Open Access Article
Open Access ArticlePhotoexcitation of (diarylmethylene)amino benziodoxolones for alkylamination of styrene derivatives with carboxylic acids†
Daichi
Okumatsu
a,
Kensuke
Kiyokawa
 *a,
Linh Tran
Bao Nguyen
b,
Manabu
Abe
*a,
Linh Tran
Bao Nguyen
b,
Manabu
Abe
 *b and
Satoshi
Minakata
*b and
Satoshi
Minakata
 *a
*a
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, Yamadaoka 2-1, Suita, Osaka 565-0871, Japan. E-mail: kiyokawa@chem.eng.osaka-u.ac.jp; minakata@chem.eng.osaka-u.ac.jp
bDepartment of Chemistry, Graduate School of Advanced Science and Engineering, Hiroshima University, Kagamiyama 1-3-1, Higashi-hiroshima, Hiroshima 739-8526, Japan. E-mail: mabe@hiroshima-u.ac.jp
First published on 12th December 2023
Abstract
The alkylamination of alkenes using pristine carboxylic acids was achieved by the photoexcitation of (diarylmethylene)amino benziodoxolones (DABXs), which serve as both an oxidant and an aminating reagent (an iminyl radical precursor). The developed method is a simple photochemical reaction without the need for external photosensitizers and shows a broad substrate scope for aliphatic carboxylic acids leading to the formation of primary, secondary, and tertiary alkyl radicals, thus enabling the facile synthesis of various structurally complex amines. Mechanistic investigations including transient absorption spectroscopy measurements using a laser flash photolysis (LFP) method disclosed the unique photochemical reactivity of DABXs, which undergoes homolysis of their I–N bonds to give an iminyl radical and ortho-iodobenzoyloxy radical, the latter of which participates in the single-electron oxidation of carboxylates.
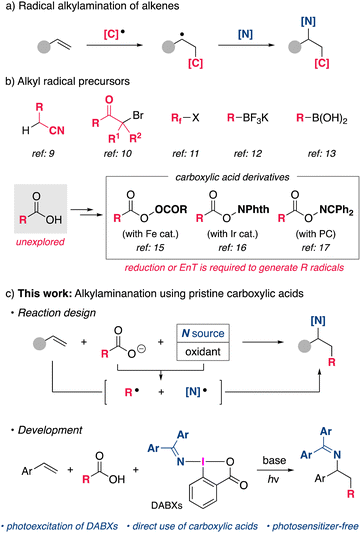
Introduction
Nitrogen-containing organic molecules are an important class of compounds that have wide applications in organic synthesis, medicinal chemistry, and material science. Therefore, the development of simple and efficient methods for the synthesis of such compounds is an important research topic. The intermolecular carboamination of readily available alkene feedstocks, in which C–C and C–N bonds are formed simultaneously, is an attractive strategy for the rapid synthesis of structurally complex amines.1 Although the transition-metal-catalyzed non-annulative carboamination of alkenes, mainly aryl- and alkenylamination, has been successfully developed in this area,2–7 the method of alkylamination has rarely been explored even though it would further expand the utility of carboamination for the synthesis of various nitrogen-containing organic molecules.5a,bA strategy involving alkyl radical species has emerged as a useful approach for the alkylamination of alkenes (Scheme 1a).8 Early examples of such reactions required the use of structurally specific alkyl radical precursors such as alkyl nitriles,9 α-halocarbonyl compounds,10 and perfluoroalkyl halides11 (Scheme 1b). Although alkylborane derivatives have recently been utilized as alkyl radical precursors,12,13 they still lack availability and versatility in terms of introducing the desired alkyl group. As a general alkyl radical precursor, aliphatic carboxylic acids are highly attractive because they are stable, non-toxic, and ubiquitous in Nature.14 Despite these advantages, due to their relatively high redox potential, the difficulty of selectively oxidizing carboxylic acids to generate alkyl radicals in the presence of more readily oxidized amines prevents their direct use in alkylamination reactions. Therefore, existing methods rely on the use of preactivated carboxylic acid derivatives with a higher oxidation state. For example, alkylamination reactions using diacyl peroxides15 or redox-active esters16 through a single electron reduction by a transition metal or a photoredox catalyst have been reported. Most recently, an elegant reaction system was reported by Glorius, in which a bifunctional oxime ester serves as both an alkyl radical and a nitrogen-centered radical through a photo-induced triplet energy transfer mechanism.17,18 However, these methods require additional steps for the preparation of alkyl radical precursors, which reduces the efficiency of the net reaction, and the use of appropriate catalysts. In order to realize a more straightforward alkylamination using pristine carboxylic acids, a new strategy employing a suitable aminating reagent and an oxidant that allows single-electron oxidation of carboxylic acids should be explored (Scheme 1c).
Our group previously developed (diarylmethylene)amino benziodoxolones (DABXs) as a unique oxidative aminating reagent that functions as a single-electron oxidant as well as an iminyl radical source.19 Accordingly, we envisioned that DABXs would be promising bifunctional reagents that could oxidize a carboxylate and simultaneously generate an alkyl radical and an iminyl radical species, which would then participate in the regioselective alkylamination of alkenes (Scheme 1c). Herein, we report on a new types of reactivity of DABXs that proceeds under conditions of photoirradiation, enabling the intermolecular alkylamination of styrene derivatives with simple carboxylic acids. The developed photochemical reaction proceeds effectively without the need for external photosensitizers.
Results and discussion
Reaction development
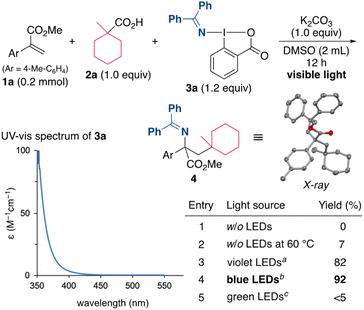
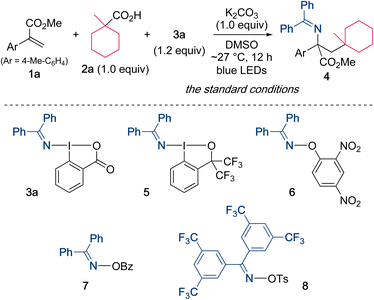
Our initial efforts focused on the alkylamination of methyl 2-(p-tolyl)acrylate (1a) with 1-methylcyclohexane-1-carboxylic acid (2a) and DABX 3a in DMSO in the presence of K2CO3 (Scheme 2). However, the target reaction without irradiation did not proceed well even under the heating conditions (entries 1 and 2). Indeed, a cyclic voltammetry (CV) measurement of 3a indicated that the reduction potential of 3a (−1.52 V vs. SCE in CH2Cl2)19a is not sufficient to oxidize the carboxylate (ca. +1.2 V vs. SCE). We then turned our attention to the photoirradiation conditions because photoexcited molecules generally behave as a better oxidant (reductant) than those in the ground state.20 Waser recently reported that the excitation of ethynylbenziodoxolones (EBXs) with visible light increases their oxidation ability.21 The UV-vis spectrum of 3a (0.05 M in DMSO) was then measured, and the results showed a weak absorption in the visible light region. Based on this result, the use of a violet LED (390 nm) was examined, and, under these conditions, the alkylamination proceeded efficiently to give the desired 4 in 82% yield (entry 3). A brief screening of the wavelength of the light source revealed that the yield of 4 increased to 92% when a blue LED (467 nm) was used (entry 4), while the use of a green LED (525 nm) resulted in a very low yield of the product (entry 5). It should be noted that the present alkylamination features a photosensitizer-free reaction.The reaction conditions including bases, solvents, and aminating reagents were then surveyed (Table 1). The alkylamination reaction also proceeded with almost the same efficiency when Na2CO3 and Cs2CO3 were used instead of K2CO3 (entries 2 and 3). In the absence of K2CO3, the yield of the product was significantly decreased, indicating that the formation of a carboxylate is necessary to promote the reaction (entry 4). Examining the reaction in other polar solvents showed that DMSO was suitable for this alkylamination (entries 5 and 6). Other oxidative aminating reagents were also investigated. The use of the benziodoxole-based reagent 5 resulted in the low efficiency, suggesting the importance of a benziodoxolone scaffold in 3a (entry 7). The O-aryl oxime 6 was also found to be unsuitable for this reaction (entry 8).22 The use of the oxime ester 7 and the O-sulfonyl oxime 8, which are used as electrophilic aminating reagents,23 failed to afford the target product (entries 9 and 10).
| Entry | Variation from the standard condition | Yieldb (%) |
|---|---|---|
| a Reactions were performed on a 0.2 mmol scale (0.1 M) under irradiation using Kessil PR160L 467 nm (max 40 W). The reaction temperature within the reaction vial was maintained around 27 °C. b Determined by 1H NMR analysis of the crude product using 1,1,1,2-tetrachloroethane as an internal standard. c Isolated yield on a 0.4 mmol scale. | ||
| 1 | None | 92 (84)c |
| 2 | Na2CO3 instead of K2CO3 | 92 |
| 3 | Cs2CO3 instead of K2CO3 | 87 |
| 4 | Without K2CO3 | 18 |
| 5 | MeCN instead of DMSO | 32 |
| 6 | DMF instead of DMSO | 78 |
| 7 | 5 (1.0 equiv.) instead of 3a | 17 |
| 8 | 6 (1.0 equiv.) instead of 3a | 15 |
| 9 | 7 (1.0 equiv.) instead of 3a | 0 |
| 10 | 8 (1.0 equiv.) instead of 3a | 0 |
Substrate scope and synthetic utility
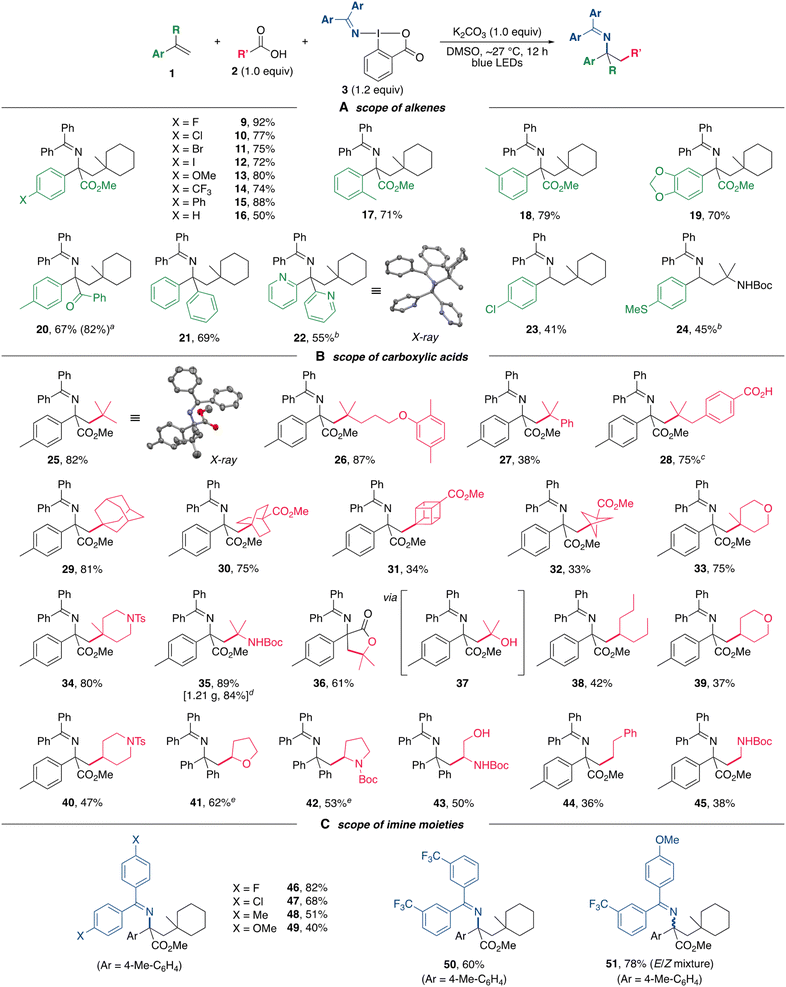
With the optimized reaction conditions in hand, the scope of alkylamination was next explored (Scheme 3). In initial experiments, a series of 2-arylacrylates bearing both electron-donating and electron-withdrawing groups on the aryl moiety were subjected to the alkylamination, and all of the reactions proceeded effectively to afford the corresponding products in good to high yields (Scheme 3A, 9–16). Several functional groups including halogens (9–12) and a trifluoromethyl group (14) on the aromatic ring were well tolerated. The presence of a substituent at the ortho and meta-positions had little effect on the reaction efficiency in the formation of 17 and 18, respectively. Substrates bearing a 3,4-methylenedioxyphenyl group also readily participated in the alkylamination (19). These reactions using 2-arylacrylates as substrates provided unnatural α-amino acid derivatives containing α-tertiary carbon centers, which are otherwise difficult to access. In addition, an α,β-unsaturated ketone (20), 1,1-di(hetero)arylethenes (21 and 22), and styrene derivatives (23 and 24) were also applicable for use in this alkylamiantion.Subsequently, the scope of carboxylic acids for the alkylamination was then investigated (Scheme 3B). A wide range of carboxylic acids bearing an α-quaternary carbon center could be applied to the present alkylamination, thus allowing the introduction of bulky alkyl groups (25–28). A carboxyl group on the aromatic ring was found to be inert toward decarboxylation under the oxidative conditions, leading to the chemoselective formation of 28. Notably, three-dimensional scaffolds with carbon skeletons such as adamantyl (29), bicyclo[2.2.2]octyl (30), cubyl (31), and bicyclo[1.1.1]pentyl (32) groups, which are of great interest as bioisosteres of the phenyl ring in medicinal chemistry,24 could be successfully introduced. Substrates containing oxa and aza-heterocycles were also well tolerated (33 and 34). An α-NHBoc (35) and an α-hydroxy (37) carboxylic acids could also be used as alkylating reagents, and using the latter provided γ-lactone 36 through an intramolecular cyclization of the alkylaminated product 37. The synthetic utility of this alkylamination was also demonstrated by a gram-scale synthesis of 35 without any loss of yield (1.21 g, 84%). Furthermore, carboxylic acids leading to secondary and primary alkyl radicals were compatible with the alkylamination, allowing the introduction of various alkyl groups including five and six-membered heterocyclic scaffolds (38–45). In particular, the product 43 with an unprotected hydroxy group was synthesized by using L-serine. Finally, the scope of DABXs was surveyed (Scheme 3C). Derivatives containing electronically varied imine moieties could be used to deliver the corresponding products 46–51, indicating that this alkylamination was not significantly influenced by the electronic property of the (diarylmethylene)amino group albeit the yields were somewhat lower for reactions using electron-rich reagents (48 and 49). An unsymmetrical (diarylmethylene)amino group could also be introduced (51).
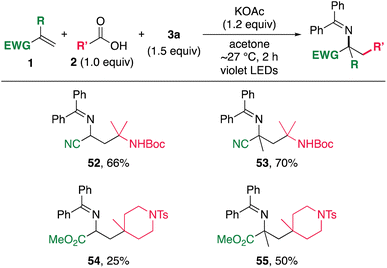
To further expand the scope of the alkylamination, the method was applied to electron-deficient alkenes. The reaction using acrylonitrile as a substrate under the standard conditions resulted in the production of only trace amounts of the target product. A brief screening of solvents and bases revealed that the use of acetone and KOAc could promote the alkylamination (Scheme 4). The reaction of acrylonitriles with 2l and 3a in the presence of KOAc in acetone under irradiation conditions proceeded effectively, affording 52 and 53. The use of a violet LED (390 nm) instead of a blue LED (467 nm) was able to significantly shorten the reaction time. In addition, acrylates were also subjected to alkylamination to afford the corresponding amines 54 and 55 in moderate yields. These results further extend the utility of the present alkylamination method for the synthesis of various nitrogen-containing organic molecules. However, simple aliphatic alkenes are unsuccessful substrates,25 probably due to the slow rate of the addition of alkyl radicals to aliphatic alkenes.26
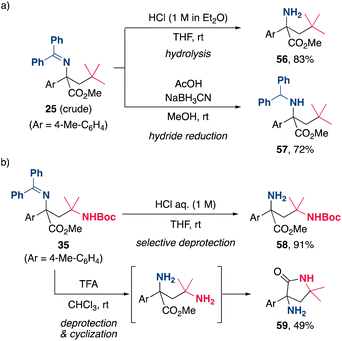
The synthetic utility of the present alkylamination was further highlighted by the derivatization of the products (Scheme 5). The facile transformation of the (diphenylmethylene)amino group of the product was demonstrated by acid hydrolysis and hydride reduction of the crude product of 25, affording α-amino ester 56 and diphenylmethylamine 57, respectively, in good yields (Scheme 5a). Furthermore, the chemoselective hydrolysis of the imine moiety of 35 was achieved by the treatment with aqueous HCl to provide 58 in excellent yield (Scheme 5b). Hydrolysis of both amino functionalities of 35 using trifluoroacetic acid (TFA) provided a diamine, which subsequently underwent cyclization to yield the γ-lactam 59, a key structural motif in pharmaceuticals and natural products. These quite simple methods provide efficient and practical processes for the synthesis of a variety of useful nitrogen-containing organic molecules.
Mechanistic investigations
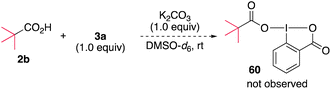
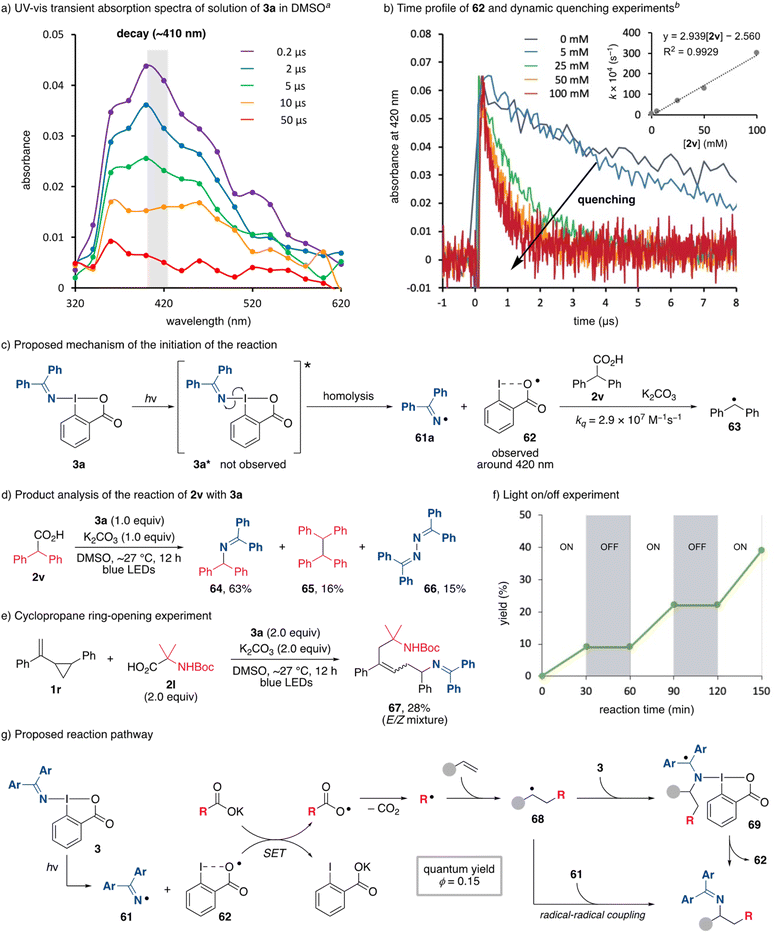
Mechanistic aspects of the alkylamination were investigated. The possibility of the ligand exchange reaction between 3a and carboxylate to form the carboxylate-substituted hypervalent iodine compound was initially examined. When a mixture of pivalic acid (2b) and 3a in the presence of K2CO3 in DMSO-d6 was monitored by 1H NMR (Scheme 6), no new signals were observed, and the starting materials remained, ruling out the possibility of ligand exchange (see ESI, Fig. S3†). In addition, density functional theory (DFT) calculations of the ligand exchange process at the M06-2X/6-311++G(d,g)-SDD(I) level of theory indicated that the formation of 60 is thermodynamically unfavorable (ΔG = +2.1 kcal mol−1), consistent with the experimental results. We next examined the possibility that an electron donor–acceptor (EDA) complex was formed between a carboxylate and DABXs. The UV-vis spectrum of the reaction mixture was nearly superimposable over that of 3a (see ESI, Fig. S4†), ruling out the participation of a photoactive EDA complex in the reaction. Based on these results, we conclude that DABXs would be directly excited and initiate the reaction.To investigate the photochemical reactivity of DABX 3a, a series of experiments and quantum chemical calculations were performed. A fluorescence emission analysis of 3a was first conducted in DMSO at room temperature. No significant emission signal was observed under these conditions (see ESI, Fig. S5†), suggesting that the singlet electronically excited state is chemically reactive, and/or that the intersystem crossing process is fast to produce the triplet state.27 We then conducted sub-microsecond transient absorption (TA) spectroscopy measurements of 3a in DMSO using a laser flash photolysis (LFP) method (266 nm or 355 nm Nd/YAG-laser, 12 ns pulse-width, 10 Hz, 6 mJ per pulse) to gain insights into the transient species generated during the photolysis of 3a (Scheme 7a). After the LFP, a transient species absorbing at 350–600 nm with λmax ∼ 410 nm was observed under air in DMSO at 298 K. The decay time-profile monitored at 420 nm could not be reproduced by a single exponential decay-equation. The half-life (τ1/2) was ∼6.8 μs at 298 K under an atmosphere of air. Under an argon atmosphere, the similar τ1/2 was found to be ∼6.6 μs at 298 K, indicating that the transient species with λmax ∼ 410 nm is not quenched by molecular oxygen (O2). These experimental results clearly suggest that the triplet excited state of 3a is not the transient species with λmax ∼ 410 nm. Interestingly, the structural optimization of triplet state of 3a at the UB3LYP/6-31G(d)-LanL2DZ(I) level of theory produced a pair of radicals, a diphenyl iminyl radical 61a and ortho-iodobenzoyloxy radical (62), suggesting that the triplet 3a spontaneously generates the radical pair after the I–N bond homolysis. In addition to the generation of the transient with λmax ∼ 410 nm, the bleaching signal at ∼300 nm and its recovering process with krec = 2.13 × 1010 M−1 s−1 were observed in the LFP experiments (see ESI, Fig. S7†). The rise in the signal is attributed to the recovering process of 3a from the pair of radicals.
The absorption spectra of 61a and 62 were computed using time-dependent density functional theory (TD-DFT) calculations at the UB3LYP/6-31G(d)-LanL2DZ(I) level of theory. The computational method well reproduced the absorption spectrum of compound 3a (see ESI, Fig. S9†). Thus, we applied the method to simulate the absorption spectra of 61a and 62. Weak electronic transitions at 491 nm (f = 0.0025, ε = ∼100), 353 nm (f = 0.015, ε = ∼1300), and 337 nm (f = 0.018, ε = ∼1300) were computed for iminyl radical 61a, where f is the oscillator strength; ε is molar extinction coefficient in M−1 cm−1 (see ESI, Fig. S10†). For radical 62, a weak transition at 758 nm (f = 0.027, ε = ∼1100) and a relatively strong one at 463 nm (f = 0.125, ε = ∼5000) were found over 300 nm at the same level of theory (see ESI, Fig. S11†). The computed results clearly suggest that the observed transient absorption at λmax ∼ 410 nm in the LFP experiments of 3a is derived from radical 62. In addition, the peroxyl radical 62_O2 was not optimized as an equilibrated structure to give a weakly bounded complex of radical 62 and O2 during the structural optimization in the doublet state, suggesting that the reaction of 62 with O2 is endothermic and slow. Indeed, the Gibbs energy of the complex was computed to be higher in energy by 5.8 kcal mol−1 than the total energy of 62 and O2 (see ESI, Fig. S13†). This computational result also supports the conclusion that the transient absorption with λmax ∼ 410 nm comes from radical 62, since the transient species with λmax ∼ 410 nm was not quenched by O2. Unfortunately, the signal of 61a could not be clearly obtained due to the low ε and the overlap with the absorption spectra of 3a and 62.
As mentioned above, the decay process of 62 was not reproduced by the first-order decay equation. However, the experimentally observed decay-signal at 420 nm was perfectly reproduced with the second-order rate equation (61a + 62 → 3a, [62] = 1/(kt + 1/[62]0), where [62]0 = [61a]0 = 3.88 × 10−6 M), to give the second-order rate constant (k) of 1.7 × 1010 M−1 s−1 (see ESI, Fig. S8†), supporting the conclusion that assignment of 62 is correct. The initial concentration of 62 was determined by the bleaching signal of 3a at 300 nm after the LFP, Abs300 = −0.0231, ε300 of 3a = 5946.7. The quantitative formation of 62 from 3a was assumed to determine the concentration.
To understand the thermal reactivity of 62, we then investigated the dynamic quenching of 62 with the carboxylate prepared from 2v with K2CO3 to determine whether it could function as an oxidant (Scheme 7b and c). Indeed, the lifetime (τ = 1/kq) of 62 was shortened by increasing the concentration of 2v. From the slope of the Stern–Volmer plot, kqversus [2v], the quenching rate constant kq was found to be 2.9 × 107 M−1 s−1. Unfortunately, the resulting diphenylmethyl radical 63 (λmax = 335 nm, ε 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900)28 formed through the fast decarboxylation was not clearly detected, although the lifetime at 370 nm was different from that at 420 nm. The optical window was more than 370 nm for the LFP experiments. To further confirm the single-electron transfer process, the reduction potentials of 61a, 62, and a carboxylate of 2v were then computed using DFT calculations at the M062X/6-31G+(d,g)-LanL2DZ(I) level of theory. Since structural optimization of the carboxyl radical species derived from 2v resulted in the formation of the diphenylmethyl radical 63 due to the barrierless decarboxylation, the calculations were carried out using a pivalate instead (see ESI†). The resulting reduction potentials for 61a, 62, and oxidation potential for a pivalate were −1.12, +1.20, and +1.37 V vs. SCE, respectively, indicating that the single-electron oxidation of pivalate by 62 is possible although the electron transfer oxidation is slightly endothermic (ΔGet = +0.17 eV). Indeed, the estimated quenching rate constant obtained by the Rehm–Weller equation, ∼1.8 × 107 M−1 s−1, is fairly consistent with the experimentally obtained value kq = 2.9 × 107 M−1 s−1 (vide supra).29
900)28 formed through the fast decarboxylation was not clearly detected, although the lifetime at 370 nm was different from that at 420 nm. The optical window was more than 370 nm for the LFP experiments. To further confirm the single-electron transfer process, the reduction potentials of 61a, 62, and a carboxylate of 2v were then computed using DFT calculations at the M062X/6-31G+(d,g)-LanL2DZ(I) level of theory. Since structural optimization of the carboxyl radical species derived from 2v resulted in the formation of the diphenylmethyl radical 63 due to the barrierless decarboxylation, the calculations were carried out using a pivalate instead (see ESI†). The resulting reduction potentials for 61a, 62, and oxidation potential for a pivalate were −1.12, +1.20, and +1.37 V vs. SCE, respectively, indicating that the single-electron oxidation of pivalate by 62 is possible although the electron transfer oxidation is slightly endothermic (ΔGet = +0.17 eV). Indeed, the estimated quenching rate constant obtained by the Rehm–Weller equation, ∼1.8 × 107 M−1 s−1, is fairly consistent with the experimentally obtained value kq = 2.9 × 107 M−1 s−1 (vide supra).29
To confirm the generation of an iminyl radical 61a as well as the alkyl radical 63, the product analysis of photochemical reaction of 2v (0.1 M) with 3a (0.1 M) was carried out in the presence of K2CO3 (Scheme 7d). As a result, the decarboxylative amination product 64 was obtained, accompanied by the formation of byproducts including 65 and benzophenone azine (66), which are apparently formed through the homocoupling of 63 and 61a, respectively.30 These results clearly demonstrate that the generation of 61a and 63 under the reaction conditions, which would couple to provide 64. Overall, the excitation of 3a led to the formation of the iminyl radical 61a and radical 62 that can oxidize a carboxylate to give an alkyl radical. Although there are several reports on the reactivity of radical 62 as a single-electron oxidant,31,32 the present study is a rare example of an experimental demonstration of its reactivity.33 A radical mechanism was further confirmed by the use of vinylcyclopropane 1r as an alkene substrate (Scheme 7e). When 1r was subjected to the standard alkylamination reaction conditions, no cyclopropane-containing product was observed, and several cyclopropane ring-opened products were obtained, one of which was determined to be 67. This result clearly indicates that the reaction proceeds through radical addition of an alkyl radical to an alkene.
Based on the experimental results reported herein, a proposed reaction pathway for the alkylamination is depicted in Scheme 7g. As an initial step, the visible-light excitation of DABXs 3 leads to the homolysis of its I–N bond to give an iminyl radical 61 and radical 62. A single-electron oxidation of a carboxylate by 62 then occurs. The resulting alkyl radical through a subsequent decarboxylation reacts with alkenes to form radical 68. Given that the relative concentration of 3 is much higher than that of 61, the radical 68 would undergo the radical addition onto 3. The Gibbs energy barrier was then computed to be 32.0 kcal mol−1 at the M06-2X/6-31G+(d,g)-LanL2DZ(I) level of theory (see ESI, Fig. S15†). The product and the radical 62 were found to be produced through the formation of the weakly bounded intermediate 69. The re-generation of radical 62 realizes the radical-chain process. However, the result of the light ON/OFF experiments (Scheme 7f) and the quantum yield Φ = 0.15 of the alkylamination do not support an efficient radical chain process, and continuous photoirradiation is therefore required for the reaction. Therefore, an alternative pathway involving a radical–radical coupling between 68 and iminyl radical 61 cannot be excluded.
Conclusions
In conclusion, the alkylamination of alkenes using pristine carboxylic acids as an alkylating reagent was enabled by photoexcitation of DABXs which function as both an oxidant and an aminating reagent. The present method has a broad substrate scope for alkenes and aliphatic carboxylic acids with good functional group tolerance. The developed alkylamination would provide a simple, scalable, and straightforward approach to accessing readily modifiable amines. The synthetic utility of the products was clearly demonstrated by the facile transformation of some of the products into valuable nitrogen-containing molecules. Experimental and computational mechanistic studies revealed the unprecedented photochemical reactivity of DABXs. Upon visible light irradiation, ortho-iodobenzoyloxy radical, in situ-generated from DABXs, oxidizes carboxylates to provide alkyl radicals. We anticipate that the present method will find wide applications in the synthesis of amines that are otherwise difficult to access.Data availability
Experimental procedures, compound characterization data, crystal data, computational details, and NMR spectra can be found in ESI.†Author contributions
D. O. performed the main part of the experiments. L. T. B. N. performed the quantum yield experiments. D. O. and K. K. wrote the draft of the manuscript, participated in compound characterization, and ESI† preparation. M. A. and S. M. supervised the research. All authors discussed the results and prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI Grant numbers JP21J20313, JP22H02078, JP22H05362, JP22K19033, JP21H01921, and JST CREST Grant number JPMJCR18R4. K. K. also thanks the research fund of the Asahi Glass Foundation.Notes and references
- X. Chen, F. Xiao and W.-M. He, Org. Chem. Front., 2021, 8, 5206–5228 RSC.
- T. Piou and T. Rovis, Nature, 2015, 527, 86–90 CrossRef CAS PubMed.
- Z. Hu, X. Tong and G. Liu, Org. Lett., 2016, 18, 1702–1705 CrossRef CAS PubMed.
- A. Lerchen, T. Knecht, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2016, 55, 15166–15170 CrossRef CAS PubMed.
- (a) Z. Liu, Y. Wang, Z. Wang, T. Zeng, P. Liu and K. M. Engle, J. Am. Chem. Soc., 2017, 139, 11261–11270 CrossRef CAS PubMed; (b) V. A. van der Puyl, J. Derosa and K. M. Engle, ACS Catal., 2019, 9, 224–229 CrossRef CAS; (c) T. Kang, N. Kim, P. T. Cheng, H. Zhang, K. Foo and K. M. Engle, J. Am. Chem. Soc., 2021, 143, 13962–13970 CrossRef CAS PubMed; (d) T. Kang, J. M. González, Z.-Q. Li, K. Foo, P. T. W. Cheng and K. M. Engle, ACS Catal., 2022, 12, 3890–3896 CrossRef CAS.
- (a) D. Wang, L. Wu, F. Wang, X. Wan, P. Chen, Z. Lin and G. Liu, J. Am. Chem. Soc., 2017, 139, 6811–6814 CrossRef CAS PubMed; (b) D. Wang, F. Wang, P. Chen, Z. Lin and G. Liu, Angew. Chem., Int. Ed., 2017, 56, 2054–2058 CrossRef CAS PubMed.
- S. Lee and T. Rovis, ACS Catal., 2021, 11, 8585–8590 CrossRef CAS PubMed.
- H. Jiang and A. Studer, Chem. Soc. Rev., 2020, 49, 1790–1811 RSC.
- (a) Y.-Y. Liu, X.-H. Yang, R.-J. Song, S. Luo and J.-H. Li, Nat. Commun., 2017, 8, 14720–14725 CrossRef PubMed; (b) N. Zhu, T. Wang, L. Ge, Y. Li, X. Zhang and H. Bao, Org. Lett., 2017, 19, 4718–4721 CrossRef CAS PubMed.
- S. N. Gockel, T. L. Buchanan and K. L. Hull, J. Am. Chem. Soc., 2018, 140, 58–61 CrossRef CAS PubMed.
- Y. Xiong, X. Ma and G. Zhang, Org. Lett., 2019, 21, 1699–1703 CrossRef CAS PubMed.
- J. J. Kennedy-Ellis, E. D. Boldt and S. R. Chemler, Org. Lett., 2020, 22, 8365–8369 CrossRef CAS PubMed.
- S. N. Gockel, S. Lee, B. L. Gay and K. L. Hull, ACS Catal., 2021, 11, 5166–5171 CrossRef CAS PubMed.
- (a) J. Xuan, Z.-G. Zhang and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 15632–15641 CrossRef CAS PubMed; (b) J. Schwarz and B. König, Green Chem., 2018, 20, 323–361 RSC.
- B. Qian, S. Chen, T. Wang, X. Zhang and H. Bao, J. Am. Chem. Soc., 2017, 139, 13076–13082 CrossRef CAS PubMed.
- X.-H. Ouyang, Y. Li, R.-J. Song and J.-H. Li, Org. Lett., 2018, 20, 6659–6662 CrossRef CAS PubMed.
- (a) T. Patra, P. Bellotti, F. Strieth-Kalthoff and F. Glorius, Angew. Chem., Int. Ed., 2020, 59, 3172–3177 CrossRef CAS PubMed; (b) H.-M. Huang, P. Bellotti, J. Ma, T. Dalton and F. Glorius, Nat. Rev. Chem., 2021, 5, 301–321 CrossRef CAS PubMed; (c) G. Tan, M. Das, H. Keum, P. Bellotti, C. Daniliuc and F. Glorius, Nat. Chem., 2022, 14, 1174–1184 CrossRef CAS PubMed.
- For exmples of other carboamination reactions, see: (a) Y. Zhang, H. Liu, L. Tang, H. J. Tang, L. Wang, C. Zhu and C. Feng, J. Am. Chem. Soc., 2018, 140, 10695–10699 CrossRef CAS PubMed; (b) J. Majhi, R. K. Dhungana, Á. Rentería-Gómez, M. Sharique, L. Li, W. Dong, O. Gutierrez and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 15871–15878 CrossRef CAS PubMed.
- (a) K. Kiyokawa, D. Okumatsu and S. Minakata, Angew. Chem., Int. Ed., 2019, 58, 8907–8911 CrossRef CAS PubMed; (b) D. Okumatsu, K. Kawanaka, S. Kainuma, K. Kiyokawa and S. Minakata, Chem.–Eur. J., 2023, 29, e202203722 CrossRef CAS PubMed; (c) A. Yoshimura, A. Saito and V. V. Zhdankin, Adv. Synth. Catal., 2023, 365, 2653–2675 CrossRef CAS.
- For recent examples, see: (a) M. Silvi, E. Arceo, I. D. Jurberg, C. Cassani and P. Melchiorre, J. Am. Chem. Soc., 2015, 137, 6120–6123 CrossRef CAS PubMed; (b) Y. Sato, K. Nakamura, Y. Sumida, D. Hashizume, T. Hosoya and H. Ohmiya, J. Am. Chem. Soc., 2020, 142, 9938–9943 CrossRef CAS PubMed.
- S. G. E. Amos, D. Cavalli, F. Le Vaillant and J. Waser, Angew. Chem., Int. Ed., 2021, 60, 23827–23834 CrossRef CAS PubMed.
- J. Davies, S. G. Booth, S. Essafi, R. A. W. Dryfe and D. Leonori, Angew. Chem., Int. Ed., 2015, 54, 14017–14021 CrossRef CAS PubMed.
- H. Tsutsui, T. Ichikawa and K. Narasaka, Bull. Chem. Soc. Jpn., 1999, 72, 1869–1878 CrossRef CAS.
- M. A. M. Subbaiah and N. A. Meanwell, J. Med. Chem., 2021, 64, 14046–14128 CrossRef CAS PubMed.
- For a list of unsuccessful substrates, see ESI†.
- H. Fischer and L. Radom, Angew. Chem., Int. Ed., 2001, 40, 1340–1371 CrossRef CAS PubMed.
- For a example of a direct S0→Tn transition in the photoreaction of hypervalent iodine compounds, see: M. Nakajima, S. Nakagawa, K. Matsumoto, T. Kuribara, A. Muranaka, M. Uchiyama and T. Nemoto, Angew. Chem., Int. Ed., 2020, 59, 6847–6852 CrossRef CAS PubMed.
- (a) G. Porter and M. W. Windsor, Nature, 1957, 180, 187–188 CrossRef CAS; (b) L. M. Hades, M. S. Platz and J. C. Scarano, J. Am. Chem. Soc., 1984, 106, 283–287 CrossRef.
- S. Farid, J. P. Dinnocenzo, P. B. Merkel, R. H. Young, D. Shukla and G. Guirado, J. Am. Chem. Soc., 2011, 133, 11580–11587 CrossRef CAS PubMed.
- (a) T. Okada, M. Kawanishi and H. Nozaki, Bull. Chem. Soc. Jpn., 1969, 32, 2981–2983 CrossRef; (b) A. R. Forrester, M. Gill and J. S. Sadd, J. Chem. Soc., Chem. Commun., 1975, 291–292 RSC.
- (a) G.-X. Li, C. A. Morales-Rivera, Y. Wang, F. Gao, G. He, P. Liu and G. Chen, Chem. Sci., 2016, 7, 6407–6412 RSC; (b) G.-X. Li, C. A. Morales-Rivera, F. Gao, Y. Wang, G. He, P. Liu and G. Chen, Chem. Sci., 2017, 8, 7180–7185 RSC; (c) F. Le Vaillant, M. D. Wordrich and J. Waser, Chem. Sci., 2017, 8, 1790–1800 RSC.
- For examples of the use of an iodanyl radical as a H-atom transfer reagent, see: (a) M. Ochiai, T. Ito, H. Takahashi, A. Nakanishi, M. Toyonari, T. Sueda, S. Goto and M. Shiro, J. Am. Chem. Soc., 1996, 118, 7716–7730 CrossRef CAS; (b) S. A. Moteki, A. Usui, T. Zhang, C. R. S. Alvarado and K. Maruoka, Angew. Chem., Int. Ed., 2013, 52, 8657–8660 CrossRef CAS PubMed; (c) J. Jiang, R. Ramozzi, S. Moteki, A. Usui, K. Maruoka and K. Morokuma, J. Org. Chem., 2015, 80, 9264–9271 CrossRef CAS PubMed.
- (a) B. L. Frey, M. T. Figgins, G. P. Van Trieste, R. Carmieli and D. C. Powers, J. Am. Chem. Soc., 2022, 144, 13913–13919 CrossRef CAS PubMed; (b) A. Maity, B. L. Frey and D. C. Powers, Iodanyl Radical Catalysis, Acc. Chem. Res., 2023, 56, 2026–2036 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2286656–2286658. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc06090j |
| This journal is © The Royal Society of Chemistry 2024 |