected elements present in both cefixime and franklinite. The franklinite composition was further confirmed using ICP-OES analysis and the atomic ratio of Zn and Fe are in complete agreement with ZnFe2O4.UV-visible analysis
Fig. 3 reveals a distinct absorption behavior for plant extract, cefixime, and franklinite (ZnFe2O4) nanostructures (bare as well as cefixime-loaded). The UV-visible spectrum of B. asiatica leaf extract displays a unique range between 250 and 400 nm, which suggests the presence of a complex mixture of phytochemicals. The cefixime spectrum shows specific absorption peaks at 300 nm and a weaker one at 280 nm indicating significant light absorption by certain functional groups. The bare ZnFe2O4 nanostructures exhibit minimal UV-visible absorbance near 300 nm, indicating the presence of both metal oxides. When cefixime is loaded onto ZnFe2O4 nanostructures, the spectrum reflects both cefixime and franklinite. Absorption at 280 nm signifies the presence of cefixime within the franklinite nanostructures while a slightly broadened peak around 300 nm hints at potential interaction between cefixime and ZnFe2O4.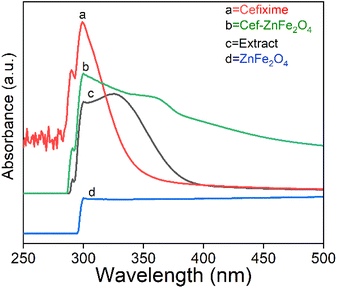 | ||
| Fig. 3 UV-visible spectroscopy analysis of plant extract, cefixime, franklinite (ZnFe2O4) and cefixime-loaded franklinite (Cef–ZnFe2O4). | ||
The functional groups such as –COOH and –NH2 in cefixime interact electrostatically with Zn2+ and Fe3+ ions on the surface of franklinite. These groups may also form hydrogen bonds with hydroxyl groups on the surface of nanostructures, changing the surface environment and affecting UV absorption. Additionally, donor atoms such as nitrogen and oxygen in cefixime can coordinate with the metal ions on the franklinite's surface and weak van der Waals forces between cefixime molecules and the nanostructures further contribute towards the broadening of the peak. These results are consistent with the earlier findings41 related to doxorubicin-conjugated iron oxide nanoparticles for biomedical applications.
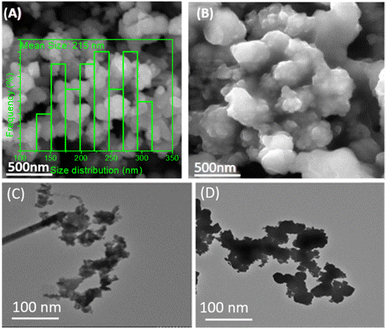
Electron microscopy analysis
Solubility measurements
Increased temperature enhances the solubility of cefixime-functionalized franklinite (ZnFe2O4) nanostructures, which could be beneficial for increasing the release rate of therapeutic agents. The solubility of Cef–ZnFe2O4 nanostructures decreases as the pH increases from 1.2 to 7.4. This trend is consistent at both 25 °C and 37 °C, indicating higher solubility in acidic conditions compared to neutral conditions. This behavior is particularly relevant for therapeutic applications of Cef–ZnFe2O4 nanostructures in body areas with acidic pH (Table 1).| pH | Temp. (°C) | S1 (mg l−1) | S2 (mg l−1) | S3 (mg l−1) | Mean ± SD (mg l−1) |
|---|---|---|---|---|---|
| 1.2 | 25 | 55.2 | 50 | 53 | 52.73 ± 2.03 |
| 1.2 | 37 | 62 | 63 | 62.8 | 62.60 ± 0.41 |
| 4.5 | 25 | 49.7 | 51 | 50.5 | 50.40 ± 0.66 |
| 4.5 | 37 | 52.3 | 55 | 53.8 | 53.70 ± 1.35 |
| 7.4 | 25 | 35.1 | 36 | 35.5 | 35.53 ± 0.45 |
| 7.4 | 37 | 43 | 45.3 | 44.2 | 44.17 ± 1.15 |
In vitro bioavailability evaluation
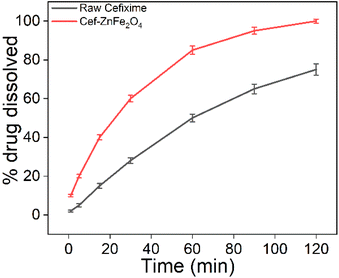
In vitro dissolution testing is an important step in the development of nanoformulation used for therapeutic purposes, particularly in assessing their potential bioavailability. Fig. 5 shows that cefixime-functionalized franklinite (Cef–ZnFe2O4) indicates a significantly faster release rate reaching 100% release by 120 minutes compared to only 75% for the raw cefixime. These results suggest that the Cef–ZnFe2O4 nanostructures could potentially enhance the bioavailability of cefixime allowing for faster and more complete absorption in a physiological environment.In vitro biocompatibility evaluation
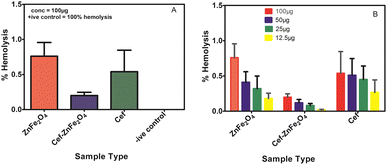
In the current research, the hemocompatibility of green-synthesized cefixime-loaded ZnFe2O4 was evaluated compared to its bare counterpart using human red blood cells at four different concentrations (12.5–100 μg ml−1). Fig. 6A shows that even at maximum concentration, the percentage of hemolysis is very low for ZnFe2O4 (both bare and cefixime loaded) as compared to the positive control. Fig. 6B shows that the decreasing concentration decreases the hemolytic percentage for Cef–ZnFe2O4 as well as its counterparts (bare composite and cefixime). All four concentrations of the synthesized bare and cefixime-loaded nanostructures show their good hemocompatibility. Remarkably, even at a maximum concentration of 100 μg ml−1, as-synthesized Cef–ZnFe2O4 nanostructures exhibited 0.49% of total hemolytic activity and there was no destruction of red blood cells. From the study's outcome, as-synthesized Cef–ZnFe2O4 nanostructures are safest to use even at higher concentrations and they might be employed for the therapeutic purpose.
| Sample type | Bald zone | Clear zone |
|---|---|---|
| ZnFe2O4 | 11 | — |
| Cef–ZnFe2O4 | — | 11 |
| Cefixime | 11 | 8 |
| Surfactin B | 22.3 | — |
| DMSO | 0 | 0 |
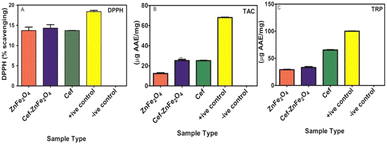
DPPH assay measures the free radical scavenging ability of an antioxidant. Fig. 7A shows that the % mean scavenging for Cef–ZnFe2O4 is 14.3 ± 0.78. This value is higher than its bare counterpart alone (13.7 ± 0.70) and cefixime alone (13.7 ± 0.1). Enhancement though modest suggests that cefixime loading improves the activity of ZnFe2O4 nanostructures. Total antioxidant capacity was determined using the phosphomolybdenum-based method where antioxidants reduce Mo(VI) to Mo(V) forming a greenish phosphate molybdate complex. Mean AAE μg per mg ± SD values for positive control, cefixime, Cef–ZnFe2O4 and bare ZnFe2O4 are 68 ± 0.24, 25.1 ± 0.25, 25.1 ± 0.27 and 12.3 ± 0.42, respectively, as shown in Fig. 7B. The significant difference between the TAC values of Cef–ZnFe2O4 nanostructures and ZnFe2O4 highlights that franklinite has its own intrinsic antioxidant capacity and the presence of cefixime enhances this capacity. TAC value of Cef–ZnFe2O4 is almost identical to cefixime alone indicating that the antioxidant capacity is retained when loaded onto ZnFe2O4 nanostructures. The total reducing power of all the tested samples was analyzed using the potassium ferricyanide colorimetric method where the sample converts Fe+3 to Fe+2 indicated by a blue color formation. TRPs of plant-synthesized pure ZnFe2O4 nanostructures, Cef–ZnFe2O4 nanostructures, and cefixime, compared to ascorbic acid at 100 μg mg−1 as a positive control, were recorded as 29 ± 0.78, 33 ± 0.7, 65 ± 0.76 and 100 ± 0, respectively, as shown in Fig. 7C. These results show that the Cef–ZnFe2O4 nanostructure has improved the reducing power compared to its bare counterpart indicating a positive effect of cefixime incorporation. However, it remains lower than that of pure cefixime possibly due to the interactions within the nanoparticles matrix that might alter the availability of cefixime active site.
These findings highlight that loading cefixime onto ZnFe2O4 nanostructures significantly enhances its antioxidant properties. This enhancement makes the cefixime-loaded ZnFe2O4 nanostructures a promising multifunctional therapeutic agent with both antimicrobial and potent antioxidant properties.
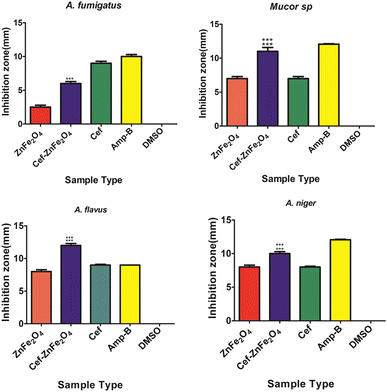
The ability of Cef–ZnFe2O4 nanostructure to exhibit significant antifungal activity against multiple strains including those that are typically more resistant such as A. fumigatus suggests a broad spectrum potential. This efficacy is crucial in clinical settings where infections are often caused by multiple fungal species simultaneously or sequentially.
Conclusions
Franklinite (ZnFe2O4) nanostructures were synthesized via the green method and successfully loaded with cefixime. Drug loading was validated and characterized using FTIR, UV, XRD, SEM, TEM and EDX techniques. In vitro hemolytic assay, antifungal, protein kinase and antioxidant activities were investigated. Results indicated that incorporating cefixime onto presynthesized ZnFe2O4 nanostructures enhances their surface area, significantly improving antifungal, antioxidant and kinase inhibition activities compared to its counterpart. The hemolytic assay demonstrated increased hemocompatibility of cefixime–franklinite. Furthermore, solubility measurements indicated increased solubility at body temperature and in acidic conditions while the dissolution test showed an enhanced dissolution rate suggesting improved in vitro bioavailability and enhanced effectiveness in the physiological environment. These findings suggest that the green synthesis of ZnFe2O4 nanostructures with cefixime loading could offer an effective strategy for enhancing the therapeutic efficacy, safety, bioavailability and solubility of cefixime under physiological conditions.Data availability
The data can be obtained from corresponding author upon reasonable request.Author contributions
Amna Munsaf performed experimentation, writing the draft and drawing all the figures and their interpretation. Muhammad Naeem Ahmed designed, conceptualized and supervised the whole project. Aroosa Zafar carried out the biological studies. Bilal Akram and Mahmoud A. A. Ibrahim performed characterization.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We are thankful to the University of Azad Jammu and Kashmir for financial support.Notes and references
- S. Frosyth, P. Penney and G. Rooney, Int. J. STD AIDS, 2011, 22, 296–297 CrossRef PubMed.
- F. Mottaghitalab, M. Farokhi, Y. Fatahi, F. Atyabi and R. Dinarvand, J. Controlled Release, 2019, 295, 250–267 CrossRef CAS PubMed.
- A. Zehra, B. Naqvi, R. Bushra and S. Ali, Jordan J. Pharm. Sci., 2010, 3, 2 Search PubMed.
- B. S. Schtaz, K. T. Karavokiros, M. A. Taeubel and G. S. Itokazu, Ann. Pharmacother., 1996, 30, 258–268 CrossRef PubMed.
- V. Weissig, T. Elbayoumi, B. Fluhmann and A. Barton, Am. J. Pharm. Educ., 2021, 85, 8331 CrossRef PubMed.
- D. W. Schaefer and R. S. Justice, Macromolecules, 2007, 40, 8501–8517 CrossRef CAS.
- S. Komarneni, J. Mater. Chem., 1992, 2, 1219–1230 RSC.
- S. K. Kumar and R. Krishnamoorti, Annu. Rev. Chem. Biomol. Eng., 2010, 1, 37–58 CrossRef CAS PubMed.
- T. Hassan, A. Salam, S. U. Khan, H. Khanzada, M. Wasim, M. Q. Khan and I. S. Kim, J. Polym. Res., 2021, 28, 36 CrossRef CAS.
- S. K. Kumar and R. Krishnamoorti, Annu. Rev. Chem. Biomol. Eng., 2010, 1, 37–58 CrossRef CAS PubMed.
- A. Sood, V. Arora, J. Shah, R. K. Kotnala and T. K. Jain, Mater. Sci. Eng., C, 2017, 80, 274–281 CrossRef CAS PubMed.
- P. D. Mangalgiri, Bull. Mater. Sci., 1999, 22, 657–664 CrossRef CAS.
- D. Zablotsky, I. Segal, A. Zablotskaya, M. Maiorov and T. A. Nguyen, Magnetic Nanoparticle-Based Hybrid Materials, Woodhead Publishing, 2021, pp. 501–527 Search PubMed.
- C. Pragathiswaran, C. Smitha, H. Barabadi, M. M. Al-Ansari, L. A. Al-Humaid and M. Saravanan, Inorg. Chem. Commun., 2020, 121, 108210 CrossRef CAS.
- S. Kumar, M. K. Shukla, A. K. Sharma, G. K. Jayaprakash, R. K. Tonk, D. K. Chellappan, S. K. Singh, K. Dua, F. Ahmed, S. Bhattacharyya and D. Kumar, MedComm, 2023, 4, e253 CrossRef CAS PubMed.
- H. Malhotra, A. Kamboj and R. K. Gautam, Metal Nanocomposites in Nanotherapeutics for Oxidative Stress-Induced Metabolic Disorders, CRC Press, 2023, pp. 327–348 Search PubMed.
- M. Pourmadadi, E. Rahmani, A. Shamsabadipur, S. Mahtabian, M. Ahmadi, A. Rahdar and A. M. Diez-Pascual, Nanomaterials, 2022, 12, 3873 CrossRef CAS PubMed.
- L. L. Beecroft and C. K. Ober, Chem. Mater., 1997, 9, 1302–1317 CrossRef CAS.
- B. Mishra, S. Varjani, D. C. Agrawal, S. K. Mandal, H. H. Ngo, M. J. Taherzadeh and W. Guo, Environ. Technol. Innovation, 2020, 20, 101063 CrossRef CAS.
- Nanocomposites-Advanced Materials for Energy and Environmental Aspects, ed. M. E. Khan, J. Aslam and C. Verma, Elsevier, 2023 Search PubMed.
- S. J. Owonubi, N. M. Malima and N. Revaprasadu, in Antibiotic Materials in Healthcare, Academic Press, 2020, pp. 287–323 Search PubMed.
- L. Gavrila-Florescu, F. Dumitrache, M. Balas, C. T. Fleaca, M. Scarisoreanu, I. P. Morjan and G. Prodan, Appl. Phys. A: Mater. Sci. Process., 2017, 123, 1–13 CrossRef.
- M. T. S. Chani, S. B. Khan, M. M. Rahman, T. Kamal and A. M. Asiri, J. King Saud Univ., Sci., 2022, 34, 101841 CrossRef.
- H. Kang, S. Mintri, A. V. Menon, H. Y. Lee, H. S. Choi and J. Kim, Nanoscale, 2015, 7, 18848–18862 RSC.
- D. Mishra, J. R. Hubenak and A. B. Mathur, J. Biomed. Mater. Res., Part A, 2013, 101, 3646–3660 CrossRef PubMed.
- S. Lee, H. Park and K. H. Yoo, Adv. Mater., 2010, 22, 4049–4053 CrossRef CAS PubMed.
- K. Letchford and H. Burt, Eur. J. Pharm. Biopharm., 2007, 65, 259–269 CrossRef CAS PubMed.
- A. Vonarbourg, C. Passairani, P. Saulnier and J. P. Benoit, Biomaterials, 2006, 27, 4356–4373 CrossRef CAS PubMed.
- A. Goyal, S. Bansal and S. Singhal, Int. J. Hydrogen Energy, 2014, 39, 4895–4908 CrossRef CAS.
- W. Gac, W. Zawadzki, G. Slowik, M. Greluk and A. Machocki, J. Therm. Anal. Calorim., 2016, 125, 1205–1215 CrossRef CAS.
- L. Han, X. Zhou, L. Wan, Y. Deng and S. Zhan, J. Environ. Chem. Eng., 2014, 2, 123–130 CrossRef CAS.
- A. Manohar, V. Vijayakanth, S. P. Vattikuti and K. H. Kim, Mater. Chem. Phys., 2022, 286, 126117 CrossRef CAS.
- S. Vijayakumar, B. Vaseeharan, B. Malaikozhundan and M. Shobiya, Biomed. Pharmacother., 2016, 84, 1213–1222 CrossRef CAS PubMed.
- J. R. Peralta-Videa, Y. Huang, J. G. Parsons, L. Zhao, L. Lopez-Moreno, J. A. Hernandez-Viezcas and J. L. Gardea-Torresdey, Nanotechnol. Environ. Eng., 2016, 1, 1–29 CrossRef.
- A. N. D. Krupa and R. Vimala, Mater. Sci. Eng., C, 2016, 61, 728–735 CrossRef CAS PubMed.
- F. T. Thema, E. Manikandan, M. S. Dhlamini and M. J. L. Maaza, Mater. Lett., 2015, 161, 124–127 CrossRef CAS.
- K. Parveen, V. Banse and L. Ledwani, AIP Conf. Proc., 2016, 1724(1), 1–7 Search PubMed.
- F. M. Arvanag, A. Bayrami, A. H. Yangjeh and S. R. Poursan, Mater. Sci. Eng., C, 2019, 97, 397–405 CrossRef PubMed.
- V. Ramalingam, P. Muthukumar Sathya, S. Thimmaarayan and H. Mohan, Life Sci., 2022, 309, 121022 CrossRef CAS PubMed.
- Y. Du, L. Xia, A. Jo, R. M. Davis, P. Bissel, M. F. Ehrich and D. G. I. Kingston, Bioconjugate Chem., 2018, 29, 420–430 CrossRef CAS PubMed.
- N. Singh, N. Millot, L. Maurizi, G. Lizard and R. Kumar, ACS Omega, 2020, 5, 16165–16175 CrossRef CAS PubMed.
- A. Imraish, T. Abu Thiab, W. Al-Awaida, H. J. Al-Ameer, Y. Bustanji, H. Hammad and A. Al-Hunaiti, J. Food Biochem., 2021, 45(6), e13730 CrossRef CAS PubMed.
- A. Al-Zabin, T. Abu Thiab, M. Zihlif, A. Al-Hunaiti, H. J. Al-Ameer, W. Al-Awaida and A. Imraish, Biomed. Rep., 2024, 20(3), 1–9 Search PubMed.
- M. Zhang, F. Zhao, Y. Yang, H. Li, H. Gao, E. Yao and Z. Jiang, J. Therm. Anal. Calorim., 2020, 141, 1413–1423 CrossRef CAS.
- H. Wu, Q. Wu, J. Zhang, Q. Gu, W. Guo, S. Rong and X. Jing, Sci. Rep., 2021, 11(1), 17113 CrossRef CAS PubMed.
- V. M. Gandhi and K. M. Cherian, Toxicol. In Vitro, 2000, 14, 513–516 CrossRef CAS PubMed.
- R. Kannaiyan and D. Mahadevan, Expert Rev. Anticancer Ther., 2018, 18, 1249–1270 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |