Open Access Article
Open Access ArticleDeveloping Ni0.5Zn0.5Fe2O4 ferrite with controlled particle size and morphology through optimized processing conditions of low energy solid state reaction†
Sarah Baayyada,
Fatima-Zahra Semlali a,
El Kébir Hlilc,
Tarik Mahfoudb,
Hassan El Moussaoui*b and
Mounir El Achaby
a,
El Kébir Hlilc,
Tarik Mahfoudb,
Hassan El Moussaoui*b and
Mounir El Achaby *a
*a
aMaterials Science, Energy and Nanoengineering Department (MSN), Mohammed VI Polytechnic University (UM6P), Lot 660 – Hay Moulay Rachid, Ben Guerir, 43150, Morocco. E-mail: mounir.elachaby@um6p.ma
bMoroccan Foundation for Advanced Science, Innovation and Research (MAScIR), Rabat Design Center, Rue Mohamed El Jazouli, Madinat El Irfane, 10100 Rabat, Morocco. E-mail: h.elmoussaoui@mascir.ma
cInstitut Néel, CNRS et Université Joseph Fourier, BP 166, F-38042 Grenoble Cedex 9, France
First published on 12th November 2024
Abstract
Soft magnetic materials, like Ni0.5Zn0.5Fe2O4, require high temperatures and regulated environments for their manufacture and processing, which can be highly energy intensive. These requirements therefore result in higher production costs and energy consumption. To address this issue, the development of composite materials based on soft magnetic ferrites has become a prominent research area. The type of particles and their size distribution, shape, and dispersion within the polymer matrix can be crucial for controlling the magnetic properties. In this context, and to reduce energy consumption, the parameters of solid-state reaction (such as calcination temperature, calcination time, and milling time) were optimized in this work to produce magnetic particles with suitable shape and size for filling a thermoplastic matrix. The impact of these parameters on phase purity, morphology, particle size, and magnetic properties was thoroughly evaluated. The results highlight that the sample synthesized at 1200 °C for 6 hours achieved an impressive saturation magnetization value of 80.07 emu g−1, showcasing exceptional magnetic performance.
1. Introduction
The rapid development of modern electronic devices, telecommunication systems, and other technological applications has generated an increasing demand for soft magnetic materials with superior properties.1 These materials are essential components in a wide range of applications, from transformers and inductors to electromagnetic wave absorbers.2,3 Also they are great materials to overcome electromagnetic issues.4 Among soft magnetic materials, ferrites have emerged as highly attractive candidates due to their unique combination of properties, including high electrical resistivity, low eddy current losses, mechanical hardness, and good magnetic saturation. Nickel zinc ferrite (Ni0.5Zn0.5Fe2O4) stands out as a particularly interesting soft magnetic material due to its tunable magnetic properties, making it suitable for high-frequency applications.5 Moreover, the doping of NiFe2O4 by non-magnetic Zn results in the modification of the AB2O4-type of crystal structure by cation redistribution in the crystal lattice as well as changes in the microstructure and magnetic and electric properties. The above mentioned properties can be controlled and are strictly dependent on the amount of Zn content and the type of synthesis procedure.6 Ni–Zn ferrite is a spinel structure in which tetrahedral A sites are occupied by Zn2+ and Fe3+ ions and octahedral B sites by Ni2+ and Fe3+ ions in the spinel formula AB2O4.7 Magnetic properties of these ferrites are mainly controlled by the cation distribution of Ni, Zn and Fe along the available tetrahedral and octahedral sites.8 In the Nix−1Znx−1Fe2O4 system, Ni0.5Zn0.5Fe2O4 has the best magnetic characteristics and microwave absorption ability, and it is a suitable candidate for several applications, it possesses beneficial characteristics such as high dielectric constant, low dielectric loss, mechanical hardness, electrical resistivity, and chemical stability.9,10 Ni–Zn ferrite is usually synthesized by the sol–gel methods,11–13 co-precipitation methods,14,15 combustion method16,17 and hydrothermal synthesis.18,19 One of the most used methods for synthesizing ferrite magnetic particles is the solid-state reaction. This technique of processing ferrite materials often involves high temperatures and controlled atmospheres, making the production process energy-intensive and costly. The sintering or calcination process, which is crucial for achieving phase purity and optimal magnetic properties, typically occurs at elevated temperatures and requires extended durations.20–22 Furthermore, the mechanical and chemical characteristics of the final product are highly dependent on the precise control of the processing parameters, such as calcination time, particle size, and morphology. These challenges highlight the need for optimizing synthesis processes that can reduce energy consumption. To address the issue of high energy consumption, research has increasingly focused on developing composite materials based on soft magnetic ferrites, particularly by incorporating magnetic particles like Ni0.5Zn0.5Fe2O4 into polymer matrices. This approach combines the lightweight, flexible, and corrosion-resistant properties of polymers with the enhanced mechanical, thermal, and magnetic properties of ferrites.23,24 A key challenge is ensuring homogeneous distribution of magnetic particles within the polymer matrix, as particle size, shape, and dispersion greatly influence the composite's overall performance. This work aims to give researchers a clear guide to optimizing key synthesis parameters of low-energy solid-state reactions for producing polymer-magnetic composites with controlled magnetic properties. The study highlights how parameters like temperature, particle size, and calcination time impact the structural, morphological, and magnetic characteristics of the materials. This approach not only enhances the performance and functionality of polymer-based magnetic composites but also supports efficient and scalable manufacturing in this field.2. Material and methods
A mixture of high purity commercial oxides powders, nickel oxide, zinc oxide and iron oxide, used as starting materials to prepare Ni0.5Zn0.5Fe2O4. The processes start with mixing the precursor using Agate vials and balls for the milling process in high energy planetary mill Pulverisette 4 (Fritsch). The ball to powder mass ratio was 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The rotation speed was 500 rpm, with the variation of milling time from 30 min to 60 min. After milling, the samples were annealed in air at a temperature of 900 °C, 1000 °C, 1100 °C and 1200 °C, for 6 and 12 hours. Table 1 summarizes samples names and synthesis conditions.
1. The rotation speed was 500 rpm, with the variation of milling time from 30 min to 60 min. After milling, the samples were annealed in air at a temperature of 900 °C, 1000 °C, 1100 °C and 1200 °C, for 6 and 12 hours. Table 1 summarizes samples names and synthesis conditions.
| Sample's number | Samples | Ball milling speed 500 (rpm) | Calcination conditions | |
|---|---|---|---|---|
| Time (min) | Temperature (°C) | Time (h) | ||
| a NZF2C2B: this sample was subjected to double ball milling, each for 30 min, followed by a 6 hours calcination at 1200 °C. | ||||
| 1 | NZF2C2Ba | 30 | 1200 | 6 |
| 2 | NZFC12 | 30 | 1200 | 12 |
| 3 | NZF-1-12 | 60 | 1200 | 12 |
| 4 | NZF-1-6 | 60 | 1200 | 6 |
| 5 | NZF1200 | 30 | 1200 | 6 |
| 6 | NZF1100 | 30 | 1100 | 6 |
| 7 | NZF1000 | 30 | 1000 | 6 |
| 8 | NZF900 | 30 | 900 | 6 |
The formation of spinel structure of Ni0.5Zn0.5Fe2O4 was investigated by D8-Discover diffractometer, Bruker (Nanotechnology Platform, MSN Laboratory). The CuK radiation (λ = 1.5418°) is used to scan the samples in the range of 5–80° with a step size of 0.01°, while the voltage and current are set at 45 kV and 100 mA, respectively.
The morphological properties of NiZnFe2O4 samples were investigated using a Zeiss EVO 10 scanning electron microscope (Carl Zeiss Microscopy, GmbH, Jena, Germany).
For magnetic properties High temperature extraction magnetometer BS1 (Louis Néel Magnetism Laboratory, CNRS, Grenoble) was used, with a resolution of 5 × 10−6, and a temperature of 300 K to 800 K, and the field strength range is ±7 teslas.
3. Results and discussion
3.1. Processing information
Solid state reaction is a widely used industrial method for processing ceramic materials, including ferrite magnetic particles. This process involves several steps, such as ball milling for extended periods, pellet pressing, and multiple high-temperature, long duration calcinations. These steps make the process highly complex and energy intensive. To reduce the energy consumption during the processing of ferrite particles using solid state reaction, it is crucial to optimize reaction parameter, including ball milling time, calcination time, and temperature. The goal is to produce ferrite magnetic particles with a pure spinel phase, optimal stoichiometry, and a well-distributed size and shape of microparticles. Optimizing these parameters involves key adjustments. First, adjust the duration of ball milling to achieve a fine and homogeneous mixture without over-grinding. Adjusting the calcination parameters to ensure complete phase formation while minimizing energy usage. Ni0.5Zn0.5Fe2O4 is particularly advantageous due to its superior magnetic characteristics and microwave absorption ability, as well as its high dielectric constant, low dielectric loss, mechanical hardness, electrical resistivity, and chemical stability. These desirable properties make it an ideal candidate for synthesis via a low-energy solid state reaction. In this work, the approach involved synthesizing Ni0.5Zn0.5Fe2O4 using a low-energy solid state reaction. The initial step involved mixing the raw materials at a milling speed of 500 rpm for 30 min. This was followed by calcination at 1200 °C for 6 hours. After this calcination, an additional ball milling and calcination under the same condition were performed to study their effects on the purity of the phase and the shape and size of the particles. For the second sample, the same ball milling conditions were used, but the calcination duration was extended to 12 hours to study the effect of prolonged calcination on the magnetic particles. Additionally, the impact of mixing time on the properties of the particles was also investigated by increasing the ball milling time from 30 min to 60 min for both samples 3 and 4. The last parameter optimized in this study was the calcination temperature. The goal was to determine the minimum temperature required to achieve a pure phase and good distribution of particle sizes. For samples 5, 6, 7, and 8, the calcination temperature was systematically decreased from the highest temperature of 1200 °C to 900 °C. Optimizing these parameters is crucial for producing ferrite magnetic particles with excellent magnetic properties and a well distributed particle size and shape. These optimized particles can be used as fillers in polymers matrices to produce homogenous polymer magnetic composite materials.3.2. Structural characteristics
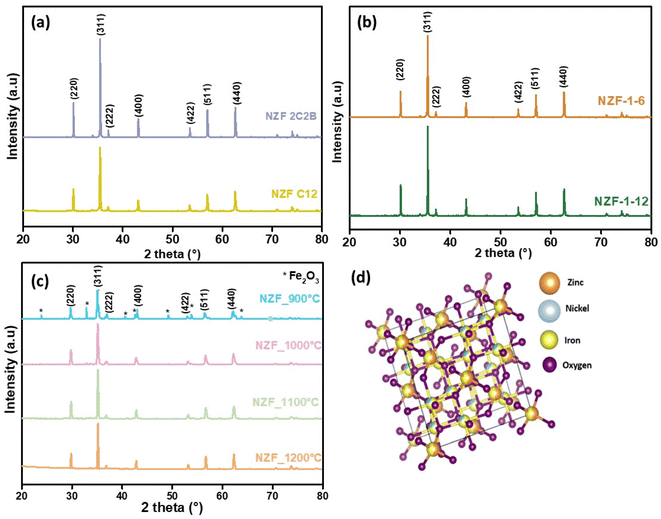
The phase purity of our samples depends on the reaction take place by diffusion, which depends directly on annealing time, temperature and mixing conditions.25 So, it is necessary to modify these factors for good diffusion. The impact of annealing time on the purity of the crystalline structure was examined in Fig. 1a. In accordance with the joint committee on powder diffraction standards (JCPDS), the existence of (220), (311), (400), (422), (511), and (440) in XRD patterns is consistent with cubic structure. All the samples show a typical single-phase, without any impurity phases. Fig. 1b shows that varying the milling time from 30 min to 60 min did not have any noticeable effect on the structure purity. After examining the effect of calcination time and mixing on the purity of the structure, the effect of calcination temperature, which also significantly affects phase purity, was studied. The results for whole samples with temperature ranging from 900 to 1200 °C are shown in Fig. 1c. All the samples show a typical single-phase, without any impurity phases, except for NZF900, which show the formation of α-Fe2O3 as secondary phase (JCPDA No. 87-1166). Y. B. Kannan et al.26 have also reported the presence of α-Fe2O3 in Ni–Zn ferrite, which mean that these experimental conditions of calcination are not sufficient to complete the chemical reaction between the total amount of oxides. Table 2 displays the approximate crystallite size and lattice parameters of the spinel powders calcined at different calcination duration and temperatures. The strong and high intensity diffraction peak (311) was selected for the crystallite size measurement, using Scherer's equation eqn (1), were K is the shape factor of spherical particles, λ is the X-ray wavelength corresponding to Cu Ka. β denotes the full width at half-maximum of the peak in radian and θ is the Bragg's angle. Crystallite size of NZF2C2B where a double grinding and double annealing was performed, was found to be higher, with a value of 113.76 nm. The crystallite size of the Ni0.5Zn0.5Fe2O4 spinel annealed at different temperatures in the range of 900 °C to 1200 °C with an increase of 100 °C has shown insignificant differences, indicating that this range of temperature exerted an insignificant influence on the crystal growth of the spinel powders. However, the increase in the annealing temperatures from 6 h to 12 h at 1200 °C increase the crystallite size from 33.73 nm to 52.56 nm.
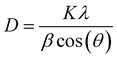 | (1) |
| Samples number | Samples name | Crystallite size (nm) | Lattice parameters (Å) | Volume of cell (Å3) | X-ray density (g cm−3) |
|---|---|---|---|---|---|
| 1 | NZF 2C2B | 113.764 | 8.398 | 592.390 | 5.331 |
| 2 | NZF C12 | 52.563 | 8.387 | 590.154 | 5.352 |
| 3 | NZF-1-6 | 54.900 | 8.381 | 588.762 | 5.364 |
| 4 | NZF-1-12 | 53.209 | 8.387 | 589.965 | 5.353 |
| 5 | NZF 1200 | 33.730 | 8.388 | 590.351 | 5.350 |
| 6 | NZF 1100 | 33.690 | 8.393 | 591.223 | 5.342 |
| 7 | NZF 1000 | 33.660 | 8.396 | 592.043 | 5.335 |
| 8 | NZF 900 | 33.608 | 8.412 | 595.379 | 5.305 |
The relation between crystal plan distance in cubic crystal lattice and lattice parameter and Bragg equation is explained below, where a is a lattice parameter, (hkl) Miller indices and d interplanar distance.
| a = d(h2 + k2 + l2)1/2 | (2) |
nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (3) |
The lattice constant of the synthesized powder had ranged from 8.387 to 8.412 Å, which agrees with the JCPDS results.
The studied Ni0.5Zn0.5Fe2O4 particles crystallize in the mixed spinel structure. As a result Ni2+ preferentially occurs in B-octahedral sites. At A-tetrahedral and B-octahedral sites Fe3+ is evenly distributed. The Zn2+, which is nominally substituted in place of 50% of Ni2+ is preferentially dispersed across the A-tetrahedral site pushing some of the Fe3+ ion to migrate to the B-octahedral site. Fig. 1d represents the mixed spinel ferrite unit cell generated based on the crystal structure carried out with the use of Vesta software.
3.3. Morphological properties
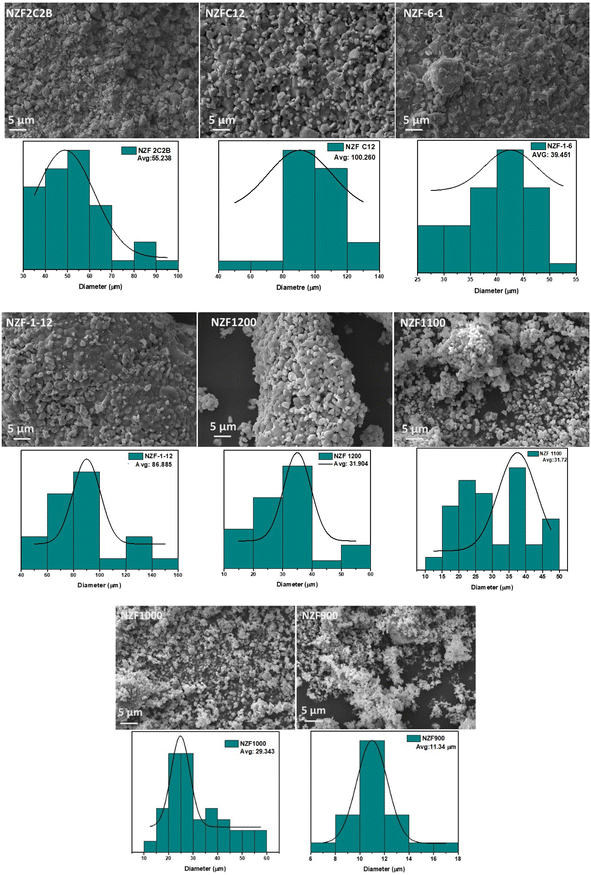
Morphological study of all samples is carried out with a scanning electron microscope (SEM) shown in Fig. 2. Most of the particles exhibited a non-spherical (roughly spherical) shape, consistent with findings in the literature.27 Notably, the samples display strong agglomeration, as observed in the figure, due to interaction between the magnetic materials.28 The level of agglomeration increased with higher temperatures and prolonged sintering times. It can be seen from the image the particles were fine and uniform, with a size of approximately 11 μm at 900 °C. As the temperature increased, the particle continued to grow and became larger. Interestingly, we found that the particle size of the sample is significantly larger than the crystallite size mentioned earlier, which is agglomerated to extent and which can be attributed to the fact that magnetic crystallites are more likely to aggregate during the regrowth to form larger particles.29 With an increase in sintering time at constant temperature of 1200 °C, a substantial growth in particle size was observed. On the other hand, varying the milling time from 30 to 60 min had no discernible impact on particles size, as evident in samples NZF-1-6 and NZF-1-12. In conclusion, sample number 5 (NZF1200) exhibited a better distribution of particle size and morphology compared to the other samples. The elemental composition of all the synthesized samples was studied by EDS. Energy dispersive spectroscopy (EDS) gives a useful analysis of elemental composition that can be used to determine the stoichiometry of the material. The EDS spectra of all prepared samples were shown in Fig. S1.† It was evident that the powders contained mainly Fe, Ni, and Zn. Quantitative EDS analysis result showed that the atomic percentage of metallic ions in the Ni0.5Zn0.5Fe2O4 has good agreement with theatrical designed stoichiometry.3.4. Magnetic properties
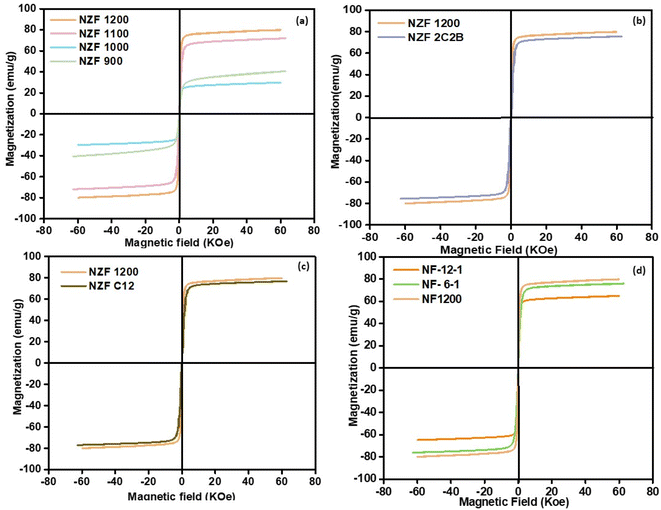
The room temperature magnetic hysteresis loop of the synthesized ferrite has been measured by VSM and the results of the Ni0.5Zn0.5Fe2O4 samples were presented in Fig. 3. The magnetic parameters such as saturation magnetization, coercivity, and remanence of the samples are listed in Table S1.† It was found that the magnetic properties of Ni0.5Zn0.5Fe2O4 ferrites were related to the sintering temperature and time. All the synthesized ferrites belong to the category of soft ferrites indication low coercivity. Additionally, the coercivity was reduced with the increase of annealing temperature and time.30,31 It is well known that coercivity is highly dependent on major parameters such as the particle size and anisotropy constant and can be also altered by heat treatment or deformation. Regarding the SEM images, the particle size is increased by increasing the time and sintering temperature and consequently influences coercivity.32 Fig. 3a shows hysteresis loops of ferrite samples sintered at 1200, 1100, 1000, and 900 °C temperature for 6 h. It can be found from Table S1† that the saturation magnetization decreases first from 900 to 1000 °C, and then increases from 1000 to 1200 °C. This change in saturation magnetization is largely dependent on the particle size and the distribution of cations in the crystal.33 The calcination temperature affects the distribution of Ni2+, Zn2+, and Fe3+ ions in tetrahedral (A) and octahedral (B) sites. At lower temperatures, an unfavorable cation distribution may occur, reducing magnetic interactions. As the temperature increases, more favorable cation distribution can take place, improving the magnetic properties. The initial drop in Ms value from 900 °C to 1000 °C may be due to the displacement of Zn2+ ions into the tetrahedral site, reducing the net magnetization. As the temperature further increases, Zn2+ relocates to optimal positions, enhancing the magnetic properties.34 In addition at lower calcination temperatures (900–1000 °C), incomplete crystallization or the presence of secondary phases might occur, reducing the magnetic properties. As the calcination temperature increases, more crystalline and uniform ferrite structures are formed, improving the magnetic interactions. Between 1000 °C and 1200 °C, increased grain growth and elimination of secondary phases enhance the magnetic properties, leading to an increase in saturation magnetization at higher temperature.35,36 The particles size is directly proportional to magnetic domain size. As the magnetic domain size increases, the atomic spin numbers increase and align themselves in the direction of the applied magnetic field, thereby enhancing the magnetic saturation of the materials.37 As the particles size increase with the increase of calcination temperature, SEM images showed, the size of magnetic domains increases. When magnetic domain grows, more atomic spins are involved, and they tend to align more easily with an external magnetic field. This alignment boosts the overall magnetization of the material, leading to higher saturation magnetization.38 Fig. 3b illustrates that applying double grinding and double calcination does not exert any additional influence on the magnetic saturation Ms of the prepared material. This observation highlights that elevated Ms values (Ms = 80.07 emu g−1) are achieved through calcination at 1200 °C, which is higher than what is reported in the literature.39,40 From Fig. 3c and d we can conclude that milling time variation from 30 min to 60 min had no effect on magnetic properties of the samples, but the increase of calcination time from 6 h to 12 h has a great impact on Ms, which decreased with the increase of calcination time. This change on magnetic properties with the increase of calcination temperature and time is related to the increase of agglomeration, which is already discussed in SEM images, which show that for sample calcined at 1200 °C for 6 h (NZF1200) the particles are more dispersed with a defined shape and smooth surface. In contrast when we increase the temperature and time of calcination the agglomeration increases, since the saturation magnetization of magnetic particles is directly proportional to the number of magnetic domains and their alignment, the reduction in the number of domains due to agglomeration leads to a decrease in saturation magnetization.35 Fig. 4 illustrate the magnetic mechanisms in ferromagnetic materials. The Curie temperature is the thermal energy required to completely destroy the magnetic interaction between neighboring magnetic ions, leading to a paramagnetic material.41 The variation of Curie temperature Tc is presented in Fig. 5 which show an increase with the increase of sintering temperature due to the increase in particle size, and structural parameters Table S1.† By decreasing the lattice parameters, the distance between the interacting ions in the A and B sites decreases and as a result, the power of interactions increases. Therefore, the thermal energy required to offset the alignment of magnetic moment increases which lead to an increase in Curie temperature.42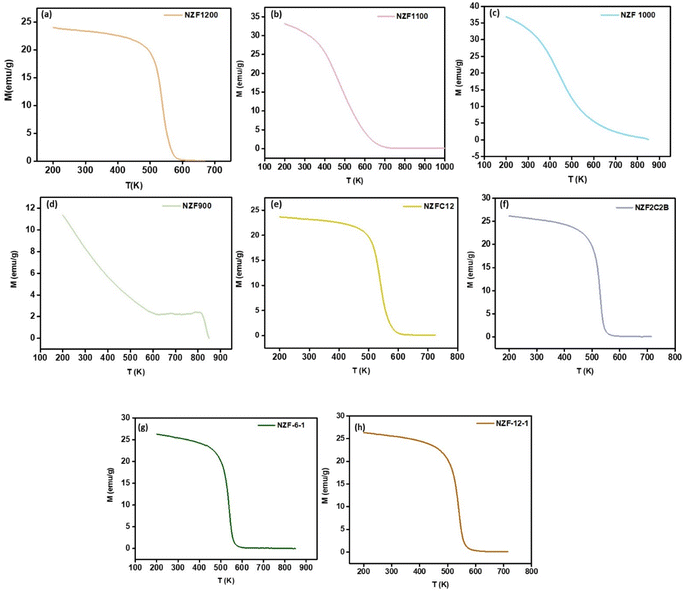 | ||
| Fig. 5 Measured magnetization versus temperature curve of nickel zinc ferrite (Ni0.5Zn0.5Fe2O4) particles. | ||
4. Conclusion
To conclude, this study focused on the optimization of low-energy-solid-state-reaction parameters to reduce the energy consumption in the fabrication of magnetic materials, and to achieve optimal particles size and shape of magnetic fillers in polymers matrices, thus producing magnetic polymers composites materials. The synthesis of Ni0.5Zn0.5Fe2O4 ferrite magnetic particles via solid-state reaction was successfully achieved. XRD analysis revealed that temperature significantly influences the purity of the spinel structure, with all samples exhibiting a single-phase spinel structure at temperatures above 1000 °C. Despite the non-spherical morphology of the particles, the absence of impurities indicates the effectiveness of the synthesis method. Furthermore, both particle size and agglomeration levels increased with higher calcination temperatures and longer durations. The magnetic properties of ferrite particles, characterized by low coercivity and high saturation magnetization, were found to be closely linked to the sintering condition. Specifically, the sample synthesized at 1200 °C for 6 hours exhibited the highest saturation magnetization value of 80.07 emu g−1, with uniform particle size and shape. Thus, the optimal condition conditions of solid-state reaction synthesis are identified as 30 minutes of mixing and the calcination temperature of 1200°c for 6 hours, resulting in a pure spinel structure with desirable morphology and enhanced magnetic properties. These characteristics make them excellent fillers material for polymer matrices, leading to high-performance magnetic polymer composite materials.Data availability
The data that support the findings of this study are available from the corresponding author, Mounir El Achaby, upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research receives support from the Moroccan Ministry of Higher Education, Scientific Research, and Innovation, along with funding from the OCP Foundation through the APRD research program.References
- Q. Li, et al., Electrostatically fabricated heterostructure of interfacial-polarization-enhanced Fe3O4/C/MXene for ultra-wideband electromagnetic wave absorption, J. Colloid Interface Sci., 2024, 662, 796–806 CrossRef CAS PubMed.
- A. Anagha, et al., Structural, optical and magnetic properties of MgFe2O4 and Ni0.5Zn0.5Fe2O4, Mater. Chem. Phys., 2024, 313, 128746 CrossRef CAS.
- Y. Chen and Y. Wang, Integrating Sulfur Doping with a Multi-Heterointerface Fe7S8/NiS@C Composite for Wideband Microwave Absorption, Small, 2024, 20(36), e2401618 CrossRef.
- K. Nan, et al., Integrated design of MOFs-derived 0D/1D/2D/3D hierarchical network for high-efficiency electromagnetic wave absorption, Carbon, 2024, 224, 119049 CrossRef CAS.
- T. J. Shinde, A. B. Gadkari and P. N. Vasambekar, Magnetic properties and cation distribution study of nanocrystalline Ni-Zn ferrites, J. Magn. Magn. Mater., 2013, 333, 152–155 CrossRef CAS.
- A. Bajorek, Microstructural and magnetic characterization of Ni0.5Zn0.5Fe2O4 ferrite nanoparticles, J. Phys. Chem. Solids, 2019, 1–12 CrossRef CAS.
- A. S. Fawzi, A. D. Sheikh and V. L. Mathe, Structural, dielectric properties and AC conductivity of Ni (1-x)ZnxFe2O4 spinel ferrites, J. Alloys Compd., 2010, 502, 231–237 CrossRef CAS.
- R. K. Singh, C. Upadhyay, S. Layek and A. Yadav, Cation distribution of Ni0.5Zn0.5Fe2O4 nanoparticles, Int. J. Eng. Sci. Technol., 2010, 2, 104–109 Search PubMed.
- S. Hasan and B. Azhdar, Effect of annealing temperature, annealing time, and hydrogen potential on the physical properties of Ni0.5Zn0.5Fe2O4 nanoparticles, Ceram. Int., 2023, 49, 5371–5381 CrossRef CAS.
- N. H. Abdullah, M. S. Mustaffa, M. N. Hamidon, F. N Shafie, I. Ismail and I. R Ibrahim, et al., Isochronal recovery behaviour on electromagnetic properties of polycrystalline nickel zinc ferrite (Ni0.5Zn0.5Fe2O4) prepared via mechanical alloying, Sci. Rep., 2021, 11, 1–11 CrossRef PubMed.
- P. Gao, et al., Structural investigations and magnetic properties of sol-gel Ni0.5Zn0.5Fe2O4 thin films for microwave heating, J. Appl. Phys., 2010, 107, 8 Search PubMed.
- B. S. Yoo, et al., Effects of solution concentration on the structural and magnetic properties of Ni0.5Zn0.5Fe2O4 ferrite nanoparticles prepared by sol-gel, J. Magn., 2013, 18, 230–234 CrossRef.
- L. Ben Tahar, M. Jaip, A. Abd, E. Abualreish and A. Noubigh, Optimization of reaction variables in the sol-gel synthesis of Ni0.5Zn0.5Fe2O4 nanoparticles as a very fast adsorbent of methylene blue, Desalin. Water Treat., 2024, 317, 100052 CrossRef.
- S. Kumar, V. Singh, S. Aggarwal, U. K. Mandal and R. K. Kotnala, Synthesis of nanocrystalline Ni0.5Zn0.5Fe2O4 ferrite and study of its magnetic behavior at different temperatures, Mater. Sci. Eng. B, 2010, 166, 76–82 CrossRef CAS.
- A. Bajorek, et al., Microstructural and magnetic characterization of Ni0.5Zn0.5Fe2O4 ferrite nanoparticles, J. Phys. Chem. Solids, 2019, 129, 1–21 CrossRef CAS.
- V. D. Sudheesh, et al., Investigation of structural and magnetic properties of Ni0.5Zn0.5Fe2O4 nano powders prepared by self combustion method, Mater. Res. Bull., 2013, 48, 698–704 CrossRef CAS.
- H. Aali, et al., High antibacterial and photocatalytic activity of solution combustion synthesized Ni0.5Zn0.5Fe2O4 nanoparticles: effect of fuel to oxidizer ratio and complex fuels, Ceram. Int., 2019, 45, 19127–19140 CrossRef CAS.
- N. Wang, et al., Two-phase magnetic nanospheres with magnetic coupling effect encapsulated in porous carbon to achieve lightweight and efficient microwave absorbers, J. Colloid Interface Sci., 2024, 671, 56–66 CrossRef CAS PubMed.
- M. Zhang, et al., Porous Ni0.5Zn0.5Fe2O4 Nanospheres: Synthesis, Characterization, and Application for Lithium Storage, Electrochim. Acta, 2014, 147, 143–150 CrossRef CAS.
- Y. M. Kwon, M. Y. Lee, M. Mustaqima, C. Liu and B. W. Lee, Structural and magnetic properties of Ni0.6Zn0.4Fe2O4 ferrite prepared by solid state reaction and sol-gel, J. Magn., 2014, 19, 64–67 CrossRef.
- T. F. Marinca, I. Chicinas, O. Isnard, V. Pop and F. Popa, Synthesis, structural and magnetic characterization of nanocrystalline nickel ferrite - NiFe2O4 obtained by reactive milling, J. Alloys Compd., 2011, 509, 7931–7936 CrossRef CAS.
- C. A. Palacio Gómez, C. A. Barrero Meneses and A. Matute, Structural parameters and cation distributions in solid state synthesized Ni-Zn ferrites, Mater. Sci. Eng. B, 2018, 236–237, 48–55 CrossRef.
- S. Baayyad, et al., High-density polyethylene composites filled with micro- and nano-particles of nickel ferrite: magnetic, mechanical, and thermal properties, RSC Adv., 2024, 14, 18750–18763 RSC.
- A. Damnjanović and N. Kovačević, Influence of Magnet Particle Shape on Magnetic and Environmental Stability of FDM Polymer-Bonded Magnets, Materials, 2023, 16, 2993 CrossRef.
- Mechanisms and Reactions in the Solid State, pp. 129–189 Search PubMed.
- Y.B. Kannan, R. Saravanan and N. Srinivasan, Sintering effect on structural, magnetic and optical properties of Ni0.5Zn0.5Fe2O4 ferrite nano particles, J. Magn. Magn. Mater., 2017, 423, 217–225 CrossRef CAS.
- S. M. Yasin, Superparamagnetic Ni0.5Zn0.5Fe2O4 nanoparticles prepared by ball-milling, Appl. Phys. A: Mater. Sci. Process., 2023, 129, 24–27 CrossRef.
- R. V. Bharathi, et al., Enhanced DC electrical resistivity and magnetic properties of transition metal cobalt substituted spinel MgFe2O4 ferrite system, Inorg. Chem. Commun., 2023, 158, 111713 CrossRef CAS.
- C. Chen, Y. Yang, D. Chen, Y. Zhang and Y. Meng, Micro-structural and magnetic analysis of spinel high entropy oxides synthesized by two-step pressureless sintering provides insight into high entropy ceramics, Mater. Today Commun., 2023, 35, 106122 CrossRef CAS.
- S. Akhter, et al., Magnetic Properties of Cu1-xZnxFe2O4 Ferrites with the Variation of Zinc Concentration, J. Mod. Phys., 2012, 03, 398–403 CrossRef CAS.
- B. Shahbahrami, S. M. Rabiee, R. Shidpour and H. Salimi-Kenari, Influence of calcination parameters on the microstructure, magnetic and hyperthermia properties of Zn-Co ferrite nanoparticles, J. Electroceram., 2022, 48, 157–168 CrossRef CAS.
- A. Ghasemi and M. Mousavinia, Structural and magnetic evaluation of substituted NiZnFe2O4 particles synthesized by conventional sol-gel method, Ceram. Int., 2014, 40, 2825–2834 CrossRef CAS.
- Y. Han, A. Sun, X. Pan, W. Zhang and X. Zhao, Effect of Different Sintering Temperatures on Structural and Magnetic Properties of Zn–Co Ferrite Nanoparticles, J. Supercond. Nov. Magnetism, 2019, 32, 3823–3830 CrossRef CAS.
- H. Nikmanesh, M. Eshraghi and S. karimi, Cation distribution, magnetic and structural properties of CoCrxFe2-xO4: Effect of calcination temperature and chromium substitution, J. Magn. Magn. Mater., 2019, 471, 294–303 CrossRef CAS.
- S. M. Olhero, et al., Co-precipitation of a Ni-Zn ferrite precursor powder: Effects of heat treatment conditions and deagglomeration on the structure and magnetic properties, J. Eur. Ceram. Soc., 2012, 32, 2469–2476 CrossRef CAS.
- B. Purnama, A. T. Wijayanta and Suharyana, Effect of calcination temperature on structural and magnetic properties in cobalt ferrite nano particles, J. King Saud Univ. Sci., 2019, 31, 956–960 CrossRef.
- S. J. Olusegun, E. T. F. Freitas, L. R. S. Lara, H. O. Stumpf and N. D. S. Mohallem, Effect of drying process and calcination on the structural and magnetic properties of cobalt ferrite, Ceram. Int., 2019, 45, 8734–8743 CrossRef CAS.
- I. Panneer Muthuselvam and R. N. Bhowmik, Mechanical alloyed Ho3+ doping in CoFe2O4 spinel ferrite and understanding of magnetic nanodomains, J. Magn. Magn. Mater., 2010, 322, 767–776 CrossRef CAS.
- K. Kim, K. W. Jeon, K. W. Moon, M. K. Kang and J. Kim, Effects of Calcination Conditions on Magnetic Properties in Strontium Ferrite Synthesized by the Molten Salt Method, IEEE Trans. Magn., 2016, 52, 8–11 Search PubMed.
- R. Masrour, et al., Synthesis and magnetic properties of bulk ferrites spinels Ni0.5Zn0.5Fe2O4: Experimental an Ab-initio study, J. Supercond. Nov. Magnetism, 2014, 27, 177–181 CrossRef CAS.
- H. Huili, B. Grindi, G. Viau and L. Ben Tahar, Effect of cobalt substitution on the structure, electrical, and magnetic properties of nanorcrystalline Ni0.5Zn0.5Fe2O4 prepared by the polyol process, Ceram. Int., 2014, 40, 16235–16244 CrossRef CAS.
- X. Pan, A. Sun, Y. Han, W. Zhang and X. Zhao, Effects of different sintering temperature on structural and magnetic properties of Ni-Cu-Co ferrite nanoparticles, Mod. Phys. Lett. B, 2018, 32, 1067–1076 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07076c |
| This journal is © The Royal Society of Chemistry 2024 |