Open Access Article
Open Access ArticleTiO2/CuWO4 heterojunction photocatalyst in the preparation of cardiovascular chromeno[4,3-b]chromene drugs†
Jiaqi Sun and
Jiahao Song *
*
Department of Cardiology, The Second Affiliated Hospital of Xinjiang Medical University, Urumqi City, 830000, China. E-mail: songjiahaourumqi@outlook.com
First published on 17th December 2024
Abstract
Chromeno[4,3-b]chromenes have shown potential as effective drugs against cardiovascular diseases. This study centered on a novel heterojunction nanocomposite TiO2/CuWO4, which incorporates titanium dioxide (TiO2) and copper tungstate(VI) to improve photocatalytic efficiency. The characterization of the TiO2/CuWO4 photocatalyst was performed using various techniques, including FT-IR, XRD, TEM, DRS, SEM, EDS, and XPS. TiO2/CuWO4 demonstrated significant effectiveness as a photocatalyst for the synthesis of chromeno[4,3-b]chromene derivatives, which show potential antibacterial and antifungal properties beneficial for oral health concerns such as dental caries and periodontal disease. While TiO2 can absorb light to produce electrons and holes under direct illumination, its photocatalytic efficiency is improved when paired with CuWO4. The research also explored the influence of several factors, including the quantity of photocatalyst, reaction duration, temperature, solvent selection, and the reusability of the nanocomposite. Optimal reaction conditions were found to involve 1 mmol of dimedone, benzaldehyde, and 4-hydroxycoumarin, using 12 mg of TiO2/CuWO4 in 4 mL of ethanol, subjected to irradiation from a green laser at room temperature for 30 minutes. The results indicate that the TiO2/CuWO4 heterojunction ranks among the most effective options for photocatalytic synthesis of chromeno[4,3-b]chromene derivatives.
1. Introduction
Chromeno[4,3-b]chromenes are a class of compounds that have garnered attention for their potential therapeutic effects, including activities against cardiovascular diseases. These compounds have shown antihypertensive effects, which could be beneficial in managing cardiovascular diseases. Moreover, chromenes are noted for their antioxidative capabilities, and can play a crucial role in cardiovascular health by reducing oxidative stress, atherosclerosis and hypertension.1–3 Chromeno[4,3-b]chromenes may exhibit anti-inflammatory effects that could mitigate the inflammatory processes associated with diseases like coronary artery disease and heart failure. Therefore, the synthesis of chromeno[4,3-b]chromenes is significant in drug design due to their diverse biological activities and structural versatility, making them valuable scaffolds for developing new therapeutic agents. The ability to synthesize chromeno[4,3-b]chromenes through various efficient methodologies enhances their accessibility for drug development.Heterojunction photocatalysis has attracted considerable attention from researchers in recent years for several important reasons. A main issue with conventional semiconductor photocatalysts is the swift recombination of photogenerated electron–hole pairs, which reduces their efficiency.4–7 Heterojunction systems effectively enhance the separation and movement of these charge carriers, resulting in improved photocatalytic performance. By combining various semiconductors, we can develop structures that promote more efficient charge transfer and minimize recombination rates.8 Additionally, heterojunctions can be tailored to capture a broader spectrum of solar energy, particularly in the visible range, which is essential for effective applications in environmental cleanup and energy conversion.9–13 TiO2/CuWO4 heterojunction is an efficient photocatalyst due to its unique structural and electronic properties. The effectiveness of TiO2/CuWO4 as a photocatalyst primarily stems from its ability to facilitate the separation of photogenerated charge carriers. The unique band alignment between TiO2 and CuWO4 allows for efficient transfer of these charges, minimizing recombination rates. CuWO4 can accept electrons from TiO2 due to its more positive conduction band edge potential, which enhances the overall photocatalytic efficiency by extending the lifetime of the charge carriers.
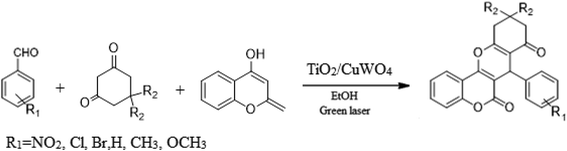
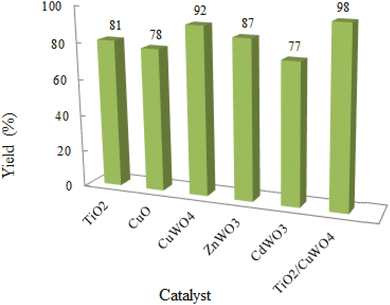
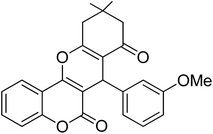
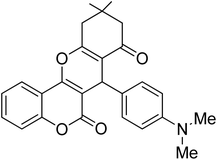
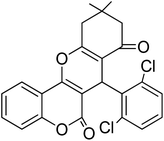
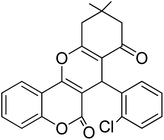
In this research, the synthesis of chromeno[4,3-b]chromenes was selected as a model for a multi-component reaction.14,15 The cyclocondensation process used to generate these compounds is a significant transformation in organic synthesis, yielding well to excellent results. This study thoroughly examines the influence of various factors, such as the quantity of photocatalyst, reaction duration, temperature, and solvent choice, along with the reusability of the nanocomposite. The addition of CuWO4 to TiO2 alters the overall efficiency of the photocatalyst and improves its performance. The enhanced quality of the final TiO2/CuWO4 is attributed to the fact that TiO2 mainly absorbs UV light, which restricts its effectiveness under visible light. In contrast, CuWO4 broadens the light absorption into the visible spectrum, facilitating improved photocatalytic activity. Additionally, the carried out modification can increase the production of reactive oxygen species, including hydroxyl radicals and superoxide anions. Thus, the integration of TiO2 and CuWO4 may lead to synergistic effects that enhance reaction pathways beyond what either component could accomplish independently. This study highlights the potential of TiO2/CuWO4 as a reusable catalyst for the efficient and sustainable synthesis of chromeno[4,3-b]chromene derivatives (Fig. 1).
2. Experimental
2.1 Materials and methods
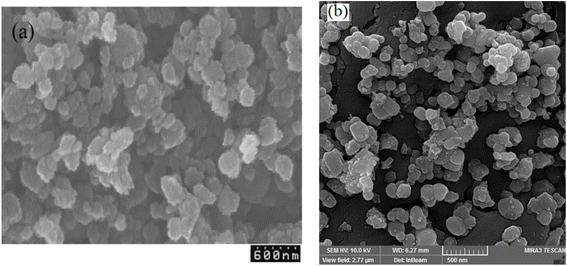
Titanium isopropoxide (C12H28O4Ti) was obtained from commercial suppliers, along with potassium and sodium hydroxides, copper(II) nitrate (Cu(NO3)2·6H2O), sodium tungstate dihydrate (Na2WO4·2H2O), and several organic compounds. The morphology of the photocatalyst was assessed using FE-SEM Mira 3-XMU. FT-IR spectra were collected using a PerkinElmer spectrophotometer, spanning the range of 400–4000 cm−1. UV-Vis spectra were obtained with a Spekol 2000 UV-visible spectrophotometer. Powder X-ray diffraction (XRD) analysis was conducted using an Xpert MPD diffractometer, employing Cu Kα radiation at 30 mA and 40 keV, with scans ranging from 5° to 80° at a speed of 3° per minute. The optical characteristics of TiO2/CuWO4 were analyzed using diffuse reflectance spectroscopy (DRS) conducted with a Shimadzu UV-2450 spectrophotometer across a wavelength range of 800–200 nm. For transmission electron microscopy (TEM) analysis, a PHILIPS TECNAI 10 microscope was employed, complemented by energy dispersive X-ray spectroscopy (EDS). X-ray photoelectron spectroscopy (XPS) was also conducted using a Thermo Fischer Scientific ESCALAB instrument.2.2 Synthesis of titanium dioxide (TiO2)
To prepare titanium oxide nanoparticles, a 30 mL solution of 0.1 M titanium isopropoxide was placed in a cold water bath, kept at around 5 °C. Gradual addition of 5.0 M KOH was performed until the pH reached 7.0. After stirring the mixture for roughly 15 minutes, a white colloidal solid was formed. The volume of final solution was brought to 60 mL by adding distilled water. This mixture was then placed in a 120 mL Teflon autoclave and heated to 270 °C for 12 hours. The resulting precipitate was dried at 400 °C for 2 hours, yielding TiO2 particles.16,172.3 Synthesis of titanium dioxide/copper(II) tungstate (TiO2/CuWO4)
Initially, 15 mL of 1 M copper(II) nitrate was mixed with 1.5 mL of 0.5 M sodium tungstate dihydrate. After that, 0.25 g of titanium dioxide was added to this solution and stirred magnetically for 1.5 hours. The mixture was then sonicated for 25 minutes. Following this step, 25 mL of 4 M sodium hydroxide was added slowly to the mixture. Finally, the resulting solid was dried in an oven at 115 °C and calcined at 350 °C for 4 hours.18,192.4 Synthesis of chromeno[4,3b]chromenes
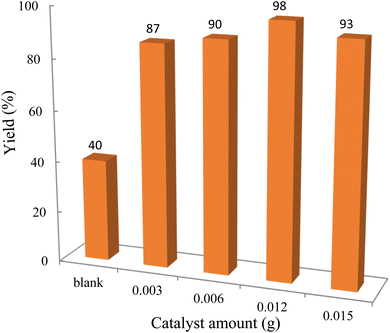
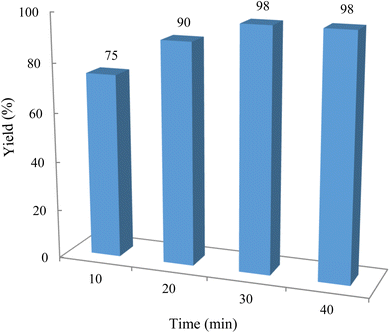
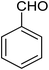
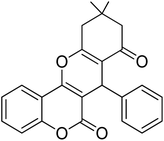
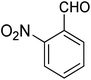
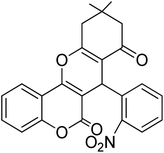
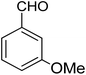
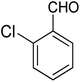
For the synthesis of chromeno[4,3-b]chromenes, a test tube was prepared with 140 mg (1 mmol) of dimedone, 162 mg (1 mmol) of 4-hydroxycoumarin, and 100 mg (1 mmol) of benzaldehyde. To this mixture, 0.012 g of TiO2/CuWO4 was added as the catalyst. The mixture was then subjected to irradiation from a commercial green-light laser for 30 minutes at room temperature. The progress of the reaction was assessed using thin-layer chromatography (TLC). After the reaction was complete, 8 mL of water and 4 mL of ethyl acetate were introduced to the mixture to dissolve the product. The ethyl acetate layer was then separated, and the aqueous layer was extracted three times with EtOAc. The combined organic layer was then dried using sodium sulfate, and the final product was purified through column chromatography.20,213. Results and discussion
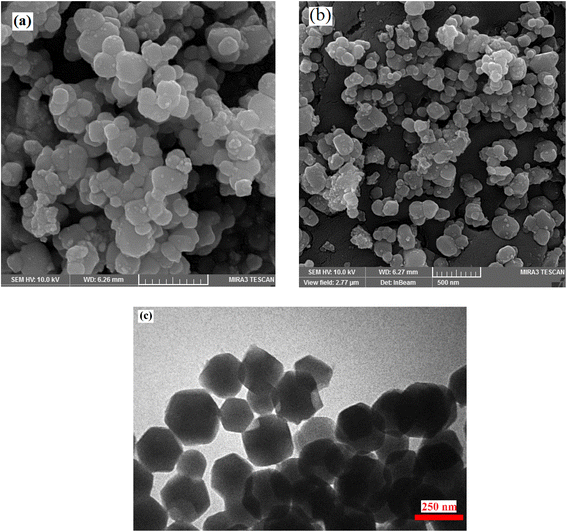
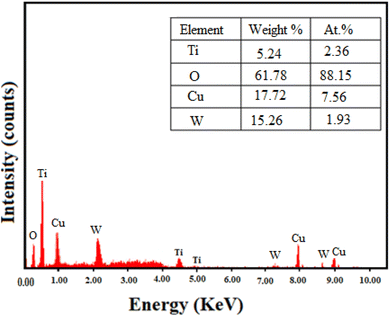
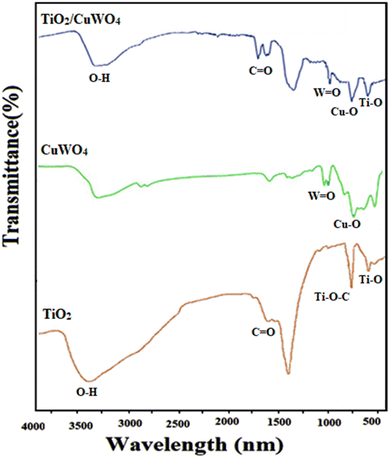
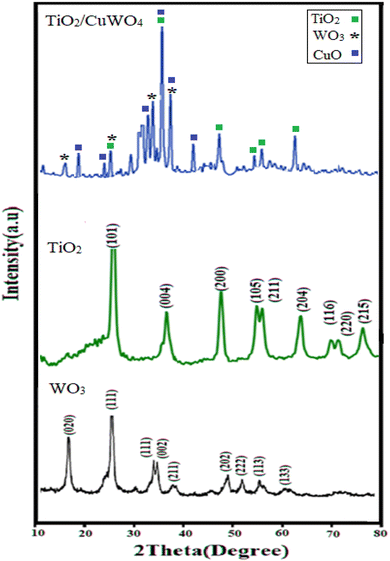
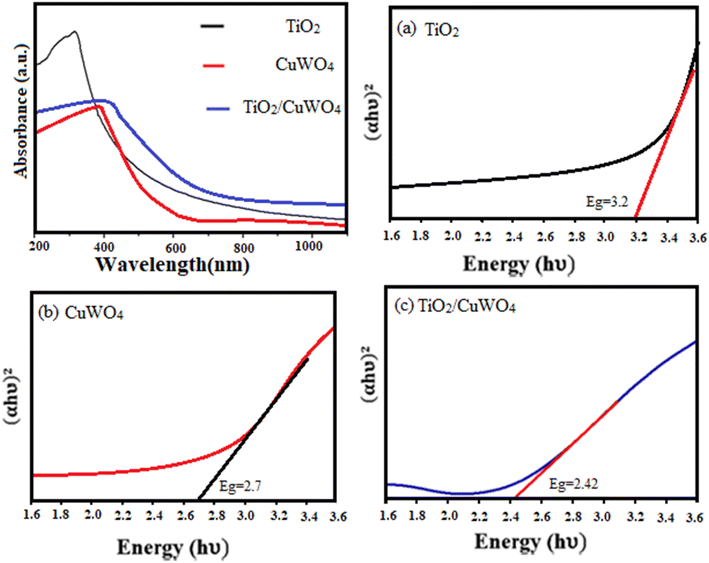
3.1 Characterization of TiO2/CuWO4
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O groups, respectively.25
C–O groups, respectively.253.2 Catalytic tests
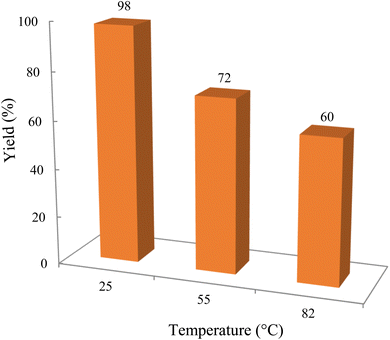
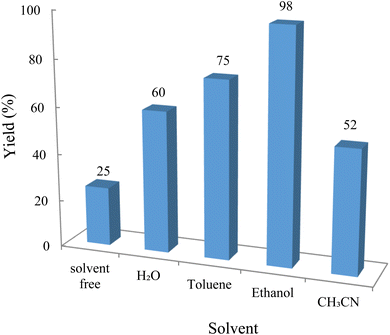
The reaction involving dimedone (140 mg, 1 mmol), benzaldehyde (100 mg, 1 mmol), and 4-hydroxycoumarin (162 mg, 1 mmol) was carried out with TiO2/CuWO4 (0.012 g) in 4 mL of ethanol under the irradiation of a green laser at 25 °C for 30 minutes.
4. Conclusion
Chromeno[4,3-b]chromenes represent a versatile and biologically active scaffold with significant implications for cardiovascular health. Their antioxidant and anti-inflammatory properties, along with potential vasodilatory effects, highlight their therapeutic promise. Therefore, it is crucial to create innovative, eco-friendly synthetic methods for these compounds. Heterojunction photocatalysts present numerous benefits compared to conventional homogeneous photocatalysts, such as improved charge separation, a wider spectrum of light absorption, greater stability and longevity, adjustable properties, and synergistic interactions. Titanium dioxide is recognized for its remarkable photocatalytic efficiency when compared to various other semiconductors, thanks to its extensive surface area and porous lattice structure. Nevertheless, its wide band gap restricts its absorption capability. To overcome this challenge, TiO2/CuWO4 has been introduced to improve the performance of both TiO2 and CuWO4. The synthesized photocatalyst underwent characterization utilizing a range of techniques, including FT-IR, XRD, UV-Vis, DRS, FE-SEM, TEM, EDS, and XPS. The TiO2/CuWO4 photocatalyst was subsequently utilized for the photocatalytic synthesis of substituted chromeno[4,3-b]chromenes and showed greater efficiency than the individual components. The study closely examined various factors, including the quantity of photocatalyst, reaction duration, temperature, choice of solvent, and the reusability of the photocatalyst. The results highlighted the importance of TiO2/CuWO4 heterojunction material in photocatalytic synthesis compared to other similar materials introduced in the field.Data availability
The data that support the findings of this study are available from the authors.Conflicts of interest
There are no conflicts to declare.References
- A. T. Benny, S. D. Arikkatt, C. G. Vazhappilly, S. Kannadasan, R. Thomas, M. S. Leelabaiamma, E. K. Radhakrishnan and P. Shanmugam, Chromone, a privileged scaffold in drug discovery: developments in the synthesis and bioactivity, Mini-Rev. Med. Chem., 2022, 22(7), 1030–1063 CrossRef PubMed.
- B. Jadhaw, B. Gandhi, M. Jhansi, S. Misra and S. S. Kaki, Synthesis and Biological Evaluation of Novel Lipophilic Chromene Based 1,3,4-Oxadiazoles for Anti-cancer and Anti-inflammatory Activity, Arabian J. Sci. Eng., 2024, 49(1), 221–230 CrossRef.
- D. H. Dawood, A. M. Srour, D. O. Saleh, K. J. Huff, F. Greco and H. M. Osborn, New pyridine and chromene scaffolds as potent vasorelaxant and anticancer agents, RSC Adv., 2021, 11(47), 29441–29452 RSC.
- Y. Zhang, R. Jiang, L. Lei, Y. Yang and T. Hu, Drug delivery systems for oral disease applications, J. Appl. Oral Sci., 2022, 30, e20210349 CrossRef PubMed.
- R. K. Kushwaha, K. Singh, P. Kumar and D. Chandra, Review on Chromen Derivatives and Their Pharmacological Activities, Res. J. Pharm. Technol., 2019, 12(11), 5566–5574 CrossRef.
- V. Raj and J. Lee, 2H/4H-Chromenes-a versatile biologically attractive scaffold, Front. Chem., 2020, 8, 623 CrossRef CAS PubMed.
- E. M. Rojas, H. Zhang, S. E. Velu and H. Wu, Tetracyclic homoisoflavanoid (+)-brazilin: a natural product inhibits c-di-AMP-producing enzyme and Streptococcus mutans biofilms, Microbiol. Spectrum, 2024, 12(5), e02418–e02423 CrossRef PubMed.
- W. Y. Teoh, J. A. Scott and R. Amal, Progress in heterogeneous photocatalysis: from classical radical chemistry to engineering nanomaterials and solar reactors, J. Phys. Chem. Lett., 2012, 3(5), 629–639 CrossRef CAS PubMed.
- H. Yang, Short review on heterojunction photocatalysts: carrier transfer behavior and photocatalytic mechanisms, Mater. Res. Bull., 2021, 142, 111406 CrossRef CAS.
- G. Huang, Z. Jalili, R. Tayebee, Z. Shi, X. Zhang, L. Liu, L. Wang, D. Sun, X. Sun, L. Liu and W. Zhao, Zinc oxide-cadmium(II) sulfide heterostructure as a potential photocatalyst for preparing substituted chromenes and its anti-liver cancer activity, Appl. Organomet. Chem., 2024, 38(5), e7410 CrossRef CAS.
- B. Li, R. Tayebee, E. Esmaeili, M. S. Namaghi and B. Maleki, Selective photocatalytic oxidation of aromatic alcohols to aldehydes with air by magnetic WO3ZnO/Fe3O4. In situ photochemical synthesis of 2-substituted benzimidazoles, RSC Adv., 2020, 10(67), 40725–40738 RSC.
- S. Abbaspour, R. Zhou, F. M. Zonoz and R. Tayebee, Advanced CuO-Ag2WO4-Ni nanophotocatalyst in the synthesis of some chromeno[4,3-b]chromenes as effective lung cancer drugs, Inorg. Chem. Commun., 2024, 170, 113284 CrossRef CAS.
- A. Balapure, J. Ray Dutta and R. Ganesan, Recent advances in semiconductor heterojunctions: a detailed review of the fundamentals of photocatalysis, charge transfer mechanism and materials, RSC Appl. Interfaces, 2024, 1, 43–69 RSC.
- M. Karami, A. Hasaninejad, H. Mahdavi, A. Iraji, S. Mojtabavi, M. A. Faramarzi and M. Mahdavi, One-pot multi-component synthesis of novel chromeno[4,3-b]pyrrol-3-yl derivatives as alpha-glucosidase inhibitors, Mol. Diversity, 2021, 1–3 Search PubMed.
- F. Kamali and F. Shirini, Melamine: an efficient promoter for some of the multi-component reactions, Polycyclic Aromat. Compd., 2019, 41, 73–94 CrossRef.
- M. Aravind, M. Amalanathan and M. S. Mary, Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties, SN Appl. Sci., 2021, 3, 409 CrossRef.
- B. Abdi, R. Tayebee, E. Rezaei-seresht, F. M. Zonoz and Z. Jalili, TiO2/AgSbO3 nanophotocatalyst with improved photocatalytic performance in the synthesis of some benzimidazole derivatives, J. Mol. Struct., 2025, 1321, 140037 CrossRef.
- F. Ahmadi, M. Rahimi-Nasrabadi and M. Eghbali-Arani, The synthesize of CuWO4 nano particles by a new morphological control method, characterization of its photocatalytic activity, J. Mater. Sci.: Mater. Electron., 2017, 28, 5244–5249 CrossRef.
- P. Raizada, S. Sharma, A. Kumar, P. Singh, A. A. Khan and A. M. Asiri, Performance improvement strategies of CuWO4 photocatalyst for hydrogen generation and pollutant degradation, J. Environ. Chem. Eng., 2020, 8(5), 104230 CrossRef.
- Z. Jalili, R. Tayebee and F. M. Zonoz, Eco-friendly synthesis of chromeno[4,3-b]chromenes with a new photosensitized WO3/ZnO@NH2-EY nanocatalyst, RSC Adv., 2021, 11(29), 18026–18039 RSC.
- M. Jarrahi, B. Maleki and R. Tayebee, Magnetic nanoparticle-supported eosin Y salt [SB-DABCO@eosin] as an efficient heterogeneous photocatalyst for the multi-component synthesis of chromeno[4,3-b]chromene in the presence of visible light, RSC Adv., 2022, 12(45), 28886–28901 RSC.
- Z. Barzgari, S. Z. Askari and A. Ghazizadeh, Fabrication of nanostructured CuWO4 for photocatalytic degradation of organic pollutants in aqueous solution, J. Mater. Sci.: Mater. Electron., 2017, 28, 3293–3298 CrossRef.
- S. Zhou, Y. Wang, G. Zhao, C. Li, L. Liu and F. Jiao, Enhanced visible light photocatalytic degradation of rhodamine B by Z-scheme CuWO4/gC3N4 heterojunction, J. Mater. Sci.: Mater. Electron., 2021, 32, 2731–2743 CrossRef.
- J. L. Gole, J. D. Stout, C. Burda, Y. Lou and X. Chen, Highly efficient formation of visible light tunable TiO2-xNx photocatalysts and their transformation at the nanoscale, J. Phys. Chem. B, 2004, 108(4), 1230–1240 CrossRef.
- X. Zhu, G. Wen, H. Liu, S. Han, S. Chen, Q. Kong and W. Feng, One-step hydrothermal synthesis and characterization of Cu-doped TiO2 nanoparticles/nanobucks/nanorods with enhanced photocatalytic performance under simulated solar light, J. Mater. Sci.: Mater. Electron., 2019, 30, 13826–13834 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07857h |
| This journal is © The Royal Society of Chemistry 2024 |