Open Access Article
Open Access ArticleA catalytic approach for the dehydrogenative upgradation of crude glycerol to lactate and hydrogen generation†
Satabdee Tanaya Sahoo,
Anurita Sinku and
Prosenjit Daw *
*
Department of Chemical Sciences, Indian Institute of Science Education and Research Berhampur Transit Campus, (Govt. ITI Building), Engineering School Junction, Berhampur, 760010, Odisha, India. E-mail: pdaw@iiserbpr.ac.in
First published on 20th November 2024
Abstract
The ambiguous nature of non-innocent ligand catalysts provides an excellent strategy for developing an efficient catalyst system featuring extended applicability in sustainable catalysis. In this study, we unveil the catalytic activity of an NNN-Ru catalyst for lactic acid synthesis from a mixture of glycerol, ethylene glycol, and methanol. The developed strategy was also implemented to synthesize lactate (up to 80% yield) with good selectivity via the dehydrogenative upgradation of a crude glycerol and ethylene glycol mixture. As an extended utility, the method was utilized for lactate synthesis from triglyceride directly with hydrogen gas generation.
Introduction
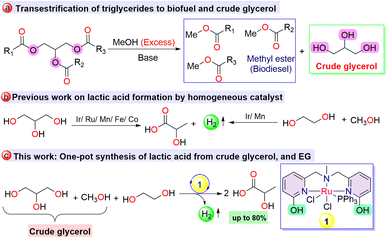
A large excess of crude glycerol waste (∼10 wt% of biodiesel production), which is produced during the transesterification of renewable feedstocks like vegetable oil, cooking oil, recycled waste grease, and tallow, has appeared on the global market with the increasing demand for biodiesel production (Scheme 1a).1,2 According to a report of the Organisation for Economic Corporation and Development (OECD), in 2030, the world biodiesel market is expected to reach nearly 50 billion liters, leading to ∼15 billion liters of crude glycerol.3 However, the high viscosity and high flash point of crude glycerol discourage its utilization as an individual fuel, except when co-combusted with other fuels.4 Most industries utilize only refined glycerol as a raw material for various value-added products, whereas crude glycerol remains as a leftover.5 The purification of this low-value crude glycerol requires high energy input, high capital investment, and maintenance costs, which further limit the low yield.6 In order to support the production and utilization of biodiesel, it is essential to develop sustainable transformation techniques for the practical utilization of crude glycerol comprised of 50–60% glycerol, in addition to residual methanol, methyl ester, and alkali, to give value-added products.7 Upcycling this sustainable surplus of glycerol into more-profitable chemicals such as lactic acid and hydrogen fuel can considerably contribute to the circular economy.8,9 The increasing market demand for lactic acid in several sectors, such as food, pharmaceuticals, cosmetics, biomedicals, and polymers, may not be attained by long-term chemical and microbial fermentative processes.10,11 In this regard, to achieve efficient production of lactic acid, great attention has been paid to developing several heterogeneous catalysts for converting glycerol into lactic acid via an alcohol dehydrogenation reaction, either in the presence of a hydrogen acceptor or via the release of molecular hydrogen.12,13 However, they are limited to energy-intensive, harsh reaction conditions.To achieve sustainable glycerol transformation, new technical routes with innovative catalyst designs are urgently needed. With a rational understanding of the structure–activity relationship in molecular homogeneous catalysts, various Ir and Ru complexes have been utilized for glycerol-to-lactic acid conversion through acceptorless dehydrogenation.14–21 In addition to the innocent ligand framework for the dehydrogenation of glycerol, a seminal development was made by Beller et al., utilising a non-innocent PNP ligand-based Ru catalyst for lactate synthesis with up to 67% selectivity at 140 °C.22 Recently, the Li group demonstrated bipyridonate ligand-based water-soluble Ir catalysts for glycerol-to-lactate synthesis, revealing the critical role of –OH moieties in the ligand framework.23 Furthermore, utilizing homogeneous pincer-based base metal catalysts, the Hazari, Fu, and Kumar groups disclosed glycerol-to-lactate conversion.24–28 Very recently, the Xu group utilized glycerol as a hydrogen source for the hydrogenation of CO2 to formate with the co-generation of lactate employing a bifunctional Ru-based catalyst.29 In addition to glycerol, lactic acid can also be synthesized via the dehydrogenative coupling of ethylene glycol and methanol. In this regard, the Tu,30 Jang,31 and Maji32 groups demonstrated the synthesis of lactate from a mixture of ethylene glycol and methanol using homogeneous molecular catalysts (Scheme 1b and Fig. S20†).
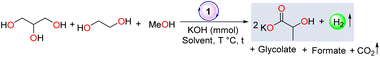
In the context of designing non-innocent cooperative ligands, the synthesis of hydroxy-bearing ligand systems has attracted extensive attention owing to their intriguing chemical participation.33,34 Further expanding the beneficial effect of the hydroxy arm in ligand architecture, in our previous report, we demonstrated a proton-responsive arm bearing stable NNN-donor Ru complex 1 to produce hydrogen and glycolate selectively in up to 94% yield from ethylene glycol, facilitating a cooperative mechanism through acceptorless dehydrogenation.35 As part of our ongoing interest in developing a sustainable strategy to utilize renewable feedstocks for hydrogen fuel and commodity chemical production, we envisaged the use of catalyst 1 for the synthesis of lactic acid via the dehydrogenation of a mixture of waste glycerol (containing glycerol and ∼25% residual methanol) and ethylene glycol in a single pot (Scheme 1c). As far as we are concerned, no literature has been reported on the conversion of a mixture of glycerol, ethylene glycol, and methanol to lactate, which may open up a new approach for upgrading crude glycerol and reforming triglycerides directly for lactate synthesis with the evolution of hydrogen gas.
Results and discussion
Our preliminary investigation began to envisage catalyst 1 for synthesizing lactate from glycerol. After investigating various reaction parameters, an efficient protocol was identified. Under reaction conditions with glycerol (1 mmol) in tBuOH (1 mL), KOH (1.5 mmol), and 1 (1 mol%) at 120 °C, a yield of 73% with 96% selectivity was attained, with the evolution of an H2 gas volume of 16 mL after 24 h (Table S1, entry 1, Fig. S23 and S24†). The evolved gas was collected by the inverted burette technique double-passed through an alkali solution, and the purity of H2 was analyzed using GC-TCD (Fig. S4†). Further, the catalytic activity of 1 was appraised to determine the feasibility of lactate formation from a mixture of ethylene glycol and methanol. After initial optimization, we achieved a lactate yield of 67% under reaction conditions of 1 (1 mol%), KOH (3.5 mmol), ethylene glycol (1 mmol), and MeOH (5 mmol) at 120 °C after 48 h with the evolution of 55 mL of H2; where 98% ethylene glycol conversion was obtained with glycolate and formate as side products (Table S2, entry 3, Fig. S31 and S32†). The lower selectivity for lactate could be adequately demonstrated by the slower dehydrogenation of methanol compared to ethylene glycol (see details in ESI, Fig. S1–S3†).After having initially optimized reaction conditions, we focused on exploring the production of lactate from a mixture of glycerol, ethylene glycol, and methanol in a single pot using complex 1. A reaction was performed with a mixture of glycerol (1 mmol), ethylene glycol (1 mmol) in the presence of 1 (1 mol% with regard to glycerol), and KOH (2.5 mmol) at 120 °C in tBuOH (1 mL)/MeOH (5 mmol) for 24 h, resulting in a 43% (0.86 mmol) yield of lactate and 12% yield of glycolate (Table 1, entry 1; considering 2 mmol of lactate will be formed from a mixture of 1 mmol of glycerol and 1 mmol of ethylene glycol in the presence of MeOH following eqn (S5),† the yield of glycolate was calculated with regard to ethylene glycol following eqn (S6), in the ESI†). Further increasing the KOH loading to 3.5 mmol, a 52% (1.04 mmol) lactate yield was obtained with the evolution of 54 mL H2 (entry 2, Fig. S35†). However, with further increasing the KOH loading to 4.5 mmol, no increase in lactate formation was observed (entry 3). Nevertheless, the reaction time was found to have a profound effect (entries 4, 5); specifically, when the reaction was continued for 72 h with KOH (3.5 mmol) and 1 (1 mol%), yields of 72% (1.44 mmol) lactate and 22% glycolate (with regard to ethylene glycol) and up to 99% carbon balance were achieved with the evolution of 65 mL (2.89 mmol) of H2 (entry 5, Fig. S36 and S37†). Although increasing the temperature to 140 °C resulted in a 78% (1.58 mmol) lactate yield, a reduced carbon balance (92%) was observed (entry 6, Fig. S40†). Thus, further optimization was examined with 3.5 mmol KOH at 120 °C for 72 h. Additionally, various solvents and bases were examined; however, no significant improvement in lactate yield could be established (entries 7–10).
| Entry | KOH (mmol) | T (°C)/t (h) | Gly conv. (%) | EG conv. (%) | Lactate yieldb (%) | H2 (mL) |
|---|---|---|---|---|---|---|
| a Glycerol (1 mmol), ethylene glycol (1 mmol), MeOH (5 mmol), 1 (1 mol%), KOH (in mmol), tBuOH (1 mL), 120 °C, 24 h.b Lactate yield was calculated from 1H NMR using 2,6-lutidine as an internal standard.c NaOH (3.5 mmol) as a base.d CsOH·H2O (3.5 mmol) as a base.e Solvent: tAmOH.f Solvent: dioxane.g Methanol 1 mmol.h Without catalyst 1.i Reactions were repeated two times, and an average of the results is reported with an error limit of 6%. | ||||||
| 1i | 2.5 | 120/24 | 47 | 51 | 43 | 36 |
| 2i | 3.5 | 120/24 | 54 | 69 | 52 | 54 |
| 3 | 4.5 | 120/24 | 55 | 71 | 51 | 53 |
| 4 | 3.5 | 120/48 | 60 | 85 | 60 | 56 |
| 5i | 3.5 | 120/72 | 75 | 91 | 72 | 65 |
| 6 | 3.5 | 140/72 | 83 | 100 | 78 | 86 |
| 7c,i | — | 120/72 | 74 | 84 | 70 | 87 |
| 8d | — | 120/72 | 26 | 63 | 31 | 58 |
| 9e | 3.5 | 120/72 | 68 | 93 | 69 | 63 |
| 10f | 3.5 | 120/72 | — | — | 30 | 40 |
| 11g,i | 3.5 | 120/72 | 76 | 95 | 62 | 56 |
| 12h | 3.5 | 120/72 | — | — | — | — |
Notably, when an equimolar mixture of glycerol, ethylene glycol, and methanol was subjected to the above reaction conditions, no appreciable decrease in the lactate yield (62%, 1.24 mmol) was detected (entry 11, Fig. S41†). Furthermore, no lactate formation was observed in KOH (3.5 mmol) without catalyst 1 (entry 12, Fig. S42 and S43†). When the catalyst loading was decreased to 0.5 mol%, considerable lactate formation (70%, 1.4 mmol) was observed, whereas a further decrease in catalyst loading to 0.1 mol% led to diminished lactate yield (36%, 0.72 mmol) with the evolution of 38 mL of H2 (Table S3, entries 13, 14, Fig. S44 and S45†). In addition, under neat conditions, lower catalytic efficiency was observed with a lactate yield of 25% (0.5 mmol) (Table S3, entry 15, Fig. S46†). Also the mercury experiment excluded the formation of metal nanoparticles, resulting in 71% (1.42 mmol) of lactate with 63 mL of H2 gas under the optimized conditions (Fig. S8†).
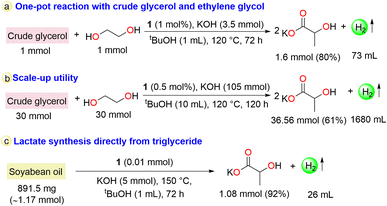
To demonstrate its practical applicability, the developed protocol was implemented for reforming crude glycerol (0.235 mL, 1 mmol; analyzed by 1H NMR: Fig. S21 and S22†) and ethylene glycol (1 mmol) in the presence of 1 (1 mol%) and KOH (3.5 mmol) in tBuOH at 120 °C, which resulted in a lactate yield of 80% (1.6 mmol) with an H2 gas volume of 73 mL. The gas purity was checked using GC-TCD analysis (Scheme 2a, Fig. S47, S48 and S7†). In contrast, the use of only crude glycerol under reaction conditions of 1 (1 mol%) and KOH (1.5 mmol) in tBuOH (1 mL) at 120 °C after 24 h resulted in a 69% (0.69 mmol) yield of lactate with good selectivity (Table S1, entry 7, Fig. S26 and S27†). To examine the scale-up utility, a reaction with a mixture of crude glycerol (7.05 mL, 30 mmol) and ethylene glycol (30 mmol) was demonstrated with 1 (0.5 mol%) (Scheme 2b), yielding 61% (36.56 mmol) lactate and 22% glycolate (with regard to ethylene glycol) with liberation of 1680 mL H2 gas (Fig. S49†). Further, triglyceride (soyabean oil composition was analyzed by NMR and mass spectrometry: Fig. S50–S54†) was used as a substrate to produce lactate and hydrogen directly through performing base hydrolysis followed by dehydrogenation in a single pot in the presence of catalyst 1. Subjecting only soyabean oil (891.5 mg) to base-hydrolysis without any catalyst in the presence of KOH (5 mmol), ∼1.17 mmol of glycerol was obtained in addition to the formation of long-chain fatty acid (Fig. S55 and S56†). When the reaction was performed with soybean oil (891.5 mg) in the presence of catalyst 1 in tBuOH (1 mL) at 150 °C for 72 h, 1.08 mmol (92%) of lactate resulted, with the evolution of 26 mL of H2 gas, and the same long chain fatty acid was observed as detected during the base-hydrolysis (Scheme 2c, Fig. S57 and S58†).
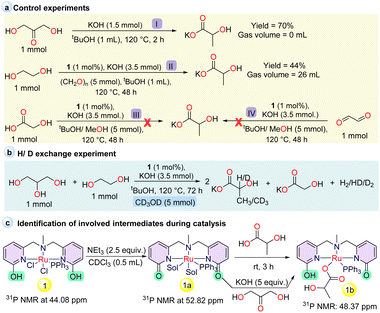
Having evaluated the catalytic activity of 1, several stoichiometric reactions and catalytic studies were performed to investigate the reaction pathway and intermediates involved during the examined process (Scheme 3). The synthesis of lactate can involve the formation of a common intermediate glyceraldehyde (which is in equilibrium with dihydroxyacetone), formed either by the dehydrogenation of glycerol or from the coupling of glycolaldehyde and formaldehyde generated during the dehydrogenation of ethylene glycol and methanol (Fig. 1B and S19B†). Additionally, glycolate and formate can also be produced as side products during the further dehydrogenation of the formed glycolaldehyde and formaldehyde with the evolution of additional hydrogen molecules.30 When dihydroxyacetone was subjected to react in the presence of KOH without catalyst 1, a 70% yield of lactate was obtained at 120 °C within 2 h, indicating that after the first dehydrogenation, a base-mediated Cannizzaro reaction took place to produce lactate (Scheme 3a, I, and Fig. S9†).25 Moreover, when ethylene glycol in the presence of 1 was subjected to react with paraformaldehyde at 120 °C for 48 h (Scheme 3a, II), the formation of 44% lactate with 26 mL of H2 supported the involvement of formaldehyde in the reaction pathway (Fig. S10†). When glycolic acid was subjected to react with methanol in the presence of 1 (Scheme 3a, III, and Fig. S11†), no lactate formation was observed. A similar result was also obtained when glyoxal was treated with methanol in the presence of catalyst 1, resulting in the formation of glycolic acid (45%) and formate (14%) with the evolution of 35 mL H2 gas (Scheme 3a, IV, and Fig. S12†), which depicted the involvement of intermediates formed before the formation of glycolate and glyoxal from ethylene glycol during the dehydrogenative coupling to lactate and supporting the formation of glycolate as a by-product that affects the selectivity towards lactate formation. When a H/D exchange experiment was conducted with CD3OD under the optimized reaction conditions, no appreciable deuterium scrambling was observed for C–H at the C2 carbon of lactate (Scheme 3b, Fig. S13 and S14†).
Continuing from our previous report on the identification of the involved intermediates,35,36 the deprotonated intermediate 1a was allowed to react with lactic acid in CDCl3 (Scheme 3c). A peak at 48.37 ppm appeared in the 31P NMR spectrum, which can be assigned as a lactic acid-coordinated intermediate (Fig. S15†). This was further supported by HRMS with the detection of peaks at 698.1193 (1a + lactic acid + H+), 608.1011 (1a + H+) and 649.1235 (1a + CH3CN + H+) (Fig. S16†). Additionally, when the in situ generated intermediate 1a was subjected to react with dihydroxyacetone in the presence of KOH, a small peak for the lactic acid-coordinated intermediate was observed at 48.37 ppm in the 31P NMR spectrum (Fig. S17†). Further, to illustrate the catalytically active intermediates, a reaction was performed with a mixture of complex 1, glycerol, and KOH on the NMR scale and monitored by using 31P NMR. After heating at 120 °C, the deprotonated intermediate 1a was observed as a significant peak without any lactate-coordinated species (48.37 ppm in 31P NMR). Additionally, an unidentified signal at 39.25 ppm appeared in the 31P NMR spectrum, which was also observed during lactic acid and dihydroxyacetone treatment (Fig. S18†). This result indicates that the intermediate 1a can be a potential active species during the dehydrogenation of glycerol, and that high KOH concentration may facilitate the discoordination of lactic acid from the active site. However, the isolation of intermediate 1a remained unsuccessful.
Based on the previous literature and the catalytic studies, a possible reaction pathway is proposed in Fig. 1.35,36 The cooperative ligand backbone can undergo lactam–lactim tautomerization during the key steps of the catalytic reactions. The initial reaction step can involve the formation of intermediate A (1a, as a deprotonated intermediate) from 1 in the presence of a base, which can act as an active intermediate. Furthermore, substrate interaction at the active catalyst center can lead to the formation of intermediate B, which may possess a hydrogen bonding interaction. Intermediate B can undergo β-hydride elimination and subsequent molecular hydrogen elimination to regenerate active intermediate A via intermediate C to afford dehydrogenated organic carbonyl intermediates. The formed carbonyl intermediates, either from the direct dehydrogenation of glycerol or dehydrogenative coupling of glycolaldehyde and formaldehyde formed from the dehydrogenation of ethylene glycol and methanol, can undergo a base-mediated rearrangement or condensation reaction to produce the lactate (Fig. 1B).
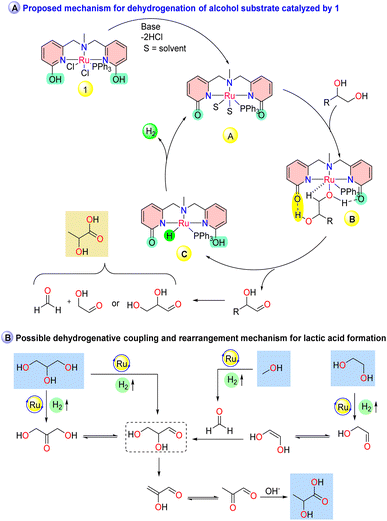 | ||
| Fig. 1 Proposed mechanism for the dehydrogenation of the alcohol substrate for the synthesis of lactate catalyzed by 1. | ||
Conclusions
In summary, incorporating proton-shuttling scaffolds in the ligand framework plays a remarkable role in the design of non-innocent-ligand-based catalysts. The developed Ru catalyst was identified as suitable for the efficient synthesis of lactate from a mixture of glycerol, ethylene glycol, and methanol under relatively benign conditions. The strategy was utilized to synthesize lactate from a mixture of crude glycerol and ethylene glycol, as well as from triglycerides directly via base hydrolysis, followed by dehydrogenation in a single pot. This coherent approach for synthesizing lactic acid may establish a new straightforward route for the more-sustainable disposal of waste glycerol produced during biodiesel production. For practical utility, further scale-up of this catalytic strategy is in progress.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
PD is grateful for financial support from SERB, DST India (CRG/2023/001880), and IISER Berhampur. STS is thankful to CSIR, New Delhi, for fellowship. The authors acknowledge CAIF IISER Berhampur for the instrumental facility.References
- S. Ao, S. P. Gouda, L. Saikia, B. Gurunathan and S. L. Rokhum, Sci. Rep., 2024, 14, 1–17 CrossRef PubMed.
- R. H. Crabtree, Chem. Rev., 2017, 117, 9228–9246 CrossRef CAS PubMed.
- OECD/FAO, OECD-FAO Agricultural Outlook 2021–2030, 2021 Search PubMed.
- M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi and C. Della Pina, Angew. Chem., Int. Ed., 2007, 46, 4434–4440 CrossRef CAS PubMed.
- P. Intasian, K. Prakinee, A. Phintha, D. Trisrivirat, N. Weeranoppanant, T. Wongnate and P. Chaiyen, Chem. Rev., 2021, 121, 10367–10451 CrossRef CAS PubMed.
- C. J. Clarke, W. C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- M. Tao, D. Zhang, H. Guan, G. Huang and X. Wang, Sci. Rep., 2016, 6, 1–13 CrossRef PubMed.
- A. Cheruvathoor Poulose, M. Medveď, V. R. Bakuru, A. Sharma, D. Singh, S. B. Kalidindi, H. Bares, M. Otyepka, K. Jayaramulu, A. Bakandritsos and R. Zbořil, Nat. Commun., 2023, 14, 1–10 Search PubMed.
- A. Kumar, P. Daw and D. Milstein, Chem. Rev., 2022, 122, 385–441 CrossRef CAS PubMed.
- Y. Ren, X. Wang, Y. Li, Y. Y. Li and Q. Wang, Sustainability, 2022, 14, 14434 CrossRef CAS.
- A. O. Ojo and O. de Smidt, Processes, 2023, 11, 688 CrossRef CAS.
- K. Mohan, S. D. K. R. Pai, B. S. Reghunath and D. Pinheiro, ChemistrySelect, 2023, 8, e202204501 CrossRef CAS.
- A. Kumar, M. Kumar Awasthi, B. Priya and S. Kumar Singh, ChemCatChem, 2022, 14, e202101951 CrossRef CAS.
- A. Bisarya, S. Karim, H. Narjinari, A. Banerjee, V. Arora, S. Dhole, A. Dutta and A. Kumar, Chem. Commun., 2024, 60, 4148–4169 RSC.
- L. S. Sharninghausen, J. Campos, M. G. Manas and R. H. Crabtree, Nat. Commun., 2014, 5, 1–9 Search PubMed.
- Z. Sun, Y. Liu, J. Chen, C. Huang and T. Tu, ACS Catal., 2015, 5, 6573–6578 CrossRef CAS.
- Z. Lu, I. Demianets, R. Hamze, N. J. Terrile and T. J. Williams, ACS Catal., 2016, 6, 2014–2017 CrossRef CAS.
- M. Finn, J. A. Ridenour, J. Heltzel, C. Cahill and A. Voutchkova-Kostal, Organometallics, 2018, 37, 1400–1409 CrossRef CAS.
- M. Dutta, K. Das, S. J. Prathapa, H. K. Srivastava and A. Kumar, Chem. Commun., 2020, 56, 9886–9889 RSC.
- Y. J. Cheong, K. Sung, J. A. Kim, Y. K. Kim and H. Y. Jang, Eur. J. Inorg. Chem., 2020, 2020, 4064–4068 CrossRef CAS.
- M. V. Jiménez, A. Ojeda-Amador, R. Puerta-Oteo, J. Martínez-Sal, V. Passarelli and J. Pérez-Torrente, Molecules, 2022, 27, 7666 CrossRef PubMed.
- Y. Li, M. Nielsen, B. Li, P. H. Dixneuf, H. Junge and M. Beller, Green Chem., 2015, 17, 193–198 RSC.
- X. Xu, H. You, B. Dong, Y. He and F. Li, Inorg. Chem., 2024, 63, 12929–12934 CrossRef CAS PubMed.
- L. S. Sharninghausen, B. Q. Mercado, R. H. Crabtree and N. Hazari, Chem. Commun., 2015, 51, 16201–16204 RSC.
- C. Deng, J. Deng and Y. Fu, Green Chem., 2022, 24, 8477–8483 RSC.
- H. Narjinari, S. Dhole and A. Kumar, Chem.–Eur. J., 2023, 30, e202302686 CrossRef PubMed.
- A. Bisarya, S. Dhole and A. Kumar, Dalton Trans., 2024, 53, 12698–12709 RSC.
- B. Venkateshappa, A. Bisarya, P. G. Nandi, S. Dhole and A. Kumar, Inorg. Chem., 2024, 63, 15294–15310 CrossRef CAS PubMed.
- T. Cui, H. Gong, L. Ji, J. Mao, W. Xue, X. Zheng, H. Fu, H. Chen, R. Li and J. Xu, Chem. Commun., 2024, 60, 12221–12224 RSC.
- J. Wu, L. Shen, Z. Chen, Q. Zheng, X. Xu and T. Tu, Angew. Chem., Int. Ed., 2020, 59, 10421–10425 CrossRef CAS PubMed.
- M. Lee, H. Byeon and H.-Y. Jang, J. Org. Chem., 2022, 87, 4631–4639 CrossRef CAS PubMed.
- S. Waiba, K. Maji, M. Maiti and B. Maji, Angew. Chem., Int. Ed., 2023, 62, e202218329 CrossRef CAS PubMed.
- C. M. Moore, B. Bark and N. K. Szymczak, ACS Catal., 2016, 6, 1981–1990 CrossRef CAS.
- W. H. Wang, J. T. Muckerman, E. Fujita and Y. Himeda, New J. Chem., 2013, 37, 1860–1866 RSC.
- S. T. Sahoo, A. Mohanty, R. Sharma and P. Daw, Dalton Trans., 2023, 52, 15343–15347 RSC.
- S. T. Sahoo, A. Mohanty, R. Sharma, S. R. Rout, R. Dandela and P. Daw, Organometallics, 2023, 42, 745–751 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07028c |
| This journal is © The Royal Society of Chemistry 2024 |