Open Access Article
Open Access ArticleSynthesis and structural characterization of stable coinage metal (Cu, Ag, Au) cyclopentadienyl complexes†
Robin
Sievers
a,
Marc
Reimann
 b,
Nico G.
Kub
a,
Susanne M.
Rupf
b,
Nico G.
Kub
a,
Susanne M.
Rupf
 a,
Martin
Kaupp
a,
Martin
Kaupp
 b and
Moritz
Malischewski
b and
Moritz
Malischewski
 *a
*a
aFreie Universität Berlin, Fabeckstraße 34/36, 14195 Berlin, Germany. E-mail: moritz.malischewski@fu-berlin.de
bTechnische Universität Berlin, Straße des 17. Juni 135, 10623 Berlin, Germany
First published on 16th January 2024
Abstract
The electron withdrawing and oxidatively stable perfluorinated Cp* ligand [C5(CF3)5]− allowed for the isolation of rare and unusually stable coinage metal complexes [M(C5(CF3)5)(PtBu3)] (M = Cu, Ag, Au), representing the first complete and structurally comparable series of group 11 Cp coordination compounds. Full characterization and structure analysis revealed distinct and partly unknown coordination motifs with hapticities ranging from η1, η3/η1 and η3/η2 for gold, silver and copper, respectively. Quantum-chemical studies using DFT methods confirm these findings and connect them to the unique electronic structure of the given ligand system.
Introduction
Within the great variety in organometallic chemistry, cyclopentadienyls (Cp) are among the most common carbon-based ligands. While coordination compounds with lanthanide and main group elements are well studied, their original and still current domain is found for the transition metals with over 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 catalogued systems according to the Cambridge Crystallographic Data Centre (CCDC).1 To a significant extent, this enormous number of structures is due to the adaptability of Cp-metal bonding to the chemical environment by diverse binding modes and hapticities ranging from η1 to η5. Consequently, hundreds and thousands of examples for almost every transition metal exist with structurally defined cyclopentadienyl binding motifs. However, a considerable gap becomes apparent for the coinage metal (Cu, Ag, Au) cyclopentadienyls, due to their pronounced instability towards moisture, air and light, and even their thermal lability at ambient conditions. Thus, with only 47 hits according to CCDC in total, group 11 is the by far least structurally characterized transition metal group in this context.1 Among these 47, the majority (39) belongs to copper complexes,2–9 while silver10 and gold11–17 cyclopentadienyls are extraordinarily rare, with only one and seven structures, respectively.1 For copper, η5-coordination is observed exclusively (with two heterobimetallic exceptions)18,19 as for example in [Cu(C5H5)(PPh3)].4,5 Silver, in comparison, may adopt η5 or η3 hapticities, both observed with [Ag(C5(SiMe3)3H2)(PnBu3)],10 while gold predominantly tends to η1 with distortions towards η3 as in [Au(C5Ph4H)(PPh3)].11 Moreover, there is no complete structurally comparable series known for group 11 cyclopentadienyl complexes at all, as either differing Cp substitution patterns or co-ligands exercise crucial influence on the bonding situation. In addition to this gap of knowledge regarding their structures, new synthetic approaches to metal Cp complexes with increased stabilities are needed. So far, attempts to overcome the stability issues have mainly focused on sterical shielding in combination with high electron affinity Cp derivatives, preventing oxidative decomposition. This was, for example, impressively demonstrated by Rubin et al. with structurally related fulleride based coinage metal complexes. However, this resulted in very large structures with atypically non-planar and highly conjugated Cp moieties.20 As demonstrated in seminal works of Menjón and Dias, both CF3 ligands and organic ligands containing CF3 groups (e.g. scorpionates) are ideal to successfully stabilize coinage metal complexes in various oxidation states and coordination geometries.21–37 As a consequence, we considered application of the perfluorinated Cp* analogue [C5(CF3)5]−, whose initial coordination and reactivity was recently investigated.38–40 Being sterically demanding, but also oxidatively stable, this Cp ligand appeared to be a suitable candidate for the elucidation of coinage metal Cp coordination chemistry.
000 catalogued systems according to the Cambridge Crystallographic Data Centre (CCDC).1 To a significant extent, this enormous number of structures is due to the adaptability of Cp-metal bonding to the chemical environment by diverse binding modes and hapticities ranging from η1 to η5. Consequently, hundreds and thousands of examples for almost every transition metal exist with structurally defined cyclopentadienyl binding motifs. However, a considerable gap becomes apparent for the coinage metal (Cu, Ag, Au) cyclopentadienyls, due to their pronounced instability towards moisture, air and light, and even their thermal lability at ambient conditions. Thus, with only 47 hits according to CCDC in total, group 11 is the by far least structurally characterized transition metal group in this context.1 Among these 47, the majority (39) belongs to copper complexes,2–9 while silver10 and gold11–17 cyclopentadienyls are extraordinarily rare, with only one and seven structures, respectively.1 For copper, η5-coordination is observed exclusively (with two heterobimetallic exceptions)18,19 as for example in [Cu(C5H5)(PPh3)].4,5 Silver, in comparison, may adopt η5 or η3 hapticities, both observed with [Ag(C5(SiMe3)3H2)(PnBu3)],10 while gold predominantly tends to η1 with distortions towards η3 as in [Au(C5Ph4H)(PPh3)].11 Moreover, there is no complete structurally comparable series known for group 11 cyclopentadienyl complexes at all, as either differing Cp substitution patterns or co-ligands exercise crucial influence on the bonding situation. In addition to this gap of knowledge regarding their structures, new synthetic approaches to metal Cp complexes with increased stabilities are needed. So far, attempts to overcome the stability issues have mainly focused on sterical shielding in combination with high electron affinity Cp derivatives, preventing oxidative decomposition. This was, for example, impressively demonstrated by Rubin et al. with structurally related fulleride based coinage metal complexes. However, this resulted in very large structures with atypically non-planar and highly conjugated Cp moieties.20 As demonstrated in seminal works of Menjón and Dias, both CF3 ligands and organic ligands containing CF3 groups (e.g. scorpionates) are ideal to successfully stabilize coinage metal complexes in various oxidation states and coordination geometries.21–37 As a consequence, we considered application of the perfluorinated Cp* analogue [C5(CF3)5]−, whose initial coordination and reactivity was recently investigated.38–40 Being sterically demanding, but also oxidatively stable, this Cp ligand appeared to be a suitable candidate for the elucidation of coinage metal Cp coordination chemistry.
Results and discussion
To offer a structurally comparable series of [M(C5(CF3)5)(L)] (M = Cu, Ag, Au) coinage metal complexes, a uniform composition with suitable co-ligands was envisaged. Previous works towards cyclopentadienyl coordination chemistry predominantly demonstrated stabilization by different phosphines such as, e.g. PPh3 for copper,2–9 silver,10 or gold.11–17However, in this case synthetic efforts with PPh3 based precursors were unsuccessful, resulting in an undefined reaction mixture, containing product traces without any Cp coordination, such as [Au(PPh3)2][C5(CF3)5]. In fact, the great oxidative stability of the [C5(CF3)5]− ligand is accompanied by a generally weakly bonding character, but also by pronounced δ-acceptor properties,38 compared to ordinary electron rich Cp ligands. Thus, the strongly electron donating and sterically demanding PtBu3 was considered as a suitable co-ligand instead. Due to the extraordinary acidity of HC5(CF3)5 (pKa = −2.2),41,42 which was in situ generated from [NEt4][C5(CF3)5] and H2SO4,38,43–45 [M(PtBu3)(OAc)] (M = Cu, Ag,46 Au) complexes were used for coordination attempts, as the weaker acid CH3COOH could easily be displaced and removed from the reaction mixture. Consequently, all [M(C5(CF3)5)(PtBu3)] complexes were successfully synthesized in quantitative yield, demonstrating the preparation of the first complete series in Cp coinage metal coordination chemistry (Scheme 1).
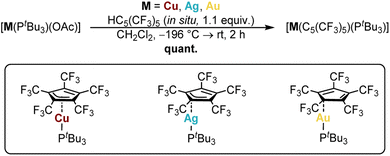 | ||
| Scheme 1 Synthesis of [M(C5(CF3)5)(PtBu3)] from [M(PtBu3)(OAc)] and HC5(CF3)5 in quantitative yield (M = Cu, Ag, Au). | ||
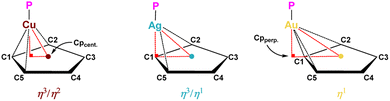
For [Au(C5(CF3)5)(PtBu3)] the NMR spectroscopic results are in agreement with expectations of a rotationally unhindered PtBu3 ligand. However, the 19F and 13C{1H}NMR spectra mimic a η5-coordination of [C5(CF3)5]− towards the Au(I) center with C5-symmetry, due to the equivalence of its carbon and fluorine atoms. As all structurally characterized Au–Cp complexes are either definitely, or at least slightly distorted η1-coordinated, this observation is readily explained by fast metallotropic shifts in solution.11–17 Subsequently, it was possible to obtain colorless single crystals and the corresponding molecular structure in the solid state by slowly cooling solutions in n-pentane/ortho-difluorobenzene (oDFB) to −35 °C (Fig. 1, right). [Au(C5(CF3)5)(PtBu3)] crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group and indeed exhibits a distinct η1-coordination towards the Au(I) center. The by far shortest distance of 2.206(7) Å is obtained for the Au–C1 σ-bond, while the further gold–carbon distances increase in a very symmetrical fashion, being 2.754(7) or 2.737(7) Å for Au–C2 and Au–C5 and 3.400(7) or 3.393(7) Å for Au–C3 and Au–C4 (Table 1, right). In contrast to some known Au–Cp complexes there is no evidence for any distortion towards η2- or η3-coordination.11–13,15,47 Consequently, the Cp-ring is assumed to have lost its aromatic stabilization, as demonstrated also by the significantly elongated C1–C2, C3–C4 and C5–C1 bonds of 1.468(10), 1.423(12), and 1.442(10) Å, respectively. In contrast, the C2–C3 and C4–C5 bonds are shortened and close to regular unconjugated C
space group and indeed exhibits a distinct η1-coordination towards the Au(I) center. The by far shortest distance of 2.206(7) Å is obtained for the Au–C1 σ-bond, while the further gold–carbon distances increase in a very symmetrical fashion, being 2.754(7) or 2.737(7) Å for Au–C2 and Au–C5 and 3.400(7) or 3.393(7) Å for Au–C3 and Au–C4 (Table 1, right). In contrast to some known Au–Cp complexes there is no evidence for any distortion towards η2- or η3-coordination.11–13,15,47 Consequently, the Cp-ring is assumed to have lost its aromatic stabilization, as demonstrated also by the significantly elongated C1–C2, C3–C4 and C5–C1 bonds of 1.468(10), 1.423(12), and 1.442(10) Å, respectively. In contrast, the C2–C3 and C4–C5 bonds are shortened and close to regular unconjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds, having 1.383(9) and 1.371(9) Å.48 As a further consequence, the σ-bonded C1 shows a well pronounced pyramidalization. Therefore, the adjacent CF3-group is significantly tilted out of the Cp-plane, with an Cpcent.–C1–CF3 angle of 148.37(58)° (180° for full planarity). The given hapticity is emphasized when comparing the relative position of the Au(I) center compared to the cyclopentadienyl ligand. Due to the mentioned σ-bonding, the orthogonal projection (Cpperp.; see Table 1) of the gold atom is already located outside of the Cp plane giving a Cpcent.–Cpperp. distance of 1.541(6) Å (for comparison Ccent.–C1 = 1.237(7) Å) and a significantly widened Au–C1–Cpcent. angle of 97.29(39)°. The overall distortion is negligible given the very similar C1–Au–C2 and C1–Au–C5 angles of 31.99(23) and 32.16(22)°, respectively. These values were also verified by DFT structure optimizations (r2SCAN-3c), providing an undistorted η1-coordination with quite comparable values, as for example an Au–C1 bond length of 2.214 Å and a Cpcent.–C1–CF3 angle of 148° (see ESI, Table S4†).
C double bonds, having 1.383(9) and 1.371(9) Å.48 As a further consequence, the σ-bonded C1 shows a well pronounced pyramidalization. Therefore, the adjacent CF3-group is significantly tilted out of the Cp-plane, with an Cpcent.–C1–CF3 angle of 148.37(58)° (180° for full planarity). The given hapticity is emphasized when comparing the relative position of the Au(I) center compared to the cyclopentadienyl ligand. Due to the mentioned σ-bonding, the orthogonal projection (Cpperp.; see Table 1) of the gold atom is already located outside of the Cp plane giving a Cpcent.–Cpperp. distance of 1.541(6) Å (for comparison Ccent.–C1 = 1.237(7) Å) and a significantly widened Au–C1–Cpcent. angle of 97.29(39)°. The overall distortion is negligible given the very similar C1–Au–C2 and C1–Au–C5 angles of 31.99(23) and 32.16(22)°, respectively. These values were also verified by DFT structure optimizations (r2SCAN-3c), providing an undistorted η1-coordination with quite comparable values, as for example an Au–C1 bond length of 2.214 Å and a Cpcent.–C1–CF3 angle of 148° (see ESI, Table S4†).
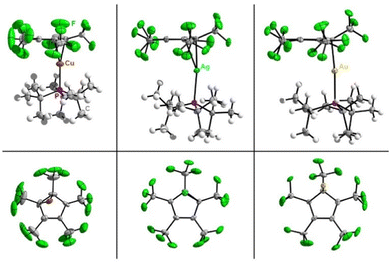 | ||
| Fig. 1 Molecular structures in the solid state of [M(C5(CF3)5)(PtBu3)] (M = Cu, Ag, Au), side-on view at the top and bottom view below. Disorder is omitted for clarity (see ESI, Fig. S31†). Ellipsoids are depicted with 50% probability level. Color code: white-hydrogen, grey-carbon, green-fluorine, purple-phosphorous, brown-copper, blue-silver, yellow-gold. | ||
| [M(C5(CF3)5)(PtBu3)] | Cu | Ag | Au |
|---|---|---|---|
| M–P | 2.201(15) | 2.367(1) | 2.268(2) |
| M–C1 | 2.119(6) | 2.304(4) | 2.206(7) |
| M–C2 | 2.589(10) | 2.687(5) | 2.754(7) |
| M–C3 | 2.947(8) | 3.253(5) | 3.400(7) |
| M–C4 | 2.803(8) | 3.277(5) | 3.393(7) |
| M–C5 | 2.299(6) | 2.728(5) | 2.737(7) |
| C1–C2 | 1.475(15) | 1.429(6) | 1.468(10) |
| C2–C3 | 1.401(8) | 1.403(7) | 1.383(9) |
| C3–C4 | 1.376(10) | 1.412(6) | 1.423(12) |
| C4–C5 | 1.412(9) | 1.394(6) | 1.371(9) |
| C5–C1 | 1.468(9) | 1.439(6) | 1.442(10) |
| M–Cpcent. | 2.265(1) | 2.609(1) | 2.674(1) |
| Cpcent.–Cpperp. | 0.871(7) | 1.212(4) | 1.541(6) |
| P–M–C1 | 167.80(18) | 173.55(10) | 175.98(18) |
| C1–M–C2 | 34.72(27) | 32.11(13) | 31.99(23) |
| C1–M–C5 | 38.54(22) | 31.84(13) | 32.16(22) |
| M–C1–Cpcent. | 80.82(30) | 90.31(22) | 97.29(39) |
| Cpcent–C1–CF3 | 163.72(60) | 157.63(40) | 148.37(58) |
[Ag(C5(CF3)5)(PtBu3)] represents a very rare example of Ag–Cp coordination without any supportive secondary coordinative interactions (as seen in, e.g., [Ag(C5(CO2Me)5)(PPh3)]).49 Again, fast metallotropic shifts mimic a η5-hapticity towards the Ag(I) center in the 19F and 13C{1H} NMR spectra. Surprisingly, pronounced direct 107Ag (1JP,Ag = 651.6 Hz) and 109Ag (1JP,Ag = 752.2 Hz) coupling is visible in the 31P{1H} spectrum, due to significant hindrance by the sterically demanding PtBu3 ligand, resulting in two distinct doublets (absent for copper and gold).32,33 This phenomenon is also observable by additional resonances in the 13C{1H} spectrum, due to geminal and vicinal Ag(I) couplings. Single crystals were obtained by slow cooling of a [Ag(C5(CF3)5)(PtBu3)] solution in n-pentane/CH2Cl2 to −75 °C. The corresponding molecular structure in the solid state revealed crystallization in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Fig. 1, middle). In contrast to [Au(C5(CF3)5)(PtBu3)] the bonding situation is less definite. It shows a strong tendency towards σ-bonding (Ag–C1 bond length 2.304(4) Å), but with some tilt towards alkene π-bonding (Table 1, middle). Here the Ag(I) center is slightly distorted, giving a shortened Ag–C2 distance of 2.687(5) Å, while the Ag–C5 distance is 2.728(5) Å, indicating secondary interactions. Consequently, the conjugation of the aromatic system is expected to be more pronounced. Indeed, the C2–C3 and C4–C5 bonds are significantly elongated, with 1.403(7) or 1.394(6) Å, and show less double-bond character compared to [Au(C5(CF3)5)(PtBu3)]. In contrast, C1–C2, C3–C4 and C5–C1 distances are shortened, with 1.429(6), 1.412(6) and 1.439(6) Å, respectively. Additionally, the Cpcent.–C1–CF3 angle decreases to 157.63(40)°, indicating minor pyramidalization at C1. Considering the relative position of silver towards the cyclopentadienyl ligand a significant deviation from η1-coordination is demonstrated. The Ag–C1–Cpcent. angle reduces to 90.31(22)°, showing an almost perpendicular orientation of silver towards C1 and a strongly shortened Cpcent.–Cpperp. distance of 1.212(4) Å compared to [Au(C5(CF3)5)(PtBu3)]. Considering the comparable values for C1–Ag–C2 and C1–Ag–C5 angles of 32.11(13) and 31.84(13)°, asymmetric coordination patterns are excluded, and the bonding situation is properly described as η3/η1 (η3 with significant distortion towards η1). These findings are further emphasized by our DFT structure optimizations (r2SCAN-3c) of [Ag(C5(CF3)5)(PtBu3)], providing for example distances of 2.272, 2.698, and 2.719 Å for Ag–C1, Ag–C2, Ag–C5, respectively (see ESI, Table S4†). Compared to the structurally characterized [Ag(C5(SiMe3)3H2)(PnBu3)], which exhibits either η5- or η3-hapticity,10 the reduced η3/η1-hapticity of [Ag(C5(CF3)5)(PtBu3)] stands out and represents a so far unknown coordination mode for Ag–Cp complexes.
space group (Fig. 1, middle). In contrast to [Au(C5(CF3)5)(PtBu3)] the bonding situation is less definite. It shows a strong tendency towards σ-bonding (Ag–C1 bond length 2.304(4) Å), but with some tilt towards alkene π-bonding (Table 1, middle). Here the Ag(I) center is slightly distorted, giving a shortened Ag–C2 distance of 2.687(5) Å, while the Ag–C5 distance is 2.728(5) Å, indicating secondary interactions. Consequently, the conjugation of the aromatic system is expected to be more pronounced. Indeed, the C2–C3 and C4–C5 bonds are significantly elongated, with 1.403(7) or 1.394(6) Å, and show less double-bond character compared to [Au(C5(CF3)5)(PtBu3)]. In contrast, C1–C2, C3–C4 and C5–C1 distances are shortened, with 1.429(6), 1.412(6) and 1.439(6) Å, respectively. Additionally, the Cpcent.–C1–CF3 angle decreases to 157.63(40)°, indicating minor pyramidalization at C1. Considering the relative position of silver towards the cyclopentadienyl ligand a significant deviation from η1-coordination is demonstrated. The Ag–C1–Cpcent. angle reduces to 90.31(22)°, showing an almost perpendicular orientation of silver towards C1 and a strongly shortened Cpcent.–Cpperp. distance of 1.212(4) Å compared to [Au(C5(CF3)5)(PtBu3)]. Considering the comparable values for C1–Ag–C2 and C1–Ag–C5 angles of 32.11(13) and 31.84(13)°, asymmetric coordination patterns are excluded, and the bonding situation is properly described as η3/η1 (η3 with significant distortion towards η1). These findings are further emphasized by our DFT structure optimizations (r2SCAN-3c) of [Ag(C5(CF3)5)(PtBu3)], providing for example distances of 2.272, 2.698, and 2.719 Å for Ag–C1, Ag–C2, Ag–C5, respectively (see ESI, Table S4†). Compared to the structurally characterized [Ag(C5(SiMe3)3H2)(PnBu3)], which exhibits either η5- or η3-hapticity,10 the reduced η3/η1-hapticity of [Ag(C5(CF3)5)(PtBu3)] stands out and represents a so far unknown coordination mode for Ag–Cp complexes.
[Cu(C5(CF3)5)(PtBu3)], the last representative of the three coinage metal complexes, is again suggested to be η5-coordinated by the 19F and 13C{1H} NMR spectra. In contrast to gold and silver, a variety of exclusively η5-coordinated monometallic Cu–Cp complexes is known.2–9 Thus, the apparent C5 symmetry could not a priori be attributed to fast metallotropic shifts in solution, such as for [M(C5(CF3)5)(PtBu3)] (M = Ag, Au). Single crystals and the corresponding molecular structure in the solid state were obtained from solutions of [Cu(C5(CF3)5)(PtBu3)] in n-pentane/CH2Cl2, slowly cooled to −75 °C (Fig. 1, left). [Cu(C5(CF3)5)(PtBu3)] crystallizes in the monoclinic P21/n space group and surprisingly did not exhibit η5-bonding. Instead, [C5(CF3)5]− binds in a highly unsymmetrical fashion to the Cu(I) center, with a Cu–C1 bond length of about 2.119(6) Å, and Cu–C2 and Cu–C5 distances of 2.589(10) or 2.299(11) Å, respectively (Table 1, left). This trend continues for the remote carbon atoms, as shown by Cu–C3 and Cu–C4 of 2.947(8) and 2.803(8) Å, respectively. Similar to the Cu–C distances, the C–C bond length vary strongly but still support the trend of a decreasing σ-bonding character compared to the heavier homologues and pronounced conjugation within the ligand. This is shown by the slightly elongated C2–C3 and C4–C5 bonds (1.401(8) and 1.412(9) Å), but also by the diminished Cpcent.–C1–CF3 angle of 163.72(60)°, indicating less pyramidalization at C1. Regarding the relative position of copper towards the cyclopentadienyl ligand, the given trend of gold and silver is continued. The reduced Cu–C1–Cpcent. angle of 80.82(30)° and a shortened Cpcent.–Cpperp. distance of 0.871(7) Å show the orthogonal projection of copper to be distinctly located within the Cp plane. However, in contrast to [Au(C5(CF3)5)(PtBu3)] and [Ag(C5(CF3)5)(PtBu3)] and as already indicated by the Cu–C bond lengths, the C1–Cu–C2 and C1–Cu–C5 angles differ significantly, being 34.72(27) and 38.54(22)°, respectively. These findings clearly exclude symmetric coordination modes and affirm a highly unusual coordination motif, describable as η3/η2 (η3 with significant distortion towards η2). Except for two exotic examples of heterobimetallic complexes this is the only known Cu–Cp complex known without the common η5-coordination.18,19 Unfortunately, the overall data quality of the copper structure suffered from significant crystallographic disorder. However, the absence of a η5-coordination was also demonstrated by structure optimizations (r2SCAN-3c) of the complex, providing a very similar distorted η3-coordination with Cu–C1, Cu–C2 and Cu–C5 distances of 2.057, 2.502 and 2.201 Å, respectively (see ESI, Table S4†).
The unusual stability of the prepared [M(C5(CF3)5)(PtBu3)] (M = Cu, Ag, Au) coinage metal complexes is particularly intriguing. While most Au–Cp complexes such as [Au(C5Ph4H)(PPh3)] show significant air sensitivity,11 and the sterically less shielded [Au(C5H5)(PPh3)] additionally thermal lability at room temperature,50 [Au(C5(CF3)5)(PtBu3)] decomposes only slowly at ambient conditions. It is even more fascinating that [Ag(C5(CF3)5)(PtBu3)] is not only thermostable and relatively insensitive against air, but it is also storable without the exclusion of light. The structurally non-characterized [Ag(C5H5)(PPh3)] in contrast decomposes within minutes at room temperature under an argon atmosphere.51,52 [Cu(C5(CF3)5)(PtBu3)] is also significantly more stable than related Cu–Cp complexes, which often have pronounced sensitivity against air.2,4,7 By displaying the space-filling van der Waals spheres of [M(C5(CF3)5)(PtBu3)] (M = Cu, Ag, Au), the relevant steric demand becomes apparent for both [C5(CF3)5]− and PtBu3 (see ESI, Fig. S34†). Pronounced shielding of the coinage metal centers is observed, which is thought to partly suppress decomposition reactions. However, the spatial demand is only minor compared to the outstandingly well shielded fulleride complexes of Rubin et al., and stabilization of these coinage metal complexes by sterical shielding alone appears insufficient.20 [C5(CF3)5]− offers the additional advantage of an extraordinarily high electron affinity and oxidative stability, showing a calculated ionization energy of 4.84 eV compared to only 1.73 eV for [C5H5]− (at the PNO-CCSD(T)-F12b/cc-pVTZ-F12//r2SCAN-3c level, the value for [C5H5]− agrees very well with high-quality estimates of 1.79 eV)53 Thus, even coordination chemistry with highly oxidative metal centers, such as Ag(I) and Au(I) is feasible, and oxidative decomposition pathways involving single-electron transfer are effectively impeded.
To further understand the trends in bonding between the [C5(CF3)5]− ligand and the coinage metals, scalar-relativistic ZORA-r2SCAN-3c54 DFT calculations have been carried out. To allow for a simpler analysis, we replaced the tBu groups of the phosphine ligand by methyl groups. While the effects of this truncation on the bond lengths are only minor in the Au and Ag complexes, the differences become significant in the Cu complex, reducing the Cu–C3 and Cu–C2 distance by about 0.152 and 0.173 Å, respectively (see ESI, Table S6†). The obtained structure is then very similar to that of the Ag complex, which indicates that the experimentally observed differences between the Ag and the Cu complexes are to a large extent due to secondary interactions between the tBu groups of the phosphine and the CF3 groups of the Cp ligand in the copper case. These interactions are much less pronounced in the silver and gold complexes due to the larger metal-to-ligand distances (see Table 1). The remaining difference between the Ag and the Au complexes can be explained by the well-known, enhanced covalency of Au–C bonds due to the relativistic stabilization and contraction of the 6s orbitals.55–58 This effect can also be observed in the charges obtained from natural population analysis (NPA; see ESI, Table S8†). Removing scalar relativistic effects from the calculation results in all three metal centers exhibiting almost identical η3/η1 coordination motifs (see ESI, Table S7†).
This leaves the question, why all these metal centers, and especially copper prefer such a coordination motif, in contrast to the typically observed η5-coordination for Cp or Cp*. Using the PMe3 model systems, we find that the standard Cp* ligand indeed prefers a fivefold coordination, which means that the origin of the uncommon motif is due to the unique electronic properties of [C5(CF3)5]−. For a closer analysis, we have obtained η5 and η3/η1 structures of the [C5(CF3)5]− and Cp* systems, respectively, by replacing CH3 groups by CF3 groups (and vice versa) in the minimum structures and re-optimizing the complexes while freezing the C5 core and the M–PMe3 unit. Using these four structures, we have performed standard energy decomposition analyses (EDA)59 for the bonding between a [Cu(PMe3)]+ and a [C5(CX3)5]− (X = H, F) fragment (Table 2). While the Cp* ligand has a clear preference of 40 kJ mol−1 for η5 coordination, the difference practically vanishes for [C5(CF3)5]−. These preferences translate into the total energy differences, where [Cu(C5(CF3)5)(PMe3)] prefers the η3/η1 motif by 11 kJ mol−1, while the preference of [Cu(C5(CH3)5)(PMe3)] for the η5 structure is three times larger (32 kJ mol−1). In a η3/η1 structure, we find overall reduced interactions between the fragments. This is true both for the destabilizing Pauli-repulsion (ΔEPauli) and the stabilizing electrostatic (ΔEElstat.) and orbital-interaction (ΔEOrb.Int.) terms. For the Cp* ligand these lowered interactions are unfavorable, as the increase in stabilization in a η5 structure outweighs the increased Pauli-repulsion. For the [C5(CF3)5]− ligand, this is no longer the case. Here, the reduction in Pauli-repulsion with lower coordination number is significantly larger than for the Cp* ligand. At the same time, the gain in orbital interaction energies in an η5 binding motif is reduced for [C5(CF3)5]− due to it decreased donor ability.38 For the Ag complexes, the analogous results are provided in Table S9,† showing a qualitatively similar picture but a clearer preference for the η3/η1 motif with [C5(CF3)5]−. We note in passing that, unlike [C5H5]−, the structure of the free [C5(CF3)5]− ligand appears to be slightly non-planar due to hyperconjugation and pyramidalization effects of the negative charge.60,61 The corresponding energy differences are, however, small compared to the interaction energies in the metal complexes.
| [C5(CX3)5]− | ΔEPauli | ΔEElstat. | ΔEOrb.Int. | ΔETotal |
|---|---|---|---|---|
| X = H (η5) | 457.9 | −826.2 | −375.3 | −750.2 |
| X = H (η3/η1) | 386.9 | −783.1 | −305.3 | −710.1 |
| X = F (η5) | 441.8 | −623.3 | −311.7 | −502.8 |
| X = F (η3/η1) | 349.5 | −587.5 | −253.2 | −502.4 |
Conclusions
Due to the extraordinarily high electron affinity and oxidative stability of [C5(CF3)5]− it was possible to prepare the first complete and structurally comparable series of cyclopentadienyl coinage metal coordination species. All coinage metal complexes [M(C5(CF3)5)(PtBu3)] (M = Cu, Ag, Au) were structurally characterized62 and revealed distinct coordination modes, with hapticities ranging from η1, η3/η1 to η3/η2 for gold, silver and copper, respectively. In contrast to earlier experience, these coinage metal Cp complexes demonstrated not only significant stability at room temperature, but also towards moisture, air and light. Quantum-chemical calculations support the coordination motifs observed in the crystal structures, connecting them to the specific electronic structure of the new ligand system arising from the extremely electron-withdrawing CF3-substituents.Data availability
Data supporting this manuscript is available within the ESI† and available on request.Author contributions
RS: investigation, formal analysis, writing (original draft); MR: investigation, formal analysis, writing (original draft); NGK: investigation; SMR: formal analysis; MK: supervision, writing (review and editing); MM: conceptualization, supervision, project administration, writing (review and editing).Conflicts of interest
There are no conflicts to declare.Acknowledgements
Gefördert durch die Deutsche Forschungsgemeinschaft (DFG) – Projektnummer 387284271 – SFB 1349. Computing time was made available by High-Performance Computing at ZEDAT/FU Berlin. The authors acknowledge the assistance of the Core Facility BioSupraMol supported by the DFG. Robin Sievers thanks the Fonds of the Chemical Industry (FCI) for a Kekulé PhD Fellowship.Notes and references
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- G. Wilkinson and T. S. Piper, J. Inorg. Nucl. Chem., 1956, 2, 32–37 CrossRef CAS.
- F. A. Cotton and T. J. Marks, J. Am. Chem. Soc., 1969, 91, 7281–7285 CrossRef CAS.
- F. A. Cotton and J. Takats, J. Am. Chem. Soc., 1970, 92, 2353–2358 CrossRef CAS.
- L. T. Delbaere, D. W. McBride and R. B. Ferguson, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1970, 26, 515–521 CrossRef CAS.
- T. P. Hanusa, T. A. Ulibarri and W. J. Evans, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1985, 41, 1036–1038 CrossRef.
- Q. T. Anderson, E. Erkizia and R. R. Conry, Organometallics, 1998, 17, 4917–4920 CrossRef CAS.
- G. A. Carriedo, J. A. K. Howard and F. G. A. Stone, Dalton Trans., 1984, 1555–1561 RSC.
- C. Zybill and G. Müller, Organometallics, 1987, 6, 2489–2494 CrossRef CAS.
- H. G. Stammler, P. Jutzi, W. Wieland and B. Neumann, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, 54, IUC9800064 CrossRef.
- E. G. Perelova, K. J. Grandberg, V. P. Dyadchenko and T. V. Baukova, J. Organomet. Chem., 1981, 217, 403–413 CrossRef.
- M. I. Bruce, J. K. Walton, B. W. Skelton and A. H. Wheite, Dalton Trans., 1983, 809–814 RSC.
- H. Werner, H. Otto, T. Ngo-Khac and C. Bruschka, J. Organomet. Chem., 1984, 262, 123–136 CrossRef CAS.
- Y. T. Struchkov, Y. L. Slovokhotov, L. Yu, D. N. Kravtsov, T. V. Baukova, E. G. Perelova and K. J. Grandberg, J. Organomet. Chem., 1988, 338, 269–280 CrossRef CAS.
- H. Schumann, F. H. Görlitz and A. Dietrich, Chem. Ber., 1989, 122, 1423–1426 CrossRef CAS.
- M. I. Bruce, P. A. Humphrey, M. L. Williams, B. W. Skelton and A. H. White, Aust. J. Chem., 1989, 42, 1847–1857 CrossRef CAS.
- L. G. Kz'mina, A. V. Churakov, K. I. Grandberg and V. S. Kuz'min, Koord. Khim., 1997, 23, 170–176 Search PubMed.
- N. Martínez-Espada, M. Mena, M. E. G. Mosquera, A. Pérez-Redondo and C. Yélamos, Organometallics, 2010, 29, 6732–6738 CrossRef.
- R. Batcup, F. S. N. Chiu, V. T. Annibale, J. E. U. Huh, R. Tan and D. Song, Dalton Trans., 2013, 16343–16350 RSC.
- H. Halim, R. D. Kennedy, M. Suzuki, S. I. Khan, P. L. Diaconescu and Y. Rubin, J. Am. Chem. Soc., 2011, 133, 6841–6851 CrossRef PubMed.
- S. Martínez-Salvador, J. Forniés, A. Martín and B. Menjón, Angew. Chem., Int. Ed., 2011, 50, 6571–6574 CrossRef PubMed.
- S. Martínez-Salvador, L. R. Falvello, A. Martín and B. Menjón, Chem.–Eur. J., 2013, 19, 14540–14552 CrossRef PubMed.
- A. Pérez-Bitrián, M. Baya, J. M. Casas, L. R. Falvello, A. Martín and B. Menjón, Chem.–Eur. J., 2017, 23, 14918–14930 CrossRef PubMed.
- D. Joven-Sancho, M. Baya, A. Martín and B. Menjón, Chem.–Eur. J., 2018, 24, 13098–13101 CrossRef CAS PubMed.
- D. Joven-Sancho, M. Baya, A. Martín, J. Orduna and B. Menjón, Chem.–Eur. J., 2020, 26, 4471–4475 CrossRef CAS PubMed.
- D. Joven-Sancho, L. Demonti, A. Martín, N. Saffon-Merceron, N. Nebra, M. Baya and B. Menjón, Chem. Commun., 2023, 59, 4166–4168 RSC.
- A. Pérez-Bitrián, S. Alvarez, M. Baya, J. Echeverría, A. Martín, J. Orduna and B. Menjón, Chem.–Eur. J., 2023, 29, e202203181 CrossRef PubMed.
- J. S. Lakhi, M. R. Patterson and H. V. R. Dias, New J. Chem., 2020, 44, 14814–14822 RSC.
- H. V. R. Dias, C. S. P. Gamage, N. B. Jayaratna and C. V. Hettiarachchi, New J. Chem., 2020, 44, 17079–17087 RSC.
- M. Vanga, A. Noonikara-Poyil, J. Wu and H. V. R. Dias, Organometallics, 2022, 41, 1249–1260 CrossRef CAS.
- M. Vanga, A. Muñoz-Castro and H. V. R. Dias, Dalton Trans., 2022, 51, 1308–1312 RSC.
- W. Jin, H. J. Kim, H. L. Lu and H. V. R. Dias, Inorg. Chem., 1996, 35, 2317–2328 CrossRef PubMed.
- S. Singh and H. V. R. Dias, Inorg. Chem., 2004, 43, 7396–7402 CrossRef PubMed.
- J. Mehara, B. T. Watson, A. Noonikara-Poyil, A. O. Zacharias, J. Roithová and H. V. R. Dias, Chem.–Eur. J., 2022, 28, e202103984 CrossRef CAS PubMed.
- R. G. Browning, S. A. Richey, C. J. Lovely and H. V. R. Dias, Organometallics, 2004, 23, 1200–1202 CrossRef.
- G. Wang, A. Noonikara-Poyil, I. Fernández and H. V. R. Dias, Chem. Commun., 2022, 58, 3222 RSC.
- M. Vanga, A. Muñoz-Castro and H. V. R. Dias, Chem.–Eur. J., 2023, e202303339 Search PubMed.
- R. Sievers, M. Sellin, S. M. Rupf, J. Parche and M. Malischewski, Angew. Chem., Int. Ed., 2022, e202211147 CAS.
- J. Parche, S. M. Rupf, R. Sievers and M. Malischewski, Dalton Trans., 2023, 52, 5496–5502 RSC.
- R. Sievers, J. Parche, N. G. Kub and M. Malischewski, Synlett, 2023, 34, 1079–1086 CrossRef CAS.
- G. Paprott and K. Seppelt, J. Am. Chem. Soc., 1984, 106, 4061–4062 CrossRef.
- E. D. Laganis and D. M. Lemal, J. Am. Chem. Soc., 1980, 102, 6633–6634 CrossRef CAS.
- R. D. Chambers, S. J. Mullins, A. J. Roche and J. F. S. Vaughan, Chem. Commun., 1995, 841–842 RSC.
- R. D. Chambers, A. J. Roche and J. F. S. Vaughan, Can. J. Chem., 1996, 74, 1925–1929 CrossRef CAS.
- R. Alberto, personal communication.
- R. G. Goel and P. Pierre, Inorg. Chem., 1978, 17, 2876–2879 CrossRef CAS.
- T. V. Baukova, Y. L. Slovokhotov and Y. T. Struchkov, J. Organomet. Chem., 1981, 220, 125–137 CrossRef CAS.
- D. R. Lide, Tetrahedron, 1962, 17, 125–134 CrossRef CAS.
- M. I. Bruce, M. L. Williams, B. W. Skelton and A. H. White, Dalton Trans., 1983, 799–808 RSC.
- R. Hüttel, U. Raffay and H. Reinheimer, Angew. Chem., 1967, 19, 859–860 CrossRef.
- H. K. Hofstee, J. Boersma and G. J. M. van der Kerk, J. Organomet. Chem., 1976, 120, 313–317 CrossRef CAS.
- D. W. Macomber and M. D. Rausch, J. Am. Chem. Soc., 1983, 105, 5325–5329 CrossRef CAS.
- P. K. Lo and K. C. Lau, J. Phys. Chem., 2014, 118, 2498–2507 CrossRef CAS PubMed.
- T. Gasevic, J. B. Stückrath, S. Grimme and M. Bursch, J. Phys. Chem., 2022, 23, 3826–3838 CrossRef PubMed.
- P. Pyykkö, Chem. Rev., 1988, 88, 563–594 CrossRef.
- P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412–4456 CrossRef PubMed.
- P. Pyykkö, Inorg. Chim. Acta, 2005, 358, 4113–4130 CrossRef.
- P. Pykkö, Chem. Soc. Rev., 2008, 37, 1967–1997 RSC.
- T. Ziegler and A. Rauk, Theor. Chim. Acta, 1977, 46, 1–10 CrossRef CAS.
- J. I. Wu, F. A. Evangelista and P. v. R. Schleyer, Org. Lett., 2010, 12, 768–771 CrossRef CAS PubMed.
- W. T. Borden, Chem. Commun., 1998, 1919–1925 RSC.
- Deposition Numbers 2309684–2309686 contain the supplementary crystallographic data for this paper.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, spectroscopic and computational details. CCDC 2309684–2309686. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc06299f |
| This journal is © The Royal Society of Chemistry 2024 |