Open Access Article
Open Access ArticleRedox stabilization of Am(V) in a biphasic extraction system boosts americium/lanthanides separation efficiency†
Xue
Dong
,
Huaixin
Hao
 ,
Jing
Chen
,
Zhipeng
Wang
* and
Chao
Xu
,
Jing
Chen
,
Zhipeng
Wang
* and
Chao
Xu
 *
*
Institute of Nuclear and New Energy Technology, Tsinghua University, 100084, Beijing, China. E-mail: xuchao@tsinghua.edu.cn
First published on 26th December 2023
Abstract
Americium (Am) is a key radioactive element in consideration in nuclear waste treatment. Separation of Am from the fission products, lanthanides, is a prerequisite to minimize the hazardous impact of Am and make utilization of rare Am isotopes, but it represents a great challenge due to the chemical similarity between the two groups of elements. Herein, we realize the separation by first oxidizing Am(III) to high valent Am(VI) and then converting it to Am(V) in situ in a biphasic extraction system with Bi(V) oxidant incorporated in an organic phase. Am(V) is highly stabilized during the separation process and this leads to record high Ln/Am separation factors (>105) in a single contact over a wide range of acidities.
The development of nuclear energy generates a large amount of used nuclear fuel containing U, Np, Pu, Am, Cm and a variety of fission products. This highly radioactive and chemically diverse mixture represents about one third of the elements in the periodic table and must be properly managed and processed to reduce its hazardous impact as well as to recover the useful resources. Americium, a long-lived α-emitter and one of the greatest contributors to the radiotoxicity in used nuclear fuel waste, is suggested to be recovered from the waste and then undergo transmutation to minimize its long-term radiotoxicity in advanced nuclear fuel cycles.1–3 Unfortunately, the coexistence of lanthanides (Ln), which represent about 40% of the mass of all fission products, has proven to be problematic for Am recovery and transmutation because these lanthanides bear high neutron capture cross-sections and would compete with Am for neutrons in the transmutation process. Separation of Am from lanthanides is thus a prerequisite for Am transmutation. However, this separation task remains a great challenge due to the chemical similarity between Am and lanthanides.4–6
Previous efforts to separate Am from lanthanides have relied mainly on the design and use of ligands bearing softer N and S donor atoms, which exhibit higher chemical affinity to relatively softer Am(III) than to harder Ln(III) in solvent extraction.5–13 This separation approach has been widely demonstrated but it still encounters obstacles such as slow kinetics, ligand instability, and narrow operation acidities.5,6,14 Another less-explored approach to realize the separation is to take advantage of the different redox properties between Am and lanthanides. While Am(III) in aqueous solution can be oxidized to high valent americyl forms Am(V) and Am(VI) under highly oxidizing conditions, the lanthanides remain as spherical Ln(III) or Ln(VI) ions. The significant difference between the linear americyl ion and the spherical Ln ion in terms of both steric configuration and charge density offers a great opportunity for efficient separation.15–20 The greatest challenge here is how to stabilize the high valent Am during the separation process. In most previous studies, the contact of americyl ions with organic reagents used for separation led to fast reduction of these high valent Am ions, causing significant deterioration in Am/Ln separation efficiency.21–26
Recently, we proved that the incorporation of oxidative Bi(V) species in an organic solvent containing TODGA (N,N,N′,N′-tetraoctyl diglycolamide) would greatly overcome the reduction issue by oxidizing Am(III) to Am(V) in the organic solvent and result in efficient separation of Am from lanthanides and curium (Cm).27,28 High Ln/Am separation factors have been maintained for a few hours during the extraction and then decreased gradually. We attribute the decrease in Ln/Am separation factors to the persistent consumption of Bi(V) by Am(III) and other reducing products in the solution. The consumption of Bi(V) and decrease of Ln/Am separation factors become more significant when relatively high concentrations of Am are present, and this might be problematic when dealing with real waste containing Am at the mM level.27 To slow down the consumption rate of Bi(V) and the reducing rate of Am(V) and thus to improve the applicability of this separation method in dealing with high concentrations of Am, herein we demonstrated a new strategy for more efficient generation and stabilization of Am(V) in a biphasic system by first oxidizing Am(III) to Am(VI) in an aqueous solution and then contacting it with a Bi(V)-incorporating organic solvent. Accordingly, record high Ln/Am separation efficiency was achieved over a wide range of acidities.
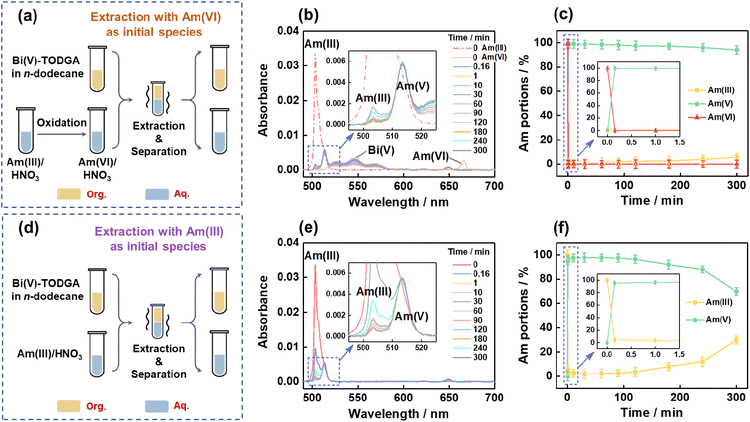
To prove the feasibility of the proposed strategy, we prepared an Am(VI)/HNO3 solution by the well-known NaBiO3 oxidation method24–26 and then contacted this solution with the Bi(V)-incorporating TODGA/n-dodecane organic solution (Fig. 1a). The variation of absorption spectra of the aqueous phase at different time intervals was monitored to probe the Am speciation change (Fig. 1b). As can be seen, Am(VI) ions (666.0 nm) in the initial HNO3 solution were quickly reduced to Am(V) (513.6 nm) in 10 s and negligible Am(III) could be observed at 1 min after the biphasic contact. Am(V) accounts for over 99.5% of the total Am in the aqueous phase in 10 s of contact and remains as the dominant Am species over a long time duration (>98% after 3 hours and ∼95% after 5 hours of contact, Fig. 1c). Meanwhile, a comparative test following the strategy in our previous work by mixing Am(III)/HNO3 solution with Bi(V)-incorporating organic solvent was also performed (Fig. 1d).27 The results suggest ∼95% of Am(III) was converted to Am(V) in 10 s of contact and this portion value dropped to ∼92% and <70% after 3 and 5 hours of contact, respectively (Fig. 1e and f). Obviously, the new strategy is much more efficient in generating and stabilizing Am(V) than the previous one.
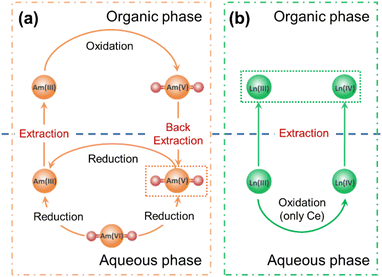
The highly efficient generation and stabilization of Am(V) in this system can be explained by examining the reduction process of Am(VI) and the oxidation process of Am(III). First, Am(VI) is very unstable in the case of contacting with organic reagents.24–26,29 When we contacted an Am(VI)/HNO3 aqueous solution with a TODGA/n-dodecane organic solution, the absorption bands of Am(VI) at 666.0 nm disappeared rapidly and the absorption bands of Am(V) at 513.4 nm emerged concurrently in the aqueous phase, meanwhile the absorption bands of Am(III) at 506.6 nm appeared gradually in the organic phase (Fig. S1†). This observation suggests Am(V) can be generated from the reduction of Am(VI). On the other hand, in our previous work we have demonstrated that Am(III) in the aqueous phase can be oxidized efficiently to Am(V) by contacting with a Bi(V)-incorporating TODGA/n-dodecane organic solution.27 Furthermore, when we contact an Am(VI)/HNO3 aqueous solution with a Bi(V)-incorporating TODGA/n-dodecane organic solution, the interplay of Am(VI) reduction and Am(III) oxidization leads to fast and quantitative generation of Am(V) and this can be illustrated by Scheme 1. In brief, Am(VI) will be reduced to Am(V) and Am(III) in the biphasic system, but any Am(III) will be extracted by TODGA and oxidized to Am(V) by Bi(V) in the organic phase immediately and then Am(V) will transfer back to the aqueous phase. Apparently, much less Bi(V) will be consumed by starting with Am(VI) solution than with Am(III) solution, thereby enhancing the stability of Am(V) over a long duration.
On the basis of quantitative generation and superior stabilization of Am(V) using Am(VI) as the starting species, highly efficient separation of Am from the lanthanides has been achieved through biphasic extraction. As shown in Fig. 2, when we contacted an aqueous solution containing 241Am(VI) and 152,154Eu(III) with a Bi(V)-incorporating TODGA/n-dodecane organic solution, 241Am stayed exclusively in the aqueous phase while 152,154Eu were extracted into the organic phase. Record high separation factors of Eu and Am (SFEu/Am) of >105 can be well maintained for over 4 hours and the value is more than 103 even after 12 hours (Fig. 2a). In contrast, if we started the extraction with Am(III) using our previous strategy, both the separation factors and stability are apparently less superior (Fig. 2b). These results are well consistent with the observations in spectral analysis (Fig. 1). Moreover, high SFEu/Am values (>104) could be obtained over a wide range of acidity from 1.0 to 14.0 M HNO3 (Fig. 2c), proving the ability of this separation strategy to deal with real nuclear waste usually of high acidity.30 To further assess the applicability of the present separation strategy in practical radioactive waste treatment, we performed a test for the separation of simulated Am/Ln waste containing 1.0 mM 241Am that is comparable to the concentration of Am in real waste and a variety of lanthanides (La, Ce, Pr, Nd, Sm, Eu and Gd). As shown in Fig. 2d, all the lanthanides were well separated from Am, and an unprecedented SFEu/Am value of 2.59 × 105 and SFCe/Am value over 1.0 × 106 through a single contact were obtained. It should be noted that during the reviewing of this work two anonymous reviewers raised an issue on the final purification of Am, since quite a portion of Bi will coexist in the aqueous phase with Am after Am/Ln separation. Considering both Am and Bi will eventually exist in the thermodynamically stable trivalent states, we expect Am(III)/Bi(III) separation can be well achieved by selective extraction using ligands such as tributyl phosphate (TBP) or NTAamide, both of which show much higher affinity to Bi(III) than to Am(III).31–33
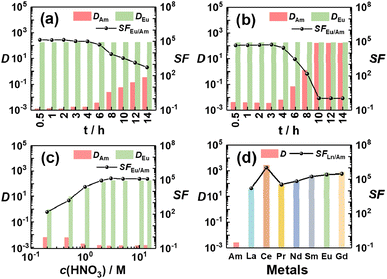 | ||
| Fig. 2 Separation results of 241Am and Ln. Effect of contact time with (a) Am(VI) or (b) Am(III) as the starting species. (c) Effect of HNO3 concentration. (d) Separation of simulated Am/Ln waste. Initial organic phase: (a–c) 0.1 M TODGA in Bi(V)-incorporating n-dodecane; (d) 0.5 M TODGA in Bi(V)-incorporating n-dodecane. Initial aqueous phase: trace amount of pre-oxidized 241Am (∼10−8 M) and 152,154Eu (∼10−9 M) in (a) 3.0 M HNO3 or (c) different concentrations of HNO3; (b) trace amount of 241Am (∼10−8 M) and 152,154Eu (∼10−9 M) in 3.0 M HNO3; (d) pre-oxidized simulated Am/Ln waste solution containing 1.0 mM 241Am and ∼mM level of Ln (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd) in 3.0 M HNO3 (see Table S1† for detailed composition). Contact time for (c) and (d) is 1 min. | ||
In conclusion, by exploiting the unique properties of Am(VI) reduction and Am(III) oxidation in a deliberately designed biphasic extraction system with Bi(V) incorporated in the organic phase, Am(V) was efficiently generated, stabilized, and separated from lanthanides with record high efficiency. The findings from this work not only provide an extremely efficient Am/Ln separation method to support the advanced nuclear fuel cycle, but also enrich our understanding of the less-explored redox chemistry of the highly radioactive element Am.
Data availability
Additional data supporting this article have been uploaded as part of the ESI.†Author contributions
Z. W. and C. X. conceived the experiments, supervised the studies and wrote the manuscript. J. C. participated in the discussion. X. D. and Z. W. executed the experiments, and collected and analyzed the data. H. H. assisted in the radiological experiments.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. X. is thankful for the financial support from the National Natural Science Foundation of China (22325603) and Beijing Natural Science Foundation (JQ20041). Z. W. is thankful for the financial support from the National Natural Science Foundation of China (22006090 and 22376116).References
- R. A. Wigeland, T. H. Bauer, T. H. Fanning and E. E. Morris, Separations and transmutation criteria to improve utilization of a geologic repository, Nucl. Technol., 2006, 154, 95–106 CrossRef CAS.
- M. Salvatores and G. Palmiotti, Radioactive waste partitioning and transmutation within advanced fuel cycles: achievements and challenges, Prog. Part. Nucl. Phys., 2011, 66, 144–166 CrossRef CAS.
- Y. A. Kazansky and M. I. Romanov, Transmuting minor actinides with thermal reactor neutrons, Nucl. Energy Technol., 2015, 1, 208–212 CrossRef.
- R. Malmbeck, C. Nourry, M. Ougier, P. Souček, J. Glatz, T. Kato and T. Koyama, Advanced fuel cycle options, Energy Procedia, 2011, 7, 93–102 CrossRef CAS.
- P. J. Panak and A. Geist, Complexation and extraction of trivalent actinides and lanthanides by triazinylpyridine N-donor ligands, Chem. Rev., 2013, 113, 1199–1236 CrossRef CAS PubMed.
- A. Leoncini, J. Huskens and W. Verboom, Ligands for f-element extraction used in the nuclear fuel cycle, Chem. Soc. Rev., 2017, 46, 7229–7273 RSC.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, A. Geist, V. N. Kozhevnikov, P. Distler and J. John, Hydrophilic sulfonated bis-1, 2, 4-triazine ligands are highly effective reagents for separating actinides(III) from lanthanides(III) via selective formation of aqueous actinide complexes, Chem. Sci., 2015, 6, 4812–4821 RSC.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, J. F. Desreux, G. Vidick, N. Bouslimani, G. Modolo, A. Wilden, M. Sypula, T. H. Vu and J. P. Simonin, Highly efficient separation of actinides from lanthanides by a phenanthroline-derived bis-triazine ligand, J. Am. Chem. Soc., 2011, 133, 13093–13102 CrossRef CAS PubMed.
- M. P. Jensen and A. H. Bond, Comparison of covalency in the complexes of trivalent actinide and lanthanide cations, J. Am. Chem. Soc., 2002, 124, 9870–9877 CrossRef CAS PubMed.
- A. Bhattacharyya and P. K. Mohapatra, Separation of trivalent actinides and lanthanides using various ‘N’, ‘S’ and mixed ‘N,O’donor ligands: a review, Radiochim. Acta, 2019, 107, 931–949 CrossRef CAS.
- Y. Y. Liu, Y. Kang, M. J. Bao, H. Cao, C. Q. Weng, X. Dong, H. X. Hao, X. Y. Tang, J. Chen, L. Wang and C. Xu, Hydroxyl-group functionalized phenanthroline diimides as efficient masking agents for Am(III)/Eu(III) separation under harsh conditions, J. Hazard. Mater., 2024, 462, 132756 CrossRef CAS PubMed.
- P. Ren, P. W. Huang, X. F. Yang, Y. Zou, W. Q. Tao, S. L. Yang, Q. Y. Wu, L. Y. Yuan, Z. F. Chai and W. Q. Shi, Hydrophilic sulfonated 2,9-diamide-1,10-phenanthroline endowed with highly effective ligand for separation americium(III) from europium(III): extraction, spectroscopy, and DFT calculations, Inorg. Chem., 2021, 60, 357–365 CrossRef CAS PubMed.
- D. S. Tian, Y. Y. Liu, Y. Kang, Y. Zhao, P. C. Li, C. Xu and L. Wang, A simple yet efficient hydrophilic phenanthroline-based ligand for selective Am(III) separation under high acidity, ACS Cent. Sci., 2023, 9, 1642–1649 CrossRef CAS PubMed.
- I. Lehman-Andino, J. Su, K. E. Papathanasiou, T. M. Eaton, J. W. Jian, D. Dan, T. E. Albrecht-Schmitt, C. J. Dares, E. R. Batista, P. Yang, J. K. Gibson and K. Kavallieratos, Soft-donor dipicolinamide derivatives for selective actinide(III)/lanthanide(III) separation: the role of S- vs. O-donor sites, Chem. Commun., 2019, 55, 2441–2444 RSC.
- W. H. Runde and B. J. Mincher, Higher oxidation states of americium: preparation, characterization and use for separations, Chem. Rev., 2011, 111, 5723–5741 CrossRef CAS PubMed.
- C. J. Dares, A. M. Lapides, B. J. Mincher and T. J. Meyer, Electrochemical oxidation of 243Am(III) in nitric acid by a terpyridyl-derivatized electrode, Science, 2015, 350, 652–655 CrossRef CAS PubMed.
- M. J. Lopez, M. V. Sheridan, J. R. McLachlan, T. S. Grimes and C. J. Dares, Electrochemical oxidation of trivalent americium using a dipyrazinylpyridine modified ITO electrode, Chem. Commun., 2019, 55, 4035–4038 RSC.
- H. L. Zhang, A. Li, K. Li, Z. P. Wang, X. C. Xu, Y. X. Wang, M. V. Sheridan, H. S. Hu, C. Xu, E. V. Alekseev, Z. Y. Zhang, P. Yan, K. C. Cao, Z. F. Chai, T. E. Albrecht-Schmitt and S. A. Wang, Ultrafiltration separation of Am(VI)-polyoxometalate from lanthanides, Nature, 2023, 616, 482–487 CrossRef CAS PubMed.
- S. Matsuda, K. Yokoyama, T. Yaita, T. Kobayashi, Y. Kaneta, M. Simonnet, T. Sekiguchi, M. Honda, K. Shimojo, R. Doi and N. Nakashima, Marking actinides for separation: resonance-enhanced multiphoton charge transfer in actinide complexes, Sci. Adv., 2022, 8, eabn1991 CrossRef CAS PubMed.
- M. V. Sheridan, J. R. Gonzalez-Moya, J. R. McLachlan, T. S. Grimes and C. J. Dares, Photocatalytic conversion of Am(III) to Am(VI) using a TiO2 electrode, ACS Appl. Energy Mater., 2021, 4, 11854–11857 CrossRef CAS.
- M. Kamoshida, T. Fukasawa and F. Kawamura, Valence control and solvent extraction of americium in the presence of ammonium phosphotungstate, J. Nucl. Sci. Technol., 1998, 35, 185–189 CrossRef CAS.
- M. Kamoshida and T. Fukasawa, Solvent extraction of americium(VI) by tri-n-butyl phosphate, J. Nucl. Sci. Technol., 1996, 33, 403–408 CrossRef CAS.
- Y. Koma, A. Aoshima, M. Kamoshida and A. Sasahira, Extraction of Am(VI) from nitric acid solution containing phosphate anion by TBP, J. Nucl. Sci. Technol., 2002, 39, 317–320 CrossRef.
- B. J. Mincher, L. R. Martin and N. C. Schmitt, Tributylphosphate extraction behavior of bismuthate-oxidized americium, Inorg. Chem., 2008, 47, 6984–6989 CrossRef CAS PubMed.
- L. Martin, B. Mincher and N. Schmitt, Extraction of americium(VI) by a neutral phosphonate ligand, J. Radioanal. Nucl. Chem., 2009, 282, 523–526 CrossRef CAS.
- B. J. Mincher, L. R. Martin and N. C. Schmitt, Diamylamylphosphonate solvent extraction of Am(VI) from nuclear fuel raffinate simulant solution, Solvent Extr. Ion Exch., 2012, 30, 445–456 CrossRef CAS.
- Z. P. Wang, J. B. Lu, X. Dong, Q. Yan, X. G. Feng, H. S. Hu, S. A. Wang, J. Chen, J. Li and C. Xu, Ultra-efficient americium/lanthanide separation through oxidation state control, J. Am. Chem. Soc., 2022, 144, 6383–6389 CrossRef CAS.
- Z. P. Wang, X. Dong, Q. Yan, J. Chen and C. Xu, Separation of americium from curium through oxidation state control with record efficiency, Anal. Chem., 2022, 94, 7743–7746 CrossRef CAS PubMed.
- G. P. Horne, T. S. Grimes, W. F. Bauer, C. J. Dares, S. M. Pimblott, S. P. Mezyk and B. J. Mincher, Effect of ionizing radiation on the redox chemistry of penta-and hexavalent americium, Inorg. Chem., 2019, 58, 8551–8559 CrossRef CAS PubMed.
- D. Magnusson, B. Christiansen, J. P. Glatz, R. Malmbeck, G. Modolo, D. Serrano-Purroy and C. Sorel, Demonstration of a TODGA based extraction process for the partitioning of minor actinides from a PUREX raffinate: part III: centrifugal contactor run using genuine fuel solution, Solvent Extr. Ion Exch., 2009, 27, 26–35 CrossRef CAS.
- J. L. Fan, G. Wang, S. Xu, J. L. Zhu, J. Zhang, J. W. Chen, L. Zheng, J. G. Pan, R. L. Wang and Y. Y. Hao, Removal of impurities from bismuth pickling solution using solvent extraction with TBP, Hydrometallurgy, 2022, 207, 105779 CrossRef CAS.
- B. Sreenivasulu, A. Suresh, N. Sivaraman and P. R. Vasudeva Rao, Solvent extraction studies with some fission product elements from nitric acid media employing tri-iso-amyl phosphate and tri-n-butyl phosphate as extractants, J. Radioanal. Nucl. Chem., 2015, 303, 2165–2172 CAS.
- Y. Sasaki, Y. Tsubata, Y. Kitatsuji, Y. Sugo, N. Shirasu, Y. Morita and T. Kimura, Extraction behavior of metal ions by TODGA, DOODA, MIDOA, and NTAamide extractants from HNO3 to n-dodecane, Solvent Extr. Ion Exch., 2013, 31, 401–415 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc06240f |
| This journal is © The Royal Society of Chemistry 2024 |