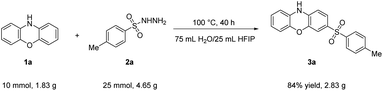
Open Access Article
Open Access ArticleCatalyst-free regioselective sulfonylation of phenoxazine with sulfonyl hydrazides in H2O/HFIP†
Yanan Li *a,
Lin Lia,
Jing Tana,
Chenpei Yanga,
Yifei Wanga,
Feiyang Lib,
Chenyu Liub,
Xiaohui Wu*c and
Jianan Sun
*a,
Lin Lia,
Jing Tana,
Chenpei Yanga,
Yifei Wanga,
Feiyang Lib,
Chenyu Liub,
Xiaohui Wu*c and
Jianan Sun *b
*b
aSchool of Chemical and Blasting Engineering, Anhui Province Key Laboratory of Specialty Polymers, Anhui University of Science and Technology, Huainan 232001, China. E-mail: liyanan@mail.ustc.edu.cn
bSchool of Biomedical Engineering, School of Health Management, Anhui Medical University, Hefei 230032, China. E-mail: jnsun@ahmu.edu.cn
cHenan Key Laboratory of Rare Earth Functional Materials, Zhoukou Normal University, Zhoukou 466001, China. E-mail: wuxiaohuixinyi@163.com
First published on 3rd October 2024
Abstract
A facile catalyst-free sulfonylation of phenoxazine with sulfonyl hydrazides was efficiently developed in H2O/HFIP without any catalyst. This metal- and catalyst-free protocol is suitable for aromatic and aliphatic reagents to afford the corresponding sulfones in moderate to high yields. A gram-scale experiment was performed to assess the practicability of the method.
Introduction
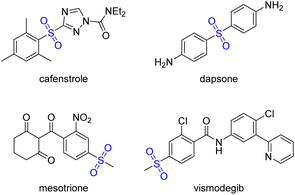
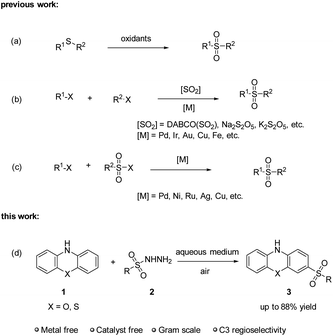
Green and sustainable chemistry represents a transformative approach in modern chemical practices, focusing on minimizing the use and generation of harmful substances while efficiently synthesizing target products.1 To achieve this goal, chemists have made remarkable strides in developing innovative approaches, particularly in metal-free synthesis2 and aqueous-phase reactions.3 Metal-free synthesis has gained significant traction in green chemistry for its environmental friendliness and cost-effectiveness. By eliminating the need for often toxic and expensive metal catalysts, this approach reduces both environmental contamination and production costs. Simultaneously, water as a solvent offers many advantages in chemical processes. Water is not only safe and abundantly available but also non-flammable and non-toxic, making it an ideal medium for sustainable reactions. Thus, conducting sulfonylation reactions in aqueous medium under metal-free conditions is highly anticipated as a green synthesis pathway.Sulfones are widely distributed in nature products and pharmaceutical drugs,4 such as cafenstrole, dapsone, mesotrione and vismodegib (Scheme 1).5 Over the past decades, numerous methodologies have been developed to synthesize sulfones, including oxidation of sulfides,6 sulfur dioxide insertion,7 and corresponding cross coupling reactions,8 etc. However, these methods usually do not meet the requirements of green chemistry, as they frequently involve the use of strong oxidants, metal catalysts, or are conducted in organic solvents. Therefore, there is a growing requirement to develop a facile, green, and economical friendly protocol for synthesizing sulfones. Sulfonyl hydrazides are ideal sulfonylation agents due to their ease of handling, stability, moisture compatibility, noncorrosive nature and eco-friendly conversion.9 To best of our knowledge, the sulfonylation reaction of phenoxazine has not been explored yet. Herein, we reported our recent work to develop a sulfonylation reaction of phenoxazine with sulfonyl hydrazides in aqueous medium under metal-free conditions (Scheme 2). This sulfonylation reaction exhibits a good tolerance to air and water. Notably, in this sulfonylation reaction, regioselective C3–SO2 bond is formed instead of N–SO2 bond.
Results and discussion
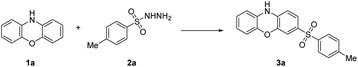
We commenced our initial trials by selecting phenoxazine 1a and 4-methylbenzenesulfonohydrazide 2a as the model substrates (Table 1). The reaction was carried out in water at 100 °C in a Schlenk tube, affording the desired product 3a in 48% yield (entry 1). When water was replaced with organic solvents (entries 2–10), the yields of 3a decreased, except when using HFIP (entry 10), which afforded a 65% yield of the target product 3a. Therefore, we investigated the influence of mixed solvents on the reaction (entries 11–13), H2O/HFIP (v/v 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) was identified as the optimum choice (entry 13), giving 3a in 85% yield. Changing the temperature did not improve the yield of 3a (entries 14–15). Conducting this reaction under nitrogen atmosphere negatively affected the yield (entry 16). Thus, the optimal reaction conditions (entry 13) were determined as described below: the sulfonylation reaction was carried out in H2O/HFIP (v/v 1.5
0.5) was identified as the optimum choice (entry 13), giving 3a in 85% yield. Changing the temperature did not improve the yield of 3a (entries 14–15). Conducting this reaction under nitrogen atmosphere negatively affected the yield (entry 16). Thus, the optimal reaction conditions (entry 13) were determined as described below: the sulfonylation reaction was carried out in H2O/HFIP (v/v 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) under air atmosphere at 100 °C in a Schlenk tube.
0.5) under air atmosphere at 100 °C in a Schlenk tube.
| Entry | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|
| a Unless otherwise noted, all reactions were performed with 1a (0.2 mmol), 2a (0.5 mmol), solvent (2.0 mL) in a Schlenk tube for 24 h.b Isolated yield.c Under nitrogen atmosphere. | |||
| 1 | H2O | 100 | 48 |
| 2 | CH3CN | 100 | 18 |
| 3 | EtOAc | 100 | 15 |
| 4 | Toluene | 100 | 12 |
| 5 | DMF | 100 | n.d. |
| 6 | THF | 100 | 11 |
| 7 | CH3OH | 100 | 16 |
| 8 | DMSO | 100 | 8 |
| 9 | CH2Cl2 | 100 | 29 |
| 10 | HFIP | 100 | 65 |
| 11 | 0.5 mL H2O/1.5 mL HFIP | 100 | 63 |
| 12 | 1.0 mL H2O/1.0 mL HFIP | 100 | 77 |
| 13 | 1.5 mL H2O/0.5 mL HFIP | 100 | 85 |
| 14 | 1.5 mL H2O/0.5 mL HFIP | 90 | 73 |
| 15 | 1.5 mL H2O/0.5 mL HFIP | 110 | 85 |
| 16c | 1.5 mL H2O/0.5 mL HFIP | 100 | 52 |
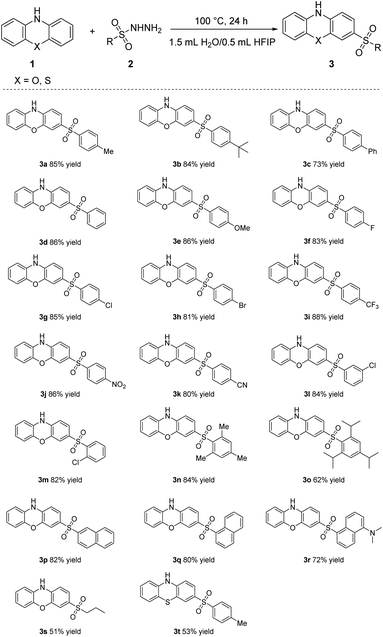
With the optimized conditions established, the substrate scope for the sulfonylation reactions was explored (Table 2). Initially, the electronic effect of the substrates was investigated by varying the para position substituent groups of the phenyl ring of R. Substrates with hydrogen (3d), electron-donating groups, such as methyl (3a) tert-butyl (3b) and methoxy (3e) groups, electron-withdrawing groups, such as halogens (3f–3h), or strong electron-withdrawing groups, such as trifluoromethyl (3i), nitro (3j) and nitrile (3k) groups provided the corresponding products in 80% to 88% yields. These results demonstrated that the electronic effect has minimal influence on this reaction. Afterwards, the steric effect on the phenyl ring was investigated. A broad range of benzenesulfonohydrazides substituted with a chlorine group in any position (ortho, meta, para) furnished the corresponding sulfone products in high yields (3g, 3l, 3m). The Substrate bearing a phenyl group (3c) affording the target product in 73% yield. Substrates bearing multi- substituted groups, such as tri-methyl (3n), tri-isopropyl (3o) groups on the phenyl ring provided the corresponding products in 84% and 62% yields, respectively, due to the steric effect. When R were 1-naphthyl (3q) and 2-naphthyl (3p) groups, the corresponding products were obtained in high yields. Changing R to N,N-dimethyl-1-naphthyl-5-amine group (3r) was tolerated but resulted in a slightly lower yield. Pleasingly, with a substrate bearing an aliphatic group, n-propyl group, target product 3s was isolated in moderate yield, which increased the significance of this sulfonylation strategy. Moreover, phenothiazine (3t) was also compatible with this reaction system, giving the corresponding product in 53% yield.
To demonstrate the synthetic application of this sulfonylation protocol, a gram scale reaction of phenoxazine 1a and 4-methylbenzenesulfonohydrazide 2a was carried out (Table 3). To our delight, the target product 3a was gained with an 84% isolated yield.
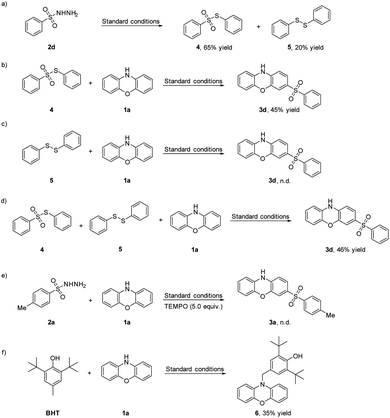
To gain further insight into the reaction mechanism, several control experiments were conducted (Scheme 3). First, when the reaction was carried out under standard conditions without phenoxazine 1a, S-phenyl benzenesulfonothioate 4 and 1,2-diphenyldisulfane 5 were obtained from benzenesulfonohydrazide 2d in 65% and 20% yields, respectively (Scheme 3a). Replacing the substrate 2d by S-phenyl benzenesulfonothioate 4 provided the corresponding product 3d in 45% yield (Scheme 3b). However, when the substrate 2d was replaced by 1,2-diphenyldisulfane 5, the target product 3d was not detected (Scheme 3c). Then the substrate 2d was replaced by S-phenyl benzenesulfonothioate 4 and 1,2-diphenyldisulfane 5, the target product 3d was obtained in 46% yield (Scheme 3d). These results indicated 5 was not involved to generate 3d. These results indicated that 4 may be generated as an intermediate in the reaction. When the model reaction was conducted in the presence of radical scavenger 2,2,6,6-tetramethylpiperidinooxy (TEMPO, 5.0 equiv.) under standard conditions, no 3a was detected (Scheme 3e), suggesting that the reaction involves a radical process. When the substrate 2 was replaced by BHT under standard conditions, 6 was obtained in 35% yield, implying N-centered radical was produced (Scheme 3f).
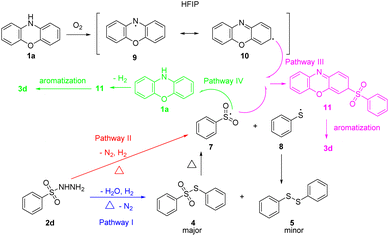
Based on the aforementioned experimental results and previous reports,10 a plausible mechanism is proposed (Scheme 4). First, two plausible competitive reaction pathways to generate intermediate 7 are proposed. According to control experiments Scheme 3a–c, pathway I is proposed. Initially, benzenesulfonohydrazide 2d transforms into minor product 5 and major intermediate 4 which can dissociate into radicals 7 and 8 under heating conditions. Due to the yields of product 3d were decreased in control experiments 3b and 3d, competitive pathway II is proposed. Benzenesulfonohydrazide 2d transforms into intermediate 7 under heating conditions directly. Afterwards, two plausible competitive reaction pathways to generate product 3d are also proposed. According to control experiment Scheme 3f, pathway III is proposed. Under oxygen atmosphere, 1a was oxidated to generate to intermediate 9. The intermediate 9 transforms into C-centered radical 10 immediately, and then reacts with SO2-radical 7 to form transition state 11. Subsequently, transition state 11 transforms into final product 3d via aromatization. HFIP plays a role in stabilizing radicals.11 According to entry 16 in Table 1, competitive pathway IV is proposed. Without oxygen, the SO2-radical 7 reacts with 1a directly to form transition state 11. Subsequently, transition state 11 transforms into final product 3d via aromatization.
Conclusions
In conclusion, we have developed a sulfonylation reaction of phenoxazine with sulfonyl hydrazides in aqueous medium under metal-free conditions. This sulfonylation can be performed under environmentally benign conditions without the need for any catalyst and ligand. A variety of corresponding sulfones were obtained in moderate to high yields. Moreover, a gram scale of the sulfonylation reaction was achieved.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the Education Bureau of Anhui Province (Grant 2023AH051220), the Open Research Fund Program of Anhui Province Key Laboratory of Specialty Polymers (Grant AHKLSP23-10), the Scientific Research Foundation for High-level Talents of Anhui University of Science and Technology (Grant 13220021), the Key Project of Natural Science Research of Anhui Provincial Department of Education (Grant KJ2021A0250) and the Natural Science Foundation of Henan (Grant 222300420396).Notes and references
- (a) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford Press, 2000 CrossRef; (b) The Role of Green Chemistry in Biomass Processing and Conversion, ed. H. B. Xie and N. Gathergood, Wiley, 2013 Search PubMed.
- (a) A. B. Cuenca, R. Shishido, H. Ito and E. Fernández, Chem. Soc. Rev., 2017, 46, 415 RSC; (b) M. Z. Rahman, M. G. Kibria and C. B. Mullins, Chem. Soc. Rev., 2020, 49, 1887 RSC; (c) S. Roy, S. Panja, S. R. Sahoo, S. Chatterjee and D. Maiti, Chem. Soc. Rev., 2023, 52, 2391 RSC.
- (a) C. J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS; (b) Y. Hayashi, Angew. Chem., 2006, 118, 8281 CrossRef; (c) Y. Jung and R. A. Marcus, J. Am. Chem. Soc., 2007, 129, 5492 CrossRef CAS PubMed; (d) C. F. Pan and Z. Y. Wang, Coord. Chem. Rev., 2008, 252, 736 CrossRef CAS; (e) M. O. Simon and C. J. Li, Chem. Soc. Rev., 2012, 41, 1415 RSC; (f) A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Commun., 2014, 50, 12800 RSC; (g) Y. N. Li, Y. K. Huang, Y. Gui, J. N. Sun, J. D. Li, Z. G. Zha and Z. Y. Wang, Org. Lett., 2017, 19, 6416 CrossRef CAS PubMed; (h) M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237 RSC; (i) B. H. Lipshutz, Green Chem., 2024, 26, 739 RSC.
- (a) M. H. Feng, B. Q. Tang, S. H. Liang and X. F. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200 CrossRef CAS PubMed; (b) K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 CrossRef PubMed.
- (a) Y. M. Ma, R. H. Liu, X. Y. Gong, Z. Li, Q. C. Huang, H. S. Wang and G. H. Song, J. Agric. Food Chem., 2006, 54, 7724 CrossRef CAS PubMed; (b) R. Beaudegnies, A. J. F. Edmunds, T. E. M. Fraser, R. G. Hall, T. R. Hawkes, G. Mitchell, J. Schaetzer, S. Wendeborn and J. Wibley, Bioorg. Med. Chem., 2009, 17, 4134 CrossRef CAS PubMed; (c) A. Chakraborty, A. K. Panda, R. Ghosh and A. Biswas, Arch. Biochem. Biophys., 2019, 665, 107 CrossRef CAS PubMed.
- (a) A. Ivachtchenko, E. Golovina, M. Kadieva, O. Mitkin, S. Tkachenko and I. Okun, Bioorg. Med. Chem., 2013, 21, 4614 CrossRef CAS PubMed; (b) R. Fu, W. J. Hao, Y. N. Wu, N. N. Wang, S. J. Tu, G. G. Li and B. Jiang, Org. Chem. Front., 2016, 3, 1452 RSC; (c) N. W. Liu, S. Liang and G. Manolikakes, Synthesis, 2016, 48, 1939 CrossRef CAS.
- (a) G. Y. S. Qiu, K. D. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561 RSC; (b) S. Q. Ye, G. Y. S. Qiu and J. Wu, Chem. Commun., 2019, 55, 1013 RSC; (c) J. Zhang, P. Q. Wang, Y. Z. Li and J. Wu, Chem. Commun., 2023, 59, 3821 RSC.
- (a) C. Shen, P. F. Zhang, Q. Sun, S. Q. Bai, T. S. A. Hor and X. G. Liu, Chem. Soc. Rev., 2015, 44, 291 RSC; (b) Y. Q. Li and Y. H. Fan, Synth. Commun., 2019, 49, 3227 CrossRef CAS; (c) D. Joseph, M. A. Idris, J. J. Chen and S. Lee, ACS Catal., 2021, 11, 4169 CrossRef CAS.
- (a) Y. Yang, L. Tang, S. Zhang, X. F. Guo, Z. G. Zha and Z. Y. Wang, Green Chem., 2014, 16, 4106 RSC; (b) L. Tang, Y. Yang, L. X. Wen, X. K. Yang and Z. Y. Wang, Green Chem., 2016, 18, 1224 RSC; (c) Y. Yang, Y. J Bao, Q. Q. Guan, Q. Sun, Z. G. Zha and Z. Y. Wang, Green Chem., 2017, 19, 112 RSC; (d) F. L. Yang and S. K. Tian, Tetrahedron Lett., 2017, 58, 487 CrossRef CAS; (e) A. Hosseinian, S. Arshadi, S. Sarhandi, A. Monfared and E. Vessally, J. Sulfur Chem., 2019, 40, 289 CrossRef CAS; (f) S. T. Zhao, K. J. Chen, L. Zhang, W. G. Yang and D. Y. Huang, Adv. Synth. Catal., 2020, 362, 3516 CrossRef CAS.
- (a) G. C. Senadi, B. C. Guo, W. P. Hu and J. J. Wang, Chem. Commun., 2016, 52, 11410 RSC; (b) Y. Yang, S. Zhang, L. Tang, Y. Hu, Z. Zha and Z. Wang, Green Chem., 2016, 18, 2609 RSC; (c) B. Wang, L. Tang, L. Liu, Y. Li, Y. Yang and Z. Wang, Green Chem., 2017, 19, 5794 RSC; (d) J. Meesin, M. Pohmakotr, V. Reutrakul, D. Soorukram, P. Leowanawat and C. Kuhakarn, Org. Biomol. Chem., 2017, 15, 3662 RSC; (e) B. Wang, Z. C. Yan, L. Y. Liu, J. W. Wang, Z. G. Zha and Z. Y. Wang, Green Chem., 2019, 21, 205 RSC.
- (a) A. A. Folgueiras-Amador, K. Philipps, S. Guilbaud, J. Poelakker and T. Wirth, Angew. Chem., Int. Ed., 2017, 56, 15446 CrossRef CAS PubMed; (b) I. Colomer, ACS Catal., 2020, 10, 6023 CrossRef CAS; (c) S. Ghosh, Z. W. Qu, S. Pradhan, A. Ghosh, S. Grimme and I. Chatterjee, Angew. Chem., Int. Ed., 2022, 61, e202115272 CrossRef CAS PubMed; (d) W. J. Wang, X. X. Yang, R. H. Dai, Z. X. Yan, J. L. Wei, X. D. Dou, X. Qiu, H. L. Zhang, C. Wang, Y. M. Liu, S. Song and N. Jiao, J. Am. Chem. Soc., 2022, 144, 13415 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra06625a |
| This journal is © The Royal Society of Chemistry 2024 |