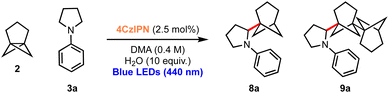Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
α-Amino bicycloalkylation through organophotoredox catalysis†
Jeremy
Nugent‡
a,
Adrián
López-Francés‡
 b,
Alistair J.
Sterling‡
a,
Min Yi
Tay
a,
Nils
Frank
a,
James J.
Mousseau
c,
Fernanda
Duarte
b,
Alistair J.
Sterling‡
a,
Min Yi
Tay
a,
Nils
Frank
a,
James J.
Mousseau
c,
Fernanda
Duarte
 *a and
Edward A.
Anderson
*a and
Edward A.
Anderson
 *a
*a
aDepartment of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: fernandaduartegonzalez@chem.ox.ac.uk; edward.anderson@chem.ox.ac.uk
bDepartment of Organic Chemistry I, Faculty of Pharmacy and Lascaray Research Center, University of the Basque Country, UPV/EHU, Paseo de la Universidad 7, 01006, Vitoria-Gasteiz, Spain
cPfizer Worldwide Research and Development, Eastern Point Road, Groton, Connecticut 06340, USA
First published on 4th June 2024
Abstract
Bridged bicycloalkanes such as bicyclo[1.1.1]pentanes (BCPs) and bicyclo[3.1.1]heptanes (BCHeps) are important motifs in contemporary drug design due to their potential to act as bioisosteres of disubstituted benzene rings, often resulting in compounds with improved physicochemical and pharmacokinetic properties. Access to such motifs with proximal nitrogen atoms (i.e. α-amino/amido bicycloalkanes) is highly desirable for drug discovery applications, but their synthesis is challenging. Here we report an approach to α-amino BCPs and BCHeps through the visible-light enabled addition of α-amino radicals to the interbridgehead C–C bonds of [1.1.1] and [3.1.1]propellane respectively. The reaction proceeds under exceptionally mild conditions and displays broad substrate scope, providing access to an array of medicinally-relevant BCP and BCHep products. Experimental and computational mechanistic studies provide evidence for a radical chain pathway which depends critically on the stability of the α-amino radical, as well as effective catalyst turnover.
Introduction
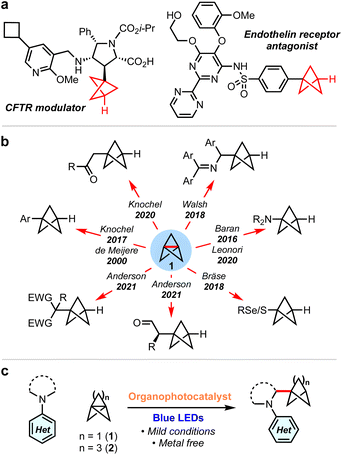
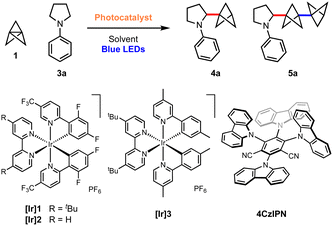
sp3-Rich ‘cage’ hydrocarbons are becoming increasingly commonplace in contemporary drug design due to their beneficial physiochemical properties compared to ‘classic’ drug functionalities such as benzene rings.1–3 Compounds featuring these rigid scaffolds often exhibit improved pharmacological profiles compared to their parent structure, such as resistance to metabolism, while increasing three-dimensionality.4–7 For example, 1,3-disubstituted BCPs are often deployed as bioisosteres for para-substituted arenes and alkynes, as they retain the specific positioning of substituents (180°),8–12 while their use as general property-enhancing motifs is also emerging.13–15 Similarly, monosubstituted BCPs are desirable as surrogates for phenyl and t-butyl groups (Fig. 1a).16,17 Recently, we reported the generation of the homologous bicyclo[3.1.1]heptanes (BCHeps), and described their use as potential bioisosteres of meta-substituted arenes, in which the bridgehead substituent vectors faithfully replicate those of the aromatic ring (∼120°).18These important rigid scaffolds are typically derived from [n.1.1]propellanes, which are convenient building blocks due to the diversity of functionality that can be introduced during ring-opening of the central C–C bond, especially using radicals19–32 and, for [1.1.1]propellane, anions.33–38 In the case of mono-substituted BCPs, synthetic approaches are most commonly anionic in nature (Fig. 1b); examples include the addition to 1 of aryl Grignard reagents,12 turbo amides,34,35 enolates,39 dithiane36 and azaallyl37,38 anions. While these methods provide ready access to valuable BCP building blocks, they are moisture-and/or air-sensitive and thus display limited functional group tolerance. Single electron strategies also enable the synthesis of monosubstituted BCPs, but have generally been limited to electron-deficient or thiyl (and related) radicals.24–28
α-Amino BCPs are highly desirable in medicinal chemistry as analogues of benzylamines – motifs found in many pharmaceuticals.40 The synthesis of these potentially valuable compounds has been mostly overlooked, with the few reported examples requiring lengthy reaction sequences, pyrophoric/strongly basic reagents, or being limited to 1° amines.37,41–44 As such, the synthesis of α-amino BCPs or BCHeps directly from [1.1.1]propellane 1 and [3.1.1]propellane 2 respectively represents an attractive yet unexplored route – especially in the latter case, as anionic additions to 2 are unfeasible.37 While these propellanes are well-established to react efficiently with electrophilic radicals,13,25,45,46 the addition of nucleophilic radicals is less studied.47 We questioned whether the direct addition of nucleophilic α-amino radicals (generated via photoredox-catalysed oxidation of simple N,N-dialkylanilines)48–58 to propellanes 1 or 2 could generate these useful α-amino bicycloalkanes in a single step. Here we report the successful development of this methodology, which represents the first examples of the ring-opening of [1.1.1] and [3.1.1]propellanes using α-amino radicals. We complement the development of this chemistry with a detailed mechanistic study that investigates the role of each reaction component, including the source of the BCP/BCHep bridgehead hydrogen atom.
Results and discussion
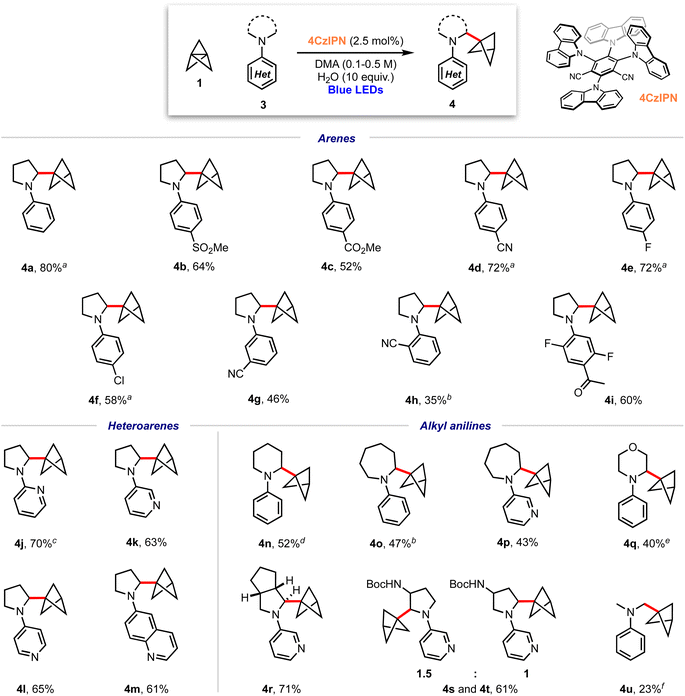
We began our investigations with the reaction of [1.1.1]propellane (1) with N-phenylpyrrolidine (3a, 5 equiv.) in the presence of the moderately oxidising photocatalyst Ir[(dF(CF3)ppy)2dtbbpy]PF6 ([Ir]1, E°(Ir(III)*/Ir(II)) = +1.21 V vs. SCE)59 in MeCN (0.5 M) under 455 nm blue LED irradiation. We were pleased to find that the desired BCP product 4a was delivered in 24% yield, along with 6% of the ‘staffane’ product 5a, which results from addition of the initially formed BCP radical to another molecule of 1 (Table 1, entry 1). A solvent screen revealed that DMF, DCE and DMA gave improved yields of 4a (36–43%, entries 2–4). Various other oxidising iridium and organophotocatalysts were investigated; while the use of [Ir]2E°(Ir(III)*/Ir(II) = +1.32 V vs. SCE)59 or [Ir]3 led to decreased yields (entries 5 and 6), the organophotocatalyst 4CzIPN (E° = 1.35 V vs. SCE)60 afforded 4a in 45% yield with 11% of 5a (entry 7). Additives including a range of H-atom sources and bases were not beneficial (see the ESI, Table S1†), but pleasingly an enhanced yield of product (60%, entry 8) and ratio of 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a (6
5a (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) could be obtained by increasing the stoichiometry of aniline 3a to 10 equiv. Changing to a stronger light source (30 W, 440 nm) further increased the yield of 4a and permitted a shorter reaction time (65%, entry 9). The addition of 10 equiv. of water marginally increased the yield of the desired product to 70% (entry 10). Conducting the reaction under an atmosphere of air resulted in slightly reduced yields (entry 11), while control experiments demonstrated that both photocatalyst and light were required to afford high yields of 4a (entries 12 and 13). We found that, if desired, unreacted amine 3a could be recovered in near quantitative yield via chromatographic purification.
1) could be obtained by increasing the stoichiometry of aniline 3a to 10 equiv. Changing to a stronger light source (30 W, 440 nm) further increased the yield of 4a and permitted a shorter reaction time (65%, entry 9). The addition of 10 equiv. of water marginally increased the yield of the desired product to 70% (entry 10). Conducting the reaction under an atmosphere of air resulted in slightly reduced yields (entry 11), while control experiments demonstrated that both photocatalyst and light were required to afford high yields of 4a (entries 12 and 13). We found that, if desired, unreacted amine 3a could be recovered in near quantitative yield via chromatographic purification.
| Entry | PC | Solvent | Amine eq. | Light source | Time (h) | Yielda (%) (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a) 5a) |
|---|---|---|---|---|---|---|
| a Yield determined by 1H NMR spectroscopy using trimethoxybenzene as internal standard. b 1 mol% of catalyst. c 2.5 mol% of catalyst. d 10 equiv. of water added. e Under air. PC = photocatalyst. | ||||||
| 1 |
[Ir]1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
MeCN | 5 | 18 W 455 nm | 48 | 25 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 2 |
[Ir]1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
DMF | 5 | 18 W 455 nm | 48 | 42 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 3 |
[Ir]1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
DCE | 5 | 18 W 455 nm | 48 | 43 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 4 |
[Ir]1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
DMA | 5 | 18 W 455 nm | 48 | 36 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 5 |
[Ir]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
DMA | 5 | 18 W 455 nm | 48 | 15 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 6 |
[Ir]3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
DMA | 5 | 18 W 455 nm | 48 | 35 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 7 |
4CzIPN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
DMA | 5 | 18 W 455 nm | 48 | 45 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 8 |
4CzIPN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
DMA | 10 | 18 W 455 nm | 48 | 60 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 9 |
4CzIPN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
DMA | 10 | 30 W 440 nm | 24 | 65 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 10 |
4CzIPN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) c
, c
,
|
DMA | 10 | 30 W 440 nm | 24 |
70 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
| 11 |
4CzIPN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c,d,e c,d,e |
DMA | 10 | 30 W 440 nm | 24 | 62 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 12 |
4CzIPN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c,d c,d |
DMA | 10 | None | 24 | <5 |
| 13 | Noned | DMA | 10 | 30 W 440 nm | 24 | 20 (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
With optimised conditions in hand, the scope of the aniline coupling partner was investigated, focussing first on variation of the N-arene substituent. We found this method of BCP installation to be successful with diversely functionalised (hetero)arylpyrrolidine substrates, with the α-amino BCP products generally obtained in good yields (Scheme 1). Electron-neutral and electron-poor para-substituted aniline substrates are well-suited to this reaction and gave good-to-excellent yields of the desired α-amino BCP products (4a–f, 52–80%). Substitution at the meta- and ortho-positions was also tolerated (4g–h, 35–46%), as were trisubstituted anilines (4i, 60%). The synthesis of BCPs substituted with heteroaryl dialkylanilines would be of high interest in a pharmaceutical context; pleasingly, we found that 2-, 3-, and 4-pyrrolidinopyridines were excellent substrates for this reaction, affording BCP products in high yields (4j–l, 63–70%); similarly a pyrrolidine–quinoline derivatives gave the BCP product 4m in good yield (61%).
We next investigated substrates in which the dialkylamine was varied. Pleasingly, piperidine (4n, 52%), azepane (4o–p, 43–47%) and morpholine (4q, 40%) substituted (hetero)arene BCPs were isolated in good yields, albeit with a slight increase in the amount of staffane side-product. Substrates bearing substituted pyrrolidines proved more challenging: while hexahydrocyclopenta[c]pyrrole 4q was obtained in excellent yield (71%), the use of non-symmetric substrates resulted in mixtures of product regioisomers, although high yields were still obtained (e.g.4r and 4s, 61%). For reasons that are unclear, acyclic dialkylamine systems generally resulted in low yields, with significant amounts of staffane formation (4u, 23%).
Recent studies suggest that nicotine may exert neuroprotective effects inducing defence mechanisms against pathologies associated with Alzheimer's or Parkinson's disease.19–28 Pleasingly, use of nicotine (6, Scheme 2) as a substrate for this α-aminobicyclopentylation reaction led to the corresponding BCP–nicotine derivative 7 (37%), highlighting the applicability of the chemistry to drug molecules. C–H abstraction occurs preferentially at the 2° position adjacent to the nitrogen atom due to the stability of the resulting α-amino radical.61 While a 3° radical at the opposing α-position should be significantly more stable than any of the 1° or 2° radicals that could be formed, we suggest that steric repulsion encountered during the C–H abstraction process prevents the formation of this radical, rationalising the observed regioselectivity of the reaction.
 | ||
Scheme 2 Bicyclopentylation of nicotine (6, 10 equiv.); 7 was isolated as a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture with the corresponding BCP-staffane. 1 mixture with the corresponding BCP-staffane. | ||
Considering the excellent results obtained in the bicyclopentylation reactions of 1 to form α-amino BCPs, and the high relevance of recently discovered BCHeps as potential bioisosteres of meta-substituted arenes,13 we questioned whether [3.1.1]propellane 2 would also perform well in this nucleophilic radical addition chemistry. We first studied the stability of 2 under blue LED irradiation (440 nm), which confirmed that 2 is stable for several hours.61 Under the optimised conditions developed for the bicyclopentylation, we explored the reaction of 2 with aniline 3a. The use of 1 equiv. of amine 3a under the optimized reaction conditions afforded the desired product 8a in low yield (18%, Table 2, entry 1), as a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture with the corresponding BCHep staffane 9a (which notably constitutes the first example of this type of ‘dimer’ formation for [3.1.1]propellane).13 An increase in yield but a similar product-staffane ratio was observed using 5 equiv. of amine 3a (47% (4
1 mixture with the corresponding BCHep staffane 9a (which notably constitutes the first example of this type of ‘dimer’ formation for [3.1.1]propellane).13 An increase in yield but a similar product-staffane ratio was observed using 5 equiv. of amine 3a (47% (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), entry 2), while the use of 10 equiv. of amine resulted in enhancement of both the yield and product
1), entry 2), while the use of 10 equiv. of amine resulted in enhancement of both the yield and product![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) staffane ratio (82% (5
staffane ratio (82% (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), entry 3).
1), entry 3).
These conditions were applied to a range of amine substrates (Scheme 3). We observed that anilines featuring neutral and electron-withdrawing N-aryl groups were well-tolerated, affording the corresponding α-amino BCHeps in good to excellent yields (8a–c, 52–82%), while more electron-deficient N-aryls (para-fluorine or meta-cyano substitution) led to low yields of BCHep product (8d–e, 20–21%). Notably, the replacement of the N-aryl and pyrrolidine rings with pyridine or morpholine motifs respectively was successful, generating BCHeps that feature multiple ‘drug-like’ functionalities (8f–g, 43–56%). Interestingly, only phenyl, para-fluorophenyl and pyridine corresponding BCHep staffanes (9a, 9d, 9f); staffane formation was not observed for other substrates.
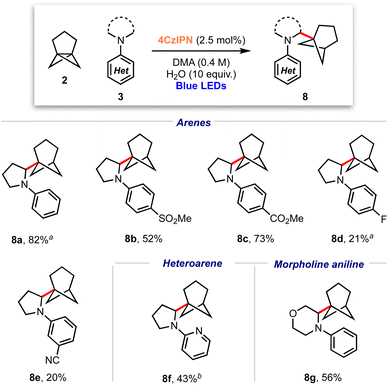 | ||
Scheme 3 Substrate scope for α-amino bicyclo[3.1.1]heptane reaction; isolated yields shown. a Isolated as a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture with 9a (from 8a) or 9d (from 8d). b Isolated as a 10 1 mixture with 9a (from 8a) or 9d (from 8d). b Isolated as a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture with 9f. 1 mixture with 9f. | ||
Mechanistic studies
A mechanistic cycle is proposed in Fig. 2a. Initial excitation of the photocatalyst gives a highly oxidising species (E° = 1.35 V vs. SCE)62 which is capable of oxidising the amine 63 followed by deprotonation of the resulting radical cation 10 (by excess 3a) to form an α-amino radical 11. This proposal is supported by a Stern–Volmer quenching study in which the amine 3a quenches the luminescence of the photocatalyst with >40 times the efficiency of 1 (Fig. 2b). The resulting α-amino radical can add to the inter-bridgehead bond of [1.1.1]propellane to form a bridgehead BCP radical 12, which can then either abstract an H atom from the α-position of a second molecule of the amine (to propagate a chain process), or from radical cation 10, or from the solvent; these HAT processes are in competition with staffane formation. Catalyst turnover can then be achieved by reduction of iminium ion 14. An alternative fate for the BCP radical could be reduction by the reduced photocatalyst (E° = −1.21 V vs. SCE)62 to complete the catalytic cycle, followed by quenching of the BCP anion by water present in the reaction; however, calculations suggest that reduction of BCP radical by the 4CzIPN radical anion would be an approximately thermoneutral process (ΔEcalc = 0.17 V, Fig. 2c) and may therefore be outcompeted by alternative low-barrier processes such as HAT. Further calculations (Fig. 2d) identified transition state barriers for hydrogen-atom transfer to a methyl-substituted BCP radical 15 with N-phenylpyrollidine (3a) (ΔG‡ = 13.1 kcal mol−1), Et2O (ΔG‡ = 14.8 kcal mol−1) and DMA (ΔG‡ = 15.4 kcal mol−1) at potential hydrogen atom sources.64 The lower barrier of the former of these (3a) can be attributed to the greater stability of the developing α-amino radical over the corresponding Et2O/DMA radicals. The importance of this radical stability is shown by the use of dimethylaniline 3u as the HAT source: its 1° α-amino radical is 1.0 kcal mol−1 less stable than the 2° radical derived from 3a, and the barrier to HAT increases to 14.5 kcal mol−1. The radical chain process is then in closer competition with HAT from the solvent (ΔΔG‡ = 0.3 kcal mol−1), which we hypothesise could decrease the radical chain length, and may be the cause of the poorer yield (of 4u, 23%), and product
63 followed by deprotonation of the resulting radical cation 10 (by excess 3a) to form an α-amino radical 11. This proposal is supported by a Stern–Volmer quenching study in which the amine 3a quenches the luminescence of the photocatalyst with >40 times the efficiency of 1 (Fig. 2b). The resulting α-amino radical can add to the inter-bridgehead bond of [1.1.1]propellane to form a bridgehead BCP radical 12, which can then either abstract an H atom from the α-position of a second molecule of the amine (to propagate a chain process), or from radical cation 10, or from the solvent; these HAT processes are in competition with staffane formation. Catalyst turnover can then be achieved by reduction of iminium ion 14. An alternative fate for the BCP radical could be reduction by the reduced photocatalyst (E° = −1.21 V vs. SCE)62 to complete the catalytic cycle, followed by quenching of the BCP anion by water present in the reaction; however, calculations suggest that reduction of BCP radical by the 4CzIPN radical anion would be an approximately thermoneutral process (ΔEcalc = 0.17 V, Fig. 2c) and may therefore be outcompeted by alternative low-barrier processes such as HAT. Further calculations (Fig. 2d) identified transition state barriers for hydrogen-atom transfer to a methyl-substituted BCP radical 15 with N-phenylpyrollidine (3a) (ΔG‡ = 13.1 kcal mol−1), Et2O (ΔG‡ = 14.8 kcal mol−1) and DMA (ΔG‡ = 15.4 kcal mol−1) at potential hydrogen atom sources.64 The lower barrier of the former of these (3a) can be attributed to the greater stability of the developing α-amino radical over the corresponding Et2O/DMA radicals. The importance of this radical stability is shown by the use of dimethylaniline 3u as the HAT source: its 1° α-amino radical is 1.0 kcal mol−1 less stable than the 2° radical derived from 3a, and the barrier to HAT increases to 14.5 kcal mol−1. The radical chain process is then in closer competition with HAT from the solvent (ΔΔG‡ = 0.3 kcal mol−1), which we hypothesise could decrease the radical chain length, and may be the cause of the poorer yield (of 4u, 23%), and product![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) staffane ratio (3
staffane ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), observed for this substrate.
1), observed for this substrate.
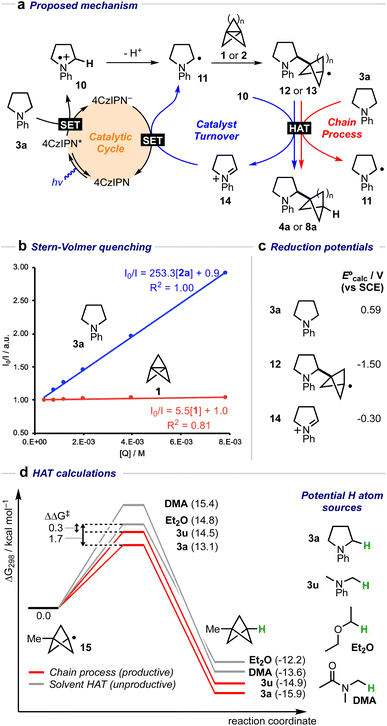 | ||
| Fig. 2 (a) Proposed mechanistic cycle for the formation of α-amino BCPs from amines and [1.1.1]propellane 1 (n = 1) or [3.1.1]propellane 2 (n = 3). (b) Stern–Volmer quenching plot for amine 3a and [1.1.1]propellane 1 with 4CzIPN. (c) Calculated E° values (V vs. SCE) for 3a, 12 and 14. (d) Calculated H-atom transfer barriers using amines 3a, 3u, DMA, and Et2O.64 Free energies were calculated at 298.15 K, and the standard concentration of each species was adjusted for the experimental molar ratios (3a/3u: 10.0 equiv., DMA: 21.6 equiv. Et2O: 12.0 equiv.). | ||
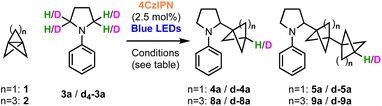
The preference for HAT transfer from 3a, rather than the solvent or H2O, was further explored using deuterium-labelling studies (Table 3). We first confirmed that under the standard conditions, no deuterium incorporation was observed in the presence of D2O, ruling out the reduction of the BCP radical as a catalyst turnover step (entry 2). Use of d7-DMF (as a surrogate for DMA) also led to no product deuteration (entry 3). However, 80% D-incorporation was observed using d4-N-phenylpyrrolidine (entry 4, d4-3a), albeit this reaction proceeded in very low yield. A significantly greater amount of staffane was observed, which is consistent with the slower rate of deuterium atom transfer compared to HAT with h4-3a (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a = 1.3
5a = 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 6.4
1 vs. 6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Only 34% D-incorporation was observed when using d4-N-phenylpyrollidine in combination with a DMA/Et2O solvent mixture (entry 5), suggesting that these solvents may also act as H-atom sources in the presence of deuterated substrate.
1). Only 34% D-incorporation was observed when using d4-N-phenylpyrollidine in combination with a DMA/Et2O solvent mixture (entry 5), suggesting that these solvents may also act as H-atom sources in the presence of deuterated substrate.
| Entry | Substrates | Solvent | Additive | % D | Yield (%) (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a)/(8a 5a)/(8a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9a) 9a) |
|---|---|---|---|---|---|
| a d4-3a = 98% D. | |||||
| 1 | 1 + h4-3a | DMA/Et2O | H2O | 0 | 70 (6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 2 | 1 + h4-3a | DMA/Et2O | D2O | 0 | 70 (6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 3 | 1 + h4-3a | d7-DMF/pentane | D2O | 0 | 70 (6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 4a | 1 + d4-3a | d7-DMF/pentane | D2O | 80 | 7 (1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 5a | 1 + d4-3a | DMA/Et2O | D2O | 34 | 10 (1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 6a | 1 + d4-3a | d7-DMF/Et2O | D2O | 63 | 10 (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 7 | 2 + h4-3a | DMA/n-Bu2O | H2O | 0 | 82 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 8a | 2 + d4-3a | DMA/n-Bu2O | H2O | 38 | 19 (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 9 | 2 + h4-3a | DMA/n-Bu2O | D2O | 21 | 79 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 10a | 2 + d4-3a | DMA/n-Bu2O | D2O | 49 | 10 (3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| 11a | 2 + d4-3a | d7-DMF/n-Bu2O | D2O | 74 | 8 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Equivalent deuteration studies were next performed using [31.1]propellane 2 as acceptor, which confirmed that the substrate 3a is again a capable H atom donor, with 38% D-incorporation using d4-N-phenylpyrrolidine, DMA and H2O (entries 7 and 8). However, additional experiments revealed that in the case of 2, use of D2O resulted in a surprising 21% D-incorporation and a much superior yield (entry 9). Furthermore, 49% deuteration was observed using a combination of d4-3a and D2O (entry 10), and the introduction of d7-DMF further increased the extent of deuteration to 74%, confirming the participation of multiple H-atom sources, including the solvent (entry 11). The BCHep/staffane ratios gradually decreased from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entries 7 and 8), progressing to 3.5
1 (entries 7 and 8), progressing to 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entries 9 and 10), and finally reaching 2
1 (entries 9 and 10), and finally reaching 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 11), showing that the bicycloheptylation reaction also features a fine balance between HAT and staffane formation.
1 (entry 11), showing that the bicycloheptylation reaction also features a fine balance between HAT and staffane formation.
Additional evidence for our mechanistic proposal was obtained using the kinetic isotope effect (KIE) observed for the HAT step in the reactions of 1. Since staffane formation is independent of the deuteration state of the amine, the product:staffane ratio for h4- and d4-N-phenylpyrollidine (Table 3, entries 1 vs. 4) should approximate the HAT KIE, i.e. kH/kD ≃ (4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a)/(d-4a
5a)/(d-4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d-5a) (see ESI† for further discussion). Using this approach, a KIE of 4.9 ± 0.5 was obtained, which is in reasonable agreement with the value obtained from computation (kH/kD = 6.1).
d-5a) (see ESI† for further discussion). Using this approach, a KIE of 4.9 ± 0.5 was obtained, which is in reasonable agreement with the value obtained from computation (kH/kD = 6.1).
While a chain process is evidently possible, the low quantum yield of 0.84 obtained for this reaction suggests catalyst turnover is important.61 Since reduction of the BCP radical is not viable to achieve turnover (at least for [1.1.1]propellane, as evidenced by the lack of deuteration of 4a in the presence D2O for this propellane), it may be that a fast HAT process between BCP radical 12 and low-concentration radical cation 10 could occur that would lead to the formation of iminium ion 14. This iminium ion could then be reduced by 4CzIPN− to reform the neutral organophotoredox catalyst, and an α-amino radical 11 (ΔEcalc = +0.88 V).
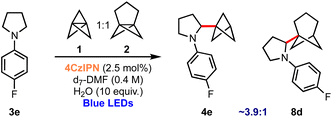
Finally, the successful use of different propellanes in this aminobicycloalkylation offers the opportunity to compare the relative propensity of the two to undergo ring-opening. A competition experiment was therefore undertaken in which the reaction was conducted using an equimolar mixture of 1 and 2; this experiment revealed that the formation of BCP derivative 4e is ∼3.9 times faster than BCHep product 8d as judged by NMR monitoring of the proportions of products formed during the reaction.61 This reveals an enhanced reactivity of [1.1.1]propellane 1 compared to [3.1.1]propellane 2, at least in this particular setting of nucleophilic radical addition chemistry (Scheme 4).
Conclusions
In conclusion, we have developed an organocatalysed photoredox approach for the synthesis of α-amino BCPs and BCHeps through the addition of α-amino radicals to the strained interbridgehead of [1.1.1] and [3.1.1]propellanes respectively. The reaction displays scope that is of high relevance in medicinal chemistry research, where such motifs are of high importance. A combination of experimental and computational mechanistic studies provide evidence for a radical chain pathway, and also offer insight into the kinetics of hydrogen atom transfer steps of bridgehead bicycloalkyl radicals.Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
J. N. and E. A. conceived the project. J. N., A. L.-F., A. J. S., M. Y.-T. carried out the experimental work. A. J. S. and N. F. carried out the computational work. J. N., J. J. M., F. D. and E. A. directed the project. J. N., A. L.-F., A. J. S. and E. A. wrote the manuscript. All authors contributed to editing and revision of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. N. thanks the Marie Skłodowska-Curie actions for an Individual Fellowship (GA No. 786683). A. J. S. thanks the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine for studentships (EP/L015838/1), generously supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB and Vertex. A. L.-F. thanks the Basque Government for a Predoctoral (PRE_2022_2_0112) and Egonlabur (EP_2023_1_0047) fellowships. E. A. A. thanks the EPSRC for support (EP/S013172/1). A. J. S. and F. D. thank the EPSRC Tier-2 National HPC Facility Service (https://www.cirrus.ac.uk), and the EPSRC Centre for Doctoral Training for Theory and Modelling in Chemical Sciences (No. EP/L015722/1) for providing access to the Dirac Cluster at Oxford.Notes and references
- M. R. Bauer, P. Di Fruscia, S. C. C. Lucas, I. N. Michaelides, J. E. Nelson, R. I. Storer and B. C. Whitehurst, RSC Med. Chem., 2021, 12, 448–471 RSC.
- G. M. Locke, S. S. R. Bernhard and M. O. Senge, Chem.–Eur. J., 2019, 25, 4590–4647 CrossRef CAS PubMed.
- M. A. M. Subbaiah and N. A. Meanwell, J. Med. Chem., 2021, 64, 14046–14128 CrossRef CAS PubMed.
- N. A. Meanwell, Chem. Res. Toxicol., 2016, 29, 564–616 Search PubMed.
- P. K. Mykhailiuk, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC.
- N. A. Meanwell, J. Agric. Food Chem., 2023, 71, 18087–18122 CrossRef CAS PubMed.
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- Y. P. Auberson, C. Brocklehurst, M. Furegati, T. C. Fessard, G. Koch, A. Decker, L. La Vecchia and E. Briard, ChemMedChem, 2017, 12, 590–598 CrossRef CAS PubMed.
- A. F. Stepan, C. Subramanyam, I. V. Efremov, J. K. Dutra, T. J. O'Sullivan, K. J. DiRico, W. S. McDonald, A. Won, P. H. Dorff, C. E. Nolan, S. L. Becker, L. R. Pustilnik, D. R. Riddell, G. W. Kauffman, B. L. Kormos, L. Zhang, Y. Lu, S. H. Capetta, M. E. Green, K. Karki, E. Sibley, K. P. Atchison, A. J. Hallgren, C. E. Oborski, A. E. Robshaw, B. Sneed and C. J. O'Donnell, J. Med. Chem., 2012, 55, 3414–3424 CrossRef CAS PubMed.
- N. D. Measom, K. D. Down, D. J. Hirst, C. Jamieson, E. S. Manas, V. K. Patel and D. O. Somers, ACS Med. Chem. Lett., 2017, 8, 43–48 CrossRef CAS PubMed.
- Y. L. Goh, Y. T. Cui, V. Pendharkar and V. A. Adsool, ACS Med. Chem. Lett., 2017, 8, 516–520 CrossRef CAS PubMed.
- I. S. Makarov, C. E. Brocklehurst, K. Karaghiosoff, G. Koch and P. Knochel, Angew. Chem., Int. Ed., 2017, 56, 12774–12777 CrossRef CAS PubMed.
- S. J. Nara, S. Jogi, S. Cheruku, S. Kandhasamy, F. Jaipuri, P. K. Kathi, S. Reddy, S. Sarodaya, E. M. Cook, T. Wang, D. Sitkoff, K. A. Rossi, M. Ruzanov, S. E. Kiefer, J. A. Khan, M. Gao, S. Reddy, S. Sivaprasad Lvj, R. Sane, K. Mosure, X. Zhuo, G. G. Cao, M. Ziegler, A. Azzara, J. Krupinski, M. G. Soars, B. A. Ellsworth and D. A. Wacker, J. Med. Chem., 2022, 65, 8948–8960 CrossRef CAS PubMed.
- B. Owen, M. de Gaetano, A. Gaffney, C. Godson and P. J. Guiry, Org. Lett., 2022, 24, 6049–6053 CrossRef CAS PubMed.
- J. Weinmann, L. Kirchner, M. Engstler, L. Meinel and U. Holzgrabe, Eur. J. Med. Chem., 2023, 250, 115176 CrossRef CAS PubMed.
- R. J. Altenbach, A. Bogdan, S. Couty, E. De Lemos, N. Desroy, B. Duthion, G. A. Gfesser, S. N. Greszler, C. G. Housseman, J. R. Koenig, P. R. Kym, B. Liu, M. J. Scanio, X. Searle, X. Wang, M. C. Yeung and G. Zhao, WO2018065962A1, 2018.
- M. V. Westphal, B. T. Wolfstädter, J.-M. Plancher, J. Gatfield and E. M. Carreira, ChemMedChem, 2015, 10, 461–469 CrossRef CAS PubMed.
- N. Frank, J. Nugent, B. R. Shire, H. D. Pickford, P. Rabe, A. J. Sterling, T. Zarganes-Tzitzikas, T. Grimes, A. L. Thompson, R. C. Smith, C. J. Schofield, P. E. Brennan, F. Duarte and E. A. Anderson, Nature, 2022, 611, 721–726 CrossRef CAS PubMed.
- X. Zhang, R. T. Smith, C. Le, S. J. McCarver, B. T. Shireman, N. I. Carruthers and D. W. C. MacMillan, Nature, 2020, 580, 220–226 CrossRef CAS PubMed.
- P. Kaszynski, A. C. Friedli and J. Michl, J. Am. Chem. Soc., 1992, 114, 601–620 CrossRef CAS.
- J. Kanazawa, K. Maeda and M. Uchiyama, J. Am. Chem. Soc., 2017, 139, 17791–17794 CrossRef CAS PubMed.
- M. Kondo, J. Kanazawa, T. Ichikawa, T. Shimokawa, Y. Nagashima, K. Miyamoto and M. Uchiyama, Angew. Chem., Int. Ed., 2020, 59, 1970–1974 CrossRef CAS PubMed.
- J. Nugent, C. Arroniz, B. R. Shire, A. J. Sterling, H. D. Pickford, M. L. J. Wong, S. J. Mansfield, D. F. J. Caputo, B. Owen, J. J. Mousseau, F. Duarte and E. A. Anderson, ACS Catal., 2019, 9, 9568–9574 CrossRef CAS.
- M. L. J. Wong, J. J. Mousseau, S. J. Mansfield and E. A. Anderson, Org. Lett., 2019, 21, 2408–2411 CrossRef CAS PubMed.
- J. H. Kim, A. Ruffoni, Y. S. S. Al-Faiyz, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2020, 59, 8225–8231 CrossRef CAS PubMed.
- D. F. J. Caputo, C. Arroniz, A. B. Dürr, J. J. Mousseau, A. F. Stepan, S. J. Mansfield and E. A. Anderson, Chem. Sci., 2018, 9, 5295–5300 RSC.
- R. M. Bär, S. Kirschner, M. Nieger and S. Bräse, Chem.–Eur. J., 2018, 24, 1373–1382 CrossRef PubMed.
- H. D. Pickford, J. Nugent, B. Owen, J. J. Mousseau, R. C. Smith and E. A. Anderson, J. Am. Chem. Soc., 2021, 143, 9729–9736 CrossRef CAS PubMed.
- W. Huang, Y. Zheng, S. Keess and G. A. Molander, J. Am. Chem. Soc., 2023, 145, 5363–5369 CrossRef CAS PubMed.
- Y. Guo, J. Zhu, Y. Wang, Y. Li, H. Hu, P. Zhang, J. Xu and W. Li, ACS Catal., 2024, 14, 619–627 CrossRef CAS.
- H. D. Pickford, V. Ripenko, R. E. McNamee, S. Holovchuk, A. L. Thompson, R. C. Smith, P. K. Mykhailiuk and E. A. Anderson, Angew. Chem., Int. Ed., 2023, 62, e202213508 CrossRef CAS PubMed.
- J. Nugent, A. J. Sterling, N. Frank, J. J. Mousseau and E. A. Anderson, Org. Lett., 2021, 23, 8628–8633 CrossRef CAS PubMed.
- M. Messner, S. I. Kozhushkov and A. de Meijere, Eur. J. Org Chem., 2000, 1137–1155 CrossRef CAS.
- R. Gianatassio, J. M. Lopchuk, J. Wang, C.-M. Pan, L. R. Malins, L. Prieto, T. A. Brandt, M. R. Collins, G. M. Gallego, N. W. Sach, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, Science, 2016, 351, 241–246 CrossRef CAS PubMed.
- J. M. Lopchuk, K. Fjelbye, Y. Kawamata, L. R. Malins, C.-M. Pan, R. Gianatassio, J. Wang, L. Prieto, J. Bradow, T. A. Brandt, M. R. Collins, J. Elleraas, J. Ewanicki, W. Farrell, O. O. Fadeyi, G. M. Gallego, J. J. Mousseau, R. Oliver, N. W. Sach, J. K. Smith, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, J. Am. Chem. Soc., 2017, 139, 3209–3226 CrossRef CAS PubMed.
- N. Trongsiriwat, Y. Pu, Y. Nieves-Quinones, R. A. Shelp, M. C. Kozlowski and P. J. Walsh, Angew. Chem., Int. Ed., 2019, 58, 13416–13420 CrossRef CAS PubMed.
- R. A. Shelp and P. J. Walsh, Angew. Chem., Int. Ed., 2018, 57, 15857–15861 CrossRef CAS PubMed.
- R. A. Shelp, A. Ciro, Y. Pu, R. R. Merchant, J. M. E. Hughes and P. J. Walsh, Chem. Sci., 2021, 12, 7066–7072 RSC.
- K. Schwärzer, H. Zipse, K. Karaghiosoff and P. Knochel, Angew. Chem., Int. Ed., 2020, 59, 20235–20241 CrossRef PubMed.
- Approved pharmaceuticals featuring a benzylamine motif include benzphetamine, bethanidine, buclizine, meclizine, terbinafine and ticlopidine.
- S. Pritz, M. Pätzel, G. Szeimies, M. Dathe and M. Bienert, Org. Biomol. Chem., 2007, 5, 1789–1794 RSC.
- S. Ni, A. F. Garrido-Castro, R. R. Merchant, J. N. de Gruyter, D. C. Schmitt, J. J. Mousseau, G. M. Gallego, S. Yang, M. R. Collins, J. X. Qiao, K.-S. Yeung, D. R. Langley, M. A. Poss, P. M. Scola, T. Qin and P. S. Baran, Angew. Chem., Int. Ed., 2018, 57, 14560–14565 CrossRef CAS PubMed.
- S. O. Kokhan, A. V. Tymtsunik, S. L. Grage, S. Afonin, O. Babii, M. Berditsch, A. V. Strizhak, D. Bandak, M. O. Platonov, I. V. Komarov, A. S. Ulrich and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2016, 55, 14788–14792 CrossRef CAS PubMed.
- R. A. Shelp, A. Ciro, Y. Pu, R. R. Merchant, J. M. E. Hughes and P. J. Walsh, Chem. Sci., 2021, 12, 7066–7072 RSC.
- A. J. Sterling, A. B. Dürr, R. C. Smith, E. A. Anderson and F. Duarte, Chem. Sci., 2020, 11, 4895–4903 RSC.
- M. L. J. Wong, A. J. Sterling, J. J. Mousseau, F. Duarte and E. A. Anderson, Nat. Commun., 2021, 12, 1644 CrossRef CAS PubMed.
- K. B. Wiberg and S. T. Waddell, J. Am. Chem. Soc., 1990, 112, 2194–2216 CrossRef CAS.
- D. T. Ahneman and A. G. Doyle, Chem. Sci., 2016, 7, 7002–7006 RSC.
- Y.-Y. Gui, L.-L. Liao, L. Sun, Z. Zhang, J.-H. Ye, G. Shen, Z.-P. Lu, W.-J. Zhou and D.-G. Yu, Chem. Commun., 2017, 53, 1192–1195 RSC.
- Y.-Y. Gui, Z.-X. Wang, W.-J. Zhou, L.-L. Liao, L. Song, Z.-B. Yin, J. Li and D.-G. Yu, Asian J. Org. Chem., 2018, 7, 537–541 CrossRef CAS.
- X. Si, L. Zhang and A. S. K. Hashmi, Org. Lett., 2019, 21, 6329–6332 CrossRef CAS PubMed.
- C. Bosset, H. Beucher, G. Bretel, E. Pasquier, L. Queguiner, C. Henry, A. Vos, J. P. Edwards, L. Meerpoel and D. Berthelot, Org. Lett., 2018, 20, 6003–6006 CrossRef CAS PubMed.
- J. B. McManus, N. P. R. Onuska and D. A. Nicewicz, J. Am. Chem. Soc., 2018, 140, 9056–9060 CrossRef CAS PubMed.
- L. Ruiz Espelt, E. M. Wiensch and T. P. Yoon, J. Org. Chem., 2013, 78, 4107–4114 CrossRef CAS PubMed.
- R. A. Aycock, C. J. Pratt and N. T. Jui, ACS Catal., 2018, 8, 9115–9119 CrossRef CAS.
- P. Kohls, D. Jadhav, G. Pandey and O. Reiser, Org. Lett., 2012, 14, 672–675 CrossRef CAS PubMed.
- C. J. Pratt, R. A. Aycock, M. D. King and N. T. Jui, Synlett, 2020, 31, 51–54 CrossRef CAS PubMed.
- Y. He, Z. Zheng, J. Yang, X. Zhang and X. Fan, Org. Chem. Front., 2021, 8, 4582–4606 RSC.
- G. Duret, R. Quinlan, P. Bisseret and N. Blanchard, Chem. Sci., 2015, 6, 5366–5382 RSC.
- M. A. Bryden and E. Zysman-Colman, Chem. Soc. Rev., 2021, 50, 7587–7680 RSC.
- See the ESI† for details.
- T.-Y. Shang, L.-H. Lu, Z. Cao, Y. Liu, W.-M. He and B. Yu, Chem. Commun., 2019, 55, 5408–5419 RSC.
- H. G. Roth, N. A. Romero and D. A. Nicewicz, Synlett, 2016, 27, 714–723 CAS.
- T. A. Young, J. J. Silcock, A. J. Sterling and F. Duarte, Angew. Chem., Int. Ed., 2021, 60, 4266–4274 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, computational details, copies of 1H and 13C NMR spectra. See DOI: https://doi.org/10.1039/d4sc01368a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |