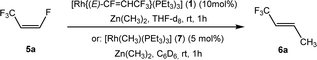Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
C–H and C–F bond activation of fluorinated propenes at Rh: enabling cross-coupling reactions with outer-sphere C–C coupling†‡
Maria
Talavera
 *ab,
Soodeh
Mollasalehi
*ab,
Soodeh
Mollasalehi
 b and
Thomas
Braun
b and
Thomas
Braun
 *b
*b
aFacultad de Química, Universidade de Vigo, Campus Universitario, 36310 Vigo, Spain. E-mail: matalaveran@uvigo.gal
bDepartment of Chemistry, Humboldt Universität zu Berlin, Brook-Taylor Straße 2, 12489 Berlin, Germany. E-mail: thomas.braun@cms.hu-berlin.de
First published on 2nd May 2024
Abstract
The reaction of [Rh{(E)-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] with Zn(CH3)2 results in the methylation of the alkenyl ligand to give [Rh{(E/Z)-C(CH3)
CHCF3}(PEt3)3] with Zn(CH3)2 results in the methylation of the alkenyl ligand to give [Rh{(E/Z)-C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3]. Variable temperature NMR studies allowed the identification of a heterobinuclear rhodium–zinc complex as an intermediate, for which the structure [Rh(CH3)(ZnCH3){(Z)-C(CH3)
CHCF3}(PEt3)3]. Variable temperature NMR studies allowed the identification of a heterobinuclear rhodium–zinc complex as an intermediate, for which the structure [Rh(CH3)(ZnCH3){(Z)-C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)2] is proposed. Based on these stoichiometric reactions, unique Negishi-type catalytic cross-coupling reactions of fluorinated propenes by consecutive C–H and C–F bond activation steps at room temperature were developed. The C–H bond activation steps provide a fluorinated ligand at Rh and deliver the fluorinated product, whereas the C–F bond activation and C–C coupling occur via outer-sphere nucleophilic attack at the fluorinated alkenyl ligand.
CHCF3}(PEt3)2] is proposed. Based on these stoichiometric reactions, unique Negishi-type catalytic cross-coupling reactions of fluorinated propenes by consecutive C–H and C–F bond activation steps at room temperature were developed. The C–H bond activation steps provide a fluorinated ligand at Rh and deliver the fluorinated product, whereas the C–F bond activation and C–C coupling occur via outer-sphere nucleophilic attack at the fluorinated alkenyl ligand.
Introduction
Fluorinated compounds are of eminent importance as they are used in a broad range of fields such as pharmaceutics, agrochemicals and materials science.1–4 Fluorinated olefins are currently indispensable, because they have applications in automobile air-conditioning systems and as monomers for polymeric compounds such as Teflon.5–7 While the synthesis of fluorinated olefins has been widely developed through fluorination reactions,8,9 the activation and functionalization of olefinic or vinylic C(sp2)–F bonds is less common,10–13 and often it is mediated by a transition metal complex.12,13A possible functionalization pathway would consist of the formation of C–C bonds by cross-coupling reactions. Such conversions have been investigated for chlorinated or brominated olefin derivatives.14,15 For fluoroalkenes, C–C couplings have been less studied and examples include group 10 catalysts and the use of gem-difluoroalkenes.16–28 The couplings proceed by an initial C–F bond oxidative addition – in some cases using lithium salts to promote the activation step – or insertion of a fluorinated olefin into a metal–carbon bond followed by β-fluorine elimination. Cao and Wu developed a Suzuki–Miyaura cross coupling arylation of gem-difluoroalkenes using a nickel catalyst,21 whereas Tsui and Liu applied a palladium catalyst.16 Xu et al. published the arylation of an in situ formed difluorovinyl ketone in the presence of a palladium catalyst and aryl boronic acids.19 Regarding rhodium catalyzed reactions, Xia and co-workers described the formation of monofluorinated dienes from gem-difluorocyclopropanes, which are employed as fluoroallyl surrogates, by C–C coupling with allylboronates.29 Negishi-type cross-coupling reactions based on vinyl C(sp2)–F bond oxidative addition steps are rare. Saeki et al. functionalized the olefin CF2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(1-naphtyl) with arylzinc derivative MeC6H4ZnCl in the presence of a palladium catalyst.30 In addition, examples using Pd or Ni catalysts and lithium salts to promote the C–F bond activation have been described by Zhang et al. and Ogoshi and co-workers developing Negishi-type C(sp2)–F bond alkylations of 2,3,3,3-tetrafluoropropene or tetrafluoroethylene, respectively.31,32
CH(1-naphtyl) with arylzinc derivative MeC6H4ZnCl in the presence of a palladium catalyst.30 In addition, examples using Pd or Ni catalysts and lithium salts to promote the C–F bond activation have been described by Zhang et al. and Ogoshi and co-workers developing Negishi-type C(sp2)–F bond alkylations of 2,3,3,3-tetrafluoropropene or tetrafluoroethylene, respectively.31,32
Recently, the reactivity of Z-1,3,3,3-tetrafluoropropene (5a) towards rhodium(I) complexes has been studied and it was shown that rhodium alkenyl complexes with a low fluoroorganyl content such as [Rh{(E)-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] can activate higher fluorinated olefins (Scheme 1). For the latter, in a C–H activation step [Rh{(E)-CF
CHCF3}(PEt3)3] can activate higher fluorinated olefins (Scheme 1). For the latter, in a C–H activation step [Rh{(E)-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] (1) and 3,3,3-trifluoropropene were produced.33
CHCF3}(PEt3)3] (1) and 3,3,3-trifluoropropene were produced.33
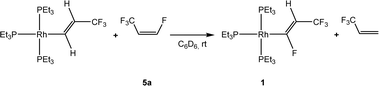 | ||
Scheme 1 Reaction of [Rh{(E)-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] with Z-1,3,3,3-tetrafluoropropene.33 CHCF3}(PEt3)3] with Z-1,3,3,3-tetrafluoropropene.33 | ||
Herein studies on catalytic C–C coupling reactions at fluorinated alkenyl ligands are described. Stoichiometric model reactions give an insight into a possible reaction mechanism. Unprecedented rhodium catalyzed Negishi-type cross-coupling reactions of fluorinated olefins have been developed, based on both C(sp2)–F and C(sp2)–H bond activation and, remarkably, involving a C–C coupling step in the outer coordination sphere.
Results and discussion
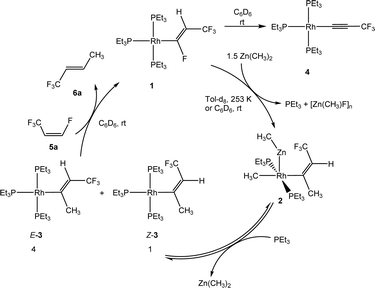
Treatment of complex [Rh{(E)-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] (1) with one equivalent of Zn(CH3)2 in C6D6 yielded after 20 min at room temperature a mixture of complexes, which included a heterobimetallic compound, for which the structure [Rh(CH3)(ZnCH3){(Z)-C(CH3)
CHCF3}(PEt3)3] (1) with one equivalent of Zn(CH3)2 in C6D6 yielded after 20 min at room temperature a mixture of complexes, which included a heterobimetallic compound, for which the structure [Rh(CH3)(ZnCH3){(Z)-C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)2] (2) is suggested, as well as the isomers [Rh{(E)-C(CH3)
CHCF3}(PEt3)2] (2) is suggested, as well as the isomers [Rh{(E)-C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] (E-3), [Rh{(Z)-C(CH3)
CHCF3}(PEt3)3] (E-3), [Rh{(Z)-C(CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] (Z-3), the alkynyl complex [Rh(C
CHCF3}(PEt3)3] (Z-3), the alkynyl complex [Rh(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCF3)(PEt3)3] (4) and an unknown product in a ratio of 3
CCF3)(PEt3)3] (4) and an unknown product in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7
0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. When the reaction was run in the presence of one equivalent of PEt3, a mixture of complexes 2 and Z-3 in a 1
1, respectively. When the reaction was run in the presence of one equivalent of PEt3, a mixture of complexes 2 and Z-3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 ratio was observed after 10 minutes. The preferential formation of the Z-3 isomer might indicate a diminished role of 2 for the reaction mechanism in this case. When the reaction was monitored at 253 K by NMR spectroscopy, after 4 h 75% conversion of complex 1 was observed to give complex 2 and a second complex (ratio 9
17 ratio was observed after 10 minutes. The preferential formation of the Z-3 isomer might indicate a diminished role of 2 for the reaction mechanism in this case. When the reaction was monitored at 253 K by NMR spectroscopy, after 4 h 75% conversion of complex 1 was observed to give complex 2 and a second complex (ratio 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which is possibly an isomer of 2, together with the release of triethylphosphine (Scheme 2). The rhodium-zinc heterobimetallic complex 2 is not stable at low temperatures, and after one day, reductive elimination of Zn(CH3)2 took place followed by coordination of phosphine to rhodium leading to the Z/E-3 isomers in a 1
1), which is possibly an isomer of 2, together with the release of triethylphosphine (Scheme 2). The rhodium-zinc heterobimetallic complex 2 is not stable at low temperatures, and after one day, reductive elimination of Zn(CH3)2 took place followed by coordination of phosphine to rhodium leading to the Z/E-3 isomers in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
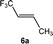
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio. Treatment of the product mixture with Z-1,3,3,3-tetrafluoropropene (5a) led then to the regeneration of complex 1 and release of E-1,1,1-trifluorobut-2-ene (6a) by C–H bond activation and formation (Scheme 2). This step comprises the replacement of a lower fluorinated ligand by a higher fluorinated ligand by C–H bond activation as a higher degree of fluorination often leads to stronger bonds.34 As previously reported, complex 1 is not stable and after a dehydrofluorination step complex 4 is obtained.33
4 ratio. Treatment of the product mixture with Z-1,3,3,3-tetrafluoropropene (5a) led then to the regeneration of complex 1 and release of E-1,1,1-trifluorobut-2-ene (6a) by C–H bond activation and formation (Scheme 2). This step comprises the replacement of a lower fluorinated ligand by a higher fluorinated ligand by C–H bond activation as a higher degree of fluorination often leads to stronger bonds.34 As previously reported, complex 1 is not stable and after a dehydrofluorination step complex 4 is obtained.33
In an alternative approach in which complex 1 was treated with LiCH3 instead of Zn(CH3)2, a mixture of complexes (Z/E)-3 and 4 was also obtained. However, in this case a heterobinuclear intermediate similar to 2 was not observed. Note that Rh–Zn bimetallic complexes have been described before.35–39
The formation of 2 followed by the generation of E/Z-3 from complex 1 can be considered as a case for a rare outer-sphere reaction where a nucleophile attacks the fluorinated ligand bonded at rhodium. Note that the attack of a fluorosilicate at the carbon atom of a fluorinated pyridyl ligand has been proposed at rhodium.40 The attack of PEt3 at the β-carbon atom of a perfluorovinyl ligand at nickel was also described.41 In addition, outer-sphere electrophilic fluorination was reported by Lynam, Slattery and co-workers.42,43 The rearrangements of the alkenyl ligands for the conversion of 2 into E-3, 1 into 2 and E-3 into 6a comprise an isomerization at the double bond. This might involve an intermediate vinylidene complex that can be formed by a reversible migration of a methyl group from the alpha carbon to the metal center. Intermediate metallacyclopropene-like species have also been discussed for such rearrangements.44–46 In addition, a negative hyperconjugation of the π-electron density into antibonding orbitals at the CF3 group might weaken the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and allow for an isomerization of the alkenyl ligands. Note that Ojima et al. proposed a zwitterionic carbene–rhodium complex as an intermediate for such a cis/trans rearrangement.47 However, another alternative route for the isomerization to give complexes 2 or E-3 could imply a rearrangement during the nucleophilic substitution. After addition of CH3− to Cα, the β-carbanion formed could show a free rotation about Cα–Cβ. A Lewis-acidic cation of the type [ZnMe]+ would then abstract the fluoride forming the oligomeric [ZnFMe]n.
C double bond and allow for an isomerization of the alkenyl ligands. Note that Ojima et al. proposed a zwitterionic carbene–rhodium complex as an intermediate for such a cis/trans rearrangement.47 However, another alternative route for the isomerization to give complexes 2 or E-3 could imply a rearrangement during the nucleophilic substitution. After addition of CH3− to Cα, the β-carbanion formed could show a free rotation about Cα–Cβ. A Lewis-acidic cation of the type [ZnMe]+ would then abstract the fluoride forming the oligomeric [ZnFMe]n.
The suggested structure of complex 2 is supported by NMR spectroscopic data, in part based on the data for the 13C labeled derivative [Rh(13CH3)(Zn13CH3){(Z)-C(13CH3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)2] (2′) (see ESI‡). Thus, in the 1H NMR spectrum of complex 2′ a resonance for the rhodium-bound methyl ligand is observed at −0.42 ppm as a doublet of triplets with coupling constants of 121.1 and 4.6 Hz due to the coupling to the carbon atom and the two phosphine ligands. In addition, a resonance at −0.21 ppm is assigned to the methyl group at the Zn atom. It appears as a doublet coupled with 1JH–C = 123.2 Hz. The 13C{1H} NMR displays two resonances at −27.5 and −9.2 ppm for the rhodium- and zinc-bonded methyl moieties, respectively. The former exhibits a doublet of pseudo quartets as a result of the coupling to rhodium (9.3 Hz), the two phosphorus atoms and the carbon atom bonded to zinc with a coupling constant of 5.2 Hz. The latter signal also shows the 5.2 Hz carbon–carbon coupling together with the extra coupling to rhodium resulting in a pseudo triplet. In addition, the geometry of complex 2 was optimized by DFT calculations using toluene as a solvent (BP86/def2-SVP, see ESI‡). The complex exhibits a tetragonal pyramidal structure at rhodium in which the methylzinc group is at the apical position. The distance between the Zn nucleus and one of the fluorine atoms of the CF3 moiety of 2.747 Å is shorter than the sum of van der Waals radii which might be the reason for the favored cis arrangement of the Zn center and the CF3 group at the moiety containing the double bond. In fact, this product is 7.4 kJ mol−1 more stable than the corresponding trans isomer.
CHCF3}(PEt3)2] (2′) (see ESI‡). Thus, in the 1H NMR spectrum of complex 2′ a resonance for the rhodium-bound methyl ligand is observed at −0.42 ppm as a doublet of triplets with coupling constants of 121.1 and 4.6 Hz due to the coupling to the carbon atom and the two phosphine ligands. In addition, a resonance at −0.21 ppm is assigned to the methyl group at the Zn atom. It appears as a doublet coupled with 1JH–C = 123.2 Hz. The 13C{1H} NMR displays two resonances at −27.5 and −9.2 ppm for the rhodium- and zinc-bonded methyl moieties, respectively. The former exhibits a doublet of pseudo quartets as a result of the coupling to rhodium (9.3 Hz), the two phosphorus atoms and the carbon atom bonded to zinc with a coupling constant of 5.2 Hz. The latter signal also shows the 5.2 Hz carbon–carbon coupling together with the extra coupling to rhodium resulting in a pseudo triplet. In addition, the geometry of complex 2 was optimized by DFT calculations using toluene as a solvent (BP86/def2-SVP, see ESI‡). The complex exhibits a tetragonal pyramidal structure at rhodium in which the methylzinc group is at the apical position. The distance between the Zn nucleus and one of the fluorine atoms of the CF3 moiety of 2.747 Å is shorter than the sum of van der Waals radii which might be the reason for the favored cis arrangement of the Zn center and the CF3 group at the moiety containing the double bond. In fact, this product is 7.4 kJ mol−1 more stable than the corresponding trans isomer.
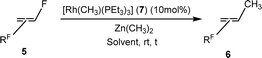
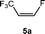
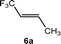
Based on the stoichiometric functionalization of the fluorinated alkenyl ligand at complex 1 to yield 6a, a process for the catalytic methylation of fluoroolefins was developed. The reaction of Z-1,3,3,3-tetrafluoropropene (5a) with Zn(CH3)2 in THF-d8 in the presence of 10 mol% [Rh{(E)-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] (1) as a catalyst gave full conversion of the olefin into the C–C coupling product E-1,1,1-trifluorobut-2-ene (6a) (Scheme 3, Table 1, entry 1).
CHCF3}(PEt3)3] (1) as a catalyst gave full conversion of the olefin into the C–C coupling product E-1,1,1-trifluorobut-2-ene (6a) (Scheme 3, Table 1, entry 1).
| Entry | Catalyst | Methyl source | Conversiona (%) |
|---|---|---|---|
| a Conversion based on the consumption of Zn(CH3)2 and determined by 1H NMR spectroscopy. b 1 h reaction time, 5 mol% of complex 7. | |||
| 1 | Complex 1 | Zn(CH3)2 | 99 |
| 2b | Complex 7 | Zn(CH3)2 | 99 |
| 3 | [Rh(F)(PEt3)3] | Zn(CH3)2 | 70 |
| 4 | [Rh(H)(PEt3)3] | Zn(CH3)2 | 70 |
| 5 | Complex 7 | MgBrCH3 | — |
| 6 | Complex 7 | LiCH3 | — |
| 7 | Complex 7 | Zn(CH3)Cl | 15 |
Complex [Rh{(E)-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] (1) is synthesized by C–H bond activation of 5a using [Rh(CH3)(PEt3)3] (7).33 Therefore, complex 7 was also tested as a pre-catalyst and full conversion to compound 6a was obtained after 1 h using only 5 mol% of catalyst (Scheme 3, Table 1, entry 2). Remarkably the transformation occurs at room temperature within 1 h. Note that Zn(CH3)2 does not react with the olefin in the absence of a rhodium catalyst.
CHCF3}(PEt3)3] (1) is synthesized by C–H bond activation of 5a using [Rh(CH3)(PEt3)3] (7).33 Therefore, complex 7 was also tested as a pre-catalyst and full conversion to compound 6a was obtained after 1 h using only 5 mol% of catalyst (Scheme 3, Table 1, entry 2). Remarkably the transformation occurs at room temperature within 1 h. Note that Zn(CH3)2 does not react with the olefin in the absence of a rhodium catalyst.
In general, there are few examples of rhodium-mediated Negishi-type cross-coupling reactions described in the literature. For these cases, C–I bond activation functionalization steps do not proceed through an outer-sphere reaction; instead they are proposed to occur via common oxidative addition/transmetalation cycles. Thus, Takagi et al. described alkyl-aryl C–C coupling reactions using [RhCl(cyclooctadiene)]2 with bidentate phosphines48 and work by Ozerov and co-workers using a PNP pincer rhodium complex as a precatalyst afforded a 7% yield in an aryl–aryl coupling.39 It might additionally be worth noting that examples on the functionalization of fluoroaromatics by cross-coupling reactions involving C–F bond cleavage have been reported, for instance Negishi-type conversions at Ni and Pd by the research groups of Love, Ogoshi and Radius.13,49–64
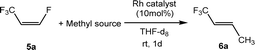
Other rhodium complexes as catalysts for the derivatization of 5a were then studied using Zn(CH3)2 as a nucleophile. Accordingly, Zn(CH3)2 methylates the Rh–F bond of [Rh(F)(PEt3)3] and in the presence of Z-1,3,3,3-tetrafluoropropene (5a), the generation of E-1,1,1-trifluorobut-2-ene (6a) with 70% conversion was achieved (Table 1, entry 3). A comparable outcome was obtained when [Rh(H)(PEt3)3] was tested as a catalyst (Table 1, entry 4). Note that the rhodium hydrido complex is known to activate preferentially vinylic C–F bonds followed by C–H bond activation of another equivalent of olefin.65–68
As an alternative to Zn(CH3)2, BrMgCH3 and LiCH3 were applied for the catalytic methylation of 5a, but the formation of the methylated derivative was not observed within one day (Table 1, entries 5 and 6). Note that an oligomerization of the nucleophilic reagents in solution can attenuate the nucleophilicity of their methyl anion. On the other hand, mono organylzinc chloride derivatives can also be used for cross-coupling reactions. Indeed, chloromethylzinc could be employed to yield 6a, but only with 15% conversion (Table 1, entry 7).
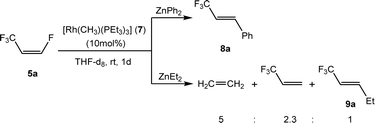
Further functionalization of Z-1,3,3,3-tetrafluoropropene (5a) was attempted using ZnEt2 and ZnPh2 as nucleophile sources. Indeed, reaction of 5a with ZnPh2 in the presence of complex 7 as a catalyst gave, within 1 d, E-3-phenyl-1,1,1-trifluoropropene (8a) with 90% conversion (Scheme 4), but after one week at room temperature, full conversion was achieved. Interestingly, ZnEt2 provided a different outcome. The reaction of 5a with ZnEt2 using complex 7 as a catalyst gave, after 1 day a mixture of ethylene, E-1,1,1-trifluoropent-2-ene (9a) and 3,3,3-trifluoropropene in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.3 ratio with 60% conversion (Scheme 4).
2.3 ratio with 60% conversion (Scheme 4).
The formation of 9a should take place through a comparable mechanism as for the generation of 6a, leading to a Rh–Zn bimetallic intermediate which would bear an ethyl group at the olefin and an ethyl ligand bound to rhodium. This intermediate can produce ethene together with [Rh(H)(PEt3)3] by β-H elimination. The rhodium hydrido complex would then react with 5a to give 3,3,3-trifluoropropene and complex [Rh{(E)-CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF3}(PEt3)3] (1) as described before in the literature.33
CHCF3}(PEt3)3] (1) as described before in the literature.33
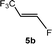
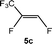
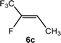
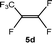
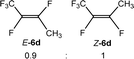
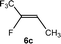
The scope of the rhodium defluorinative methylation was then investigated using other olefinic substrates and complex [Rh(CH3)(PEt3)3] (7) as a catalyst (Table 2, see also the ESI‡ for further optimizations). Thus, E-1,3,3,3-tetrafluoropropene (5b) was converted into compound 6a with 95% conversion. Higher fluorinated derivatives were also attempted and both Z-1,2,3,3,3-pentafluoropropene (5c) and perfluoropropene (5d) were used. In the first case Z-1,1,1,2-tetrafluorobut-2-ene (6c) was identified in 25% conversion as the main product, while in the second case a mixture of Z/E-1,2,3,3,3-pentafluorobut-2-ene isomers (6d) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
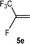
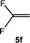
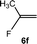
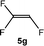
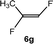
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 ratio was formed (10% conversion). The latter stoichiometric conversion and decrease in reactivity for the perfluorinated substrate could be due to the preference of complex 7 for the activation of C–H over C–F bonds.67 Then, 2,3,3,3-tetrafluoropropene (HFO-1234yf, 5e) was used as a substrate, but only 11% conversion was achieved to form 6c and unidentified compounds. Finally, to investigate the influence of a CF3 group on the catalytic methylation of fluoroolefins, 1,1-difluoroethane (5f) and trifluoroethane (5g) were employed as reagents. While 5f yielded only traces of the methylated product 2-fluoropropene (6f) even at 60 °C, compound 5g provided a mixture of E-1,2-difluoropropene (6g) and an unknown compound in a 5
0.9 ratio was formed (10% conversion). The latter stoichiometric conversion and decrease in reactivity for the perfluorinated substrate could be due to the preference of complex 7 for the activation of C–H over C–F bonds.67 Then, 2,3,3,3-tetrafluoropropene (HFO-1234yf, 5e) was used as a substrate, but only 11% conversion was achieved to form 6c and unidentified compounds. Finally, to investigate the influence of a CF3 group on the catalytic methylation of fluoroolefins, 1,1-difluoroethane (5f) and trifluoroethane (5g) were employed as reagents. While 5f yielded only traces of the methylated product 2-fluoropropene (6f) even at 60 °C, compound 5g provided a mixture of E-1,2-difluoropropene (6g) and an unknown compound in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with 17% conversion. These results also suggest a preference of the rhodium system for olefins with a geminal CHF group facilitating the initial C–H bond activation.
1 ratio with 17% conversion. These results also suggest a preference of the rhodium system for olefins with a geminal CHF group facilitating the initial C–H bond activation.
Conclusions
In conclusion, Negishi-type cross-coupling reactions of fluorinated alkenes were developed. The conversions follow an unprecedented reaction pathway: (I) the C–F bond activation and C–C coupling steps occur by an outer-sphere nucleophilic attack at the fluorinated alkenyl ligand. (II) Another crucial step comprises C–H bond activation to convert the olefinic substrate into a Rh derivative, whereas at the same time the lower-fluorinated olefinic product is released. Stoichiometric model reactions give insight into key-steps of a putative catalytic cycle. A heterobinuclear Rh/Zn complex might play a certain role for the C–C coupling step. Note that in the past Rh-catalyzed hydrodefluorination, germylation, silylation and borylation reactions of fluorinated olefins were studied, but cross coupling reactions were elusive.65–71Data availability
Details of experimental procedures, characterization of the complexes can be found in the ESI.‡Author contributions
Conceptualization, M. T. and T. B.; investigation, M. T., S. M.; writing—original draft preparation, M. T.; writing—review and editing, M. T., S. M. and T. B.; funding acquisition, T. B.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the DFG (Deutsche Forschungsgemeinschaft) for financial support (BR 2065/13-1). M. T. thanks the Spanish Government and NextGenerationEU/PRTR funds for a María Zambrano contract. We thank Dr N. Otero for fruitful discussions.Notes and references
- T. Hiyama, Organofluorine Compounds: Chemistry and Applications, Springer, Berlin, 2000 Search PubMed.
- Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity and Applications, Wiley-VCH, Weinheim, 2nd edn, 2013 Search PubMed.
- P. Javidmand and K. A. Hoffmann, Int. J. Refrig., 2016, 69, 114–135 CrossRef CAS.
- V. F. Cardoso, D. M. Correia, C. Ribeiro, M. M. Fernandes and S. Lanceros-Méndez, Polymers, 2018, 10, 161–187 CrossRef PubMed.
- R. Dams and K. Hintzer, in Fluorinated Polymers: Volume 2: Applications, ed. B. Ameduri and H. Sawada, RSC, 2017, vol. 24, pp. 1–31 Search PubMed.
- R. Britton, V. Gouverneur, J.-H. Lin, M. Meanwell, C. Ni, G. Pupo, J.-C. Xiao and J. Hu, Nat. Rev. Methods Primers, 2021, 1, 47 CrossRef CAS.
- S.-J. Lou, D.-Q. Xu and Z.-Y. Xu, Angew. Chem., Int. Ed., 2014, 53, 10330–10335 CrossRef CAS PubMed.
- H. Amii and K. Uneyama, Chem. Rev., 2009, 109, 2119–2183 CrossRef CAS PubMed.
- R. D. Chambers and J. F. S. Vaughan, in Organofluorine Chemistry. Fluorinated Alkenes and Reactive Intermediates, ed. R. D. Chambers, Springer-Verlag, Germany, 1997, vol. 192 Search PubMed.
- M. Ohashi and S. Ogoshi, Top. Organomet. Chem., 2015, 52, 197–216 CrossRef CAS.
- T. Ahrens, J. Kohlmann, M. Ahrens and T. Braun, Chem. Rev., 2015, 115, 931–972 CrossRef CAS PubMed.
- Metal-Catalyzed Cross-Coupling Reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2nd edn, 2008 Search PubMed.
- Metal-Catalyzed Cross-Coupling Reactions and More, ed. A. d. Meijere, S. Bräse and M. Oestreich, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013 Search PubMed.
- Y. Wang, X. Qi, Q. Ma, P. Liu and G. C. Tsui, ACS Catal., 2021, 11, 4799–4809 CrossRef CAS.
- W. Dai, X. Zhang, J. Zhang, Y. Lin and S. Cao, Adv. Synth. Catal., 2016, 358, 183–187 CrossRef CAS.
- R. T. Thornbury and F. D. Toste, Angew. Chem., Int. Ed., 2016, 55, 11629–11632 CrossRef CAS PubMed.
- L. Xu, Q. Zhang, Q. Xie, B. Huang, J.-J. Dai, J. Xu and H.-J. Xu, Chem. Commun., 2018, 54, 4406–4409 RSC.
- W. Heitz and A. Knebelkamp, Makromol. Chem., Rapid Commun., 1991, 12, 69–75 CrossRef CAS.
- Y. Xiong, T. Huang, X. Ji, J. Wu and S. Cao, Org. Biomol. Chem., 2015, 13, 7389–7392 RSC.
- X. Tong, S.-S. Luo, H. Shen, S. Zhang, T. Cao, Y.-P. Luo, L.-L. Huang, X.-T. Ma and X.-W. Liu, Org. Chem. Front., 2021, 8, 4533–4542 RSC.
- H. Shi, W. Dai, B. Wang and S. Cao, Organometallics, 2018, 37, 459–463 CrossRef CAS.
- T. Fujita, T. Fukuda, N. Suzuki and J. Ichikawa, Eur. J. Org. Chem., 2022, 2022, e202200600 CrossRef CAS.
- Y. Ishibashi, T. Fujita and J. Ichikawa, Org. Lett., 2022, 24, 9306–9310 CrossRef CAS PubMed.
- Q. Ma, Y. Wang and G. C. Tsui, Angew. Chem., Int. Ed., 2020, 59, 11293–11297 CrossRef CAS PubMed.
- W. Dai, J. Xiao, G. Jin, J. Wu and S. Cao, J. Org. Chem., 2014, 79, 10537–10546 CrossRef CAS PubMed.
- K. T. Keasler, M. E. Zick, E. E. Stacy, J. Kim, J.-H. Lee, L. Aeindartehran, T. Runčevski and P. J. Milner, Science, 2023, 381, 1455–1461 CrossRef CAS PubMed.
- Y. Zeng, H. Yang, J. Du, Q. Huang, G. Huang and Y. Xia, Chem. Sci., 2022, 13, 12419–12425 RSC.
- T. Saeki, Y. Takashima and K. Tamao, Synlett, 2005, 2005, 1771–1774 CrossRef.
- Y.-C. Luo, M.-K. Wang, L.-C. Yu and X. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202308690 CrossRef CAS PubMed.
- M. Ohashi, T. Kambara, T. Hatanaka, H. Saijo, R. Doi and S. Ogoshi, J. Am. Chem. Soc., 2011, 133, 3256–3259 CrossRef CAS PubMed.
- M. Talavera and T. Braun, Chem. Sci., 2022, 13, 1130–1135 RSC.
- O. Eisenstein, J. Milani and R. N. Perutz, Chem. Rev., 2017, 117, 8710–8753 CrossRef CAS PubMed.
- M. Molon, T. Cadenbach, T. Bollermann, C. Gemel and R. A. Fischer, Chem. Commun., 2010, 46, 5677–5679 RSC.
- O. Ekkert, A. J. P. White, H. Toms and M. R. Crimmin, Chem. Sci., 2015, 6, 5617–5622 RSC.
- M. D. Fryzuk, D. H. McConville and S. J. Rettig, Organometallics, 1993, 12, 2152–2161 CrossRef CAS.
- J. J. Gair, Y. Qiu, R. L. Khade, N. H. Chan, A. S. Filatov, Y. Zhang and J. C. Lewis, Organometallics, 2019, 38, 1407–1412 CrossRef CAS.
- C. J. Pell, W.-C. Shih, S. Gatard and O. V. Ozerov, Chem. Commun., 2017, 53, 6456–6459 RSC.
- A. L. Raza and T. Braun, Chem. Sci., 2015, 6, 4255–4260 RSC.
- T. Braun, B. Blöcker, V. Schorlemer, B. Neumann, A. Stammler and H.-G. Stammler, J. Chem. Soc., Dalton Trans., 2002, 2213–2218 RSC.
- L. M. Milner, N. E. Pridmore, A. C. Whitwood, J. M. Lynam and J. M. Slattery, J. Am. Chem. Soc., 2015, 137, 10753–10759 CrossRef CAS PubMed.
- L. M. Milner, L. M. Hall, N. E. Pridmore, M. K. Skeats, A. C. Whitwood, J. M. Lynam and J. M. Slattery, Dalton Trans., 2016, 45, 1717–1726 RSC.
- J. Cid, J. J. Carbó and E. Fernández, Chem.–Eur. J., 2012, 18, 1512–1521 CrossRef CAS PubMed.
- R. S. Tanke and R. H. Crabtree, J. Am. Chem. Soc., 1990, 112, 7984–7989 CrossRef CAS.
- R. J. Pawley, M. A. Huertos, G. C. Lloyd-Jones, A. S. Weller and M. C. Willis, Organometallics, 2012, 31, 5650–5659 CrossRef CAS.
- I. Ojima, N. Clos, R. J. Donovan and P. Ingallina, Organometallics, 1990, 9, 3127–3133 CrossRef CAS.
- S. Ejiri, S. Odo, H. Takahashi, Y. Nishimura, K. Gotoh, Y. Nishihara and K. Takagi, Org. Lett., 2010, 12, 1692–1695 CrossRef CAS PubMed.
- Y. P. Budiman, S. A. Westcott, U. Radius and T. B. Marder, Adv. Synth. Catal., 2021, 363, 2224–2255 CrossRef CAS.
- L. Fu, Q. Chen and Y. Nishihara, Chem. Rec., 2021, 21, 3394–3410 CrossRef CAS PubMed.
- S. Iqbal, M. Sharif and P. Langer, Synlett, 2021, 32, 1784–1795 CrossRef CAS.
- Z. Zhu, L. Lin, J. Xiao and Z. Shi, Angew. Chem., Int. Ed., 2022, 61, e202113209 CrossRef CAS PubMed.
- Y. Li and J. Zhu, J. Org. Chem., 2022, 87, 8902–8909 CrossRef CAS PubMed.
- B. C. Figula, D. L. Kane, K. Balaraman and C. Wolf, Org. Lett., 2022, 24, 8719–8723 CrossRef CAS PubMed.
- R. Doi, Y. Zhou and S. Ogoshi, Synthesis, 2023, 55, 857–867 CrossRef CAS.
- X. Lu, Y. Wang, B. Zhang, J.-J. Pi, X.-X. Wang, T.-J. Gong, B. Xiao and Y. Fu, J. Am. Chem. Soc., 2017, 139, 12632–12637 CrossRef CAS PubMed.
- T. W. Butcher, J. L. Yang, W. M. Amberg, N. B. Watkins, N. D. Wilkinson and J. F. Hartwig, Nature, 2020, 583, 548–553 CrossRef CAS PubMed.
- X. Li, Y. Li, Z. Wang, W. Shan, R. Liu, C. Shi, H. Qin, L. Yuan, X. Li and D. Shi, ACS Catal., 2023, 13, 2135–2141 CrossRef CAS.
- T. Schaub, M. Backes and U. Radius, J. Am. Chem. Soc., 2006, 128, 15964–15965 CrossRef CAS PubMed.
- O. He, B. Cula and T. Braun, Helv. Chim. Acta, 2023, 106, e202200156 CrossRef CAS.
- D. Breyer, T. Braun and P. Kläring, Organometallics, 2012, 31, 1417–1424 CrossRef CAS.
- M. Ohashi, R. Doi and S. Ogoshi, Chem.–Eur. J., 2014, 20, 2040–2048 CrossRef CAS PubMed.
- A. D. Sun, K. Leung, A. D. Restivo, N. A. LaBerge, H. Takasaki and J. A. Love, Chem.–Eur. J., 2014, 20, 3162–3168 CrossRef CAS PubMed.
- M. W. Kuntze-Fechner, C. Kerpen, D. Schmidt, M. Häring and U. Radius, Eur. J. Inorg. Chem., 2019, 2019, 1767–1775 CrossRef CAS.
- T. Ahrens, M. Teltewskoi, M. Ahrens, T. Braun and R. Laubenstein, Dalton Trans., 2016, 45, 17495–17507 RSC.
- M. Talavera, R. Müller, T. Ahrens, C. N. von Hahmann, B. Braun-Cula, M. Kaupp and T. Braun, Faraday Discuss., 2019, 220, 328–349 RSC.
- M. Talavera, C. N. von Hahmann, R. Müller, M. Ahrens, M. Kaupp and T. Braun, Angew. Chem., Int. Ed., 2019, 58, 10688–10692 CrossRef CAS PubMed.
- M. Talavera and T. Braun, Chem.–Eur. J., 2021, 27, 11926–11934 CrossRef CAS PubMed.
- M. Talavera and T. Braun, Synlett, 2020, 31, 1760–1774 CrossRef CAS.
- T. Braun, F. Wehmeier and K. Altenhöner, Angew. Chem., Int. Ed., 2007, 46, 5321–5324 CrossRef CAS PubMed.
- T. Braun, M. Ahijado Salomon, K. Altenhöner, M. Teltewskoi and S. Hinze, Angew. Chem., Int. Ed., 2009, 48, 1818–1822 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Prof. Dr Helmut Werner on the occasion of his 90th birthday. |
| ‡ Electronic supplementary information (ESI) available: Synthesis and analytics of all compounds and details of the DFT calculations. See DOI: https://doi.org/10.1039/d4sc00951g |
| This journal is © The Royal Society of Chemistry 2024 |