Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Covalency versus magnetic axiality in Nd molecular magnets: Nd-photoluminescence, strong ligand-field, and unprecedented nephelauxetic effect in fullerenes NdM2N@C80 (M = Sc, Lu, Y)†
Wei
Yang
a,
Marco
Rosenkranz
a,
Georgios
Velkos
a,
Frank
Ziegs
a,
Vasilii
Dubrovin
 a,
Sandra
Schiemenz
a,
Lukas
Spree
a,
Sandra
Schiemenz
a,
Lukas
Spree
 ab,
Matheus Felipe
de Souza Barbosa
a,
Charles
Guillemard
ab,
Matheus Felipe
de Souza Barbosa
a,
Charles
Guillemard
 c,
Manuel
Valvidares
c,
Bernd
Büchner
a,
Fupin
Liu
c,
Manuel
Valvidares
c,
Bernd
Büchner
a,
Fupin
Liu
 *a,
Stanislav M.
Avdoshenko
*a and
Alexey A.
Popov
*a,
Stanislav M.
Avdoshenko
*a and
Alexey A.
Popov
 *a
*a
aLeibniz Institute for Solid State and Materials Research (IFW Dresden), 01069 Dresden, Germany
bCenter for Quantum Nanoscience, Institute for Basic Science (IBS), Seoul, Republic of Korea
cALBA Synchrotron Light Source, E-08290 Barcelona, Spain. E-mail: f.liu@ifw-dresden.de; s.avdoshenko@ifw-dresden.de; a.popov@ifw-dresden.de
First published on 21st December 2023
Abstract
Nd-based nitride clusterfullerenes NdM2N@C80 with rare-earth metals of different sizes (M = Sc, Y, Lu) were synthesized to elucidate the influence of the cluster composition, shape and internal strain on the structural and magnetic properties. Single crystal X-ray diffraction revealed a very short Nd–N bond length in NdSc2N@C80. For Lu and Y analogs, the further shortening of the Nd–N bond and pyramidalization of the NdM2N cluster are predicted by DFT calculations as a result of the increased cluster size and a strain caused by the limited size of the fullerene cage. The short distance between Nd and nitride ions leads to a very large ligand-field splitting of Nd3+ of 1100–1200 cm−1, while the variation of the NdM2N cluster composition and concomitant internal strain results in the noticeable modulation of the splitting, which could be directly assessed from the well-resolved fine structure in the Nd-based photoluminescence spectra of NdM2N@C80 clusterfullerenes. Photoluminescence measurements also revealed an unprecedentedly strong nephelauxetic effect, pointing to a high degree of covalency. The latter appears detrimental to the magnetic axiality despite the strong ligand field. As a result, the ground magnetic state has considerable transversal components of the pseudospin g-tensor, and the slow magnetic relaxation of NdSc2N@C80 could be observed by AC magnetometry only in the presence of a magnetic field. A combination of the well-resolved magneto-optical states and slow relaxation of magnetization suggests that Nd clusterfullerenes can be useful building blocks for magneto-photonic quantum technologies.
Introduction
The partially filled 4f-shell in lanthanide compounds is an inexhaustible source of magnetic and optical phenomena with a plethora of already practical and equally numerous prospective applications. The splitting and wavefunction composition of the ligand-field (LF) levels of the lanthanide ground-state multiplet determine the magnetic anisotropy and magnetization relaxation pathways and therefore are of paramount importance for lanthanide-based molecular magnets, such as single-molecule magnets (SMMs). Optical spectroscopy can contribute to the understanding of the SMM properties by the analysis of the fine structure of f–f transitions, in particular in low-temperature photoluminescence (PL) spectra, and by the correlation of the determined level splitting with magnetic behaviour.1–5 Since the first report on the Dy(DOTA) complex by Sessoli et al. in 2012,6 the vast majority of such studies were devoted to Dy6–17 and Yb8,18–25 complexes, with rare instances of Tb,8,26–28 Ho,29 Nd,30–33 and Er SMMs.34,35 Recent trends also include a development of luminescence thermometry based on the temperature-dependent PL of Ln-SMMs,2,21,29,36,37 and the use of lanthanide complexes with a narrow optical linewidth for photonic quantum technologies.38,39Lanthanide-based endohedral metallofullerenes (Ln-EMFs) represent an important class of SMMs due to their air and thermal stability, ability to encapsulate Ln ions in rather unusual yet simple chemical environments with strong ligand fields, and stabilization of unconventional bonding interactions between lanthanide ions.40–44Ab initio computational methods are very instrumental in predicting single-ion magnetic anisotropy in Ln-EMFs,42,45–52 but verification of such predictions by direct experimental access to the LF splitting in fullerene-based Ln-SMMs is still missing. A natural prerequisite for the realization of the Ln-based PL in Ln-EMFs is that the lowest-energy fullerene excited state should have higher energy than the lanthanide emitting state. However, the optical gaps of EMFs rarely exceed 1.5 eV. For instance, recent PL studies of the YxSc3−xN@Ih-C80 (x = 0–3) family revealed that the energy of the triplet emitting state (T1) in this series depends on the Sc content in the endohedral cluster and varies from 1.51 eV in YSc2N@C80 to 1.68 eV in Y3N@C80.53,54 As M3N@Ih-C80 clusterfullerenes have the highest optical gaps among EMFs, these energies set the upper limit for Ln-emitting states in Ln-EMFs. The possibility of Ln-based PL in Ln-EMFs is thus essentially restricted to the near-infrared (NIR) range. To date, only different types of Er-EMFs exhibited well-established Er-based NIR luminescence,55–63 including the fine structure at low temperatures in some of them, and one study reported on Tm-based NIR-PL in Tm-EMFs.64 Unfortunately, Er and Tm tend to have easy-plane type magnetic anisotropy in EMFs42,65 and are thus not well suited for the design of EMF-SMMs. The vast majority of EMF-SMMs are based on Tb,41,42,66–70 Dy,43,49,51,71–73 and Ho,42,74 but none of them fulfills the criterion of low Ln-emitting state energy, and thus their Ln-based luminescence is not to be expected unless the fullerene excited states are driven to much higher energies.
Among different lanthanides, Nd seems to be a suitable candidate for Ln-based luminescence in EMF-SMMs. The free-ion energy of the Nd3+ NIR-emitting state, 4F3/2 at 1.45 eV,75 is just below the fullerene excited triplet state in M3N@Ih-C80. Nd3N@Ih-C80 cannot be produced in a reasonable yield because Nd3+ ions are too large,76 but this limitation can be circumvented by using mixed-metal analogs with smaller rare-earth ions, such as NdSc2N@Ih-C80.77 The high magnetic anisotropy in nitride clusterfullerenes is caused by the close proximity of the nitride N3− to lanthanide ions, and paramagnetic NMR studies of the LnSc2N@C80 series accompanied by point-charge ligand field calculations demonstrated that Nd3+ in NdSc2N@C80 has the easy-axis type anisotropy favorable for SMMs.65 Nd-EMFs are among the least studied Ln-EMFs, and the first example of the SMM behavior of EMFs with light lanthanides, Nd2@C80(CF3), has only recently been reported.78 This lack of studies echoes the situation with light-lanthanide SMMs in general, which are substantially less explored than their heavy-lanthanide congeners.79,80 Thus, despite the ubiquitous NIR luminescence known for NdIII complexes and solids, the reports on the combination of slow relaxation of magnetization and Nd-based luminescence remain scarce.30–33,36,81–85
In this work, we synthesized a series of NdM2N@C80 (M = Sc, Lu, Y) molecules to study if the ligand-field experienced by the Nd3+ ion and SMM performance can be increased by deliberately shortening the Nd–N bond length and increasing the internal strain induced by complementary M3+ metal ions of a different size. By detecting the finely structured Nd-based near-infrared photoluminescence of the NdM2N@C80 series, we demonstrate the largest ligand-field splitting ever reported for Nd3+ in molecular compounds. Furthermore, we show that the ligand field splitting in NdM2N@C80 is indeed modulated by the increase of the size of M3+ ions. However, we also found that shortening of the Nd–N bond not only increases the LF splitting, but also enhances the covalency, which manifests itself in the strongest nephelauxetic effect ever observed in the PL spectra of Nd compounds. The influence of the enhanced LF and covalency on the magnetic and SMM properties of NdSc2N@C80 is evaluated by SQUID magnetometry and X-ray magnetic circular dichroism.
Synthesis and molecular structure
Synthesis and isolation
Metallofullerenes NdM2N@C80 (M = Sc, Y, Lu) were synthesized by the arc-discharge method using guanidinium thiocyanate as a solid source of nitrogen (see the ESI for further details of synthesis and HPLC separation, Fig. S1–S3†).86 The increase of the metal size decreases the propensity of the nitride clusterfullerene formation and thus strongly affects the yield of the arc-discharge synthesis. Thus, in accordance with the ionic radii of M3+, the yield of NdM2N@C80 species follows Sc ≫ Lu > Y. The main efforts were therefore focused on NdSc2N@C80, which could be accumulated in sufficient amounts for its structural, spectroscopic, and AC/DC magnetic studies. For NdLu2N@C80 and NdY2N@C80, as their yield is considerably lower, we only accumulated amounts required for spectroscopic studies. All isolated compounds were characterized by LDI-TOF mass-spectrometry to ensure their compositional purity (Fig. S1–S3†). The UV-Vis-NIR absorption spectra of NdM2N@C80 showed a typical absorption pattern of M3N@C80 nitride clusterfullerenes with the Ih fullerene cage isomer (Fig. S4†); no absorption features caused by f–f transitions of Nd3+ could be identified. The vibrational spectra of NdSc2N@C80 (Fig. S5†) are also in line with the data on other M3N@C80 compounds.Single-crystal X-ray diffraction
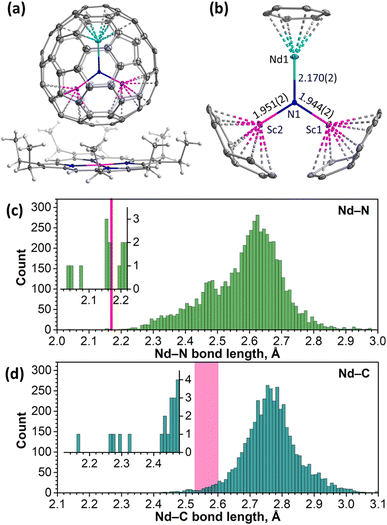
The molecular structure of NdSc2N@Ih-C80 was established by single-crystal X-ray diffraction (SC-XRD) using a co-crystal with nickel(II) octaethylporphyrin (NiOEP) grown by layering benzene solutions of the fullerene and NiOEP and allowing a slow diffusion thereof for one month. Measurements were performed at 100 K with synchrotron irradiation at the BESSY storage ring (BL14.2, Berlin-Adlershof, Germany).87 The XDSAPP2.0 suite was employed for data processing.88,89 The structure was solved by direct methods and refined with SHELXL-2018.90The NdSc2N@C80·NiOEP·2C6H6 co-crystal gives a rare example of a fully ordered EMF molecule in the crystal, allowing detailed analysis of the molecular structure (Fig. 1a, S6 and Table S1†). The NdSc2N cluster is planar and oriented perpendicular to the porphyrin backbone. Two Sc atoms are located above NiOEP nitrogens, while Nd is facing the opposite side of the fullerene. This orientation of the cluster is typical for MSc2N@C80 molecules in co-crystals with NiOEP.91–94 Nd is coordinated to the fullerene hexagon in an η6-fashion with Nd–C distances of 2.527(2)–2.604(2) Å, while the shortest Sc–C bonds of 2.228(3)–2.245(3) Å are to carbons on pentagon/hexagon edges (Fig. 1b).
The large difference of ionic radii95 of Nd3+ (0.983 Å) and Sc3+ (0.745 Å) results in a considerable distortion of the NdSc2N cluster, with a 0.320(2) Å displacement of nitrogen from the centroid of the C80 cage towards Sc ions. Sc–N bond lengths are 1.944(2) and 1.951(2) Å, while the Nd–N bond length is 2.170(2) Å. Similar structural parameters were found in LnSc2N@C80 molecules with other light lanthanides, La91 (La–N 2.196(4) Å, Sc–N 1.943(6)/1.921(7) Å) and Ce93 (Ce–N 2.184(2) Å, and Sc–N 1.942(2)/1.933(2) Å). For heavier lanthanides, the decrease of the Ln3+ ionic radius results in a systematic shortening of the Ln–N bond and increase of Sc–N bond lengths. For instance, the Dy–N bond length in DySc2N@C80 is 2.096(6) Å, while Sc–N bonds are 1.965(6) and 1.978(6) Å.94
To consider the structural parameters of NdSc2N@C80 in a broader context, we analyzed Nd–N and Nd–C bonds for all compounds deposited in the Cambridge Crystallographic Data Centre (CCDC) database. The Nd–N bond length distribution with more than 5800 entries peaks near 2.62 Å (Fig. 1c). The Nd–N bond in NdSc2N@C80 appears to have one of the shortest lengths in the whole dataset. Shorter Nd–N distances are found only in terminal imides with Nd![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds (2.04–2.08 Å)96 and some μ2-imide97,98 and amide99 complexes (2.15–2.17 Å), all reported by Anwander et al.
N double bonds (2.04–2.08 Å)96 and some μ2-imide97,98 and amide99 complexes (2.15–2.17 Å), all reported by Anwander et al.
Nd–C bond lengths fall closer to a normal range although being still rather short for their type. For comparison, the peak in the Nd–C bond length distribution (4300 entries) is near 2.75 Å (Fig. 1d). Nd–C distances in complexes with ηn-coordinated arenes span the range of 2.65–2.85 Å for variously substituted η5-cyclopentadienyls,100–102 2.60–2.72 Å for η8-C8H8,103–105 and 2.845(6)–2.915(7) for η9-C9H9.104 In another Nd-EMF characterized by SC-XRD, Nd@C2v(9)-C82, Nd is coordinated to two carbons in the η2-fashion, and the corresponding Nd–C bonds are rather short, 2.25–2.30 Å.106
DFT calculations
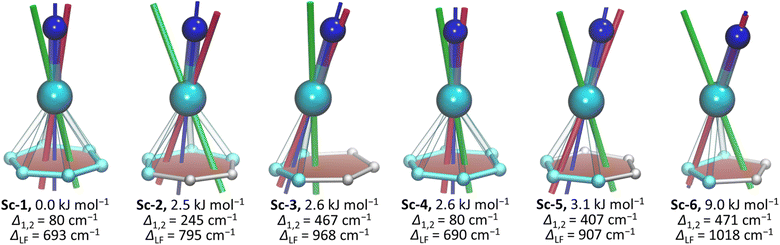
An increase of the metal size in M3N@C80 molecules leads to the accumulation of the internal strain, which at first manifests itself in the unusually short M–N bonds, and then changes the M3N cluster shape from planar to pyramidal when the M–N bonds reach their limits of shortening.107,108 The influence of the metal size on the molecular structures in the NdM2N@C80 series was analyzed with the help of DFT calculations. As the M3N cluster can have somewhat different orientations (conformers) inside the fullerene cage, we first performed a full search of conformers for isolated NdSc2N@C80 molecules. 120 uniformly distributed NdSc2N cluster orientations inside the C80 cage were generated using Fibonacci sampling109 and used as starting geometries in DFT optimization. The procedure resulted in 6 unique conformers, of which the most stable one (conformer Sc-1) is identical to the SC-XRD structure (see Fig. S7 in the ESI†). Four others are only 2–3 kJ mol−1 less stable, while the least stable one (Sc-6) has a relative energy of 9 kJ mol−1. For all conformers, DFT calculations predict a planar NdSc2N cluster, showing that a comparably small Sc3+ ion compensates for the large radius of Nd3+. DFT-optimized Nd–N bond lengths in all conformers are in the range of 2.22–2.23 Å and overestimated compared to the experimental value by 0.05 Å.When Sc3+ is substituted by larger Lu3+ (0.861 Å) and Y3+ (0.900 Å), the Nd–N bond lengths in the most stable DFT conformers of NdLu2N@C80 (Lu-1) and NdY2N@C80 (Y-1) are shortened to 2.165 Å and 2.170 Å, respectively (Fig. S8 and S9†). These similar values for metals of different sizes indicate that the Nd–N bond has reached its shortening limit. As a result, the NdM2N cluster cannot sustain a planar shape in NdM2N@C80 and becomes pyramidal. In different conformers of NdLu2N@C80 and NdY2N@C80, the nitrogen is elevated above the NdM2 plane by 0.37–0.51 Å and 0.52–0.62 Å, respectively (Fig. S8 and S9†).
Photoluminescence and ligand-field splitting
Short Ln–N bonds along with the large negative charge of the nitride ion lead to a strong axial ligand field imposed on lanthanide ions in nitride clusterfullerenes. To address the size of LF splitting in Nd-EMFs experimentally, we turned to photoluminescence spectroscopy. The photophysical behavior of MSc2N@C80 molecules without a partially filled 4f-shell in the metal can be illustrated by YSc2N@C80.53 After photoexcitation and internal conversion to S1, it undergoes a fast intersystem crossing to the T1 state located at 1.5–1.6 eV above the ground state. However, the energy difference between S1 and T1 is only 0.03 eV, facilitating inversed intersystem crossing. As a result, YSc2N@C80 exhibits thermally activated delayed fluorescence at room temperature and down to 30 K. At lower temperature, the thermal repopulation of the S1 state from T1 is frozen, leaving weak phosphorescence as the only radiational decay process.53 However, when the endohedral cluster contains Nd instead of Y, the energy can be further transferred from the fullerene-based T1 state to the 4f-shell of the lanthanide and produce Nd-based NIR luminescence with the origin at the 4F3/2 state (Fig. 2a). Indeed, our PL measurements of NdSc2N@C80 showed very characteristic Nd-centered NIR luminescence with three bands caused by 4F3/2 → 4I9/2, 4F3/2 → 4I11/2, and 4F3/2 → 4I13/2 transitions (Fig. 2b). Similar luminescence spectra were also observed for NdLu2N@C80 and NdY2N@C80 (Fig. 2b). As far as we know, this is the first observation of Nd-based luminescence in endohedral metallofullerenes.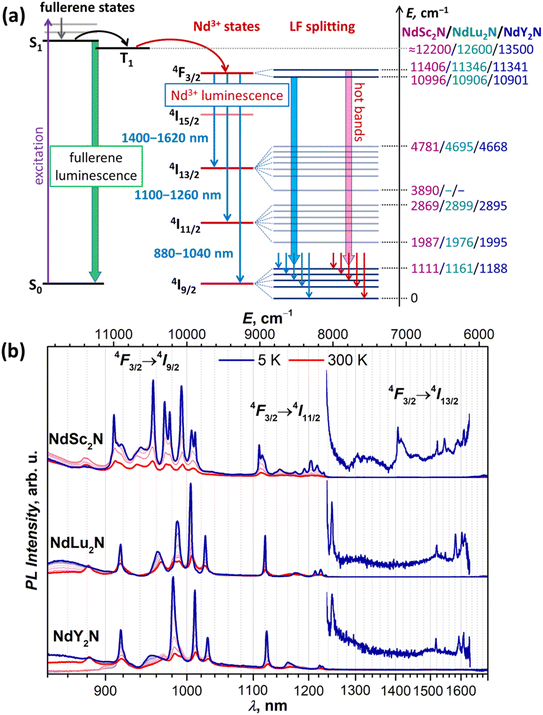 | ||
| Fig. 2 (a) Schematic description of the Nd-based luminescence in NdM2N@C80. In non-4f M3N@C80, a fullerene-based excitation is followed by an internal conversion to the singlet state S1, which then undergoes intersystem crossing to the triplet state T1, and both S1 and T1 participate in the radiational decay in the form of thermally activated delayed fluorescence and phosphorescence.53 When one M in the endohedral cluster is replaced with Nd, the energy is further transferred from the T1 state to the 4f-shell of Nd, which then emits from the 4F3/2 state giving three near-infrared 4F3/2 → 4IJ PL bands (J = 9/2, 11/2, 13/2). Their fine structure is caused by the ligand-field splitting in each 4IJ multiplet, while hot bands appear when the LF-excited state in the 4F3/2 doublet has significant thermal population. (b) Luminescence spectra of solid NdM2N@C80 (M = Sc, Lu, Y) measured at 5–300 K with 50 K steps using laser excitation at 488 nm. The energy values listed on the right scale in (a) are obtained from PL measurements at 5 K, and the LF splitting of 4F3/2 is estimated from hot bands at 300 K (see Fig. 3a and S12–S17†), while conservative estimations of fullerene T1 energies are based on the VT-PL data on YSc2N@C80 and Y3N@C80 from ref. 53 and preliminary VT-PL measurements of Lu3N@C80. | ||
The PL spectra of NdM2N@C80 are remarkable in several aspects. First, the 4F3/2 → 4IJ PL bands are shifted from 900 nm, 1060 nm, and 1350 nm, at which they are usually centered in Nd3+ complexes,110 to longer wavelengths. Particularly, the characteristic Nd3+ emission at 1060 nm used in solid-state lasers is “missing” as the 4F3/2 → 4I11/2 PL band of NdM2N@C80 occurs at 1100–1250 nm (Fig. 2b). As we discuss below, this shift of PL bands is the manifestation of a strong nephelauxetic effect. Second, the bands have atypical intensity distribution. Normally, the 4F3/2 → 4I11/2 band is the strongest one, followed by 4F3/2 → 4I9/2 with 2–4 times lower intensity, while the 4F3/2 → 4I13/2 band is the weakest. In NdM2N@C80, the latter is also very weak, but the 4F3/2 → 4I9/2 band is considerably stronger than 4F3/2 → 4I11/2. Third, the luminescence lifetimes of NdM2N@C80 are very short. PL lifetimes in Nd complexes are usually found in the 0.2–2 μs range. For the polycrystalline film of NdSc2N@C80, the PL decay is biexponential with the lifetimes of 4.5 ns/13 ns at room temperature, and 4 ns/19 ns between 200 and 5 K, with the longer component having the main contribution with a weight of 80% (Fig. S10†). To check if this fast decay can be caused by intermolecular Nd⋯Nd quenching, the fullerene was diluted in a polystyrene matrix. In the latter, the decay was mono-exponential with the lifetime near 20 ns. Polycrystalline NdLu2N@C80 and NdY2N@C80 showed monoexponential PL decay with very weak temperature dependence and lifetimes of 10.5–11.0 ns and 9.0–9.5 ns, respectively (Fig. S11†). These short lifetimes suggest a low quantum yield and explain the weak PL intensity of NdM2N@C80. Fourth, each PL band of NdM2N@C80 exhibits unusually broad and well-resolved fine structure already at room temperature. Upon cooling, the narrowing of the lines and increase of their intensity took place down to 50–100 K, while further cooling did not bring considerable changes in the spectra (Fig. 2b and S12–S17†).
At low temperatures, the 4F3/2 → 4I9/2 band of NdSc2N@C80 is reduced to seven narrow peaks, of which three are stand-alone and four form two doublets with the splitting of 70 and 50 cm−1 (Fig. 3a). We assign these peaks to five pure electronic 4F3/2(KD1) → 4I9/2(KDn) transitions. The splitting of KD3 and KD5 features into doublets is likely caused by a coexistence of two emitting sites,53,111 but the lack of analogous splitting for other KDs is not clear. Two broad peaks located between KD1 and KD2, at ΔE = 115 and 370 cm−1, can be tentatively assigned to vibronic transitions, but may also correspond to different conformers (vide infra). The low-T PL spectrum thus allows determination of experimental Δ1,2 and ΔLF values in NdSc2N@C80 as 536 cm−1 and 1061/1110 cm−1. The room-temperature PL spectrum also features a peak at ΔE = −410 cm−1, which disappears upon cooling (Fig. 3a). This hot band is likely caused by the 4F3/2(KD2) → 4I9/2(KD1) transition and thus allows estimation of the LF splitting in the 4F3/2 multiplet.
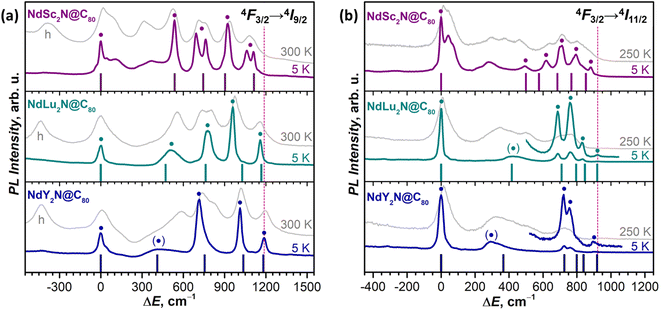 | ||
| Fig. 3 The fine structure of (a) 4F3/2 → 4I9/2 and (b) 4F3/2 → 4I11/2 photoluminescence bands of NdM2N@C80 (M = Sc, Lu, Y) measured at 5 K and 300 K (250 K). Vertical bars are the CASSCF-computed energies of LF states in 4I9/2 (a) and 4I11/2 (b) multiplets in Sc-3, Lu-3, and Y-3 conformers scaled by 1.15 to account for the systematic underestimation. The peaks assigned to pure electronic 4F3/2(KD1) → 4IJ(KDn) transitions are labeled with ● and broad peaks with uncertain assignment to KD2 are labelled with (●). The ΔE scale for each NdM2N@C80 is given versus the peak of the lowest-energy Kramers doublet in the 4I9/2 or 4I11/2 multiplet. In (a), the peaks at negative ΔE in 300 K spectra labelled with “h” are hot bands caused by the 4F3/2(KD2) → 4I9/2(KD1) transition of each NdM2N@C80, which allow estimation of Δ1,2 in the 4F3/2 multiplet. The intensity of 5 K and higher-temperature spectra is given off-scale (see Fig. 2b and S12–S17† for the real relative intensity at different temperatures). To guide the eye, the vertical dashed line marks the highest energy KD in the 4F3/2 → 4I9/2 band (NdY2N@C80) and in the 4F3/2 → 4I11/2 band (NdLu2N@C80). | ||
The interpretation of the 4F3/2 → 4I9/2 PL bands of NdLu2N@C80 and NdY2N@C80 is more straightforward as they show neither pronounced vibronic features nor additional splitting (Fig. 3 and Table S2†). The five peaks in low-T spectra are thus readily assigned to five KDs of the 4I9/2 multiplet. The only peculiarity is the strongly enhanced linewidth of the KD2 peak, which is still well-defined for NdLu2N@C80, but is barely discernible for NdY2N@C80. Such a strong broadening indicates that this state experiences very fast relaxation even at 5 K. The LF splitting of 4I9/2 in the NdM2N@C80 series increases systematically with the size of the M ion from NdSc2N@C80 (ΔLF = 1111 cm−1) to NdLu2N@C80 (1161 cm−1) and further to NdY2N@C80 (1188 cm−1). The LF splitting of the 4F3/2 multiplet is also larger for Lu and Y and amounts to 440 cm−1versus 410 cm−1 for Sc. At the same time, Δ1,2 decreases from 536 cm−1 (Sc) to 510 cm−1 (Lu) and 420 cm−1 (Y). The energies of other KDs also vary considerably in the NdM2N@C80 series. Besides, not only the energies, but also the intensities of transitions are quite different from Sc to Lu to Y, which indicates that the state composition is also considerably affected by the internal strain.
Thus, analysis of the 4F3/2 → 4I9/2 PL band demonstrates that enclosing Nd3+ in a molecular environment with a very short Nd–N bond and further increasing the internal strain by changing the size of the NdM2N cluster indeed result in a record-high LF splitting in NdM2N@C80 compounds exceeding 1100 cm−1. For comparison, a resolved structure of LF levels allowing estimation of ΔLF for the Nd-4I9/2 multiplet was observed in the optical spectra of five Nd-SMMs, including NdTp3,112,113 acetato-diphenoxo bridged {ZnNd} complex,30 dinuclear {Nd2} complexes with 9-anthracenecarboxyl and 2,2′-bipyridine31 or phenanthroline32 ligands, and in the Nd complex with N-(diphenylphosphoryl)pyrazine-2-carboxamide.33 None of them had the ΔLF exceeding 460 cm−1. Among the Nd-SMMs studied ab initio, ΔLF values exceeding 500 cm−1 were reported only for three Nd-SMMs: metallocenium complex [Nd(Cpttt)2]B(C6F5)4 (ΔLF = 672 cm−1 and Δ1,2 = 130 cm−1),100 Nd complex with equatorial metallacrowns and axial n-Bu3PO ligands in hexagonal bipyramidal coordination (ΔLF = 586 cm−1 and Δ1,2 = 104 cm−1),114 and (COT)Nd(Cpttt) sandwich complex (ΔLF = 545 cm−1 and Δ1,2 = 78 cm−1).115 Among non-SMMs, a very large LF splitting almost rivaling the results of this work was determined by Amberger et al. based on absorption and PL spectra and phenomenological crystal-field modelling in some tris(η5-cyclopentadienyl)Nd(III) complexes, such as Nd(η5-C5Me5)3 (ΔLF = 861 cm−1),116 Nd(η5-C5Me4H)3 (ΔLF = 1000 cm−1),117 Nd(η5-C5H4tBu)3 and Nd(η5-C5H4SiMe3)3 (in both, ΔLF = 1030 cm−1).118 The SMM properties of these complexes were not studied, but the ground state with the dominating |±5/2〉 term (see the ESI for results of ab initio calculations†) suggests that the slow relaxation of magnetization can hardly be expected.
In addition to the ground-state LF structure, well-resolved PL spectra also enable analysis of the 4I11/2 multiplet. For NdSc2N@C80, the experimental KD2 is found at 491 cm−1, whereas the overall ΔLF(4I11/2) splitting is 880 cm−1, considerably smaller than ΔLF(4I9/2) of 1111 cm−1. Broad features in the gap between KD1 and KD2 at 50 cm−1 and 280 cm−1 can be preliminary assigned to vibronic transitions. In NdLu2N@C80 and NdY2N@C80, the 4F3/2 → 4I11/2 band is dominated by the 4F3/2(KD1) → 4I11/2(KD1) transition, whereas other features have much lower intensity (Fig. 2b and 3b). Similar to the 4F3/2 → 4I9/2 band, the 4I11/2(KD2) peaks appear strongly broadened, which makes their assignment less certain. The ΔLF(4I11/2) splitting of 920 cm−1 in NdLu2N@C80 and 900 cm−1 in NdY2N@C80 exceed that in NdSc2N@C80.
A complete set of 4F3/2 → 4I13/2 transitions can be identified only for NdSc2N@C80 (Fig. 2b and Table S2†). Seven KDs of the 4I13/2 multiplet span the energy range of 890 cm−1 and show similar motifs to the 4F3/2 → 4I11/2 band with a large gap between KD1 and KD2 of 530 cm−1 and a dense group of other KDs at higher energy. For NdLu2N@C80 and NdY2N@C80, the position of the KD1 peak cannot be determined because of its very low intensity (Fig. 2b), which prevents further analysis of the LF splitting in 4I13/2.
The variation of the LF splitting can be correlated with the Nd-fullerene coordination motif (Fig. 4). The smallest Δ1,2 of 80 cm−1 is found for conformers Sc-1 and Sc-4, in which Nd is coordinated to the center of a fullerene hexagon in an η6-manner. Even a small shift of Nd from the hexagon center toward a C–C bond yields a 3-fold increase of Δ1,2 in Sc-2 (hapticity η4), while the more pronounced shift toward one of the carbon atoms increases Δ1,2 to 407 cm−1 in Sc-5 (η3) and further to 467 cm−1 in Sc-3 (η3). The largest Δ1,2 and ΔLF values are achieved when Nd faces one carbon atom in Sc-6 (hapticity between η1 and η3). The deviation of the quantization axis of the ground-state Kramers doublet (KD1) from the Nd–N bond axis (Fig. 4) is another manifestation of the strong influence of the Nd-fullerene coordination. The two axes coincide only in Sc-6, while the angle between them in other conformers reaches 13°.
The comparison of theoretical LF splitting to the fine structure of PL spectra shows that only conformers Sc-3 and Sc-6 agree reasonably well with experimental data (Fig. 5 and S18†), while predictions for other conformers, including the lowest-energy Sc-1, are far off. Thus, it appears that only the conformers, in which Nd is coordinated close to one C atom in the η1–η3 manner, contribute to the fine structure of PL spectra. The only features which might be attributed to other conformers of NdSc2N@C80 are broad peaks in the gap between 0 and 500 cm−1 (KD1 and KD2, Fig. 5), for which our preferred assignment is vibronic transitions. It is hard to determine at this moment whether other conformers have more efficient non-radiative decay channels and do not show up in the spectra, or the CASSCF predictions for these conformers are incorrect, or they are destabilized in powder samples. The technical aspects of CASSCF computations also indicate some untrivial behavior. For some conformers, the CASSCF wavefunction was found seamlessly, while for others, they were sensitive to initial guesses, and it took an effort to converge the wavefunction to a decent 4f-pure state.
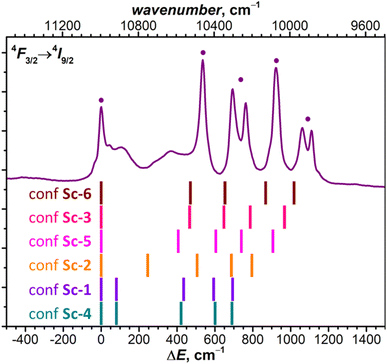 | ||
| Fig. 5 The fine structure of 4F3/2 → 4I9/2 photoluminescence bands of NdSc2N@C80 measured at 5 K compared to LF splitting of the 4I9/2 multiplet in six conformers of NdSc2N@C80 from CASSCF calculations (see Tables S3b–S3f† for numerical values). | ||
Following the calculation results on NdSc2N@C80, for NdLu2N@C80 and NdY2N@C80 we performed CASSCF calculations for two representative conformers, conformer Lu(Y)-1 with η6-type coordination of Nd, and conformer Lu(Y)-3 with η3-pentagon-coordinated Nd (see Fig. S19†). The difference between LF splitting of conformers is also considerable but less pronounced than in NdSc2@C80 (Tables S7 and S8†), and the conformer Lu(Y)-3 provides better match to the fine structure in experimental PL spectra (Fig. 3). Remarkably, while the total LF splitting in NdM2N@C80 increases with the size of M, the Δ1,2 value exhibits the opposite trend and shows a gradual decrease, which is correctly captured by CASSCF calculations for Lu(Y)-3 (Fig. 3).
| KD | Exp. Ea cm−1 | Calc. E cm−1 | % Composition in the |mJ〉 basis (J = 9/2) b | g x | g y | g z | φ° c |
|---|---|---|---|---|---|---|---|
| a Experimental values are from photoluminescence measurements at 5 K (see Fig. 3a). b For each KD, 4 main terms exceeding 5% are shown in the descending order of their contribution. c φ is the angle between the gz direction of the given KD and that of the ground state KD1. | |||||||
| CASSCF/RASSI | |||||||
| KD1 | 0 | 0 | 85|±9/2〉 + 7|±5/2〉 + 6|±3/2〉 | 0.0371 | 0.0445 | 5.6890 | — |
| KD2 | 536 | 467 | 48|±5/2〉 + 25|±7/2〉 + 19|±3/2〉 + 8|±1/2〉 | 0.5312 | 1.3555 | 3.1828 | 21 |
| KD3 | 693/763 | 648 | 31|±3/2〉 + 29|±5/2〉 + 19|±7/2〉 + 14|±9/2〉 | 0.0369 | 1.0730 | 3.6643 | 25 |
| KD4 | 923 | 786 | 48|±7/2〉 + 30|±3/2〉 + 14|±1/2〉 + 8|±5/2〉 | 2.0524 | 0.3009 | 3.7618 | 55 |
| KD5 | 1061/1110 | 968 | 70|±1/2〉 + 14|±3/2〉 + 8|±5/2〉 + 8|±7/2〉 | 4.3734 | 2.4200 | 0.4013 | 7 |
| Point-charge model | |||||||
| KD1 | 0 | 0 | 99.9|±9/2〉 | 0.0000 | 0.0000 | 6.5437 | — |
| KD2 | 536 | 466 | 99.6|±7/2〉 | 0.0013 | 0.0014 | 5.0823 | 3 |
| KD3 | 693/763 | 643 | 99.0|±5/2〉 | 0.0937 | 0.1002 | 3.6158 | 1 |
| KD4 | 923 | 770 | 95.0|±3/2〉 + 4.4|±1/2〉 | 1.4408 | 1.4927 | 2.0604 | 7 |
| KD5 | 1061/1110 | 867 | 94.8|±1/2〉 + 4.5|±3/2〉 | 5.0352 | 2.0948 | 0.6166 | 0 |
The strongly axial LF for 4f-prolate Kramers ions (Ce3+, Nd3+, and Dy3+) normally leads to the high purity of KDs in the |mJ〉 representation, with the ground state featuring the largest Jz projection. It also implies that pseudospin g-tensors of KDs have small transversal gx,y components, colinear principal gz axes, and gz values close to 2|mJ|gJ, where gJ is the Landé g-factor. The description we infer from ab initio calculations of Sc-3 is quite different (Table 1; see Table S3† for other conformers). KD1 indeed resembles the |±9/2〉 state with 85% contribution and the quantization axis aligned along the Nd–N bond with a deviation of 4.1°. The gz and gx,y values of KD1 are 5.689 and near 0.04, respectively, which can be compared to gz = 6.545 and gx,y = 0 expected for the pure |±9/2〉 doublet. However, higher-energy KDs have strongly mixed |mJ〉 composition and enhanced gx,y components, while their gz axes are rotated away from that of KD1. In contrast, the ab initio description of the isostructural DySc2N@C80 is entirely consistent with expectations for a strongly axial ligand field.45,120 The ground state KD1 has 99.7% weight of the |±15/2〉 state and gx,y is less than 10−4, while gz = 19.84 (versus 20.00 expected for the pure mJ = ±15/2 state).120 The next three KDs of DySc2N@C80 also have high contribution of one of the |±mJ〉 states (>80%), and the principal z axes of their pseudospin g-tensors are nearly collinear. The overall LF splitting in DySc2N@C80 is 1284 cm−1.
Thus, the molecular environment, in which the strong axiality is expected and indeed realized for Dy, produces quite different properties for Nd. We can recall that Nd3+ has considerably larger Steven's factor γj (−38 × 106) than Dy3+ (1.03 × 106), which increases the weight of Bqk terms with k = 6 in the ligand field. While for DyM2N@C80 molecules, Bq2, Bq4, and Bq6 contribute 65–80%, 5–10%, and 10–25% to ab initio computed LF splitting, respectively, in NdM2N@C80 the balance changes to similar contributions of Bq2 and Bq6 near 40–45%, while Bq4 terms cover 10–15%. The enhanced Bq6 weight implies the higher sensitivity of the Nd3+ ligand field to the environment, yet the deviation from axiality in NdM2N@C80 is too strong and cannot be explained by the γj factor alone. To understand if low symmetry or unobvious peculiarities of the electrostatic distribution may play a role, we obtained the LF parameters of NdSc2N@C80 using a point-charge model with LoProp121 charges from CASSCF calculations scaled by a factor of 1.08 to reproduce the ab initio Δ1,2 value. The KD energies calculated with such a LF Hamiltonian are not far from ab initio LF energies (Table 1), but the states are described by almost pure |mJ〉 functions, and KD1–KD3 have gz values close to 2|mJ|gJ. Similar results with a slightly higher degree of mJ-mixing were obtained when the model was adjusted by treating the Nd3+ ion quantum chemically at the CASSCF/RASSI level, while keeping point charges instead of all other atoms (Table S5†). From these models, we infer that the electrostatic environment around Nd3+ indeed favors LF axiality, while deviations from axiality in NdSc2N@C80 should be caused by non-electrostatic contributions, such as the covalency.122–124 Indeed, when CASSCF/RASSI computations were performed for the (NdSc2N)6+ cluster augmented with point charges mimicking the carbon atoms of the fullerene cage, a considerable state mixing was observed (Table S5c†). We thus conclude that the expansion of the valence space from Nd3+ through NdSc2N6+ to NdSc2N@C80 results in the systematic increase of the mJ mixing in Nd KDs and decrease of the axiality. Significant changes of the LF splitting induced by subtle variation of the local Nd environment in NdSc2N@C80 conformers also cannot be expected based on simple electrostatic considerations. Point-charge calculations for the conformer Sc-1 do not follow CASSCF results and predict similarly large KD2 energy as in Sc-3 (compare values in Table S5†). These facts highlight the importance of valence interactions and show that pure electrostatic models would be inappropriate for the description of Nd-EMF SMMs.
Comparison to other coordination environments, in which heavy lanthanide analogs exhibit very high magnetic axiality, reveals that the situation found here for NdSc2N@C80 is not unique for fullerenes but rather describes a common behaviour of Nd in molecular magnets. For instance, [Nd(H2O)5L2]3+ complexes with pentagonal bipyramidal coordination and axial phosphine oxide ligands,125,126 or Nd metallocenium complexes,100 all have the ground state KD with enhanced |±9/2〉 contribution (86–99%), but their higher-energy KDs are mixed considerably in the |mJ〉 representation. Likewise, the gx,y values of 0.01–0.02 found in KD1 of these complexes are small in comparison to gz values near 6.3, but appear quite large when compared to transversal g-tensor components of 10−4 in KD1 of similar Dy complexes. In other Nd-SMMs, for which ab initio calculations were performed, the degree of axiality is considerably smaller.
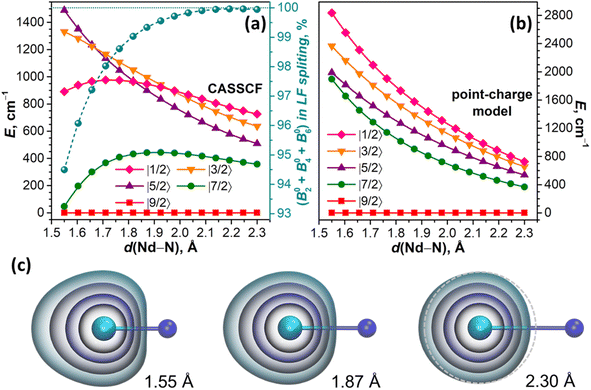
For the linear NdN molecule with the axial ligand field of C∞v symmetry, the LF Hamiltonian should include only B02, B04, and B06 terms, while LF states are described by pure |mJ〉 functions. At a Nd–N distance of 2.3 Å, the ab initio computed LF splitting is 726 cm−1, and the state energies increase in the descending order of |mJ| (Fig. 6a). The structure of states and their energies are reproduced closely by a point charge model using a LoProp charge of nitrogen (−1.513 e) scaled by a factor of 1.08 (Fig. 6b). The same factor was used then to scale the LoProp charges of nitrogen for point-charge computations at shorter Nd–N distances. According to the electrostatic model, a stepwise decrease of the Nd–N distance from 2.3 Å to 1.55 Å does not change the order of states and only gradually increases the LF splitting to 2836 cm−1, while the energies of all LF states increase monotonously (Fig. 6b). A very different Nd–N distance dependence emerges from CASSCF calculations. The energies of |±7/2〉 and |±1/2〉 doublets relative to |±9/2〉 pass through a maximum and then decrease, and |±3/2〉 also changes a curvature at short Nd–N, and only |±5/2〉 grows continuously in the whole range. The order of |mJ〉 states thus changes several times, and the overall LF splitting at 1.55 Å is almost twice smaller than predicted by the point-charge model (Fig. 6a).
From the contributions of ab initio computed Bqk terms to LF splitting provided by the Single_aniso code,122,127 we infer that the weight of the B02 term, which amounts to 87% at 2.3 Å, decreases to 49% at 1.55 Å, and the weight of B06 increases from 7% at 2.3 Å to 38% at 1.55 Å, while the sign of B06 changes from negative to positive below 2.1 Å (in the point-charge model, B06 is negative through the whole range). Furthermore, the LF Hamiltonian, comprising only B02, B04, and B06, cannot reproduce the LF splitting at short Nd–N distances, and requires addition of B08. The weight of the latter increases from negligible 0.05% at 2.3 Å to significant 5.5% at 1.55 Å (Fig. 6a). As Bqk terms with k > 6 should vanish for pure f orbitals, their non-zero contribution serves as a diagnostic criterion for the breakdown of the assumption of the isolated spherically shaped 4f shell.122 Indeed, the shape of 4f-electron density distribution changes from nearly spherical at 2.3 Å to a considerably elongated one at short Nd–N distances (Fig. 6c). This naturally leads to the notion of 4f AOs mixing with other orbitals and the overall increase of the covalency as the Nd–N distance shrinks. Of course, the distance of 1.55 Å is unrealistically short, but it serves here only for a clear-cut demonstration, whereas the effect is already present at more realistic distances (Fig. 6c). Importantly, ab initio calculations of NdM2N@C80 similarly showed that the LF Hamiltonian including Bqk terms with k ≤ 6 recovers only ∼95% of the ab initio LF splitting, while covering the remaining 5% requires Bq8 terms.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900–11
900–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1, which is lower than the usual energy of the 4F3/2 multiplet by around 1100 cm−1. Such a covalency-induced reduction of the excitation energies in coordination compounds of transition metals and lanthanides is known as the nephelauxetic effect.128
000 cm−1, which is lower than the usual energy of the 4F3/2 multiplet by around 1100 cm−1. Such a covalency-induced reduction of the excitation energies in coordination compounds of transition metals and lanthanides is known as the nephelauxetic effect.128
Numerical representation of the nephelauxetic effect is often given by nephelauxetic parameters βF = F2(compound)/F2(free ion), where F2 is the second-order Slater integral describing interelectron repulsion, or β′ = ζ4f/ζ4f(free ion), where ζ4f is the Lande spin–orbit coupling constant. The degree of covalency, defined as an admixture of ligand orbitals to f-like orbitals, is sometimes estimated as b0.5 = [(1 − β)/2]0.5, although it appears somewhat ambiguous because βF and β′ can be quite different, F2 being more sensitive to the bonding nature.129,130 For instance, the energy differences between 4IJ levels are mainly determined by ζ4f, and since the gaps between 4IJ levels in the NdM2N@C80 series are similar to other Nd compounds, we can conclude that ζ4f is not strongly affected. The energy differences between multiplets with different L, on the other hand, are more sensitive to inter-electron repulsion parameters, and the reduced 4F3/2 energy in NdM2N@C80 indicates that F2 is significantly modified.
Experimentally, ζ4f, F2 and other Fk integrals can be determined by fitting the Hamiltonian, comprising free-ion and crystal-field terms, to spectroscopic data. As such a Hamiltonian includes a large number of parameters, extended data on many multiplets in a broad energy range are required for a reliable fitting. In the absence of such data, which is the case for NdM2N@C80 and the majority of Nd-SMMs, a simplified spectroscopic nephelauxetic parameter βspec based on the transition energy between selected multiplets can be used: βspec = ΔE(compound)/ΔE(standard). For the following discussion, we define βspec as the energy difference between barycenters of 4I9/2 and 4F3/2 multiplets divided by the free ion value75 ΔE(4F3/2–4I9/2) = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 699 cm−1. When the 4F3/2 → 4I9/2 PL band is not resolved into LF components, as is the case for many Nd-SMMs, PL band energy is simply used instead of barycenters, which can introduce an uncertainty to βspec of ∼0.01.
699 cm−1. When the 4F3/2 → 4I9/2 PL band is not resolved into LF components, as is the case for many Nd-SMMs, PL band energy is simply used instead of barycenters, which can introduce an uncertainty to βspec of ∼0.01.
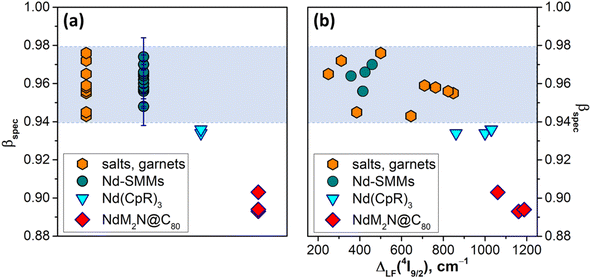
Fig. 7a plots βspec values in NdM2N@C80, all 17 PL-characterized Nd-SMMs reported before this work,30–33,36,81–85,112,131,132 selected tris(η5-cyclopentadienyl)Nd complexes with large ligand field splitting,116–118 and several inorganic salts and garnets thoroughly characterized by optical spectroscopy133–139 (see Table S9† for the compound titles, numerical values, and literature references for each entry). The latter group is the most studied experimentally, and for this analysis we selected the set of compounds encompassing the broadest range of βspec parameters, from the most ionic ones like Nd:LaF3 (ref. 139) with βspec = 0.976 to the most covalent Nd:Y2O3 (ref. 133) and Nd2S3 (ref. 134) with βspec of 0.943–0.945. A similar span of βspec from 0.948 to 0.982 is found for Nd-SMMs. It appears safe to state that the βspec range of 0.94–0.98 is where the vast majority of Nd compounds will be found. Amberger et al. demonstrated that Nd(CpR)3 complexes (CpR = C5Me5, C5Me4H, C5H4tBu, C5H4SiMe3, etc.) with enhanced metal–ligand interactions have large LF splitting and reduced nephelauxetic parameters βF (see ref. 116 for comparison of LF strength and βF in a series of Nd complexes).116–118 Likewise, their βspec values of 0.934–0.936 fall below the normal βspec range and were presumably the lowest values among Nd-compounds before our study. Analyzed in this broad context, the βspec parameters found in this work for the NdM2N@C80 series, 0.893 for NdLu2N@C80, 0.894 for NdY2N@C80, and 0.903 for NdSc2N@C80, are unprecedently small. This very strong nephelauxetic effect evidences one more time that by increasing the internal strain in metallofullerenes we achieved a much higher degree of covalency than is usually observed in Nd compounds. It is also instructive to find that there is seemingly no correlation between the nephelauxetic effect and ligand field splitting for the compounds in the normal βspec range (Fig. 7b). However, as we leave this range, the decrease of βspec starts to correlate with the increase of ΔLF. This is not surprising given that the strengthening of the ligand field is achieved by enhancing the interactions between the lanthanide ion and the ligands, but it will require more compounds with such unusual properties to establish firm correlations.
The strong nephelauxetic effect and ligand field splitting may also be among the reasons for the short luminescence lifetimes of NdM2N@C80. The low energy of the NIR-emitting state in lanthanide compounds is responsible for the efficient nonradiative vibrational relaxation. For Nd3+, the non-radiative relaxation rate is determined by the gap between 4F3/2 and 4I15/2, which typically is around 5400 cm−1. The 4I15/2 multiplet of NdM2N@C80 is not directly accessible in our experiments, but based on the experimental and computational data on other 4IJ multiplets (Table S4†), we can assume that the energy of 4I15/2 is also close to standard values for Nd3+ and that the LF splitting of the 4I15/2 multiplet amounts to ΔLF ≈ 1300 cm−1 (Table S4†). The lower than usual energy of the 4F3/2 state and the strong LF splitting of the 4I15/2 multiplet reduce the gap between the KD1 of 4F3/2 and KD8 of 4I15/2 to ≈3600 cm−1. This low gap opens the possibility for two-phonon relaxation and thus, in accordance with the energy gap law, is a plausible reason for the anomalously short Nd3+ luminescence lifetimes in NdM2N@C80 molecules.
Magnetic properties of NdSc2N@C80
While the energies of LF states are accessible via PL spectroscopy, experimental verification of the state compositions is not straightforwardly available from this technique. Complimentary information on the ground state may be given by magnetometry. The large Δ1,2 value in the 4I9/2 multiplet suggests that the low-temperature magnetic properties of NdM2N@C80 will be dominated by the ground state doublet.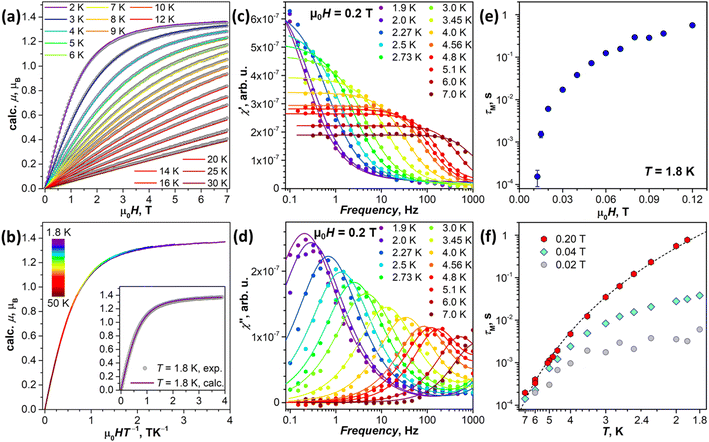 | ||
| Fig. 8 (a) Experimental magnetization curves of NdSc2N@C80 (gray dots) compared to calculations (colored lines) for pseudospin s˜ = 1/2 with the ab initio computed g-tensor corresponding to the ground-state Kramers doublet (KD1, Table 1). (b) Magnetization curves of NdSc2N@C80 measured between 1.8 K and 50 K and presented as a function of the HT−1 product; the inset shows the comparison of experimental and calculated data for T = 1.8 K. (c) and (d) AC magnetometry measurements of in-phase χ′ (c) and out-of-phase χ′′ (d) magnetic susceptibility of NdSc2N@C80 in the constant field of 0.2 T at T = 1.9–7 K; dots are experimental data, and lines are fits with the generalized Debye model, from which relaxation times are extracted. (e) Magnetic field dependence of magnetization relaxation time at T = 1.8 K. (f) Temperature dependencies of magnetization relaxation times measured in the field of 0.02 T, 0.04 T, and 0.2 T; dashed line is a fit of 0.2 T data with the function τM−1 = CTn (C = 0.0205 and n = 6.59). | ||
![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z〉 = 2.02 μB and 〈Ŝz〉 = −0.45 μB, and the magnetic moment along the beam direction, μz = 1.11 μB (see the ESI for the calculation details†). The uncertainty in the determined value of μz is mainly caused by ambiguities in integration limits for the Nd-M4 edge and is estimated to be less than ±0.1 μB. The ratio 〈Ŝz〉/〈
z〉 = 2.02 μB and 〈Ŝz〉 = −0.45 μB, and the magnetic moment along the beam direction, μz = 1.11 μB (see the ESI for the calculation details†). The uncertainty in the determined value of μz is mainly caused by ambiguities in integration limits for the Nd-M4 edge and is estimated to be less than ±0.1 μB. The ratio 〈Ŝz〉/〈![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z〉 = −0.22 is close to the ab initio prediction of −0.24 for the ground state doublet (note that a theoretical expectation for the f3 system is −0.25). For comparison, in the assumption of a disordered powder, the ab initio predicted ground-state g-tensor of NdSc2N@C80 gives a saturated magnetic moment of 1.38 μB. At T = 5.5 K and under an applied magnetic field of 6 T, the theoretical moment is reduced to 1.14 μB, which is very close to the value we determined from XMCD measurements. At the same time, the ground state with the pure mJ = ±9/2 composition would have a saturated magnetic moment of 1.65 μB, while the value at 5.5 K and 6 T would be 1.43 μB. Thus, XMCD measurements confirm the ab initio prediction of the considerable deviation of the NdSc2N@C80 ground state KD from the pure mJ = ±9/2 state.
z〉 = −0.22 is close to the ab initio prediction of −0.24 for the ground state doublet (note that a theoretical expectation for the f3 system is −0.25). For comparison, in the assumption of a disordered powder, the ab initio predicted ground-state g-tensor of NdSc2N@C80 gives a saturated magnetic moment of 1.38 μB. At T = 5.5 K and under an applied magnetic field of 6 T, the theoretical moment is reduced to 1.14 μB, which is very close to the value we determined from XMCD measurements. At the same time, the ground state with the pure mJ = ±9/2 composition would have a saturated magnetic moment of 1.65 μB, while the value at 5.5 K and 6 T would be 1.43 μB. Thus, XMCD measurements confirm the ab initio prediction of the considerable deviation of the NdSc2N@C80 ground state KD from the pure mJ = ±9/2 state.
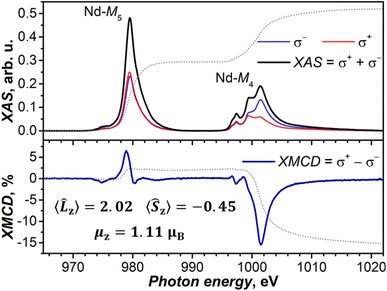 | ||
Fig. 9 XAS (upper panel) and XMCD (lower panel) spectra of NdSc2N@C80 at the Nd-M4,5 edges (3d → 4f), T ≈ 5.5 K, and μ0H = 6 T. Left-hand and right-hand polarized XAS spectra are denoted as σ+ and σ−, and non-polarized XAS spectrum is their sum, while XMCD (in %) is their difference normalized to the maximum of XAS. Dotted lines are integrals of XAS and XMCD used in the sum rule analysis, which provided 〈![[L with combining circumflex]](https://www.rsc.org/images/entities/i_char_004c_0302.gif) z〉, 〈Ŝz〉, and μz values (see the ESI for details†). Measurements were performed at the BOREAS beamline (synchrotron ALBA).140 z〉, 〈Ŝz〉, and μz values (see the ESI for details†). Measurements were performed at the BOREAS beamline (synchrotron ALBA).140 | ||
The temperature dependence of τM was measured in the fields of 0.02, 0.04, and 0.2 T (Table S11†). The QTM is fully suppressed at 0.2 T, and the temperature dependence of τM can be well described by the Raman relaxation mechanism, τM−1 = CTn, with the fitted parameters C = 0.0205 ± 0.0026 s−1 K−n and n = 6.59 ± 0.09 (Fig. 8f). The contribution of the QTM, inducing faster relaxation with flatter temperature dependence at lower temperatures, is clearly visible in smaller fields (Fig. 8f). Higher-energy LF states are thus not involved in the reorientation of NdSc2N@C80 magnetic moment as the system finds an underbarrier shortcut with the help of phonon excitations.
The relaxation behavior observed in this work for NdSc2N@C80 can be compared to that of non-fullerene Nd-SMMs. As discussed above, Nd-SMMs usually have considerable gx,y components in the ground state, which lead to fast zero-field QTM. Only three Nd complexes, two of [Nd(H2O)5L2]3+ type with pentagonal bipyramidal coordination geometry (L = tBuPO(NHiPr)2 in ref. 125 and CyPh2PO in ref. 126), and the (COT)Nd(Cpttt) sandwich diluted with the Y analog,115 exhibited slow relaxation of magnetization in zero magnetic field. In all other Nd-SMMs, the slow relaxation required the DC magnetic field to suppress the fast QTM. [Nd(H2O)5(CyPh2PO)2]3+ and (COT)Nd(Cpttt) complexes are also the only Nd-SMMs, for which a narrow magnetic hysteresis could be recorded at 2 K.115,126 For other Nd-SMMs, the slow relaxation was attested only by AC measurements, implying relaxation times shorter than 1 s. The temperature dependence of relaxation times for some Nd-SMMs was described by the Raman mechanism with an exponent of n = 6–7,100,114,126,144–146 similar to the value of n = 6.59 determined in this work for NdSc2N@C80. When instead the Orbach mechanism was assumed to describe the relaxation of Nd-SMMs, the barriers obtained from the fits of the temperature dependence were usually several times smaller than the energies of excited LF states predicted ab initio.113,114,125,126,144–146 This inconsistency is a strong indication that the Orbach mechanism is rarely operative for Nd-SMMs, while relaxation of magnetization is more often governed by the Raman mechanism, which may also involve coupling to optical phonons.
Conclusions
The synthesis of a NdM2N@C80 series with diamagnetic rare-earth metals of different sizes (M = Sc, Y, Lu) allowed us to analyze the influence of internal strain on the magnetic states and optical properties of Nd3+ in these metallofullerenes. We found that Nd3+ ions in nitride clusterfullerenes experience the strongest ligand field ever observed in molecular complexes of Nd, causing the LF splitting of the ground-state 4I9/2 multiplet reaching 1100–1200 cm−1. This strong ligand field is the result of the unusually short Nd–N bond lengths and the large negative charge of the nitride ion. At the same time, a confinement of the NdM2N cluster of a variable size inside the fullerene cage results in the increase of the strain from Sc to Lu to Y, which leads to the change of the geometrical shape of the cluster and substantial variation of the LF splitting pattern of the Nd3+ ion. The latter could be directly addressed by photoluminescence spectroscopy, which showed finely structured narrow emission lines caused by f–f transitions in Nd3+. When compared to their energies in Nd3+ compounds, the PL bands of NdM2N@C80 are considerably red-shifted, which points to the very strong nephelauxetic effect and enhanced contribution of covalency in Nd bonding.The enhanced covalency is the price for the large LF splitting and, as shown by ab initio modelling, is the reason for the reduced magnetic axiality in NdM2N@C80. Point-charge modelling demonstrates that pure electrostatic interactions in these molecules would produce highly axial LF states with sheer mJ wavefunction compositions. Experimental studies of magnetic properties revealed that NdSc2N@C80 exhibits slow relaxation of magnetization only in the presence of an external magnetic field, whereas its zero-field relaxation is dominated by the fast tunneling process. The large LF splitting also does not help to slow down the in-field relaxation of magnetization, which appears to be governed by the under-barrier Raman mechanism.
The results of this work demonstrate that pushing the LF strength to its extreme by enhancing interactions of Nd ions with surrounding atoms does not improve the magnetic axiality even in the molecular geometry, which favors such axiality for heavy lanthanides. The reason is that the larger ionic radius makes Nd more susceptible to enhanced covalency. Theoretically, the single-ion magnetic anisotropy of Nd3+ could be made highly axial by placing point charges at short distances, but in reality, the strong LF would require an interaction with strong ligands placed at close distances, which inevitably leads to the enhanced covalency and all its negative effects on the magnetic axiality discussed in this work. This dichotomy between the strong ligand field and reduced magnetic axiality appears to be a fundamental limitation for creating high-performance SMMs based on light lanthanides. Nevertheless, the large LF splitting appears very instrumental in separating f–f transitions and enabling direct optical access to individual spin states of Nd3+, which can be useful for application in magneto-photonic quantum technologies. Another possible consequence of enhanced covalency worth mentioning is that the 4f-shell can become more susceptible to manipulations by other methods like scanning tunneling microscopy, which is usually considered not amenable to probe 4f electrons in molecules due to their core-like behavior. Thus, while Nd-EMFs may not perform great as single molecular magnets, they show good prospects for optically and electronically addressable quantum bits. Importantly, their LF splitting and spin-state wavefunction can be further tuned by varying the composition of the endohedral cluster or exohedral chemical modification of metallofullerenes.
Data availability
The data supporting the findings of this study are available from the corresponding authors upon reasonable request.Author contributions
WY synthesized fullerenes with help and supervision from FL; MR and FZ performed PL measurements; VD performed DFT calculations under the supervision of SMA; GV and MB performed SQUID measurements; SS performed vibrational spectroscopic studies; MR, GV, MB, LS, CG, MV and AAP performed the XMCD study; SMA performed ab initio calculations; FL grew single-crystals and performed the SC-XRD study; AAP conceived and supervised the study; FL, SMA, and AAP wrote the manuscript with contributions from all co-authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support by the Deutsche Forschungsgemeinschaft (grants PO 1602/7-1, PO 1602/11-1, LI 3055/3-1, and AV 169/3-1) and the China Scholarship Council (Fellowship to W. Y.). Diffraction data have been collected on BL14.2 at the BESSY II electron storage ring operated by the Helmholtz-Zentrum Berlin; we would like to acknowledge the help and support of Manfred Weiss and his group members during the experiments at BESSY II. XMCD measurements at the synchrotron ALBA were performed in the framework of the beamtime proposal ID 20201035073. Computational resources were provided by the Center for High Performance Computing at the TU Dresden. We appreciate the technical support with computational resources in IFW Dresden by Ulrike Nitzsche. Dr Anja Wolter-Giraud and Sebastian Gaβ are acknowledged for the help with SQUID magnetic measurements.References
- J. Long, Y. Guari, R. A. S. Ferreira, L. D. Carlos and J. Larionova, Coord. Chem. Rev., 2018, 363, 57–70 CrossRef CAS
.
- R. Marin, G. Brunet and M. Murugesu, Angew. Chem., Int. Ed., 2021, 60, 1728–1746 CrossRef CAS PubMed
.
- J.-H. Jia, Q.-W. Li, Y.-C. Chen, J.-L. Liu and M.-L. Tong, Coord. Chem. Rev., 2019, 378, 365–381 CrossRef CAS
.
- F. Pointillart, B. le Guennic, O. Cador, O. Maury and L. Ouahab, Acc. Chem. Res., 2015, 48, 2834–2842 CrossRef CAS PubMed
.
- R. Jankowski, M. Wyczesany and S. Chorazy, Chem. Commun., 2023, 59, 5961–5986 RSC
.
- G. Cucinotta, M. Perfetti, J. Luzon, M. Etienne, P. E. Car, A. Caneschi, G. Calvez, K. Bernot and R. Sessoli, Angew. Chem., Int. Ed., 2012, 51, 1606 CrossRef CAS PubMed
.
- J. Long, R. Vallat, R. A. S. Ferreira, L. D. Carlos, F. A. Almeida Paz, Y. Guari and J. Larionova, Chem. Commun., 2012, 48, 9974–9976 RSC
.
- S. Shintoyo, K. Murakami, T. Fujinami, N. Matsumoto, N. Mochida, T. Ishida, Y. Sunatsuki, M. Watanabe, M. Tsuchimoto, J. Mrozinski, C. Coletti and N. Re, Inorg. Chem., 2014, 53, 10359–10369 CrossRef CAS PubMed
.
- Y. Bi, C. Chen, Y.-F. Zhao, Y.-Q. Zhang, S.-D. Jiang, B.-W. Wang, J.-B. Han, J.-L. Sun, Z.-Q. Bian, Z.-M. Wang and S. Gao, Chem. Sci., 2016, 7, 5020–5031 RSC
.
- E. Mamontova, J. Long, R. A. S. Ferreira, A. M. P. Botas, D. Luneau, Y. Guari, L. D. Carlos and J. Larionova, Magnetochemistry, 2016, 2, 41 CrossRef
.
- J. Long, E. Mamontova, V. Freitas, D. Luneau, V. Vieru, L. F. Chibotaru, R. A. S. Ferreira, G. Felix, Y. Guari, L. D. Carlos and J. Larionova, RSC Adv., 2016, 6, 108810–108818 RSC
.
- Y.-C. Chen, J.-L. Liu, Y. Lan, Z.-Q. Zhong, A. Mansikkamäki, L. Ungur, Q.-W. Li, J.-H. Jia, L. F. Chibotaru, J.-B. Han, W. Wernsdorfer, X.-M. Chen and M.-L. Tong, Chem.–Euro. J., 2017, 23, 5708–5715 CrossRef CAS PubMed
.
- F. Guégan, F. Riobé, O. Maury, J. Jung, B. Le Guennic, C. Morell and D. Luneau, Inorg. Chem. Front., 2018, 5, 1346–1353 RSC
.
- M. Perfetti, M. Gysler, Y. Rechkemmer-Patalen, P. Zhang, H. Taştan, F. Fischer, J. Netz, W. Frey, L. W. Zimmermann, T. Schleid, M. Hakl, M. Orlita, L. Ungur, L. Chibotaru, T. Brock-Nannestad, S. Piligkos and J. van Slageren, Chem. Sci., 2019, 10, 2101–2110 RSC
.
- D. Guettas, F. Gendron, G. Fernandez Garcia, F. Riobé, T. Roisnel, O. Maury, G. Pilet, O. Cador and B. Le Guennic, Chem.–Eur. J., 2020, 26, 4389–4395 CrossRef CAS PubMed
.
- R. A. S. Ferreira, E. Mamontova, A. M. P. Botas, M. Shestakov, J. Vanacken, V. Moshchalkov, Y. Guari, L. F. Chibotaru, D. Luneau, P. S. André, J. Larionova, J. Long and L. D. Carlos, Adv. Opt. Mater., 2021, 9, 2101495 CrossRef CAS
.
- Y. Liu, B.-H. Lyu, S.-N. Du, G.-Z. Huang, Z.-Y. Ruan, S.-G. Wu, J.-L. Liu and M.-L. Tong, Dalton Trans., 2021, 50, 6778–6783 RSC
.
- F. Pointillart, B. L. Guennic, S. Golhen, O. Cador, O. Maury and L. Ouahab, Chem. Commun., 2013, 49, 615–617 RSC
.
- K. S. Pedersen, J. Dreiser, H. Weihe, R. Sibille, H. V. Johannesen, M. A. Sørensen, B. E. Nielsen, M. Sigrist, H. Mutka, S. Rols, J. Bendix and S. Piligkos, Inorg. Chem., 2015, 54, 7600–7606 CrossRef CAS PubMed
.
- K. Soussi, J. Jung, F. Pointillart, B. Le Guennic, B. Lefeuvre, S. Golhen, O. Cador, Y. Guyot, O. Maury and L. Ouahab, Inorg. Chem. Front., 2015, 2, 1105–1117 RSC
.
- G. Brunet, R. Marin, M.-J. Monk, U. Resch-Genger, D. A. Gálico, F. A. Sigoli, E. A. Suturina, E. Hemmer and M. Murugesu, Chem. Sci., 2019, 10, 6799–6808 RSC
.
- F. Guégan, J. Jung, B. Le Guennic, F. Riobé, O. Maury, B. Gillon, J.-F. Jacquot, Y. Guyot, C. Morell and D. Luneau, Inorg. Chem. Front., 2019, 6, 3152–3157 RSC
.
- W.-B. Chen, L. Zhong, Y.-J. Zhong, Y.-Q. Zhang, S. Gao and W. Dong, Inorg. Chem. Front., 2020, 7, 3136–3145 RSC
.
- F. Gendron, S. Di Pietro, L. Abad Galán, F. Riobé, V. Placide, L. Guy, F. Zinna, L. Di Bari, A. Bensalah-Ledoux, Y. Guyot, G. Pilet, F. Pointillart, B. Baguenard, S. Guy, O. Cador, O. Maury and B. Le Guennic, Inorg. Chem. Front., 2021, 8, 914–926 RSC
.
- K. Karachousos-Spiliotakopoulos, V. Tangoulis, N. Panagiotou, A. Tasiopoulos, E. Moreno-Pineda, W. Wernsdorfer, M. Schulze, A. M. P. Botas and L. D. Carlos, Dalton Trans., 2022, 51, 8208–8216 RSC
.
- K. Yamashita, R. Miyazaki, Y. Kataoka, T. Nakanishi, Y. Hasegawa, M. Nakano, T. Yamamura and T. Kajiwara, Dalton Trans., 2013, 42, 1987–1990 RSC
.
- K. Ehama, Y. Ohmichi, S. Sakamoto, T. Fujinami, N. Matsumoto, N. Mochida, T. Ishida, Y. Sunatsuki, M. Tsuchimoto and N. Re, Inorg. Chem., 2013, 52, 12828–12841 CrossRef CAS PubMed
.
- E. A. Mikhalyova, M. Zeller, J. P. Jasinski, R. J. Butcher, L. M. Carrella, A. E. Sedykh, K. S. Gavrilenko, S. S. Smola, M. Frasso, S. C. Cazorla, K. Perera, A. Shi, H. G. Ranjbar, C. Smith, A. Deac, Y. Liu, S. M. McGee, V. P. Dotsenko, M. U. Kumke, K. Müller-Buschbaum, E. Rentschler, A. W. Addison and V. V. Pavlishchuk, Dalton Trans., 2020, 49, 7774–7789 RSC
.
- J. Wang, J. J. Zakrzewski, M. Zychowicz, V. Vieru, L. F. Chibotaru, K. Nakabayashi, S. Chorazy and S.-i. Ohkoshi, Chem. Sci., 2021, 12, 730–741 RSC
.
- I. Oyarzabal, B. Artetxe, A. Rodríguez-Diéguez, J. Á. García, J. M. Seco and E. Colacio, Dalton Trans., 2016, 45, 9712–9726 RSC
.
- B. Casanovas, S. Speed, O. Maury, M. S. El Fallah, M. Font-Bardía and R. Vicente, Eur. J. Inorg. Chem., 2018, 2018, 3859–3867 CrossRef CAS
.
- B. Casanovas, S. Speed, O. Maury, M. Font-Bardía and R. Vicente, Polyhedron, 2019, 169, 187–194 CrossRef CAS
.
- Y. H. Pham, V. A. Trush, M. Korabik, V. M. Amirkhanov and P. Gawryszewska, Dyes Pigm., 2021, 186, 108986 CrossRef CAS
.
- F. Pointillart, B. Le Guennic, T. Cauchy, S. Golhen, O. Cador, O. Maury and L. Ouahab, Inorg. Chem., 2013, 52, 5978 CrossRef CAS PubMed
.
- M. Karbowiak, C. Rudowicz, T. Nakamura, R. Murakami and T. Ishida, Chem. Phys. Lett., 2016, 662, 163–168 CrossRef CAS
.
- K. Kumar, D. Abe, K. Komori-Orisaku, O. Stefańczyk, K. Nakabayashi, J. R. Shakirova, S. P. Tunik and S.-i. Ohkoshi, RSC Adv., 2019, 9, 23444–23449 RSC
.
- D. Errulat, R. Marin, D. A. Gálico, K. L. M. Harriman, A. Pialat, B. Gabidullin, F. Iikawa, O. D. D. Couto, J. O. Moilanen, E. Hemmer, F. A. Sigoli and M. Murugesu, ACS Cent. Sci., 2019, 5, 1187–1198 CrossRef CAS PubMed
.
- D. Serrano, S. K. Kuppusamy, B. Heinrich, O. Fuhr, D. Hunger, M. Ruben and P. Goldner, Nature, 2022, 603, 241–246 CrossRef CAS PubMed
.
- D. W. Laorenza and D. E. Freedman, J. Am. Chem. Soc., 2022, 144, 21810–21825 CrossRef CAS PubMed
.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, H. Brune, S. Rusponi, F. Nolting, A. Popov, S. Yang, L. Dunsch and T. Greber, J. Am. Chem. Soc., 2012, 134, 9840–9843 CrossRef PubMed
.
- L. Spree, F. Liu, V. Neu, M. Rosenkranz, G. Velkos, Y. Wang, S. Schiemenz, J. Dreiser, P. Gargiani, M. Valvidares, C.-H. Chen, B. Büchner, S. M. Avdoshenko and A. A. Popov, Adv. Funct. Mater., 2021, 31, 2105516 CrossRef CAS
.
- F. Liu, G. Velkos, D. S. Krylov, L. Spree, M. Zalibera, R. Ray, N. A. Samoylova, C.-H. Chen, M. Rosenkranz, S. Schiemenz, F. Ziegs, K. Nenkov, A. Kostanyan, T. Greber, A. U. B. Wolter, M. Richter, B. Büchner, S. M. Avdoshenko and A. A. Popov, Nat. Commun., 2019, 10, 571 CrossRef CAS PubMed
.
- L. Spree and A. A. Popov, Dalton Trans., 2019, 48, 2861–2871 RSC
.
- W. Li, C.-R. Wang and T. Wang, Chem. Commun., 2021, 57, 10317–10326 RSC
.
- V. Vieru, L. Ungur and L. F. Chibotaru, J. Phys. Chem. Lett., 2013, 4, 3565–3569 CrossRef CAS
.
- F. Cimpoesu, N. Dragoe, H. Ramanantoanina, W. Urland and C. Daul, Phys. Chem. Chem. Phys., 2014, 16, 11337–11348 RSC
.
- M. K. Singh and G. Rajaraman, Chem. Commun., 2016, 52, 14047–14050 RSC
.
- G. Rajaraman, M. K. Singh and N. Yadav, Chem. Commun., 2015, 51, 17732–17735 RSC
.
- C.-H. Chen, D. S. Krylov, S. M. Avdoshenko, F. Liu, L. Spree, R. Yadav, A. Alvertis, L. Hozoi, K. Nenkov, A. Kostanyan, T. Greber, A. U. B. Wolter and A. A. Popov, Chem. Sci., 2017, 8, 6451–6465 RSC
.
- D. S. Krylov, F. Liu, S. M. Avdoshenko, L. Spree, B. Weise, A. Waske, A. U. B. Wolter, B. Büchner and A. A. Popov, Chem. Commun., 2017, 53, 7901–7904 RSC
.
- W. Yang, G. Velkos, F. Liu, S. M. Sudarkova, Y. Wang, J. Zhuang, H. Zhang, X. Li, X. Zhang, B. Büchner, S. M. Avdoshenko, A. A. Popov and N. Chen, Adv. Sci., 2019, 6, 1901352 CrossRef CAS PubMed
.
- L. Spree, C. Schlesier, A. Kostanyan, R. Westerström, T. Greber, B. Büchner, S. Avdoshenko and A. A. Popov, Chem.–Eur. J., 2020, 26, 2436–2449 CrossRef CAS PubMed
.
- M. Zalibera, F. Ziegs, S. Schiemenz, V. Dubrovin, W. Lubitz, A. Savitsky, S. Deng, X.-B. Wang, S. Avdoshenko and A. A. Popov, Chem. Sci., 2021, 12, 7818–7838 RSC
.
- M. Zalibera, D. S. Krylov, D. Karagiannis, P.-A. Will, F. Ziegs, S. Schiemenz, W. Lubitz, S. Reineke, A. Savitsky and A. A. Popov, Angew. Chem., Int. Ed., 2018, 57, 277–281 CrossRef CAS PubMed
.
- G. Dantelle, A. Tiwari, R. Rahman, S. R. Plant, K. Porfyrakis, M. Mortier, R. A. Taylor and A. D. Briggs, Opt. Mater., 2009, 32, 251–256 CrossRef CAS
.
- A. Tiwari, G. Dantelle, K. Porfyrakis, R. A. Taylor, A. A. R. Watt, A. Ardavan and G. A. D. Briggs, J. Chem. Phys., 2007, 127, 194504 CrossRef PubMed
.
- R. M. Macfarlane, D. S. Bethune, S. Stevenson and H. C. Dorn, Chem. Phys. Lett., 2001, 343, 229–234 CrossRef CAS
.
- M. Nie, J. Xiong, C. Zhao, H. Meng, K. Zhang, Y. Han, J. Li, B. Wang, L. Feng, C. Wang and T. Wang, Nano Res., 2019, 12, 1727–1731 CrossRef CAS
.
- M. Nie, L. Yang, C. Zhao, H. Meng, L. Feng, P. Jin, C.-R. Wang and T. Wang, Nanoscale, 2019, 11, 18612–18618 RSC
.
- Y. Ito, T. Okazaki, S. Okubo, M. Akachi, Y. Ohno, T. Mizutani, T. Nakamura, R. Kitaura, T. Sugai and H. Shinohara, ACS Nano, 2007, 1, 456–462 CrossRef CAS PubMed
.
- S. R. Plant, G. Dantelle, Y. Ito, T. C. Ng, A. Ardavan, H. Shinohara, R. A. Taylor, A. D. Briggs and K. Porfyrakis, Chem. Phys. Lett., 2009, 476, 41–45 CrossRef CAS
.
- D. Xu, Y. Jiang, Y. Wang, T. Zhou, Z. Shi, H. Omachi, H. Shinohara, B. Sun and Z. Wang, Inorg. Chem., 2019, 58, 14325–14330 CrossRef CAS PubMed
.
- H. Jin, J. Xin, W. Xiang, Z. Jiang, X. Han, M. Chen, P. Du, Y.-R. Yao and S. Yang, Adv. Mater., 2023, 35, 2304121 CrossRef CAS PubMed
.
- Z. Wang, N. Izumi, Y. Nakanishi, T. Koyama, T. Sugai, M. Tange, T. Okazaki and H. Shinohara, ACS Nano, 2016, 10, 4282–4287 CrossRef CAS PubMed
.
- Y. Zhang, D. Krylov, M. Rosenkranz, S. Schiemenz and A. A. Popov, Chem. Sci., 2015, 6, 2328–2341 RSC
.
- A. Kostanyan, R. Westerström, D. Kunhardt, B. Büchner, A. A. Popov and T. Greber, Phys. Rev. B, 2020, 101, 134429 CrossRef CAS
.
- F. Liu, S. Wang, C.-L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerström, F. Jin, S.-Y. Xie, A. A. Popov, T. Greber and S. Yang, Angew. Chem., Int. Ed., 2017, 56, 1830–1834 CrossRef CAS PubMed
.
- F. Liu, C.-L. Gao, Q. Deng, X. Zhu, A. Kostanyan, R. Westerström, S. Wang, Y.-Z. Tan, J. Tao, S.-Y. Xie, A. A. Popov, T. Greber and S. Yang, J. Am. Chem. Soc., 2016, 138, 14764–14771 CrossRef CAS PubMed
.
- G. Velkos, D. Krylov, K. Kirkpatrick, L. Spree, V. Dubrovin, B. Büchner, S. Avdoshenko, V. Bezmelnitsyn, S. Davis, P. Faust, J. Duchamp, H. Dorn and A. A. Popov, Angew. Chem., Int. Ed., 2019, 58, 5891–5896 CrossRef CAS PubMed
.
- Y. Wang, G. Velkos, N. J. Israel, M. Rosenkranz, B. Büchner, F. Liu and A. A. Popov, J. Am. Chem. Soc., 2021, 143, 18139–18149 CrossRef CAS PubMed
.
- F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Büchner and A. A. Popov, Nat. Commun., 2017, 8, 16098 CrossRef CAS PubMed
.
- R. Westerström, J. Dreiser, C. Piamonteze, M. Muntwiler, S. Weyeneth, K. Krämer, S.-X. Liu, S. Decurtins, A. Popov, S. Yang, L. Dunsch and T. Greber, Phys. Rev. B, 2014, 89, 060406 CrossRef
.
- Y. Wang, J. Xiong, J. Su, Z.-Q. Hu, F. Ma, R. Sun, X.-Y. Tan, H.-L. Sun, B. Wang, Z. Shi and S. Gao, Nanoscale, 2020, 12, 11130–11135 RSC
.
- J. Dreiser, R. Westerström, Y. Zhang, A. A. Popov, L. Dunsch, K. Krämer, S.-X. Liu, S. Decurtins and T. Greber, Chem.–Eur. J., 2014, 20, 13536–13540 CrossRef CAS PubMed
.
- J.-F. Wyart, A. Meftah, A. Bachelier, J. Sinzelle, W.-Ü. L. Tchang-Brillet, N. Champion, N. Spector and J. Sugar, J. Phys. B: At., Mol. Opt. Phys., 2006, 39, L77–L82 CrossRef CAS
.
- F. Melin, M. N. Chaur, S. Engmann, B. Elliott, A. Kumbhar, A. J. Athans and L. Echegoyen, Angew. Chem., Int. Ed., 2007, 46, 9032–9035 CrossRef CAS PubMed
.
- S. Yang, A. A. Popov, C. Chen and L. Dunsch, J. Phys. Chem. C, 2009, 113, 7616–7623 CrossRef CAS
.
- W. Yang, G. Velkos, M. Rosenkranz, S. Schiemenz, F. Liu and A. A. Popov, Adv. Sci., 2024, 1, 202305190 Search PubMed
.
- F. Pointillart, O. Cador, B. Le Guennic and L. Ouahab, Coord. Chem. Rev., 2017, 346, 150–175 CrossRef CAS
.
- A. Borah and R. Murugavel, Coord. Chem. Rev., 2022, 453, 214288 CrossRef CAS
.
- G. Huang, G. Calvez, Y. Suffren, C. Daiguebonne, S. Freslon, O. Guillou and K. Bernot, Magnetochemistry, 2018, 4, 44 CrossRef
.
- B. Casanovas, M. Font-Bardía, S. Speed, M. S. El Fallah and R. Vicente, Eur. J. Inorg. Chem., 2018, 2018, 1928–1937 CrossRef CAS
.
- B. Casanovas, S. Speed, R. Vicente and M. Font-Bardía, Polyhedron, 2019, 173, 114113 CrossRef CAS
.
- R. Vicente, À. Tubau, S. Speed, F. A. Mautner, F. Bierbaumer, R. C. Fischer and S. S. Massoud, New J. Chem., 2021, 45, 14713–14723 RSC
.
- H. Chen, L. Sun, K. Zheng, J. Zhang, P. Ma, J. Wang and J. Niu, Dalton Trans., 2022, 51, 10257–10265 RSC
.
- S. Yang, L. Zhang, W. Zhang and L. Dunsch, Chem.–Eur. J., 2010, 16, 12398–12405 CrossRef CAS PubMed
.
- U. Mueller, R. Förster, M. Hellmig, F. U. Huschmann, A. Kastner, P. Malecki, S. Pühringer, M. Röwer, K. Sparta, M. Steffien, M. Ühlein, P. Wilk and M. S. Weiss, Eur. Phys. J. Plus, 2015, 130, 141 CrossRef
.
- W. Kabsch, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 125–132 CrossRef CAS PubMed
.
- K. M. Sparta, M. Krug, U. Heinemann, U. Mueller and M. S. Weiss, J. Appl. Crystallogr., 2016, 49, 1085–1092 CrossRef
.
- G. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed
.
- S. Stevenson, C. B. Rose, J. S. Maslenikova, J. R. Villarreal, M. A. Mackey, B. Q. Mercado, K. Chen, M. M. Olmstead and A. L. Balch, Inorg. Chem., 2012, 51, 13096–13102 CrossRef CAS PubMed
.
- S. Stevenson, C. Chancellor, H. M. Lee, M. M. Olmstead and A. L. Balch, Inorg. Chem., 2008, 47, 1420–1427 CrossRef CAS PubMed
.
- X. L. Wang, T. M. Zuo, M. M. Olmstead, J. C. Duchamp, T. E. Glass, F. Cromer, A. L. Balch and H. C. Dorn, J. Am. Chem. Soc., 2006, 128, 8884–8889 CrossRef CAS PubMed
.
- D. Krylov, F. Liu, A. Brandenburg, L. Spree, V. Bon, S. Kaskel, A. Wolter, B. Buchner, S. Avdoshenko and A. A. Popov, Phys. Chem. Chem. Phys., 2018, 20, 11656–11672 RSC
.
- R. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef
.
- T. E. Rieser, R. Thim-Spöring, D. Schädle, P. Sirsch, R. Litlabø, K. W. Törnroos, C. Maichle-Mössmer and R. Anwander, J. Am. Chem. Soc., 2022, 144, 4102–4113 CrossRef CAS PubMed
.
- D. Schädle, C. Schädle, D. Schneider, C. Maichle-Mössmer and R. Anwander, Organometallics, 2015, 34, 4994–5008 CrossRef
.
- D. Schädle, C. Schädle, K. W. Törnroos and R. Anwander, Organometallics, 2012, 31, 5101–5107 CrossRef
.
- W. J. Evans, R. Anwander, J. W. Ziller and S. I. Khan, Inorg. Chem., 1995, 34, 5927–5930 CrossRef CAS
.
- J. Liu, D. Reta, J. A. Cleghorn, Y. X. Yeoh, F. Ortu, C. A. P. Goodwin, N. F. Chilton and D. P. Mills, Chem.–Euro. J., 2019, 25, 7749–7758 CrossRef CAS PubMed
.
- R. Taube, S. Maiwald and J. Sieler, J. Organomet. Chem., 2001, 621, 327–336 CrossRef CAS
.
- D. Barbier-Baudry, O. Blacque, A. Hafid, A. Nyassi, H. Sitzmann and M. Visseaux, Eur. J. Inorg. Chem., 2000, 2000, 2333–2336 CrossRef
.
- S. M. Cendrowski-Guillaume, M. Nierlich, M. Lance and M. Ephritikhine, Organometallics, 1998, 17, 786–788 CrossRef CAS
.
- L. Münzfeld, C. Schoo, S. Bestgen, E. Moreno-Pineda, R. Köppe, M. Ruben and P. W. Roesky, Nat. Commun., 2019, 10, 3135 CrossRef PubMed
.
- F. M. Sroor, C. G. Hrib, P. Liebing, L. Hilfert, S. Busse and F. T. Edelmann, Dalton Trans., 2016, 45, 13332–13346 RSC
.
- Y. Zhang, R. Guan, M. Chen, Y. Shen, Q. Pan, Y. Lian and S. Yang, Inorg. Chem., 2021, 6, 1462–1471 CrossRef PubMed
.
- M. M. Olmstead, T. Zuo, H. C. Dorn, T. Li and A. L. Balch, Inorg. Chim. Acta, 2017, 468, 321–326 CrossRef CAS
.
- C. Schlesier, F. Liu, V. Dubrovin, L. Spree, B. Büchner, S. Avdoshenko and A. A. Popov, Nanoscale, 2019, 11, 13139–13153 RSC
.
- V. Dubrovin, L.-H. Gan, B. Büchner, A. A. Popov and S. M. Avdoshenko, Phys. Chem. Chem. Phys., 2019, 21, 8197–8200 RSC
.
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC
.
- J. G. M. Morton, A. Tiwari, G. Dantelle, K. Porfyrakis, A. Ardavan and G. A. D. Briggs, Phys. Rev. Lett., 2008, 101, 013002 CrossRef PubMed
.
- H. Reddmann, C. Apostolidis, O. Walter and H.-D. Amberger, Z. Anorg. Allg. Chem., 2006, 632, 1405–1408 CrossRef CAS
.
- J. D. Rinehart and J. R. Long, Dalton Trans., 2012, 41, 13572–13574 RSC
.
- Q.-W. Li, R.-C. Wan, Y.-C. Chen, J.-L. Liu, L.-F. Wang, J.-H. Jia, N. F. Chilton and M.-L. Tong, Chem. Commun., 2016, 52, 13365–13368 RSC
.
- M. D. Korzyński, M. Bernhardt, V. Romankov, J. Dreiser, G. Matmon, F. Pointillart, B. Le Guennic, O. Cador and C. Copéret, Chem. Sci., 2022, 13, 10574–10580 RSC
.
- H.-D. Amberger, H. Reddmann, T. J. Mueller and W. J. Evans, J. Organomet. Chem., 2011, 696, 2829–2836 CrossRef CAS
.
- H.-D. Amberger and H. Reddmann, Z. Anorg. Allg. Chem., 2007, 633, 443–452 CrossRef CAS
.
- S. Jank, H. Reddmann, H.-D. Amberger and C. Apostolidis, J. Organomet. Chem., 2004, 689, 3143–3157 CrossRef CAS
.
- R. Westerström, V. Dubrovin, K. Junghans, C. Schlesier, B. Büchner, S. M. Avdoshenko, A. A. Popov, A. Kostanyan, J. Dreiser and T. Greber, Phys. Rev. B, 2021, 104, 224401 CrossRef
.
- Y. Hao, G. Velkos, S. Schiemenz, M. Rosenkranz, Y. Wang, B. Büchner, S. M. Avdoshenko, A. A. Popov and F. Liu, Inorg. Chem. Front., 2023, 10, 468–484 RSC
.
- L. Gagliardi, R. Lindh and G. Karlström, J. Chem. Phys., 2004, 121, 4494–4500 CrossRef CAS PubMed
.
- L. Ungur and L. F. Chibotaru, Chem.–Euro. J., 2017, 23, 3708–3718 CrossRef CAS PubMed
.
- M. Briganti, G. F. Garcia, J. Jung, R. Sessoli, B. Le Guennic and F. Totti, Chem. Sci., 2019, 10, 7233–7245 RSC
.
- R. Alessandri, H. Zulfikri, J. Autschbach and H. Bolvin, Chem.–Eur. J., 2018, 24, 5538–5550 CrossRef CAS PubMed
.
- S. K. Gupta, T. Rajeshkumar, G. Rajaraman and R. Murugavel, Chem. Commun., 2016, 52, 7168–7171 RSC
.
- Y.-C. Chen, X.-S. Huang, J.-L. Liu and M.-L. Tong, Inorg. Chem., 2018, 57, 11782–11787 CrossRef CAS PubMed
.
- L. F. Chibotaru and L. Ungur, J. Chem. Phys., 2012, 137, 064112 CrossRef CAS PubMed
.
- C. K. Jørgensen, Prog. Inorg. Chem., 1962, 73–124 Search PubMed
.
- S. P. Sinha and H. H. Schmidtke, Mol. Phys., 1965, 10, 7–11 CrossRef CAS
.
- D. E. Henrie, Mol. Phys., 1974, 28, 415–421 CrossRef CAS
.
- S. Chorazy, T. Charytanowicz, J. Wang, S.-i. Ohkoshi and B. Sieklucka, Dalton Trans., 2018, 47, 7870–7874 RSC
.
- R. Jankowski, J. J. Zakrzewski, M. Zychowicz, J. Wang, Y. Oki, S.-i. Ohkoshi, S. Chorazy and B. Sieklucka, J. Mater. Chem. C, 2021, 9, 10705–10717 RSC
.
- N. C. Chang, J. Chem. Phys., 1966, 44, 4044–4050 CrossRef CAS
.
- J. B. Gruber, R. P. Leavitt and C. A. Morrison, J. Chem. Phys., 1983, 79, 1664–1668 CrossRef CAS
.
- J. A. Koningstein and J. E. Geusic, Phys. Rev., 1964, 136, A711–A716 CrossRef
.
- J. B. Gruber, M. E. Hills, T. H. Allik, C. K. Jayasankar, J. R. Quagliano and F. S. Richardson, Phys. Rev. B, 1990, 41, 7999–8012 CrossRef CAS PubMed
.
- H. M. Crosswhite, H. Crosswhite, F. W. Kaseta and R. Sarup, J. Chem. Phys., 1976, 64, 1981–1985 CrossRef CAS
.
- R. M. Hammond, M. F. Reid and F. S. Richardson, J. Less-Common Met., 1989, 148, 311–319 CrossRef
.
- W. T. Carnall, H. Crosswhite and H. M. Crosswhite, Energy level structure and transition probabilities in the spectra of the trivalent lanthanides in LaF3, United States, 1978, DOI: DOI:10.2172/6417825.
- A. Barla, J. Nicolás, D. Cocco, S. M. Valvidares, J. Herrero-Martín, P. Gargiani, J. Moldes, C. Ruget, E. Pellegrin and S. Ferrer, J. Synchrotron Radiat., 2016, 23, 1507–1517 CrossRef CAS PubMed
.
- B. T. Thole, P. Carra, F. Sette and G. van der Laan, Phys. Rev. Lett., 1992, 68, 1943–1946 CrossRef CAS PubMed
.
- P. Carra, B. T. Thole, M. Altarelli and X. Wang, Phys. Rev. Lett., 1993, 70, 694–697 CrossRef CAS PubMed
.
- Y. Teramura, A. Tanaka, B. T. Thole and T. Jo, J. Phys. Soc. Jpn., 1996, 65, 3056–3059 CrossRef CAS
.
- M.-X. Xu, Y.-S. Meng, J. Xiong, B.-W. Wang, S.-D. Jiang and S. Gao, Dalton Trans., 2018, 47, 1966–1971 RSC
.
- E. Regincós Martí, A. B. Canaj, T. Sharma, A. Celmina, C. Wilson, G. Rajaraman and M. Murrie, Inorg. Chem., 2022, 61, 9906–9917 CrossRef PubMed
.
- J. Li, L. Yin, S.-J. Xiong, X.-L. Wu, F. Yu, Z.-W. Ouyang, Z.-C. Xia, Y.-Q. Zhang, J. van Tol, Y. Song and Z. Wang, iScience, 2020, 23, 100926 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental details, SC-XRD data, results of DFT and CASSCF calculations, and magnetic and spectroscopic data. CCDC 2194854. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc05146c |
| This journal is © The Royal Society of Chemistry 2024 |