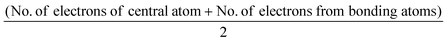Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Resources for reasoning of chemistry concepts: multimodal molecular geometry†
Nicola A.
Kiernan
 *a,
Andrew
Manches
a and
Michael K.
Seery
*a,
Andrew
Manches
a and
Michael K.
Seery
 b
b
aCentre for Research in Digital Education, Moray House School of Education, University of Edinburgh, Edinburgh, Scotland, UK. E-mail: Nicola.Kiernan@ed.ac.uk
bQuality Enhancement Directorate, Cardiff Metropolitan University, Cardiff CF5 2YB, UK
First published on 5th January 2024
Abstract
Central to conceptual understanding of STEM disciplines is visuospatial processing. Despite its acknowledged role in assuring learners’ success, less is known about the underlying reasoning students must employ when solving 3-D problems and the ways in which gaining an understanding of this can inform formative assessment and learning in STEM education. Chemists must utilise their spatial understanding when visualising 3-D structures and processes from 2-D representations and so this exploratory practitioner-researcher study sought to identify the ways in which secondary school chemistry students reason when explaining their predictions about molecular geometry, and how the use of certain modalities was linked to assessed accuracy. Coding of students’ verbal and written responses to the research task revealed that students employed multiple reasoning strategies and conceptual resources to facilitate use of analytical heuristics and imagistic reasoning. Analysis of students’ verbal responses and spontaneous gestures provided insight into the extent of imagistic vs. analytical reasoning and the finer-grained conditions which promoted their use. Importantly, it was observed that despite being instructed on the use of VSEPR theory to find analytical solutions, some students exhibited preference for alternative reasoning strategies drawing upon imagistic reasoning; showing more nuanced and varying degrees of accuracy through their verbal responses and representations gestured in 3D space. This work has pedagogical implications as use of specific reasoning strategies and the identification of key conceptual resources is not readily promoted as classroom practice for learning or assessment. This study therefore raises questions and contributes to the evidence base for attending to learners’ visuospatial thinking, as revealed through the multiple modalities they may use to assist and communicate their understanding, and highlights the significance of this to formative assessment in Chemistry and STEM Education.
Introduction
Visuospatial thinking is widely considered to be a fundamental cognitive component of problem solving in science, technology, engineering and mathematics (STEM) (National Research Council, 2006; Newcombe, 2010; Stieff et al., 2016). Similarly, spatial ability is the key skill which enables STEM learners to mentally generate, rotate and transform imagined images, and is a crucial cognitive resource most important for developing expertise in STEM disciplines (Benbow et al., 2013; Hornbuckle et al., 2014; Underwood et al., 2021).Mental visualisation and imagistic reasoning have been suggested to play a key role in learning STEM subjects at undergraduate level, with previous studies indicating that problem solvers may employ imagistic reasoning in tandem with alternative problem-solving strategies such as analytical reasoning (Cooper, 1988; Schwartz and Black, 1996; Wu et al., 2001; Stieff, 2007).
Given its significance to gaining expertise, studies in recent decades exploring the role that visuospatial thinking plays in STEM learning have started to examine the multimodality of students’ journey towards mastery (Hegarty, 2004; Lubinski, 2010; Stieff, 2011; Cooper et al., 2015). This multimodal approach considers all culturally shaped resources available and their contribution to meaning making in classroom discourse (Kress et al., 2005; Givry and Roth, 2006; Abels, 2016).
One such representational mode which exerts an intrinsic role on human communication and meaning making, is gesture. Hand gestures help convey relational, spatial and embodied concepts and the unconscious nature in which gestures often accompany speech has seen this particular mode receiving greater attention in recent STEM education studies (Alibali et al., 2011; Chue et al., 2015; Flood et al., 2014; Stieff et al., 2016; Ping et al., 2021).
Despite insightful formative studies, the unique ways in which STEM learners problem solve and communicate their visuospatial understanding by utilising the conceptual resources and multiple modalities available to them is still not well understood and leaves much to be investigated, particularly in the field of chemistry. Understanding how visuospatial thinking can enable chemistry learners to construct their subject knowledge through varied reasoning modes and problem-solving strategies is key to developing new and improved learning support materials, teaching approaches, digital teaching tools, assessment criteria and ultimately widening access to learning in chemistry and related STEM disciplines (Stieff et al., 2020; Kiernan et al., 2021).
This study builds upon prior research by Kiernan et al., 2021, examining high achieving students use of diagrammatic reasoning when learning visuospatial concepts and that of Hammer (2000), whose resources-based work defined the idea of conceptual resources for physics learning. This naturalistic study also attends to the work of Flood et al., (2014), Stieff et al., (2016) and Fiorella et al. (2017) which called for further research to systematically explore students’ spontaneous use of spatial strategies during learning and the importance of understanding the types of strategies that successful students employ spontaneously. Furthermore, to try to identify why students might use different strategies at particular moments for solving particular problems and capture the finer grained context-dependent conceptual resources which contribute to shaping their understanding.
This paper therefore, explores students’ visuospatial thinking and reasoning strategies when problem solving to identify the conceptual resources that chemistry students’ evidence when communicating their predictions to molecular geometry problems; and the implications of such strategies for teaching, learning and assessment.
This research explores the following core questions:
(1) How do secondary school chemistry students’ preferred modalities and reasoning strategies relate to the assessed accuracy of their responses to molecular geometry problems?
(2) In what ways can students’ spontaneous multimodal responses reveal key conceptual resources for chemistry learning which can facilitate problem solving when making molecular geometry predictions?
(3) To what extent can secondary school chemistry students’ verbal responses to molecular geometry problems in a naturalistic environment be used as a means of formative assessment?
Theoretical background
Resources for reasoning
Few would disagree that exploration of learners’ reasoning strategies is key to informing the promotion of students’ conceptual development; elucidating this important area of chemistry educational research is potentially transformative for both learners and educators. The way in which such research is conducted and communicated is critical to its efficacy and ultimate applicability. Previous and recent research studies in the chemistry education literature have offered helpfully detailed contributions of how students may think when developing representational competence and how the distinctive characteristics of varied textbook chemical representations can affect chemistry students’ reasoning (Talanquer, 2014; 2022). Likewise, much previous chemistry education research exploring students’ reasoning has centred around the role of identifying and overcoming student misconceptions (Griffiths and Preston, 1992; Kelly et al., 2010; Teo et al., 2014).A misconceptions theoretical perspective is relatively intuitive in its approach, which is likely why it has filtered through as routine instructional theory within science teacher training programmes. Although the chemistry education community has devoted much attention to identifying and reporting potential misconceptions, it has paid little attention to determining domain-specific aspects of chemistry learning that can lead to such misconceptions. Teachers do not necessarily consider the contextual subtleties of student reasoning and do not account for the conceptual resources students may have for improving their own understanding through misconceptions (Smith et al., 1993/94; Hammer, 1996).
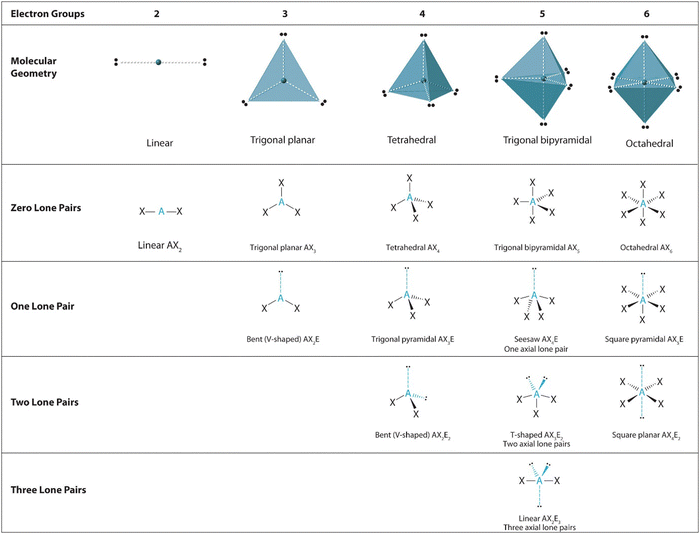
Unfortunately, the complexity of findings and control of experimental variables for some chemistry education research studies can render novel conclusions of student reasoning as substantially theoretical and not transferable to classroom instructors; therefore, despite worthy contributions to the research domain, they are not readily applicable in practice. The resources framework however, first introduced within the domain of physics education, regards all students’ naïve ideas as being capable of contributing to their conceptual understanding, providing these resources are activated within appropriate contexts (Hammer, 2000; Hammer et al., 2005). This theoretical framework considers the idea that these conceptual resources need not lead directly to student understanding to be considered productive; the activation need not necessarily be “appropriate.” Resources theory considers any activated resource as being productive, even if “wrong”, if it has the potential to help develop resources for later ‘right’ thinking (Hammer 1996; Young and Meredith, 2017). This theoretical approach which has been largely confined to physics education lends itself to classroom practice as instructors need only consider how they might identify key resources that assist with students’ thinking around the problem concept and if this might differ across other contexts. Resources by their nature vary, but are identified as fine-grained, beliefs or theories which comprise learners’ construction of knowledge. Few studies have provided examples of how to apply the resources framework or defined the grain-size range of such conceptual resources for reasoning, however formative attempts in physics education provided useful insights into transferable methods. Physics education authors identified resources for students learning about fluid dynamics and highlighted how mathematical equations (ideal gas law and kinetic energy equations), alongside more intuitive physical theories of motion and cause and effect relationships might serve as key conceptual resources for learning about fluid dynamics (Hammer 1996; Young and Meredith, 2017). The domain of physics is by its nature more ‘intuitive’ and therefore it might be expected that learners have developed prior understanding and hold pre-conceptions of physical phenomena through their day-to-day experiences which they can activate to assist with problem solving of physical principles. Chemistry offers quite a different context to apply such a theoretical framework, as much student thinking and problem solving is abstract, concerned with the submicroscopic world of atoms, sub-atomic particles, and molecules. Chemistry learners’ conceptual understanding is less likely to be supported by their own direct experiences of observable phenomena, but this does not mean that they will not similarly activate prior conceptual learning to reason when learning new concepts. Given chemistry students requirement to visualise and manipulate mental models, as Taber suggests, the domain of chemistry can offer usefully different contexts to further explore the resources framework (Taber, 2008), and particularly useful to apply to students’ reasoning when learning visuospatial concepts (Fig. 1).
Imagistic thinking – imagining the invisible
At the core of learners’ mental modelling is imagistic reasoning. This refers specifically to the process of spatial visualisation which involves generating and manipulating perceived analog image-like mental representations and perspective taking for spatial thinking; its role is considered intrinsic to STEM problem solving (Hegarty, 2004; Stieff, 2011).Imagistic reasoning strategies have been reported to be intrinsic to solving visuospatial problems in chemistry (Stieff, 2007; Cooper et al., 2017). Notably, novice chemistry students may rely upon imagistic reasoning to help mentally visualise chemical processes having not yet mastered established analytical techniques to support their understanding. Previous studies in the field of chemistry have reported that novice students often access imagistic strategies to help visualize molecular structures when translating between two dimensional and three-dimensional representations and that such translation tasks prove exceptionally troublesome for beginning students (Stieff and Raje, 2010; Stieff et al., 2014; Kiernan et al., 2021).
Despite this, few studies have considered the finer-grained complexity of the multi-modal ways in which high school chemistry students may employ and activate conceptual resources to support their imagistic reasoning when solving visuospatial problems and how attending to this may assist instructors in formatively assessing and supporting student understanding.
Analytical thinking – lessening the load
There are various ways reported to support and even circumvent imagistic reasoning; whereby students (and experts) may use specific ‘‘rules’’ for predicting, manipulating, and transforming the spatial relationships without necessarily employing imagistic strategies (Stieff, 2007). This analytical approach to reasoning about complex visuospatial transformations contrasts to that inherent of imagistic reasoning, thus provides an alternative strategy (Schwartz and Black, 1996).A typical analytical reasoning strategy introduced to chemistry students when learning about molecular geometry is valence shell electron pair repulsion theory. In VSEPR theory, pairs of negatively charged electrons that surround the central atom of a molecule are identified and arranged as far apart as possible to minimise electron–electron repulsion and thus yield the resulting molecular shape.
VSEPR theory essentially provides an algorithmic method that can predict the 3D shape of many chemical compounds using the following relationship:
More recently, analytic reasoning strategies have been considered as helping to lessen the cognitive load of visuospatial thinking through the application of rules and heuristics to spatial tasks (Chandler and Sweller, 1991; Hegarty et al., 2013; Nyachwaya and Gillespie, 2016).
Multimodal studies have revealed that the reasoning strategy adopted can be influenced by the mode of learning employed. Diagrammatic reasoning through student sketching has been considered to alleviate the potential cognitive load imposed by imagistic reasoning; therefore, students’ strategy choice and modality employed when solving spatial problems may reveal the way they are thinking, what they have learned previously, in addition to possible cognitive load they are experiencing (Kiernan et al., 2021). Goldin-Meadow et al. (2001), found that hand gesturing lightened cognitive load for adults and children solving mathematical problems.
Gestures in chemistry learning
For decades, researchers have been challenged by the complex relationships between an individual's use of gesture, verbal language used, imagery evoked and thought processed.A seminal contribution by McNeill defined four categories (or dimensions) of gesture: iconic, metaphoric, deictic, and beat (McNeill, 2005). These gesture dimensions were further defined as non-imagistic; including deictic and beat gestures (pointing movements with a finger or hand), or imagistic. Imagistic gestures are considered mostly representational gestures (Abner et al., 2015) and are used to help communicate concrete objects/actions and sometimes abstract concepts; these can include iconic or metaphoric gestures.
In recent decades, studies have attempted to understand the underlying mechanisms of iconic gestures and their integration into the accompanying verbal utterance. These gestures have been shown to convey meaning semantically related to the content of the accompanying speech and as such “gesture-speech integration” has more recently emerged as a central concept in this field (McNeill, 1992; Green et al., 2009; Kandana Arachchige et al., 2021).
The use of gestures in science education is an emerging area of research and as such there are increasingly compelling theoretical arguments for the usefulness of gestures in STEM learning. Iconic gestures have been reported as being used by STEM learners to represent spatially complex phenomena and therefore may hold significant potential for supporting spatial thinking (Morsella and Krauss, 2004; Stieff et al., 2016).
Prior research examining the functions of gestures in chemistry learning and teaching have assumed an embodied cognition perspective to consider how bodily experiences can influence cognition (Amaya et al., 2005). A formative study conducted by Flood et al. (2014) considered the role that gesture can play as an interactional resource for meaning-making, concluding that generating such opportunities for learning offered a promising avenue for pedagogical innovation and research (Flood et al., 2014). Limitations were noted in the authors’ inability to draw immediate conclusions about the generalisability of findings, and it was suggested that future studies might hope to contribute to the emergence of concrete universals, through comparisons across different cases of multimodal interaction, leading to a fuller understanding of multimodal meaning-making in chemistry teaching and learning (Flood et al., 2014).
Stieff et al. (2016) explored the role of gesturing by comparing the effectiveness of undergraduate chemistry instruction which involved watching gesture, reproducing gesture, or reading text. Results from this study indicated that students in the reproducing gesture condition produced significantly more gestures when independently problem solving than students in the other two groups and significantly outperformed these groups on study attainment measures. The authors noted that the limitations of the study's gesture analysis related to quantifying discrete instances of students’ gesture production and the analysis did not adequately measure students’ tendency to gesture, why students gestured at particular moments or precisely how students used a gesture in relation to the diagrams on the research assessment task.
Research rationale
To address some of the cited limitations and build upon previous work, this naturalistic study further explores through a resources framework lens when, how and why particular gestures may be used by students verbalising spatial problem solving and how students’ use of gestures and language can reveal activation of key conceptual resources and underlying reasoning strategies used for problem solving.Previous research has described the application of the resources framework to physics curriculum design (Wittmann et al., 2003; Redish and Hammer, 2009; Redish and Kuo, 2015; Young and Meredith, 2017). Hammer urges us to listen to student talk in more ways than just evaluating for textbook correctness, looking instead for progress and resources in their many guises. Researchers have access to such activated resources through students’ use of language or gestures and a few previous studies have attempted to identify the conceptual resources activated by undergraduate physics students as they solved problems following instruction of key physics concepts (DiSessa, 1993; Scherr, 2008; Young and Meredith, 2017).
By identifying key conceptual resources revealed through students’ use of gestures and speech while solving visuospatial molecular geometry problems in their classroom, this study hopes to contribute an exemplar naturalistic empirical exploration within the domain of chemistry education and consider how observation of such resources can fulfil and inform formative assessment criteria for attentive chemistry and STEM instructors.
Methods
Study design and context
This study was viewed through a phenomenographic lens, guided by a resources framework approach with both an exploratory and explanatory mixed-method design to yield quantitative and qualitative data. The intent of this study is not to report on attainment gains resulting from classroom interventions, but rather to provide specific examples that can enhance instructors’ awareness of students’ conceptual resources to support them in making research-informed instructional decisions.To gain insight into student thinking, an open-ended written activity designed to capture individual aspects of student reasoning in their natural classroom environment was devised; tasking participants with describing their understanding of the three-dimensional shape of molecules. This data collection tool was deemed an ecologically valid way of investigating and was intended to allow students to express their understanding using the typical and naturalistic modes employed to teach them. This paper will seek to identify and describe how, when and why students use imagistic or analytical reasoning and some of the finer-grained conceptual resources activated when describing their predictions about molecular geometry. Moreover, it will examine how specific reasoning strategies evidenced through multimodal means relate to the accuracy of these predictions and communicates their understanding to instructors.
Molecular geometry was considered a suitable chemistry context to investigate spatial reasoning due to it being a core skill that all chemistry students (at senior school and undergraduate levels) must acquire. Understanding this concept is key to comprehending a wide range of scientific topics spanning across several STEM disciplines, such as biomolecular structure, industrial catalysis, soft condensed matter engineering and quantum mechanics, (Nicoll, 2001; Erlina et al., 2018; Kiernan et al., 2021). To relate, represent and predict molecules’ submicroscopic form is difficult for learners; it requires imagining how the molecules will appear from different perspectives and as such is certainly aided by the ability to visualise and mentally manipulate (Vlacholia et al., 2017). Students have likewise been shown to have difficulty with determination of molecular geometries using the established analytical method of molecular shape determination, Valence Shell Electron Pair Repulsion Theory (VSEPR theory), commonly taught in schools and universities (Furio and Calatayud, 1996; Gillespie, 1997; Nicoll, 2001; Erlina et al., 2018). Therefore, the concept of molecular geometry offered an appropriate chemistry context to identify and explore preferred reasoning strategies shown by students as they attempted to describe each problem and identify possible conceptual resources they activated.
Participants
This research study was introduced as part of the normal working mode of senior students (aged 16–18 years) in the chemistry classroom at an independent school in Scotland. In the Scottish context, participating students were completing the final (6th) year of secondary schooling, studying towards the final year qualification assessed by the Scottish Qualifications Authority, (SQA). This (Advanced Higher) course is typically chosen by those planning to enter related undergraduate studies at university the following academic session. (SQA Advanced Higher, 2019).The study was designed in accordance with BERA (British Educational Research Association) ethical guidelines and ethical approval from the Research and Knowledge Exchange Ethics Committee at Moray House School of Education, The University of Edinburgh was granted. The primary researcher was also a teacher at the school, which helped provide an ecologically valid setting and analytical approach consistent with typical student assessment at this stage.
The molecular geometry topic is typically taught to students over a one week period of seven, 40-minute lessons involving lectures and tutorial working activities (3 double periods and 1 single). The teaching was delivered by a colleague of the primary researcher.
The opportunistic sample of participating students (N = 16) were fully aware of the research activities, having given their written consent. The study centred around a single topic and core skill typically introduced during the final year school chemistry course or first year of an undergraduate chemistry degree – Valence Shell Electron Pair Repulsion (VSEPR) Theory. The students had previously received instruction on related concepts in prior courses which included theory on the molecular shape of simple molecules and covalent molecular bonding.
Procedure
For this study, a double period (1 hour and 20 minutes) was used to carry out the data collection activities and served as an introduction to the topic for participants. Initial instruction of the analytical VSEPR method consisted of a 20 minute lecture by a chemistry teacher, delivered using a slide presentation to introduce VSEPR theory in a format consistent with typical textbook learning, including text and 2D representations. An eight question written exercise was then issued to allow for exploration of students’ visuospatial thinking as assessed by their descriptive responses and molecular geometry predictions.Video-recorded data was collected from students tasked with predicting the molecular geometries of selected compounds. Only the symbolic chemical formula of eight compounds was given, with the VSEPR Theory rules appended for reference. Students were asked to ‘describe’ their understanding of the molecular shape for each compound following VSEPR theory instruction. To ensure the data collected was ecologically valid and could yield reliable and comparable data, the complexity of the exercise questions were selected by the primary researcher and verified by two chemistry teachers to be consistent with typical SQA past examination paper questions. The term ‘describe’ rather than ‘predict’ was chosen as the question stem to allow students to show their understanding in a more open-ended way. For each SQA chemistry examination paper there are typically two open-ended questions for which there is no absolute right answer; this allows students to gain credit for more varied answers with varying degrees of understanding. The eight formulae provided were H2O, NH3, NH4, SO2, SiCl4, TeCl4, IF5 and SF6.
Participating students worked in dyads to film one another, using their tablet computers, and were given 30 minutes to complete the VSEPR task. This filming activity was a procedure students were familiar with and had used regularly in the classroom to record work audio-visually throughout their prior schooling. Students took turns to answer the molecular geometry questions in the task.
Physical models (and other visualisations) were removed, and this activity was not supported by the tangible molecular models sometimes available in the classroom when learning new concepts. This condition was imposed to be consistent with typical examination conditions and to circumvent possible imprinted dependence on concrete models as described by Stieff et al. who suggested that instruction with models appeared to benefit only those students who had concrete models available while problem solving (Stieff et al., 2016). However, paper was provided to allow students to use for rough working if required, and to note their molecular geometry predictions down for each question, similar to both exam conditions and those provided by Ping et al. (2021) in their recent study.
Data analysis
The data analysis was guided by a constant-comparative methodological approach to repeatedly analyse data and establish reliability and validity across researchers and contexts through open-coding (Corbin and Strauss, 2008; Young and Meredith, 2017).Students’ verbal and written responses to the molecular geometry exercise were analysed and coded after testing. The verbal descriptions and gestures evidenced within students’ recorded responses were transcribed into written form and open coded by the primary researcher after close, repeated observation and analysis of video data using ELAN 1.3 linguistic annotator software. Due to the small sample, the resulting coding was reviewed and revised by the chemistry teacher, two colleague teachers and two expert researchers to provide reliable agreement and reproducibility of the coding scheme rubric. This was checked by triangulating the findings from the different types of data and the different perspectives from all staff involved. Approximately 25% of the data was compared for interrater reliability.
In the process of open coding for this study, the data was further analysed and coded through a resources framework lens. The primary researcher used a similar approach to Young and Meredith (2017) to code, categorise and identify emergent student resources and underlying reasoning strategies based upon the varied symbolic, algorithmic and concept “bytes” students demonstrated through the multiple modes of expression used to think about molecular geometry.
The coding of resource bytes identified as being activated during problem solving was cross verified for validity and reliability by two independent chemistry teachers to reach agreement across coding categories. The Cohen's Kappa (k) score was found to be 0.71 (95% CI) between both chemists, after initial pilot coding of a smaller sample from two students’ responses which was 0.54. This indicated the final inter-rater reliability agreement as being substantial (>0.6) (Cohen, 1960; Landis and Koch, 1977).
The verbal and written responses were marked for accuracy in accordance with typical SQA examination guidance where the use of the established VSEPR theory and the correct molecular geometry terminology would be required to gain credit. This marking was moderated by two resident chemistry teachers as per SQA guidance to agree consensus of final scoring.
To investigate the first research question exploring the accuracy of different types of student responses, initial quantitative statistical analyses were performed. The accuracy of both written and verbal responses was compared to whether students evidenced use of VSEPR theory to solve the molecular geometry problems. Accuracy data was tested initially to verify normality and homogeneity before further parametric analysis. A Shapiro–Wilk test yielded p values of 0.183 and 0.131 for verbal and written responses respectively, thus satisfied the non-significance required to assume a normal distribution (p < 0.05). Levene's test indicated that the variance across groups was homogeneous. For all quantitative analyses, due to the opportunistic nature of sampling, a priori power analysis was conducted at the point of statistical analyses and was not <80%.
Gestures
Video data was analysed to identify instances of gesture production. A gesture was defined as the time from which the hands or body first engaged in gesturing to the time they came to rest (Stieff et al., 2016). Student gestures produced outwith the research task descriptions were not included or accounted for, including, personal gestures such as hand clasping or face touching or deictic gestures that were directed at a sketch or written working.Gesture frequency was measured as the number of gestures produced while problem solving. Gestures were identified and coded as being ‘beat’, ‘deictic’, ‘iconic’, ‘deictic-iconic’ and ‘deictic-beat’ (hybridised forms of deictic/iconic and deictic/beat gestures). Gesture types were further categorised in terms of their increasing ability to convey imagistic reasoning based upon previously reported associations that iconic gestures do so (Abner et al., 2015; Tversky, 2017). See Table 1 for examples of gesture and underlying reasoning type coding.
Verbal descriptions
Students’ video recorded responses were examined initially to identify key verbal features and speech content which could reveal aspects of the reasoning strategies employed to predict the molecular geometries. Students’ responses were coded using a similar method to Stieff and Raje (2010), where problem-specific utterances were categorised as being either analytical or imagistic.Student descriptions indicative of imagistic reasoning strategies were frequently accompanied by iconic, deictic-iconic and deictic gestures. For example, participants made such comments as, “Imagine that the central atom is here (clenched left fist to represent the central atom…), then the hydrogens go here (points with right hand to space around first), here (points to different space around fist) and here (points to 3rd location around first).” All utterances that referenced inspecting an internal image or visualisation, imagining molecular shapes, or that gave description of dynamic spatial activity were considered indicative of imagistic reasoning and therefore coded as such. Conversely, utterances coded as revealing analytical strategies made specific reference to VSEPR theory or detail provided in the teacher introduction to the molecular geometry topic. For example, some participants described the taught heuristics with utterances such as, “7 outer electrons for iodine and then 5 fluorines, is 12 and then divided by 2 is 6, so it's octahedral…”. All utterances that referenced a specific rule for predicting molecular shape were coded as analytical. Table 2 shows representative examples of each verbal response code.
| Reasoning strategies | Verbal response features | Examples |
|---|---|---|
| Analytical | Use of VSEP count | “Sulfur's got six outer electrons and two oxygens, so that's 8, divided by 2 is 4, so it's tetrahedral?” |
| Reference to diagrams or examples given in teacher introduction | “But didn’t she show an example with double bonds that wasn’t linear?” | |
| Reference to similarity of a previous question | “This is just like the first one, so its shape is angular. | |
| Imagistic | Response transcript indecipherable without accompanying gestures | “So, NH 3 , also known ammonium… is shaped…. so the N is the top here… 3… you've got H, H, H.” |
| Use of key terms or phrases which evidence visualisation | “this atom is above…” | |
| “the Cls go round and round…” | ||
| “because they need more space here…” | ||
| “Coming directly above the nitrogen…” | ||
| Use of phrases which refer to gestures | “It would look like this…” | |
| “Can I draw this in air…?” | ||
| “Hands might do better at showing this…” |
Multimodal student resources
It was assumed that if a student resource is activated it can be manifested through some multimodal means and thereby become accessible for study through the transcribed video data and written working. However, we must acknowledge that while some of the cognitive constructs students access will be explicit and, therefore, available to conscious thought, others are implicit and may influence reasoning without an individual's awareness (Taber, 2014). Likewise, it is possible that students may activate resources and quickly dismiss, therefore evade detection. The key conceptual resources that were captured were identified and coded in a similar manner to that detailed in Young and Meredith's study which used the resources framework to devise interventions for physics students learning about pressure in fluids (2017). These resources were categorised accounting for the overarching and dominant analytical and imagistic reasoning strategies which underlies their use and the modalities used to express.The nature of resources identified as being key within students’ responses varied and was agreed to ensure domain-specific interrater reliability, whilst noting that resources did not necessarily need to lead students to correct final solutions or indeed directly relate to the context of the molecular geometry problem, but rather held the potential to improve understanding in future (Hammer, 1996).
The emergent student resources identified from students’ responses are shown in Table 3. Evidence of using the “VSEPR equation” as taught through written working or verbal responses which described the mathematical method was considered a key analytical resource to determining the correct molecular geometry. Similarly, accessing conceptual resources that might offer a more primitive route to solving the VSEPR equation, for example working out to the number of outer electrons and identification of lone electron pairs that atoms in the molecules possess from an atom's “Valency” and a molecule's likelihood of achieving a stable “Octet Electron Arrangement”, emerged as key conceptual resources. These conceptual resources were of note as they were first developed during students prior learning in previous school courses and so within the context of solving molecular geometry problems, these more simplistic concepts don’t offer an obvious advantage to solving the problem. Likewise, chemists would consider the “octet rule” as not valid for all chemical systems and therefore should be used with caution. However it is well-established that typical classroom teaching of chemical bonding will give an impression that ‘‘the octet rule’’ is an exact, determining rule rather than a heuristic that is valid for limited chemical systems; this can lead to misconceptions later (Taber, 2001, 2009; Taber and Coll, 2002). These emergent conceptual resources were considered analytical in nature, as they did not directly relate or require understanding of the three-dimensional shape to access, however taking a resources perspective would suggest that these resources may help students to construct their “knowledge in pieces” and therefore could ultimately help lead to building a picture of the 3D molecular shape (DiSessa, 1993). The remaining student resources that emerged from the multimodal data were imagistic in nature and would have required students to visualise the molecule in question as they were activated.
| Reasoning strategy | Resources for molecular geometry | Multimodal coded evidence example | |
|---|---|---|---|
| Speech | Gesture | ||
| Analytical | Octet electron arrangement | “Oxygen has 6 outer electrons and hydrogen has 1 each, so it's got 8… but 2 lone pairs” | Deictic-beat |
| VSEPR equation | “Silicon has 4 outer electrons, and there's 4 Chlorines, so 4 plus 4, divided by 2 is 4. It must be tetrahedral.” | None | |
| Valency | “Nitrogen has a valency of 3, so the other 2 electrons must be a lone pair…” | None | |
| Imagistic | Electrostatic forces | “the lone pairs are trying to get as far away from each other as possible…” | Deictic-iconic |
| Spatial coordinates | “One Cl will be on the x-axis, one on the y and one on the x…like this…” | Deictic-iconic | |
| Geometric shape | “It's like two pyramids on top of each other, like that…. so, octahedral? | Iconic | |
Acknowledging the “Electrostatic Forces” of repulsion that electron lone pairs exert upon each other was identified as a prevalent imagistic resource, which although not a requirement to apply VSEPR theory could assist students with their mental models when deducing the final molecular shape. Student resources which evidenced understanding of applying a “Spatial Coordinates” system along x, y and z axes in space or the ability to determine and convey where the substituent atoms sit relative to the central atom were also coded as a key imagistic resource. This particular imagistic resource is not taught within the chemistry curriculum and so within the context of solving molecular geometry problems, this resource was used out of context and has possibly been previously employed in students’ mathematics or physics learning. Finally, activation of resources which enabled students to clearly demonstrate the visualized three-dimensional, solid geometric molecular shape was likewise coded as being imagistic. The concept of “Geometric Shape” is not taught explicitly in chemistry curricula but introduced in mathematics or possibly art learning at earlier stage’ of students' education. Familiarity with geometric shapes is not a requirement to accurately apply the VSEPR equation to solve the molecular geometry problems and does not necessarily assist students’ with solving these problems, however accessing this particular resource might be expected to support students’ visualisations of the molecular shape if relying on imagistic resources to reason. To understand when and how students used resources and varied reasoning strategies, the multimodal data was further analysed to consider how students approached specific aspects of each molecular geometry problem; to gauge an understanding of the factors that affected the nature of reasoning adopted.
Results
Both quantitative and qualitative data was collected and analysed. The quantitative data presents the nature, frequency, and absolute accuracy of students’ multimodal responses to the molecular geometry problems including written and verbal responses. The qualitative data presents individual cases to illustrate how students’ multimodal responses can reveal some of the finer-grained detail to their reasoning through the conceptual resources activated when students employ their preferred reasoning strategies and any conceptual miscomprehension evidenced through multimodal mismatches.Gestures
N = 16 students, working in 8 dyad pairings produced a total of 440 distinct gestures while describing their molecular geometry predictions. The frequency of different gesture types made by student dyads was examined. See Fig. 2.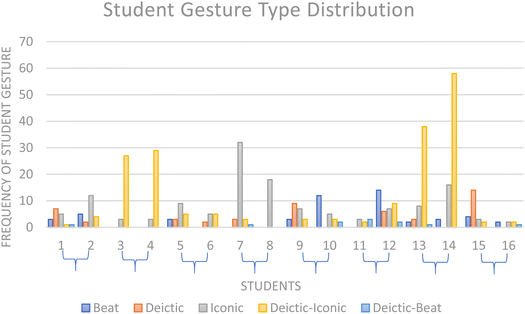 | ||
Fig. 2 Frequency of gesture codes for each student response. Where curly brackets, 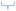 , represent student dyad pairings. , represent student dyad pairings. | ||
Students evidenced greater use of imagistic gestures than non-imagistic, with iconic and deictic-iconic gesturing featuring more across all student responses than other gestures [Iconic = 138 gestures, Deictic-Iconic = 198 gestures, Deictic = 49 gestures, Deictic-Beat = 11 gestures, Beat = 51 gestures].
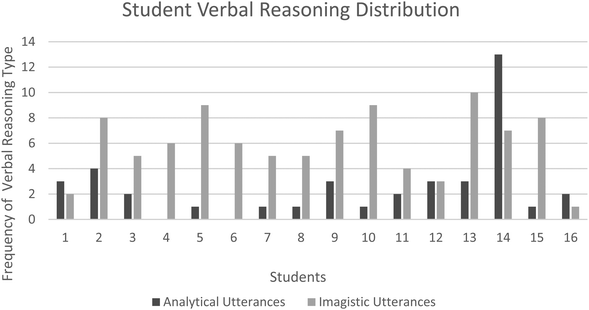
Verbal reasoning
Fig. 3 illustrates the variation in verbal reasoning strategy codes for each student response. Many students (N = 12) exhibited preference for imagistic reasoning strategies as conveyed through their coded verbal responses and accompanying gestures.Student response accuracy
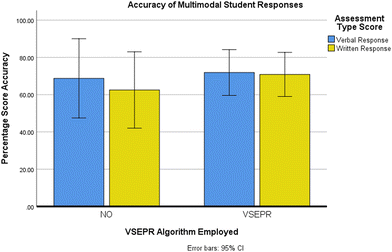
Both written and transcribed verbal responses were marked to yield percentage accuracy scores and note evidence of VSEPR Theory use, for example, evidence of employing the mathematical relationship to calculate the correct VSEP number and hence, the molecular geometry. Written tasks were marked as per SQA exam marking instructions whereby the correct terminology was required to gain credit for each written response. Verbal responses were ‘marked’ similarly as correct if the correct molecular geometry was predicted and named through use of speech, where the student verbalised the correct terminology. The results from a mixed repeat-measures Analysis of Variance (ANOVA) (see Fig. 4) revealed a significant main effect of assessment type on assessment scores (F(1,14) = 7.46, MSE = 10.696, p = 0.016, η2 = 0.348).Pairwise comparisons (with Bonferroni corrections applied) indicated that students' percentage scores were significantly higher for (verbal responses) (M = 71.09, SD = 9.21) than (written responses) (M = 68.75, SD = 8.82). These results suggest that students provided more accurate answers through their verbal and gestured responses than typical pen-on-paper written assessments.
There was also no significant main effect for VSEPR Theory use on assessment scores F(1,14) = 0.264, MSE = 747.303, p = 0.616, η2 = 0.018. The mean values of both verbal and written assessment scores for students who did not evidence use of the taught VSEPR theory was lower than those students who did evidence use of VSEPR theory (M = 68.75, SD = 29.76) and (M = 62.50, SD = 30.62) vs. (M = 71.88, SD = 16.10) and (M = 70.83, SD = 14.43) respectively. However, there was no significant interaction between VSEPR Theory use and assessment scores by type, F(1,14) = 3.80, MSE = 10.696, p = 0.71, η2 = 0.214. Therefore, despite the VSEPR algorithm being the recognised analytical method taught to students solving molecular geometry problems, evidence of its use did not assure higher attainment for either assessment type significantly.
Multimodal student resources
Fig. 5 shows the prevalence of each identified conceptual resource used by students across their multimodal responses. Students clearly evidenced imagistic resources more frequently in their responses than the analytical resources that are promoted when introducing the molecular geometry topic during instruction; with only 57 instances of “VSEPR Theory” being employed as a resource across all student responses (10%). Of note, were the most frequently used imagistic resources which evidence reasoning around the “Spatial Coordinates” of the molecule, through reference to the 3-dimensional axes x, y, z that atoms would lie in relation to the central atom and how they dictate the molecule's axes of symmetry. Likewise, imagistic conceptual resources revealing students’ visualizations of the three-dimensional, solid “Geometric Shape” that the molecule would appear to form, was evident in almost a quarter of all those recorded. | ||
| Fig. 5 Conceptual resources for reasoning about molecular geometry, where analytical resources are shown as outlined bars and imagistic resources as shaded bars. | ||
The analytical resources that tapped into thinking around the central atom's “Octet Electron Configuration” were recorded in ∼7% of the total, with this resource offering a direct route to correctly calculating the molecular geometry. The “Valency” analytical resource was evidenced in 17% of multimodal responses; a concept relating to the number of chemical bonds the central atom can make and generally introduced to students in a prior school year. The less frequently observed imagistic resource relating to “Electrostatic Forces” was observed in 9% of responses, this concept relates to where lone and bonding pairs of electrons might reside and the repulsion effects they exert upon each other which helps to dictate the molecule's shape.
Formative assessment
To illustrate how applying the resources framework to students’ multimodal reasoning can inform formative assessment of students’ problem-solving explanations, two cases are described (see Tables S4 and S5 of ESI†).Of note, was the fact that although the ammonia molecule was incorrectly named as ammonium initially (which has a tetrahedral molecular shape), the student accurately described the molecular shape of the ammonia molecule as being trigonal pyramidal. Although this mismatch could be potentially costly in a written examination, where the student incorrectly named the molecule as “ammonium” from the chemical formula, NH3; by elaborating through verbal and gestured responses, the molecular shape of the ‘ammonia’ molecule was correctly predicted, stated, and shown by the student.
Of further note, was student 15's use of deictic gestures, which although not previously acknowledged as being imagistic in the way that representational iconic gestures have been suggested (Abner et al., 2015), using deictic gestures to point to specific areas in three-dimensional space illustrated that the student was accessing “spatial coordinates” resources to help mentally visualise the molecular geometry; essentially rendering these deictic gestures as imagistic to the observer. To an observant teacher formatively assessing the student's response, the preference for accessing these imagistic conceptual resources, reliance on gesture to convey their solution and uncertainty of spoken terminology allows for rich insights into student reasoning and true accuracy of understanding which the accompanying written responses did not reveal.
Conditions for student reasoning
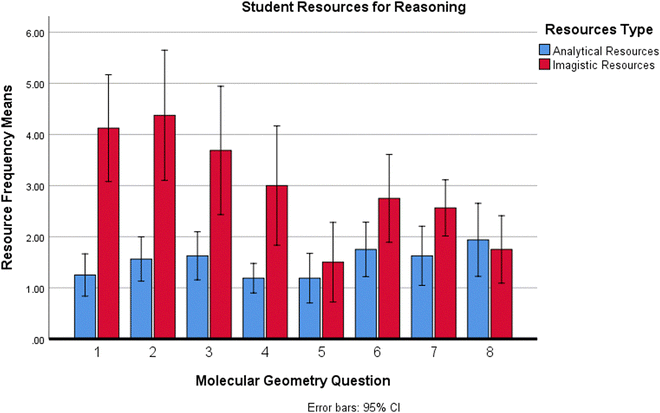
Having identified the nature of conceptual resources and underlying reasoning strategies that constructed students’ contextualised thinking, to understand when and how students used and applied specific reasoning strategies, further analysis of the conceptual resources evidenced when attempting each molecular geometry problem was conducted.Fig. 6 shows the frequency and type of reasoning employed by students evidenced through their multimodal responses for each individual question in the molecular geometry exercise. Differences in resources reasoning evidenced across the molecular geometry questions attempted were compared. A one-way repeated-measures ANOVA was performed to reveal a multivariate significant main effect for resources reasoning across questions, there was a statistically significant difference between the reasoning type of resources evidenced by students across the different molecular geometry questions attempted (F(14,208) = 5.54, p = 0.000, partial η2 = 0.27). Mauchly's test showed p values >0.05, therefore did not indicate any violation of sphericity for the main effects of resource reasoning type on molecular geometry questions attempted.
Consequently, univariate ANOVAs were conducted to reveal a significant result for imagistic reasoning evidenced F(7,105) = 9.880, MSE = 1.779, p = 0.000, partial η2 = 0.397, but no significance was found for analytical reasoning F(7,105) = 1.665, MSE = 0.748, p = 0.126, partial η2 = 0.100.
A post hoc pairwise comparison using the Bonferroni correction showed greater mean scores for resources coded as imagistic vs. means for analytical reasoning resources evidenced across molecular geometry questions 1 to 7. However, these increased mean values for imagistic reasoning resources were found to be significant only for questions 1, 2 and 3 (M = 4.13, SD = 1.96, p = 0.036), (M = 4.38, SD = 2.39, p = 0.005) and (M = 3.69, SD = 2.36, p = 0.015) respectively.
The molecules which appeared to require significantly more imagistic reasoning to predict their molecular geometry were dihydrogen oxide (water) H2O, ammonia NH3 and ammonium NH4+ respectively. Of note is the fact that the molecular geometry problems evidencing more imagistic reasoning input have fewer atoms in the molecules (≤5) and the central atoms have lone pairs of electrons. Although not found to be significant, those questions answered evidencing the greatest means for analytical thinking had a greater number of atoms in the molecular formulae (≥5).
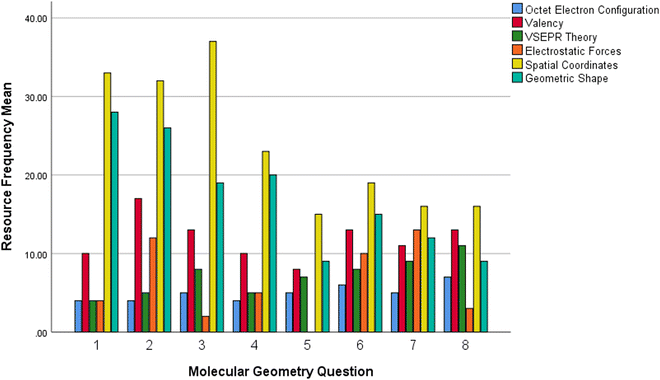
The decline in imagistic reasoning seen as students progressed through the entirety of the exercise involving increasingly larger molecules was further analysed as presented in Fig. 7, which shows how each coded conceptual resource was utilised across the different molecular geometry problems. A repeated-measures MANOVA was conducted to determine whether there was a difference between conceptual resources evidenced across the different molecular geometry questions answered by students. There was a significant main effect difference between the means of conceptual resources evidenced in the molecular geometry questions attempted by students, F(42,472) = 3.185, p = 0.000, Wilk's Lambda = 0.311, partial η2 = 0.177.
Mauchly's test showed p values >0.05, therefore did not indicate any violation of sphericity for the main effects of resources type on molecular geometry questions attempted.
Results showed significant differences for the means of imagistic reasoning resources evidenced across all questions attempted; electrostatic forces (F(7,105) = 6.728, MSE = 0.217, p = 0.000, partial η2 = 0.310), spatial coordinates (F(7,105) = 6.720, MSE = 0.729, p = 0.000, partial η2 = 0.309) and geometric shape (F(7,105) = 5.356, MSE = 0.619, p = 0.000, partial η2 = 0.263) resources.
A post hoc pairwise comparison using the Bonferroni correction showed that the electrostatic forces conceptual resource means were significantly different when comparing students answers to question 2 (M = 0.75, SD = 0.77) with those of question 5 (M = 0.000, SD = 0.00, p = 0.042); question 3 (M = 0.13, SD = 0.34) with question 7 (M = 0.81, SD = 0.40, p = 0.001); question 5 with question 6 (M = 0.62, SD = 0.62, p = 0.030) and question 7 (M = 0.81, SD = 0.40, p = 0.000); and question 7 with question 8 (M = 0.1875, SD = 0.54, p = 0.030). These significant comparisons are notable in that all but one, involve comparisons between smaller molecules with bigger molecules and molecules containing lone pairs of electrons with molecules without. Where questions involving molecules without lone pairs scored lower electrostatic forces means than questions for molecules with lone pairs; and larger molecules with lone pairs scoring higher electrostatic forces means than smaller molecules with lone pairs. Of note was the significant difference in means found between question 5 (M = 0.00, SD = 0.00) and question 6 (M = 0.63, SD = 0.62, p = 0.030), where both molecules contain the same number of atoms (5), however question 5's SiCl4 contains no lone pairs whilst question 6's TeCl4 does. The means for the spatial coordinates conceptual resource use decreased from question 1 to question 5, with the decreasing means found to be significantly different when comparing students answers to questions 3 (M = 2.31, SD = 1.99) with those of question 5 (M = 0.9375, SD = 1.48, p = 0.000). Similarly, questions 3 and 5 both contain the same number of atoms in the chemical formulae (5), however the central atom in Q5 does not have any lone pairs to consider. This trend mirrors the significant difference in the electrostatic forces resource means between question 5 and 6.
The geometric shape conceptual resource means were found to be significantly different when comparing students answers to questions 2 (M = 1.63, SD = 0.62) with those of question 5 (M = 0.56, SD = 0.96, p = 0.032), question 7 (M = 0.75, SD = 0.45, p = 0.036) and question 8 (M = 0.56, SD = 0.51, p = 0.005). In this case, the smaller molecule in question 2 (NH3), containing a lone pair of electrons, evidenced a greater mean value for this imagistic resource than the larger molecules in questions 5 (SiCl4), 7 (IF5) and 8 (SF6).
Results showed no statistically significant differences for the means of analytical reasoning resources; octet electron arrangement (F(7,105) = 0.361, MSE = 0.198, p = 0.198, partial η2 = 0.24), valency (F(7,105) = 1.890, MSE = 0.302, p = 0.78, partial η2 = 0.112) and VSEPR theory (F(7,105) = 1.789, MSE = 0.187, p = 0.97, partial η2 = 0.107) resources.
Discussion
Research question 1
Both qualitative and quantitative data analysis was conducted to address this study's core research questions relating to students’ acquisition and assimilation of the visuospatial concept, molecular geometry. To answer the first research question, “How do secondary school chemistry students’ preferred reasoning strategies and modalities relate to the assessed accuracy of their responses to molecular geometry problems?”, Table 1 outlined qualitatively how secondary school students’ gestures were coded and categorised into gesture types and associated underlying reasoning. This coding and categorisation were based upon both established and unique features evidenced within this study and prior studies’ (McNeill, 2005).These coded gestures were further contrasted and compared to previously reported associations on the use of gesture type and related reasoning employed to reveal that it was possible to deduce students’ distinct reasoning strategies from their responses (Abner et al., 2015; Tversky, 2017). The quantitative data displayed in Fig. 2, showed that students evidenced greater use of imagistic gestures than non-imagistic, with iconic and deictic-iconic gestures featuring significantly more in student responses than other gestures. Furthermore, the secondary students’ verbal responses were coded and categorised as indicated by the representative examples shown in Table 2 to reveal the frequency of verbal reasoning by type as shown in Fig. 3 to similarly favour imagistic reasoning. These findings are in some agreement with previous studies which found that novice learners tend to rely upon imagistic reasoning strategies when first introduced to a topic before discovering alternative analytical strategies, whereas ‘experts’ would presumably have mastered the analytical means to scaffold their problem solving (Schwartz and Black, 1996; Stieff, 2007). Of note from this study, however, was that the participating students were provided with this scaffolding from the outset and given reference to the algorithmic analytical method for solving the molecular geometry problems, yet still many appeared to persist with imagistic strategies as expressed and deduced through their coded multimodal responses.
This study's findings not only provided insight into students’ reasoning when solving visuospatial problems, but also the accuracy of their multimodal responses; through the mismatches evidenced through different modalities. As discussed in the example case studies (Tables S4 and S5, ESI†), it was clear that for some students, not only did they employ both imagistic and analytical reasoning in tandem as previously reported (Cooper, 1988; Schwartz and Black, 1996), but they did so through use of different modalities and of varying accuracy.
Case 1 described how Student 15 evidenced both imagistic and analytical reasoning through gesture and speech, both modalities were used cooperatively by the student to explain their molecular geometry prediction accurately. Student 3, in Case 2 however, evidenced a mismatch between the verbal and gestured response with the written response when answering question 8. Student 3 appeared to correctly use the analytical method, VSEPR theory, in their written working to calculate the molecular geometry as being octahedral. This was true for most students (N = 11) answering question 8, who used the analytical VSEPR theory when attempting to predict the shape of the largest molecule most consistently. However, on answering the question verbally, Student 3 evidently struggled to access the imagistic reasoning required to visualise the three-dimensional shape of the correctly predicted molecule in question; by incorrectly gesturing the position of substituent atoms in the molecule at 45 degrees (instead of 90 degrees) and verbalising their difficulty.
Overall, the contrary was observed when comparing formative assessment modalities (speech and gesture) with summative (written) assessments, the quantitative data analysis shown in Fig. 4 revealed there was a significant main effect of multimodal assessment type on assessment scores; with students shown to provide significantly more accurate answers through their verbal and gestured responses than typical pen-on-paper written assessments. For most, their attempted use of the VSEPR algorithm did not assure significantly higher attainment through either formative or written summative assessment types. This agrees with a previous study by Kiernan et al. (2021) which found that students use of spontaneous diagrammatic reasoning yielded greater accuracy of molecular geometry prediction than that of the typical pen-on-paper summative assessment.
Therefore, this research study was able to show that secondary school students’ multimodal responses can not only demonstrate the use of both analytical and imagistic reasoning through qualitative and quantitative analysis of speech and gesture, but that their preferred reasoning strategies and the modalities they might utilise to assist with problem solving when first introduced to the concept can more reliably assure accuracy of concept than application of the established VSEPR Theory widely promoted by chemistry instructors and curricula. Moreover, this preference exhibited by some students for imagistic strategies over the analytical strategies provided to mitigate three-dimensional visualisation, indicates that instructors might consider how to focus and tailor teaching to tap into this preference when supporting students learning of visuospatial problems. Adopting teaching approaches to support students’ imagistic reasoning in chemistry classrooms is not routine practice and existing related research in this area has yet to feedforward into mainstream practice.
Research question 2
To elucidate the conditions that influence students learning of molecular geometry concepts which could potentially inform formative assessment and tailored instructor interventions, the 2nd research question explored: “In what ways can students’ spontaneous multimodal responses reveal key conceptual resources for chemistry learning which can facilitate problem solving when making molecular geometry predictions?”. Table 3 outlined how students’ multimodal responses were coded to reveal the key conceptual resources activated and the underlying reasoning strategies employed when solving the molecular geometry problems. Fig. 5 shows the frequency with which these key conceptual resources were evidenced across all students’ responses.The 2nd case (Table S5 of ESI†) described qualitatively how student 3's verbal description in isolation was impoverished of any spatial cues, containing no information to the listener which would allow them to visualise the molecular shape from speech alone; however, such was the comprehensive use of imagistic gestures alongside speech that it was possible to capture the activation of “Spatial Coordinates” and “Geometric Shape” conceptual resources.
Of further note, was the fact that student 3 primarily used hybrid deictic-iconic pointing gestures, not just iconic gestures. So, although the accompanying verbal response contained no spatial inferences, student 3 was able to explicitly state and identify through accessing resources relating to “Spatial Coordinates”, which atoms were being referred to. The use of these hybridised deictic-iconic gestures was such that it not only allowed the observer to assess that they were incorrectly visualising the octahedral molecular shape but appeared to assist the student in realising this for themselves. This observation may be consistent with Wesp et al.'s study's findings (2001) that iconic gestures can help learners offload and transfer spatial information onto the hands to maintain spatial representations in working memory.
Following a finer grained analysis into when students might activate certain conceptual resources and related reasoning strategies, the question-by-question analysis (shown in Fig. 6) revealed that there was a significant difference between the reasoning type of resources students activated across the different questions attempted. Students more consistently used analytical resources in favour of imagistic resources when molecules were bigger (≥5 atoms) and were shown to significantly favour imagistic resources when answering questions 1–3 for smaller molecules (≤5 atoms) with lone pairs of electrons.
Possible ad hoc explanations might consider the possibility that smaller molecules may be easier for students to represent through imagistic hand gestures, than the geometrical shape of larger molecules, e.g., trigonal planar vs. octahedral.
Another possibility relates to whether the presence of lone pairs of electrons around the central atom of the molecule might exert additional cognitive loading to students’ visualisation of the overall molecular geometry. The preference for imagistic resources seen to solve problems involving smaller molecules with lone pairs of electrons was evident through students’ use of spontaneous gestures, which may have scaffolded this imagistic component of thought as well as offload spatial information onto the hands to free up cognitive resources (Goldin-Meadow et al., 2001). This could indicate that learners generally found it more helpful to attempt to visualise the three-dimensional layout of atoms within the molecule and lone pairs of electrons when determining the overall geometric shape where possible, without the requirement to employ the taught VSEPR theory or other analytical resources. With larger molecules however, fewer students have attempted to circumvent such analytical resources and have not evidenced the same degree of visualisation through imagistic resources while problem solving. This might suggest that for the larger molecules, internal visualisation was less accessible and thus activating analytical resources to help predict the molecular geometry may have been more useful.
Previous studies have investigated the ways in which chemistry representations might cause students difficulty when learning new concepts and induce cognitive load. Furio and Calatayud (1996), found that most student misconceptions observed in their study exploring students’ understanding of molecular polarity could be explained by considering the difficulties associated with three-dimensional visualization and by methodological obstacles such as the inability to identify the presence of lone pairs of electrons as a factor in determining the resultant polarity of a molecule. Similarly, Tiettmeyer et al. (2017), investigated the structural characteristics of students’ drawn Lewis structures to explore their potential to induce increased cognitive load. As with VSEPR theory sums, Lewis structure diagrams account for the bonding and lone pair electrons within a molecule and are useful diagrammatic representations for predicting reactivity, polarity and molecular geometry. The authors noted that the addition of nearly any representational or structurally complex feature to a molecule's Lewis structure, caused significant increases in students’ cognitive load.
Interestingly, in the case of questions 5 and 6, although the molecules both have 5 atoms and is therefore on the boundary condition for the favoured reasoning type employed, the fact that the SiCl4 molecule attempted in question 5 has no lone pairs and TeCl4 does, further reinforces the possibility that the additional cognitive load exerted by the internal visualisation required to determine the molecular geometry of molecules with lone pairs of electrons, requires relatively greater use of imagistic resources.
Likewise, we must consider that the decline of imagistic reasoning seen as students progressed through the entirety of the exercise may not necessarily have been specific to the problem being solved, but rather due to cognitive weariness from the task. Perhaps the load associated with imagistic reasoning had reached a critical point of cognitive exhaustion for some students. Moreover, it is also possible that some of the smaller molecules were already familiar to some students through prior knowledge and therefore there was less requirement to employ the taught algorithmic analytical method as the molecular shape had been encountered previously – certainly the shape of the introductory Q1 molecule (H2O) would likely have been encountered by students at this stage and so it is interesting that 4 students did use the analytical resource (VSEPR Theory) to verify the molecular shape of water.
This study's findings are therefore consistent with those in the cognitive sciences which have increasingly supported a resource-limited model of working memory, where working memory is not considered to be limited by an absolute number of items necessarily, but rather an absolute number of cognitive resources that are available for processing. Where problem solving may require a greater commitment of particular cognitive resources, this subsequently results in fewer, more cognitively complex problems being managed in the working memory (Tiettmeyer et al., 2017). Therefore, it was evident from this study, that students’ spontaneous multimodal responses can reveal key conceptual resources for chemistry learning which can facilitate problem solving and assist their thinking when making molecular geometry predictions as posed by research question 2.
Research question 3
To answer the 3rd research question by examining the extent to which secondary school chemistry students’ verbal responses to molecular geometry problems in a naturalistic environment can be used as a means of formative assessment; the 2 cases qualitatively described (Tables S4 and S5 of ESI†) and the quantitative findings presented in Fig. 6 and 7 shows how different modalities can reveal insight into how and when conceptual resources and reasoning strategies are applied as students attempt to problem solve. This study's findings demonstrated that not only can verbal responses reveal how students are constructing their understanding, but also capture any conflicting accuracy of students’ predictions through the different modes of expression. Case 1 showed an exemplar of how even if incorrect naming terminology has been used, the roles of speech and gesture conveyed meaning and revealed students’ visuospatial thinking and true accuracy of prediction. Each modality in isolation would not have sufficed, but the combined information across all modes employed allowed the assessor to capture a richer picture of student understanding. Conversely, case 2 demonstrated that although the student included an accurate verbal geometry prediction from the outset, they did not evidence understanding of the molecular shape through their verbal utterances. Gesture alone appeared to embody the problem-solving visualisations and allowed for formative assessment to reveal the imagistic inaccuracy of the student's visualisation, regardless of accurate verbal prediction made.Fig. 4 mapped a comparison of students’ written predictions vs. verbal predictions to reveal a significant difference between the accuracy of students’ responses depending on the assessment type. The evident mismatch between students’ understanding as conveyed in their written responses and that evidenced through speech and gesture in the discussion task (Fig. 2, 3 and 5) is not novel, but revealed a much richer picture of not just expected misconceptions around the molecular geometry topic, but offered the observer a more nuanced, multi-grained and context sensitive overview of the conceptual resources and underlying strategies employed by students.
Stieff and Raje (2010) suggested that instructors might employ a formative assessment rubric to attend to students’ utterances and gestures and guide them through ways to adopt an algorithmic strategy when imagistic reasoning is not effective. Fig. 5–7 show that by attending to the multimodality of student problem solving, it is possible to probe even finer-grained and richer detail as learners employ such reasoning strategies, to identify conceptual resources for a given threshold concept which may assist teachers in pin-pointing fundamental context dependent barriers to students’ progress. Such conceptual barriers and cognitive resources may traverse other disciplines, for example the imagistic conceptual resources identified as being preferentially activated for solving the smaller molecular geometry problems and molecules with lone pairs of electrons: electrostatic repulsive forces, 3D spatial coordinates awareness and the mathematical geometric form of molecules are conceptual resources that could similarly be met in other STEM learning contexts and therefore reinforced within other disciplines.
Likewise, the discipline-specific analytical resources evidenced more by students when predicting the geometry of larger molecules in this study; relating to atoms’ valency and octet electron arrangement could be revisited or introduced to students (in addition to VSEPR theory) ahead of new learning to make explicit their applicability and highlight their potential limitations out with the context of solving molecular geometry problems.
Conclusion
The present study's findings are pertinent not only to chemistry education, but across STEM disciplines. This naturalistic study revealed that it is possible to capture high school students’ reasoning strategies through analysis of both speech and gestures used in verbal responses to a chemistry task; with a significant proportion of the cohort preferring imagistic strategies as reported in some previous studies investigating the impact of spatial training on students’ exam attainment (Stieff et al., 2014; Castro-Alonso and Uttal, 2019).Few studies to date, have identified the conditions under which different reasoning strategies are employed, or how reasoning strategy preference might affect the accuracy of students’ responses through use of different modalities. Likewise, very few studies have adopted a multimodal approach to explore the conceptual resources that comprise such reasoning strategies. With an emphasis on difficulties and misconceptions, chemistry education research has somewhat overlooked the task of studying and describing the raw material constructed from students’ prior knowledge. This study offers a much-needed exemplar study for the application of the resources framework within a chemistry context which has scarcely been reported outside of the physics education community and shows promise as a formative assessment guide to assist instructors to devise tailored interventions which recognises the conceptual resources that can support students’ learning.
The present study's findings therefore suggest that adopting a finer grained multimodal approach should be considered by educators and may serve as a useful assessment tool which can yield greater insight into the quality and complexity of student understanding and consequently inform novel teaching and assessment approaches. This agrees with previous works which have indicated that students’ descriptions can reveal misconceptions of scientific phenomena that may not be detectable using traditional assessment instruments (Kelly et al., 2010; Cooper et al., 2015). This study's findings stretch beyond misconceptions to suggest that adopting the resources framework as an overarching pedagogical model could offer an important complimentary means of assessing students true understanding in a way that doesn’t simply focus on misconceptions and absolute scribed correctness; capturing a richer picture of the conceptual resources and reasoning strategies that can help students build a fuller and more comprehensive understanding. Identifying such key resources for given threshold concepts would allow instructors and curriculum designers to produce bespoke formative assessment instruments which can dovetail with the delivery of core learning objectives and give teachers immediate and valuable multimodal feedback from the assumptions and modes of reasoning that frame their students’ thinking.
Given the emphasis on pen-on-paper examinations for secondary school students across STEM disciplines in the UK, it would seem that multimodal formative assessment guided by a conceptual resources framework could hold the potential to initiate transformative pedagogical impact for teaching, learning and assessment if recognised by national assessment boards and promoted within teacher education programmes as the misconceptions framework has outreached previously.
In conclusion, this study's findings hold promise to be transferable; identifying key context dependent conceptual resources and reasoning strategies that teachers can recognise as crucial to enhancing learners’ understanding of troublesome concepts and the modalities with which they are expressed, is pertinent to all STEM instructors, not just chemists.
Moreover, considering the recent, rapidly adaptive, and evolving approaches to deliver and assess school learning remotely during the COVID pandemic, blended curriculum designers, digital resource developers and diligent national examination boards might also consider such inclusive pedagogical adaptations to inform and transform future science learning and teaching for all learners.
Limitations and future studies
There are limitations to this study as research findings from this cohort sample from a single classroom of high school students in their final school year may be unique to this context and therefore, we cannot draw immediate conclusions about the external generalisability of findings. The explorative nature of this study provides a starting point to catalyse further research in this field; future studies with varied and multiple measures of spatial ability might employ spatial aptitude tests as proposed by Bodner et al., to use as a pre-testing diagnostic tool to provide baseline information of students’ individual visuospatial aptitude (Bodner and Guay, 1997). Subsequent testing of student visuospatial aptitude may explore whether this ability is innate or can be developed through instructional intervention and open-ended freedom to reason in a multimodal way. Although, the scale of this study made it possible to adopt a rich analytical approach, additional research studies in different educational authorities and regions could emulate this research to collect a greater sample of data to address the underlying lack of statistical power due to small sample size.Our study sought to identify the most common conceptual resources, revealing rich detail of students reasoning within the context of a visuospatial concept, molecular geometry. We believe our findings are transferrable across other contexts within chemistry such as stereoisomerism (Ping et al., 2022) and organic reaction mechanisms. Likewise, across other STEM domains such as polymer science, materials engineering, biochemistry, and molecular biology where there is the requirement for students to solve visuospatial problems and potentially mentally manipulate macromolecular systems. Identifying the nature of students’ conceptual resources is a complex process, future studies expanding upon this work using a resources framework lens, should recognise the challenge associated with categorizing and defining the grain-size of students’ conceptual resources when applying to different learning contexts.
Although this study's approach was representative of typical chemistry classroom discourse and teaching activities, thus ensuring some degree of ecological validity, had students been interviewed separately rather than working in dyad pairings, results and insights into their reasoning may have differed. Future studies might attempt to record students’ naturalistic explanations individually to minimise possible peer mirroring. This study identified some key conceptual resources and underlying reasoning strategies through speech and gesture which can feedforward to classroom instructors teaching and assessing molecular geometry; future studies might suggest predictions of key conceptual resources for different threshold concepts and test this through students’ multimodal responses.
Moreover, this study's findings illustrate the richness of detail gleaned from attending to students’ gestures as they articulate their understanding, and the potential gestures hold to informing formative assessment protocols. Further research investigating the traditional nature of assessment and whether it adequately serves to assess true student knowledge and understanding of 3-dimensional concepts and visuospatial thinking is of importance not only to STEM instructors and students, but to assessment boards and further education providers.
Molecular models are routinely used in the chemistry classroom to assist learners; previous studies have explored the role of embodiment as students use physical models when imagining chemistry concepts (Stull et al., 2018). Future studies might explore the ways in which students interact with molecular models when predicting molecular geometries, which may offer greater insight into their visuospatial reasoning and the nature of conceptual resources activated.
Finally, the development of new classroom teaching resources and digital educational tools should consider individual learning strategies and in particular the role of multiple modalities and conceptual resources to help student reasoning and support students towards ultimate visuospatial competence. Digital chemistry education tools might incorporate interfaces and activities which require students to access key conceptual resources and interact with their learning in a multimodal way. For example, through speech recognition software which can feedback to support multimodal, three-dimensional thinking and tangible accessories with augmented reality software may be a useful way to scaffold three-dimensional manipulation of molecules to view molecular geometries from different aspects as they learn this tricky concept. Similarly, digital gesture recognition tools, enabled with Artificial Intelligence capability are increasingly accessible and could serve as a transformative digital resource not only to novice chemists to learn and self-assess from, but to provide revolutionary support for teachers, to lighten their significant workload, as they conduct crucial formative assessment of student understanding.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by The Chemistry Departments of The Edinburgh Academy and The High School of Dundee. Any findings, conclusions, opinions, or recommendations are those of the authors and do not necessarily reflect the views of The Edinburgh Academy or The High School of Dundee.References
- Abels S., (2016), The role of gestures in a teacher–student-discourse about atoms, Chem. Educ. Res. Pract., 17, 618–628.
- Abner N., Cooperrider K. and Goldwin-Meadow S., (2015), Gesture for Linguists: A Handy Primer, Lang. Linguist. Compass, 9/11, 437–449.
- Alibali M. W., Nathan M. J. and Fujimori Y., (2011), Gestures in the mathematics classroom: What's the point, in Stein N. and Raudenbush S. (ed.), Developmental cognitive science goes to school, New York, NY: Routledge, pp. 219–234.
- Amaya Becvar L., Hollan J., and Hutc E., (2005), Hands as molecules: Representational gestures used for developing theory in a scientific laboratory, Semiotica, 156, 89–112.
- Benbow C., Lubinski D. and Park G., (2013), Recognizing spatial ability, Scientific American, https://www.scientificamerican.com/article.cfm?id=recognizing-spatial-intel.
- Bodner G. M. and Guay R. B., (1997), Visualization of Rotations Test, Chem. Educ., 2, 1–17.
- Castro-Alonso J. C. and Uttal D. H., (2019), Science Education and Visuospatial Processing, in Castro-Alonso J. C. (ed.), Visuospatial Processing for Education in Health and Natural Sciences, Springer International Publishing, vol. 3, pp. 53–79.
- Chandler P. and Sweller J., (1991), Cognitive load theory and the format of instruction, Cognit. Instr., 8(4), 293–332.
- Chue S., Lee Y., and Tan K. C. D., (2015), Iconic gestures as undervalued representations during science teaching, Cogent Educ., 2, 1021554.
- Cohen J., (1960), A coefficient of agreement for nominal scales, Educ. Psychol. Meas., 20, 37–46.
- Cooper L., (1988), The role of spatial representations in complex problem solving, in Schiffer S. and Steele S. (ed.), Cognition and Representation, Boulder, CO: Westview Press, pp. 53–86.
- Cooper M. M., Williams L. C. and Underwood S. M., (2015), Student Understanding of Intermolecular Forces: A Multimodal Study, J. Chem. Educ., 92, 1288–1298.
- Cooper M. M., Stieff M. and DeSutter D., (2017), Sketching the Invisible to Predict the Visible: From Drawing to Modeling in Chemistry, Top. Cogn. Sci., 9, 902–920.
- Corbin J. and Strauss A., (2008), Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory, Los Angeles, CA: Sage Publications.
- Disessa A. A., (1993), Toward an epistemology of physics, Cognit. Instr., 10, 105.
- Ebbing D. and Gammon S. D., (2015), General Chemistry, ISBN-10: 1305580346, ISBN-13: 9781305580343.
- Erlina, Cane C. and Williams D. P., (2018), Prediction! The VSEPR Game: Using Cards and Molecular Model Building To Actively Enhance Students’ Understanding of Molecular Geometry, J. Chem. Educ., 95, 991–995.
- Fiorella L. and Mayer R. E., (2017), Spontaneous spatial strategy use in learning from scientific text, Contemp. Educ. Psychol., 49, 66–79.
- Flood V. J. et al., (2014), Paying Attention to Gesture When Students Talk Chemistry: Interactional Resources for Responsive Teaching, J. Chem. Educ., 92(1), 11–22.
- Furio C. and Calatayud M. L., (1996), Difficulties with the Geometry and Polarity of Molecules, J. Chem. Educ., 73(1), 36–41.
- Gillespie R. J., (1997), The Great Ideas of Chemistry, J. Chem. Educ., 74(7), 862–864.
- Givry D. and Roth W.-M., (2006), Toward a new conception of conceptions: interplay of talk, gestures, and structures in the setting, J. Res. Sci. Teach., 43(10), 1086–1109.
- Goldin-Meadow S., Nusbaum H., Kelly S. D., and Wagner S., (2001), Explaining Math: Gesturing Lightens the Load, Psychol. Sci., 12(6), 516–522.
- Green A., Straube B., Weis S., Jansen A., Willmes K., Konrad K., et al., (2009), Neural integration of iconic and unrelated coverbal gestures: a functional MRI study, Hum. Brain Mapp., 30, 3309–3324.
- Griffiths A. K. and Preston K. R., (1992), Grade-12 students’ misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29(6), 611–628.
- Hammer D., (1996), More than misconceptions: Multiple perspectives on student knowledge and reasoning, and an appropriate role for education research, Am. J. Phys., 64(10), 1316–1325.
- Hammer D., (2000), Student resources for learning introductory physics, Am. J. Phys., 68, S52.
- Hammer D., Elby A., Scherr R. A. and Redish E. F., (2005), Resources, framing, and transfer, in Mestre J. (ed.), Transfer of learning from a modern multidisciplinary perspective, Greenwich, CT: Information Age Publishing, pp. 89–119.
- Hegarty M., (2004), Diagrams in the mind and in the world: Relations between internal and external visualizations, in Blackwell A., Mariott K. and Shimojima A. (ed.), Diagrammatic representation and inference, Berlin: Springer-Verlag, pp. 1–13.
- Hegarty M., Stieff M. and Dixon B. L., (2013), Cognitive change in mental models with experience in the domain of organic chemistry, J. Cogn. Psychol., 25(2), 220–228.
- Hornbuckle S. F., Gobin L. and Thurman S. N., (2014), Contemporary Issues In Education Research – First Quarter, 7(1), 45–50.
- Kandana Arachchige K. G., Simoes Loureiro I., Blekic W., Rossignol M. and Lefebvre L., (2021), The Role of Iconic Gestures in Speech Comprehension: An Overview of Various Methodologies, Front. Psychol., 12, 634074.
- Kelly R. M., Barrera J. H. and Mohamed S. C., (2010), An Analysis of Undergraduate General Chemistry Students’ Misconceptions of the Submicroscopic Level of Precipitation Reactions, J. Chem. Educ., 87(1), 113–118.
- Kiernan N. A., Manches A., and Seery M. K., (2021), The role of visuospatial thinking in students’ predictions of molecular geometry, Chem. Educ. Res. Pract., 22, 626–639.
- Kress G., Jewitt C., Bourne J., Franks A., Hardcastle J., Jones K. and Reid E., (2005), English in urban classrooms, A multimodal perspective on teaching and learning, London, New York: RoutledgeFalmer.
- Landis J. R. and Koch G. G., (1977), The measurement of observer agreement for categorical data, Biometrics, 33, 159–174.
- Libretexts.org., TheVSEPRModel:https://chem.libretexts.org/@go/page/21752 [Accessed February 15, 2022].
- Lubinski D., (2010), Spatial Ability and STEM: A Sleeping Giant for Talent Identification and Development, Pers. Individ. Differ., 49, 344–35.
- McNeill D., (1992), Hand and mind: What gestures reveal about thought, Chicago, USA: University of Chicago Press.
- McNeill D., (2005), Gesture and thought, Chicago, IL: University of Chicago Press.
- Meredith and Young, Application of Resources Framework for Fluid Dynamics.
- Morsella E. and Krauss R. M., (2004), The role of gestures in spatial working memory and speech. Am. J. Psychol., 117(3), 411–424.
- National Research Council, (2006), Learning to think spatially, Washington, D.C.: National Academies Press.
- Newcombe N. S., (2010), Picture this: Increasing math and science learning by improving spatial thinking. Am. Educ., 34(2), 29.
- Nicoll G. A., (2001), Report of Undergraduates’ Bonding Misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Nyachwaya J. M. and Gillespie M., (2016), Features of representations in general chemistry textbooks: a peek through the lens of the cognitive load theory, Chem. Educ. Res. Pract., 17, 58.
- Ping R., Church M., Larson S. W., Zinchenko E. and Goldin-Meadow S., (2021), Unpacking the Gestures of Chemistry Learners: What the Hands Tell Us About Correct and Incorrect Conceptions of Stereochemistry, Discourse Processes, 58(3), 213–232.
- Ping R., Parrill F., Church R. B. and Goldin-Meadow S., (2022), Teaching stereoisomers through gesture, action, and mental imagery, Chem. Educ. Res. Pract., 23, 698.
- Redish E. F. and Hammer D., (2009), Reinventing college physics for biologists: Explicating an epistemological curriculum, Am. J. Phys., 77, 629.
- Redish E. and Kuo E., (2015), Language of physics, language of math: Disciplinary culture and dynamic epistemology, Sci. Educ., 24, 561.
- Scherr R. E., (2008), Gesture analysis for physics education researchers, Phys. Rev. Spec. Top. Phys. Educ. Res.4, 010101.
- Schwartz D. L. and Black J. B., (1996), Shuttling between depictive models and abstract rules: Induction and fallback, J. Cognit. Sci., 20(4), 457.
- Smith J., DiSessa A. and Roschelle J., (1993/94), Misconceptions reconceived: A constructivist analysis of knowledge in transition, J. Learning Sci., 3(2) 115–163.
- SQA, (2019), Advanced Higher Chemistry Course Specification: https://www.sqa.org.uk/files_ccc/AHChemistryCourseSpec.pdf.
- Stieff M., (2007), Mental rotation and diagrammatic reasoning in science, Learn. Instr., 17(2), 219–234.
- Stieff M., (2011), When Is a Molecule Three Dimensional? A Task-Specific Role for Imagistic Reasoning in Advanced Chemistry, Sci. Ed., 95, 310–336.
- Stieff M. and Raje S., (2010), Expert algorithmic and imagistic problem solving strategies in advanced chemistry, Spat. Cogn. Comput., 10(1), 53–81.
- Stieff M., Dixon B. L., Ryu M., Kumi B. and Hegarty M., (2014), Strategy training eliminates sex differences in STEM spatial problem solving, J. Educ. Psychol., 106(2), 390–402.
- Stieff M., Lira M. E. and Scopelitis S. A., (2016), Gesture Supports Spatial Thinking in STEM, Cogn. Instr., 34(2), 80–99.
- Stieff M., Werner S., DeSutter D., Franconeri S. and Hegarty M., (2020), Visual Chunking as a Strategy for Spatial Thinking in STEM, Cognit. Res.: Princ. Implic., 5, 18.
- Stull A. T., Gainer M. J. and Hegarty M., (2018), Learning by enacting: The role of embodiment in chemistry education. Learn. Instr., 55, 80–92.
- Taber K. S., (2001), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ. Res. Pract., 2, 123–158.
- Taber K. S. and Coll R., (2002), Bonding, in Gilbert J. K., Jong O. D., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical education: towards research-based practice, Dordrecht: Kluwer, pp. 213–234.
- Taber K. S., (2008), Conceptual resources for learning science: issues of transience and grain-size in cognition and cognitive structure, Int. J. Sci. Educ., 30(08), 1027–1053.
- Taber K. S., (2009), College students’ conceptions of chemical stability: the widespread adoption of a heuristic rule out of context and beyond its range of application, Int. J. Sci. Educ., 31, 1333–1358.
- Taber K. S., (2014), The Significance of Implicit Knowledge in Teaching and Learning Chemistry, Chem. Educ. Res. Pract., 15, 447–461.
- Talanquer V., (2014), Threshold Concepts in Chemistry: The Critical Role of Implicit Schemas, J. Chem. Educ.92, 3–9.
- Talanquer V., (2022), The Complexity of Reasoning about and with Chemical Representations, JACS Au, 2022, 2, 2658–2669.
- Teo T. W., Goh M. T. and Yeo L. W., (2014), Chemistry education research trends: 2004–2013, Chem. Educ. Res. Pract., 15, 470–487.
- Tiettmeyer J. M., Coleman A. F., Balok R. S., Gampp T. W., Duffy P. L., Mazzarone K. M., and Grove N. P., (2017), Unraveling the Complexities: An Investigation of the Factors That Induce Load in Chemistry Students Constructing Lewis Structures, J. Chem. Educ., 94, 282–288.
- Tversky B., (2017), Gestures can create diagrams (that are neither imagistic nor analog), Behav. Brain Sci., 40, E73.
- Underwood S. M., Kararo A. T. and Gadia G., (2021), Investigating the impact of three-dimensional learning interventions on student understanding of structure–property relationships. Chem. Educ. Res. Pract., 22(2), 247–262.
- Vlacholia M., Vosniadou S., Roussos P., Salta K., Kazi S., Sigalase M. and Tzougraki C., (2017), Changes in visual/spatial and analytic strategy use in organic chemistry with the development of expertise, Chem. Educ. Res. Pract., 18(763–773), 763.
- Wesp R., Hesse J., Keutmann D. and Wheaton K., (2001), Gestures maintain spatial imagery, Am. J. Psychol., 114(4), 591–60.
- Wittmann M. C., Sternberg R. N., and Redish E. F., (2003), Understanding and affecting student reasoning about sound waves, Int. J. Sci. Educ., 25, 991.
- Wu H.-K., Krajcik J. S. and Soloway E., (2001), Promoting conceptual understanding of chemical representations: students’ use of a visualization tool in the classroom, J. Res. Sci. Teach., 38(7), 821–842.
- Young D. E. and Meredith D. C., (2017), Using the resources framework to design, assess, and refine interventions on pressure in fluids, Phys. Rev. Phys. Educ. Res., 13, 010125.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3rp00186e |
| This journal is © The Royal Society of Chemistry 2024 |