ChemPren: a new and economical technology for conversion of waste plastics to light olefins
Anne
Gaffney
*a,
Debtanu
Maiti
 a,
Debasish
Kuila
b and
Gennaro
Mafia
c
a,
Debasish
Kuila
b and
Gennaro
Mafia
c
aIdaho National Laboratory, 1955 Fremont Avenue, Idaho Falls, ID 83415, USA. E-mail: anne.gaffney@inl.gov
bDepartment of Applied Science and Technology, North Carolina Agricultural and Technical State University, Greensboro, NC 27411, USA
cDepartment of Chemical Engineering, Manhattan College, 4513 Manhattan College Parkway, Riverdale, NY 10471, USA
First published on 7th October 2024
Abstract
With the ever-increasing demand for plastics, sustainable recycling methods are key necessities. The current plastics industry can manage to recycle only 10% of the 400 million metric tons of plastic produced globally. Waste plastics, in the current infrastructure, land up mostly in landfills. Although a lot of research efforts have been spent on processing and recycling co-mingled mixed plastics, energy-efficient sustainable and scalable routes for plastic upcycling are still lacking. Catalytic valorization of waste plastic feedstock is one of the potential scalable routes for plastic upcycling. Silica-alumina based materials, and zeolites have shown a lot of promise. A major interest lies in restricting catalyst deactivation, and refining product selectivity and yield for such catalytic processes. This article highlights ChemPren technology as a clean energy solution to waste plastic recycling. Co-mingled, mixed plastic feedstock along with spray dried, attrition resistant, ZSM-5 containing catalysts is preprocessed with an extruder to form optimally sized particles and fed into a fluidized bed reactor for short contact times to produce selectively and in high yields ethylenes, propylenes and butylenes. This techno-economic perspective indicates that the ChemPren technology can produce propylene at $0.16 per lb, whereas the current selling price of virgin propylene is $0.54 per lb. This technology can serve as a platform for mixed plastic upcycling, with more advancements necessary in the form of robust and resilient catalysts and reactor operation strategies for tuning product selectivity.
Introduction
The rate of plastic production across the globe is currently quite high. The global production of plastics reached 400.3 million metric tons in 2022.1 This reflects about 1.6 percent increase from the previous year. Unfortunately, only 10% of global plastic waste is recycled, mostly due to challenges in sorting and depolymerization.2 More specifically, lightweight packaging (LWP) comprises about 50% of the total plastic consumption and consists mainly of single and multilayer films and containers. Polyethylene (PE) and polypropylene (PP) make up more than 65% of the LWP that end up in waste materials recovery facilities. The North America LWP market is estimated at $45.9 billion in 2024 and is expected to grow to $52.6 billion by 2029 at a CAGR (compound annual growth rate) of 2.8%.3 It is difficult to recycle LWP as it is heterogeneous and contaminated with other undesired materials.4,5Current recycling approaches of LWP are not adequate to address the growing volume of packaging plastics. Mechanical recycling generally degrades, or downcycles the polymer and cannot be used in food contact packaging because of the low level of impurity and contaminant removal. Chemical recycling converts polymers to molecular intermediates that can be used to make new products that have virgin-like performance, thereby creating new value chains for what is currently a waste stream. However, present high TRL methods like pyrolysis and gasification are highly energy intensive and require multiple steps to make polyolefin feedstock like ethylene and propylene. One of the predominant routes involves pyrolysis and cleanup/hydrotreatment, followed by steam cracking and gasification to syngas. The syngas is finally converted to high-value hydrocarbons either via Fischer–Tropsch or a combination of syngas to methanol and methanol to olefins. The main challenges for pyrolysis of plastic waste are: (1) unavailability and inconsistent quality of feedstock; (2) inefficient and hence costly sorting; (3) non-existent markets citing lack of standardized products; and (4) unclear regulations around plastic waste management. Possible solutions could include tight cooperation between feedstock providers and converters for securing steady quantity and quality of feedstock.
Polymer upcycling, in contrast, aims at selectively deconstructing polymers directly into monomers, high value chemicals or materials.6 Thus, there is a great opportunity to do fundamental research and generate foundational knowledge that will advance this field. It is of utmost interest to develop an industrially scalable, single step, lower energy consumption technology that enables targeted production of high value products like ethylenes, propylenes, butylenes (C2–C4 olefin) and polyols from LWP waste.7
To address these challenges, we highlight ChemPren technology for the conversion of waste polyolefins into monomers, intermediates, new polymers and value-added chemicals. Our vision is to improve thermal catalytic deconstruction (TCD) to offer an improvement in efficiency, cumulative energy demand and selectivity over conventional recycling processes and, in the long term, a viable environmentally friendly solution to plastic recycling.
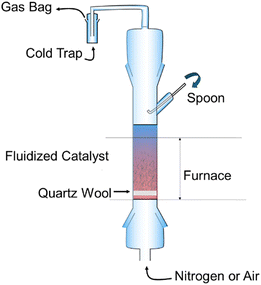
The ChemPren process, shown in Fig. 1, essentially consists of a fluidized bed reactor with zeolite catalysts to enable cracking of waste plastics. In a laboratory-scale reactor, the pellets of plastics (comprising 50 wt% polyethylene, 20 wt% polypropylene, 15 wt% polystyrene, and 15 wt% polyethylene) mixed with the ZSM-5 catalyst were dropped into the fluidized bed through a spoon once the reaction temperature of 550 °C was reached. The residence time of the gases (air or N2) is about 1 second, and the cracking step takes about 1 minute. The composition of the product stream was detected using gas chromatography. This perspective focuses on the techno-economic feasibility of this process and encourages more research work for further development of this technology. The advantage of this approach lies in the controlled tuning of product selectivity from waste mixed plastics based on the selection of catalysts and appropriate cracking conditions, instead of expensive pretreatment processes. While thermocatalytic decomposition of plastics has been studied at large, the main challenge lies in developing catalysts that are resistant to coking and/or developing energy-efficient catalyst regeneration processes. There are some excellent articles that review the current state of the art practices on thermocatalytic plastic recycling.8–11 Plas-TCat™, developed by Anellotech, is another one-step process for the thermocatalytic conversion of mixed plastic waste into basic chemicals such as olefins and aromatics.12
Techno-economic analysis
Techno-economic analysis (TEA) is an important tool to evaluate the economic performance of industrial processes, such as those found in refineries and chemical plants. It is especially valuable in the assessment of novel unit operations and research and development. In this current assessment of the cost of production (COP) for the upcycling of waste plastics, a single number can be calculated and used for comparison with a variety of new technologies. Thus, TEA presents an opportunity to weigh the performance of competing technologies and valuation of recycled products. The components of TEA can be broken down into several items and then reassembled to gain insight into the sensitivities of novel technologies in waste plastic upcycling.The COP can be evaluated in economic segments, such as variable costs, raw materials (including catalysts), energy balances (including utilities), fixed costs, and capital recovery. Subsets of the COP are especially important in the analysis and can be isolated in categories such as cash cost, after tax return on investment (ATROI), net income (before and after tax), minimum acceptable rate of return and payback period. Other categories are used by a variety of companies, such as the present value (PV). The present value at 10% before tax (PV10, or 10% after tax) is particularly valuable in the comparison of competing technologies. The sum of the categories above provides a required netback, which is very valuable in the assessment of new technologies. COP and TEA are calculated from process models assembled on software packages such as ASPEN Plus, HYSYS 12.1v, and several others.
We calculated yields of propylene and ethylene from the original ChemPren data13,14 and converted yields into stoichiometry for uploading into process models. We developed an ASPEN-HYSYS model for the processing of polyethylene and polypropylene and strategized the recovery of monomers and higher valued chemicals by utilizing several recovery options: (1) one tower option; (2) three tower option; (3) seven tower option; (4) adsorption to replace some distillation options.
We developed TEA models and sensitivity assessments and discovered strong value from recovered monomers and by-products making Chem-Pren an established and potentially profitable technology. We developed a cost of production technique to assess the economics, including operational expenses (OPEX) and capital expenses (CAPEX). While reused propylene has a value of $0.166 per pound ($332 per ton), virgin propylene has a selling price of $1200 per ton.
Waste plastic upcycling
The distribution of municipal solid waste (MSW)15 is shown in Table 1 below and amounts to about 300 million tons per year or 5.5 pounds per person per day. Of the MSW generated, approximately 69 million tons were recycled, and 25 million tons were composted. These account for 92% of all US plastic sales with 61% municipal and 39% non-municipal. Almost 77% of PVC goes to non-municipal solid waste, and ca. 80% of the plastics in municipal solid waste comprises PE, PS and PP.| Components | % |
|---|---|
| LDPE | 17.4 |
| PVC | 17.2 |
| HDPE | 14.8 |
| PP | 12 |
| PS | 10.8 |
| Phenolic | 6.7 |
| PU | 6.3 |
| PET and PBT | 3.3 |
| Urea and melamine | 3.3 |
| Unsaturated polyester | 3.0 |
| ABS | 2.5 |
| Acrylic | 1.6 |
| Nylon | 1 |
Chemical recycling methods like pyrolysis could significantly increase the circular rates. A combination of advanced technology and tight cooperation between feedstock providers and converters can pave the way for securing steady quantity and quality of feedstock. Advanced pretreatment, such as the use of plasma from a modular extruder could provide the basis for cost-effective recycling, producing higher valued products. The classification of pyrolysis liquid as a product instead of waste is possible, and the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) registration should be carried out to standardize the liquid oil as a product.
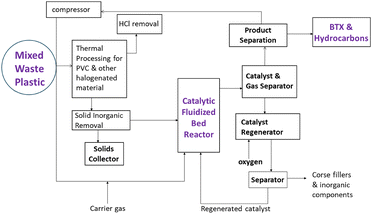
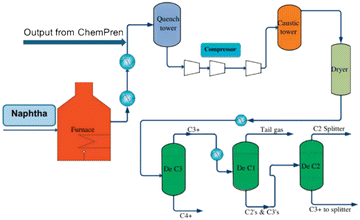
A block flow diagram of the ChemPren process is depicted in Fig. 2. Using the yields from the original ChemPren work for polypropylene, an initial PFD has been developed as shown in Fig. 3. Several cases have been analyzed with different levels of purification. The case presented, here, has light gases, NGLs, and liquids. We have developed a cost of production technique that assesses the economics, including OPEX and CAPEX.
Due to the large, diverse yields of chemicals from the ChemPren process, there is an extensive distillation system with eight towers as in a typical olefin plant liquid cracker or refinery. This is unlike the grouped products, which will require only three towers. The PFD presented above is for the three-tower system, with potential integration of ChemPren into an existing liquid cracking unit. The pre-treatment process involves an extruder to form optimally sized particles to be fed into the fluid bed reactor. This provides the basis for cost-effective recycling, producing higher valued products. The production of high value light olefins, ethylenes, propylenes and butenes in high selectivity and overall yields of 70% is demonstrated.
Some noteworthy features of the aPP baseline process are:
1. Carbon yield at 2% issued in the heat balance.
2. Only trace acetylenes and diolefins.
3. Significant BTX (27%).
4. Significant C6–C8 non-aromatics.
5. C2–C4 mono-olefins, including ethene and propene at 20%.
6. The product streams can be interfaced with the reactor effluent from a liquid cracking plant.
The yields and capacity of the ChemPren technology is presented in Table 2. Chemical grade (CG) propylene (93% propylene) can be upgraded to polymer grade (99.5% purity). Inside battery limits (ISBL) refers to the area within the physical boundary of the plant where the primary process equipment is located. This is the cost used by process engineers, project engineers, and cost professionals as they approach the initial development of process plant projects in terms of geographical boundaries. Outside battery limits (OSBL) is a semi-arbitrary boundary surrounding the main process equipment. This process equipment is considered outside battery limits and includes utilities, storage, and other auxiliary services. The value of OSBL is often referred to as 40% of the ISBL during the initial economic calculations. Before tax payback period (BTPB), which is the number of years to recover the capital cost of a chemical plant, is estimated to be four years. Total installed cost (TIC) represents the total capital cost of the process equipment and is often referred to as CAPEX.
| Capacity (lb per year of propylene) | 1.00 × 108 |
| Plastics (lb per year of plastics) | 7.69 × 108 |
| Inside battery limits (ISBL) for 2022 | $300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Outside battery limits (OSBL) for 2022 | $100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Before tax payback period (BTPB), (year) | 4.00 |
| Total installed cost (TIC) | $4.00 × 108 |
| Capital recovery ($ per lb propylene) | 1.00E+00 |
As noted earlier, a vast array of hydrocarbons is produced. From lower chain products such as C2–C4 olefins, to higher chain BTX, and oxygenated intermediates are produced from polyolefin packaging waste streams. The product distribution is presented in Table 3. Propylene selectivity is the highest amongst low-carbon hydrocarbons. The cost of production is directly linked to the cost of downstream process separation units with several options such as a single column, a multiple of 3 columns, and 8 columns similar to that in conventional ethylene plants. Thorough analysis of the material and energy balances has been performed on the ChemPren process. The summary of the economics is shown in Table 4. More significantly, the upcycled propylene produced is priced at $0.16 per pound as compared to virgin propylene selling at $0.54 per pound, a 70% reduction in cost.
| Product | Symbol | Moles | % |
|---|---|---|---|
| CH4 | C1 | 1 | 0.9634 |
| C2H4 | C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) |
6 | 5.7803 |
| C2H6 | C2 | 0.2 | 0.1927 |
| C3H6 | C3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) |
13.5 | 13.0058 |
| C3H8 | C3 | 6.5 | 6.2620 |
| C4H8 | C4 | 16 | 15.4143 |
| C5H10 | C5 | 4 | 3.8536 |
| C6H6 | B | 6.825 | 6.5751 |
| C7H8 | T | 13.65 | 13.1503 |
| C8H10 | X | 6.825 | 6.5751 |
| C7H14 | C7 | 27.3 | 26.3006 |
| C | C | 2 | 1.9268 |
| SUM | 103.8 | 100 |
| Parameters | $ per lb propylene |
|---|---|
| VC (variable costs) | −$1.2972 |
| FC (fixed costs) | $0.4608 |
| CR (capital recovery) | $1.0000 |
| Sum of required realizations | $0.1636 |
| SG&A@1.50% | $0.0025 |
| RNB (required netback) | $0.1661 |
Summary and future directions/perspectives
Ideally, a techno-economic model represents the best current understanding of the system being modelled. TEA can be used to anticipate whether a process will be sufficiently profitable under a certain set of assumptions. Catalyst and catalytic process development research when combined with sensitivity analyses TEA and life cycle analysis (LCA) can be used to identify targets with the greatest potential to improve profitability and environmental sustainability. Thus, it can help companies bring their technologies to the market more efficiently. To quantify uncertainty and risks, sensitivity analyses like tornado diagrams and Monte Carlo analysis can be used to quantify economic uncertainty in the model results. This brief perspective showcases the economic viability of the ChemPren approach of mixed waste plastic upcycling to valuable products.Future research directions must focus on both fundamental scientific advances in this process and rigorous economic and life cycle analysis of this process under different implementation scenarios. Improvements in terms of product yields, and precise product selectivity can be targeted via application of dynamic catalyst science principles, plasma-assisted ChemPren technology, and more energy-efficient electric-field induced approaches. Research for better process scale-up strategies with respect to robust catalyst designs, multiphysics models for transport processes, and improved process integration with existing technologies are important. Prolonged experiments must be performed under real feed conditions to learn about potential catalyst deactivation pathways and developing ways for the mitigation. Process optimization to account for variable feedstocks using intermittent renewable energy sources can encourage application of this technology for distributed modular chemical manufacturing from waste plastics. Case-specific models for successful integration with different industries will also attract commercial partners. This perspective article is, thus, an effort to present ChemPren technology as a potential solution to the waste plastic recycling challenge, with further research work only expected to improve this process.
Data availability
The data for the ChemPren process and the techno-economic analysis will be shared upon request.Author contributions
A. G. – conceptualization, data analysis, and writing; D. M. – writing; D. K. and G. M. – data analysis and writing.Conflicts of interest
There are no conflicts to declare.Notes and references
- S. R. Department, Global plastic production 1950-2022, 2024 Search PubMed.
- UNEP, Single-use plastics: A roadmap for sustainability, 2018 Search PubMed.
- M. Intelligence, North America Flexible Packaging Market Size & Share Analysis - Growth Trends & Forecasts (2024-2029), 2024 Search PubMed.
- R. Voss, R. P. Lee and M. Fröhling, Circ. Econ. Sustainability, 2022, 2, 1369–1398 CrossRef CAS.
- J. Schmidt, M. Auer, J. Moesslein, P. Wendler, S. Wiethoff, C. Lang-Koetz and J. Woidasky, Chem. Ing. Tech., 2021, 93, 1751–1762 CrossRef CAS.
- P. F. Britt, G. W. Coates, K. I. Winey, J. Byers, E. Chen, B. Coughlin, C. Ellison, J. Garcia, A. Goldman, J. Guzman, J. Hartwig, B. Helms, G. Huber, C. Jenks, J. Martin, M. McCann, S. Miller, H. O'Neill, A. Sadow, S. Scott, L. Sita, D. Vlachos and R. Waymouth, Report of the Basic Energy Sciences Roundtable on Chemical Upcycling of Polymers, United States, 2019 Search PubMed.
- A. Eschenbacher, R. J. Varghese, M. S. Abbas-Abadi and K. M. Van Geem, Chem. Eng. J., 2022, 428, 132087 CrossRef CAS.
- A. J. Martín, C. Mondelli, S. D. Jaydev and J. Pérez-Ramírez, Chem, 2021, 7, 1487–1533 Search PubMed.
- L. O. Mark, M. C. Cendejas and I. Hermans, ChemSusChem, 2020, 13, 5808–5836 CrossRef CAS.
- V. K. Soni, G. Singh, B. K. Vijayan, A. Chopra, G. S. Kapur and S. S. V. Ramakumar, Energy Fuels, 2021, 35, 12763–12808 CrossRef CAS.
- H. Li, H. A. Aguirre-Villegas, R. D. Allen, X. Bai, C. H. Benson, G. T. Beckham, S. L. Bradshaw, J. L. Brown, R. C. Brown, V. S. Cecon, J. B. Curley, G. W. Curtzwiler, S. Dong, S. Gaddameedi, J. E. García, I. Hermans, M. S. Kim, J. Ma, L. O. Mark, M. Mavrikakis, O. O. Olafasakin, T. A. Osswald, K. G. Papanikolaou, H. Radhakrishnan, M. A. Sanchez Castillo, K. L. Sánchez-Rivera, K. N. Tumu, R. C. Van Lehn, K. L. Vorst, M. M. Wright, J. Wu, V. M. Zavala, P. Zhou and G. W. Huber, Green Chem., 2022, 24, 8899–9002 RSC.
- Anellotech, Plas-TCat™ for Chemical Recycling of Mixed Waste Plastic, https://anellotech.com/plas-tcat-mixed-plastic-recycling.
- A. M. Gaffney, US Pat., US10987661B2, 2021 Search PubMed.
- F. Associates, Characterization of Plastic Products in Municipal Solid Waste, Final Report. Prepared for Council for Solid Waste Solutions, Prairie Village, KS, 1990 Search PubMed.
- B. A. Hegberg, W. H. Hallenbeck and G. R. Brenniman, Resour., Conserv. Recycl., 1993, 9, 89–107 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |