Open Access Article
Open Access ArticleCross-linked K0.5MnO2 nanoflower composites for high rate and low overpotential Li–CO2 batteries†
Jiawei
Wu‡
ac,
Jian
Chen‡
 b,
Xiaoyang
Chen
b,
Yang
Liu
b,
Xiaoyang
Chen
b,
Yang
Liu
 *ab,
Zhe
Hu
*ab,
Zhe
Hu
 d,
Feijian
Lou
*a,
Shulei
Chou
d,
Feijian
Lou
*a,
Shulei
Chou
 e and
Yun
Qiao
e and
Yun
Qiao
 *ab
*ab
aSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, China. E-mail: liuy986@htu.edu.cn; loufeijian@htu.edu.cn
bSchool of Environment and Chemical Engineering, Shanghai University, Shanghai 200444, China. E-mail: yunqiao@shu.edu.cn
cSinopec Petroleum Engineering Zhongyuan Co. Ltd, Natural Gas Technology Center, Zhengzhou, Henan 450000, China
dCollege of Materials Science and Engineering, Shenzhen University, Shenzhen 518055, China
eInstitute for Carbon Neutralization, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou, Zhejiang 325035, China
First published on 14th May 2024
Abstract
Rechargeable Li–CO2 batteries are deemed to be attractive energy storage systems, as they can effectively inhale and fix carbon dioxide and possess an extremely high energy density. Unfortunately, the irreversible decomposition of the insoluble and insulating Li2CO3 results in awful electrochemical performance and inferior energy efficiency of Li–CO2 batteries. Furthermore, the low energy efficiency will exacerbate the extra waste of resources. Therefore, it is vital to design novel and efficient catalysts to enhance the battery performance. Herein, a facile, one-step strategy is introduced to design cross-linked, ultrathin K0.5MnO2 nanoflowers combined with CNTs (K0.5MnO2/CNT) as a highly efficient cathode for Li–CO2 batteries. Impressively, the Li–CO2 battery based on the K0.5MnO2/CNT cathode achieves a low overpotential (1.05 V) and a high average energy efficiency (87.95%) at a current density of 100 mA g−1. Additionally, the K0.5MnO2/CNT cathode can steadily run for over 100 cycles (overpotential < 1.20 V). Moreover, a low overpotential of 1.47 V can be obtained even at a higher current density of 1000 mA g−1, indicating the superior rate performance of K0.5MnO2/CNT. This strategy offers new insight and guidance for the development of low-cost and high-performance Li–CO2 batteries.
Introduction
The massive consumption of fossil fuels has led to a surge in carbon dioxide (CO2) emissions, which has caused a severe greenhouse effect.1–3 A straightforward strategy to alleviate this concern is converting CO2 into renewable energy. Using Li–CO2 batteries has been considered as one of the most promising approaches for sustainable utilization and reduction of CO2 to produce electrochemical energy.4–7 Generally, the electrochemical reaction mechanism of Li–CO2 batteries is based on the following reaction: 4Li + 3CO2 ↔ 2Li2CO3 + C (Eo = 2.80 V versus Li/Li+).8–11 Unfortunately, Li2CO3 as the main discharge product is an insoluble and wide bandgap insulator with thermodynamic stability.12,13 Consequently, the irreversible decomposition of Li2CO3 will lead to large polarization, inferior cycle stability, low energy efficiency, and even battery failure, which are the dominant obstacles in the development of high-performance Li–CO2 batteries.14–17 In this regard, exploration and fabrication of effective catalysts is a great choice to improve the CO2 reduction and evolution reaction.To date, many efforts have been taken to design efficient catalysts with high catalytic activity. Noble metals, such as Ru, Ir and their oxides, have been demonstrated to enable the reversible formation and decomposition of carbonate at low overpotential, and thus can enhance the energy efficiency of Li–CO2 batteries,18–21 whereas, their large-scale application in Li–CO2 batteries is still limited by the high cost and scarcity of noble metals. Notably, transition metal oxides are regarded as attractive candidates. Recently, Wang and co-workers demonstrated a Mn based metal organic framework with catalytic metal centers and porous structure, which can facilitate the complete and efficient decomposition of the nanostructured Li2CO3 as well as reducing the charge overpotential.22 Thus, introducing low-cost Mn as catalytic metal centers is a promising approach to decompose the discharge product of Li2CO3.
In particular, MnO2 materials have emerged as catalysts, which may enhance the reaction dynamics and reduce the overpotentials for Li–CO2 batteries. Ge et al. first introduced Co-interstitial α-MnO2 nanowires as a cathode for Li–CO2 batteries, which can deliver a good capacity of 8160 mA h g−1 at 100 mA g−1, high efficiency and high energy density.23 Meanwhile, IrO2-decorated few-layered δ-MnO2 was obtained, in which the IrO2 acts as catalytically active centers for CO2 reduction, and δ-MnO2 has a co-catalytic effect for conformal growth of amorphous Li2CO3.24 However, the electrocatalytic performances are still finite for MnO2 materials as catalytic electrodes in Li–CO2 batteries. It is commonly recognized that birnessite MnO2 exhibits the best activity among various MnO2, while studies on layered MnO2 as a catalyst in Li–CO2 batteries are still in their infancy stage.25–27 Therefore, it is imperative to develop K-birnessite MnO2 as a feasible catalyst for Li–CO2 batteries with high performance, taking advantage of the catalytic manganese centers and large interlayer space.
Herein, we adopted a facile method to synthesize ultrathin K0.5MnO2 nanoflowers combined with CNTs (K0.5MnO2/CNT) via a hydrothermal process. As a cathode for Li–CO2 batteries, the K0.5MnO2/CNT electrode exhibits a low overpotential (1.05 V), high average energy efficiency (87.95%) and excellent rate performance (up to 1000 mA g−1). Meanwhile, the cathode can be steadily operated for 100 cycles and the terminal discharge and charge voltages are preserved between 2.72 and 3.92 V. This superb electrochemical performance is ascribed to the outstanding catalytic activity of K0.5MnO2/CNT. Fig. 1 displays the schematic diagram of the typical Li–CO2 battery with microstructural K0.5MnO2/CNT as a cathode, and the corresponding catalytic reactions for CO2 reduction and evolution. Of note, the introduction of CNTs can not only improve the electronic conductivity of the composite, but also facilitate the formation of ultrathin K0.5MnO2 nanoflowers with small size and high separation. Simultaneously, the cross-linked ultrathin K0.5MnO2 nanoflowers can effectively shorten the electron/Li+ transport distances, and also expose ample catalytic active sites. Furthermore, K+ occupies the interlayer space of K0.5MnO2, which can not only achieve support and charge balance, but also significantly improve the conductivity and the diffusion rate of Li+. Therefore, K0.5MnO2/CNT can be used as a highly efficient cathode for Li–CO2 batteries, which is helpful for the realization of ultra-low overpotential, ultra-high energy efficiency, and excellent cycling performance of Li–CO2 batteries.
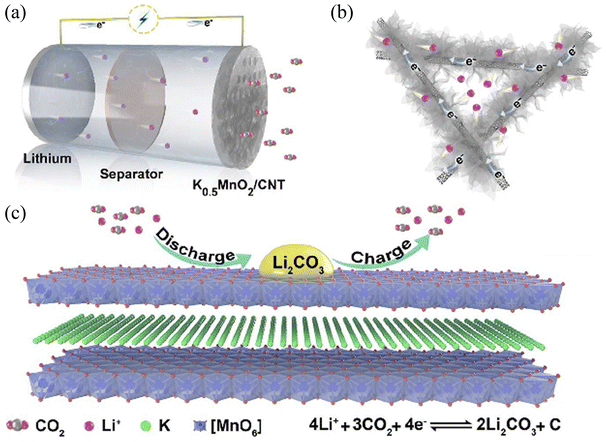 | ||
| Fig. 1 (a) Schematic diagram of the Li–CO2 battery with the K0.5MnO2/CNT cathode. (b) The microstructure of K0.5MnO2/CNT and (c) the catalytic mechanism of K0.5MnO2. | ||
Results and discussion
The morphology and microstructure were first characterized through field emission scanning electron microscopy (FE-SEM). Pristine CNTs with an average diameter of about 20–30 nm (Fig. S1†) were first treated with mixed acid, and then the K0.5MnO2/CNT composite was achieved via a facile, one-step hydrothermal process. The X-ray diffraction (XRD) patterns of the sample are presented in Fig. S2.† In which, the distinct peaks at 12.5°, 25.2°, 37.3°, and 65.6° in the K0.5MnO2/CNT composite can be well indexed to the (001), (002), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) and (020) planes for monoclinic potassium birnessite (JCPDS No. 80-1098). As shown in Fig. 2a, K0.5MnO2 nanoflowers with diameters of ∼200 nm are assembled by ultrathin nanosheets and cover the surface of CNTs, which are cross-linked with each other to form a network void structure. It is of note that the intrinsic voids in the K0.5MnO2/CNT composite with an interconnected network structure can expose more active sites and provide sufficient space for the discharge deposition. The typical transmission electron microscopy image in Fig. 2b clearly discloses that K0.5MnO2 nanoflowers are composed of interconnected nanoflakes alongside CNTs, which can conventionally increase the electronic conductivity of the composite. The high-resolution transmission electron microscopy (HRTEM) image shows that the thickness of the nanosheets ranges from 2.70 to 3.30 nm (Fig. 2c), which is coincident with 4–5 layers of MnO2 in the (001) direction with an interplanar spacing of ∼0.67 nm. Moreover, the layered structure is further proved by the high-angle-annular dark-field scanning transmission electron microscopy (HAADF-STEM) image in Fig. 2d. The edge-shared [MnO6] octahedral (bluish violet dots) and K ions (green dots) are alternately distributed between the interlayers, which correspond to 2D layered MnO2 in the inset of Fig. 2d.28,29 Two diffraction rings are observed in selected area electron diffraction (SAED) patterns, which can be indexed to the (
11) and (020) planes for monoclinic potassium birnessite (JCPDS No. 80-1098). As shown in Fig. 2a, K0.5MnO2 nanoflowers with diameters of ∼200 nm are assembled by ultrathin nanosheets and cover the surface of CNTs, which are cross-linked with each other to form a network void structure. It is of note that the intrinsic voids in the K0.5MnO2/CNT composite with an interconnected network structure can expose more active sites and provide sufficient space for the discharge deposition. The typical transmission electron microscopy image in Fig. 2b clearly discloses that K0.5MnO2 nanoflowers are composed of interconnected nanoflakes alongside CNTs, which can conventionally increase the electronic conductivity of the composite. The high-resolution transmission electron microscopy (HRTEM) image shows that the thickness of the nanosheets ranges from 2.70 to 3.30 nm (Fig. 2c), which is coincident with 4–5 layers of MnO2 in the (001) direction with an interplanar spacing of ∼0.67 nm. Moreover, the layered structure is further proved by the high-angle-annular dark-field scanning transmission electron microscopy (HAADF-STEM) image in Fig. 2d. The edge-shared [MnO6] octahedral (bluish violet dots) and K ions (green dots) are alternately distributed between the interlayers, which correspond to 2D layered MnO2 in the inset of Fig. 2d.28,29 Two diffraction rings are observed in selected area electron diffraction (SAED) patterns, which can be indexed to the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) and (020) planes of K0.5MnO2 (Fig. 2e). As shown in Fig. 2f, Mn, O, C, and K elements are detected in energy-dispersive X-ray spectra (EDS) of K0.5MnO2/CNT, further confirming the existence of K ions. Moreover, HAADF-STEM mapping images in Fig. 2g–j clearly reveal that K, Mn, and O elements are homogeneously distributed in layered MnO2 nanoflowers. The presence of K+ can not only improve the structural stability and charge balance of K0.5MnO2, but also boost the catalytic activity of the electrode.30 The existence of carbon further confirms that K0.5MnO2 nanoflowers cover the CNTs substrate (Fig. 2k).
11) and (020) planes of K0.5MnO2 (Fig. 2e). As shown in Fig. 2f, Mn, O, C, and K elements are detected in energy-dispersive X-ray spectra (EDS) of K0.5MnO2/CNT, further confirming the existence of K ions. Moreover, HAADF-STEM mapping images in Fig. 2g–j clearly reveal that K, Mn, and O elements are homogeneously distributed in layered MnO2 nanoflowers. The presence of K+ can not only improve the structural stability and charge balance of K0.5MnO2, but also boost the catalytic activity of the electrode.30 The existence of carbon further confirms that K0.5MnO2 nanoflowers cover the CNTs substrate (Fig. 2k).
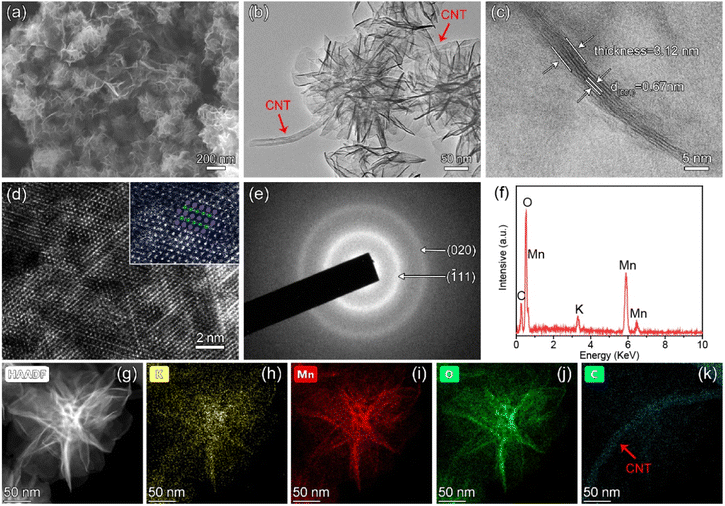 | ||
| Fig. 2 (a) SEM, (b) TEM, (c) HRTEM, and (d) high-magnification HAADF-STEM images of K0.5MnO2/CNT (inset of Fig. 2d: bluish violet dots stand for [MnO6] and green dots represent K+). (e) SAED patterns and (f) EDS spectrum of K0.5MnO2/CNT. (g–k) HAADF-STEM image and the corresponding EDS elemental mapping results of K0.5MnO2/CNT. | ||
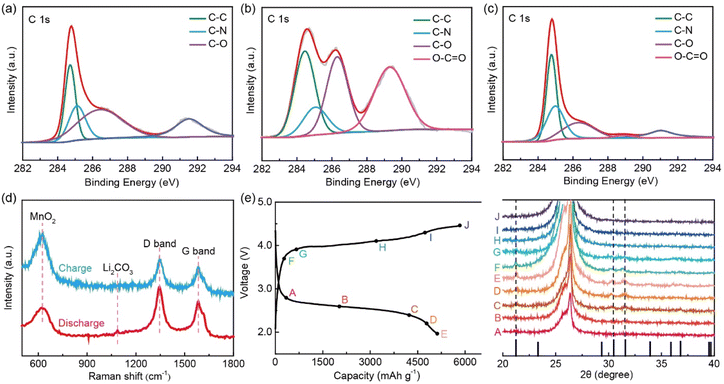
Inductively coupled plasma (ICP) analysis was used to investigate the chemical composition of K0.5MnO2/CNT, where the K/Mn molar ratio in K0.5MnO2/CNT is about 0.5/1.0 (Table S1†). Therefore, the molecular formula of K0.5MnO2/CNT can be identified as K0.5MnO2. The thermogravimetric result reveals that the proportion of CNTs in the K0.5MnO2/CNT composite is 16.0% (Fig. S3†). The full X-ray photoelectron spectrum (XPS) of K0.5MnO2/CNT shows distinct characteristic peaks of K, Mn, O and C elements, further indicating the presence of K0.5MnO2 and CNTs (Fig. S4†). In Fig. S5a,† the high-resolution C 1s XPS spectrum can be deconvoluted into three peaks centered at 284.7, 285.5 and 286.8 eV, which can be ascribed to nonoxygenated ring carbon (C–C), C–N and the C–O bonds, respectively.31–33 Fig. S5b† shows the high-resolution XPS spectrum of Mn 2p; the two peaks at 654.2 and 642.4 eV correspond to the binding energy of Mn 2p1/2 and Mn 2p3/2 for K0.5MnO2, respectively. Their spin-energy separation is 11.8 eV, consistent with a previous report.27 Furthermore, the spectrum of Mn 2p can be resolved into three peaks, confirming the existence of a mixed-valence state of Mn4+ (642.5 eV) and Mn3+ (644.9 and 654.1 eV) ions in the MnO6 subunits.34,35 The high-resolution XPS spectra of Mn 3s exhibit a splitting of the doublet, because of the parallel spin coupling of 3s and 3d electrons during the photoelectron ejection (Fig. S5c†).36,37 The energy difference of 4.7 V among the two peaks is attributed to the existence of Mn4+. These mixed-valence states of Mn could enhance the catalytic performance of MnO2 with different oxide species. As shown in Fig. S5d,† the O 1s spectra display three fitted peaks at 529.9, 531.5 and 532.8 eV, which are assigned to the Mn–O–Mn, Mn–O–H, and H–O–H (adsorbed water) bonds, respectively.38 These results prove the successful synthesis of K-birnessite-type MnO2/CNT.
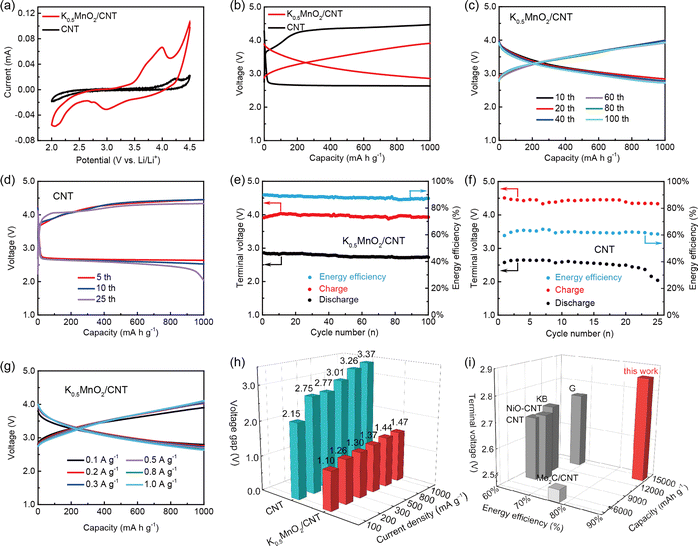
To evaluate the electrochemical behavior of the K0.5MnO2/CNT cathode in Li–CO2 batteries, the cyclic voltammetry (CV) measurement was first carried out between 2.0 and 4.5 V at a scan rate of 0.2 mV s−1. As shown in Fig. 3a, the K0.5MnO2/CNT cathode displays an obvious cathodic peak at 2.98 V, indicating the CO2 reduction reaction. In contrast, none of the significant peaks are found for the CNT cathode. Upon the anodic scan, the K0.5MnO2/CNT cathode exhibits a significantly lower onset potential (≈3.0 V) than that of the CNT cathode (≈3.4 V). The stronger oxidation peak at 3.95 V for the K0.5MnO2/CNT cathode can be ascribed to the CO2 evolution reaction (CO2ER) with high activity, implying the remarkably boosted kinetic reaction of Li2CO3 decomposition, Li+ and CO2 transport. Of note, the K0.5MnO2/CNT cathode also endows the larger integral area and higher peak intensity in the CV curves than that of the CNT cathode, suggesting that the synergistic effect between K0.5MnO2 and CNTs significantly improves the catalytic activity of the CO2 reduction reaction (CO2RR) and CO2ER. Subsequently, deep discharge/charge tests of Li–CO2 cells with K0.5MnO2/CNT and CNTs as cathodes were also carried out at 100 mA g−1 between 2.0 and 4.5 V (Fig. S6†). During the discharge process, the CNT cathode delivers a discharge capacity of 5191 mA h g−1. It is noteworthy that the K0.5MnO2/CNT cathode achieves an increased discharge capacity of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408 mA h g−1, which is 2.58 times that of the CNT cathode. Upon the recharge process, the K0.5MnO2/CNT cathode delivers a charge capacity of 14
408 mA h g−1, which is 2.58 times that of the CNT cathode. Upon the recharge process, the K0.5MnO2/CNT cathode delivers a charge capacity of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267 mA h g−1, which is significantly higher than that of the CNT cathode (3926 mA h g−1). The charge capacity is larger than the discharge capacity of the K0.5MnO2/CNT cathode, which can be assigned to minor side reactions during the deep charge and discharge processes. On the basis of the above evidence, the K0.5MnO2/CNT nanoflowers as a catalyst play a significant role in enhancing the electrocatalytic activity of the cathode.
267 mA h g−1, which is significantly higher than that of the CNT cathode (3926 mA h g−1). The charge capacity is larger than the discharge capacity of the K0.5MnO2/CNT cathode, which can be assigned to minor side reactions during the deep charge and discharge processes. On the basis of the above evidence, the K0.5MnO2/CNT nanoflowers as a catalyst play a significant role in enhancing the electrocatalytic activity of the cathode.
Fig. 3b shows the first discharge–charge curves of K0.5MnO2/CNT and CNT cathodes at 100 mA g−1 with a capacity limited to 1000 mA h g−1. The first discharge and charge terminal voltages are 2.86 and 3.91 V, respectively. The corresponding overpotential is as low as 1.05 V, while the overpotential for the CNT electrode is up to 1.84 V, consistent with the CV results. The subsequent discharge/charge profiles at various cycles prove that the Li–CO2 battery with K0.5MnO2/CNT as the cathode can be steadily operated over 100 cycles (Fig. 3c). More importantly, the terminal discharge and charge voltages are maintained between 2.72 and 3.92 V with a minimal overpotential of 1.20 V (Fig. 3e). In contrast, the overpotential for the CNT cathode is up to 1.80 V with an inferior energy efficiency of about 60% and the battery can only run for 25 cycles (Fig. 3d and f).
It is worth mentioning that the average energy efficiency for the K0.5MnO2/CNT cathode is as high as 87.95%, which is higher than that of most reported Li–CO2 batteries (60–80%).39–43 These results demonstrate that K0.5MnO2/CNT as a cathode exhibits an incredible electrochemical performance with low overpotential, high energy efficiency and excellent cycling stability. To the best of our knowledge, the K0.5MnO2/CNT cathode delivers the best electrochemical performance among the non-previous catalysts for Li–CO2 batteries reported to date. Fig. 3g shows the charge/discharge curves of Li–CO2 batteries with the K0.5MnO2/CNT cathode at various current densities. The battery maintains stable discharge and charge plateaus without significant change from 0.1 to 1.0 A g−1, implying its superior rate performance. The overpotentials of the Li–CO2 battery based on the K0.5MnO2/CNT cathode are 1.10, 1.26, 1.30, 1.37, 1.44, and 1.47 V at current densities of 0.1, 0.2, 0.3, 0.5, 0.8, and 1.0 A g−1, respectively. It is noteworthy that the terminal discharge and charge voltages are stable at 2.64 and 4.11 V, respectively, even when the current density is up to 1.0 A g−1, implying its outstanding rate performance. Concurrently, the decomposition of electrolyte and other side reactions can be effectively avoided due to its low terminal charge voltage.44,45 It can be intuitively observed that the battery with the K0.5MnO2/CNT cathode displays much lower overpotentials than that with CNTs as the cathode at various current densities (Fig. 3h and S7†). The electrochemical impedance spectroscopy (EIS) tests were performed for K0.5MnO2/CNT and CNT cathodes after the first discharge and charge processes, as shown in Fig. S8.† It can be seen that the charge transfer resistance (Rct) value for K0.5MnO2/CNT after charge is smaller than that of the value after discharge, indicating that K0.5MnO2/CNT delivers a superior catalytic activity in promoting the decomposition of Li2CO3. Furthermore, the K0.5MnO2/CNT cathode also delivers evidently smaller Rct values than that of CNTs, suggesting that the synergistic effect between K0.5MnO2 and CNTs can expose ample catalytically active sites, shorten the electron/Li+ transport distances and facilitate Li2CO3 nucleation and decomposition.
In the development of Li–CO2 batteries, the short cycle life, large overpotential, poor rate performance and inferior energy efficiency are the most urgent and tricky troubles to be solved before commercial application in the future. However, it is also a great challenge to completely solve the above problems. Fortunately, the as-synthesized K0.5MnO2/CNT catalyst can substantially overcome those obstacles. For a more intuitive observation, we compare the electrochemical performance of the K0.5MnO2/CNT cathode with other current reports (Fig. 3i). Besides, the more systematic and detailed comparison is available in Table S2.† Attractively, the Li–CO2 battery with the K0.5MnO2/CNT cathode presents the overwhelming superiority in the voltage gap, energy efficiency and full discharge capacity. In consequence, K0.5MnO2/CNT is a promising candidate to convert CO2 into electrical energy using aprotic Li–CO2 batteries.
To further probe the electrochemical reaction process and mechanism upon the charge–discharge processes, ex situ measurements were performed, such as XPS, Raman and XRD. The deconvoluted C 1s spectra for the K0.5MnO2/CNT cathode at different stages are shown in Fig. 4a–c. Compared with the original cathode (Fig. 4a), the high-resolution C 1s XPS spectra of the fully discharged cathode exhibit a distinct peak at 289.3 eV related to the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in Li2CO3 (Fig. 4b). The peak at 291.2 eV for the pristine cathode is attributed to the C–F bond in the PVDF binder. After the discharge process, the peak disappears, as the nucleation of Li2CO3 covers the cathode surface, whereas, a relatively smaller peak at ∼291.0 eV reappears after the charge process. It is worth noting that the reversible generation and disappearance of Li2CO3 after discharge and charge processes indicate that the K0.5MnO2/CNT cathode possesses excellent catalytic reversibility and activity (Fig. 4b and c). These results are further verified by Raman spectroscopy, as shown in Fig. 4d. The Raman peak near 631 cm−1 corresponds to the Mn–O bond in the K-birnessite-type MnO2, indicating the stability of K0.5MnO2/CNT during the cycling process. Additionally, the two main peaks detected at 1346 and 1586 cm−1 are attributed to the D and G bands of the CNT, respectively. Importantly, the characteristic peak at 1087 cm−1 corresponding to Li2CO3 emerges after the discharge process, and it disappears after a recharge process. The ex situ XRD patterns of the K0.5MnO2/CNT electrode at different states were recorded to further reveal the electrochemical reaction process and mechanism (Fig. 4e). The intensity of three additional peaks at 21.2°, 30.5° and 31.6° is gradually enhanced in the discharge process, which are attributed to the (110), (
O bond in Li2CO3 (Fig. 4b). The peak at 291.2 eV for the pristine cathode is attributed to the C–F bond in the PVDF binder. After the discharge process, the peak disappears, as the nucleation of Li2CO3 covers the cathode surface, whereas, a relatively smaller peak at ∼291.0 eV reappears after the charge process. It is worth noting that the reversible generation and disappearance of Li2CO3 after discharge and charge processes indicate that the K0.5MnO2/CNT cathode possesses excellent catalytic reversibility and activity (Fig. 4b and c). These results are further verified by Raman spectroscopy, as shown in Fig. 4d. The Raman peak near 631 cm−1 corresponds to the Mn–O bond in the K-birnessite-type MnO2, indicating the stability of K0.5MnO2/CNT during the cycling process. Additionally, the two main peaks detected at 1346 and 1586 cm−1 are attributed to the D and G bands of the CNT, respectively. Importantly, the characteristic peak at 1087 cm−1 corresponding to Li2CO3 emerges after the discharge process, and it disappears after a recharge process. The ex situ XRD patterns of the K0.5MnO2/CNT electrode at different states were recorded to further reveal the electrochemical reaction process and mechanism (Fig. 4e). The intensity of three additional peaks at 21.2°, 30.5° and 31.6° is gradually enhanced in the discharge process, which are attributed to the (110), (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 02) and (002) peaks of Li2CO3 (JCPDS No. 09–0359). Subsequently, these peaks disappear after the charge process, indicating the complete decomposition of Li2CO3. These results demonstrate that K0.5MnO2/CNT as the cathode is a promising candidate to propel the reversible reduction and evolution of carbon dioxide.
02) and (002) peaks of Li2CO3 (JCPDS No. 09–0359). Subsequently, these peaks disappear after the charge process, indicating the complete decomposition of Li2CO3. These results demonstrate that K0.5MnO2/CNT as the cathode is a promising candidate to propel the reversible reduction and evolution of carbon dioxide.
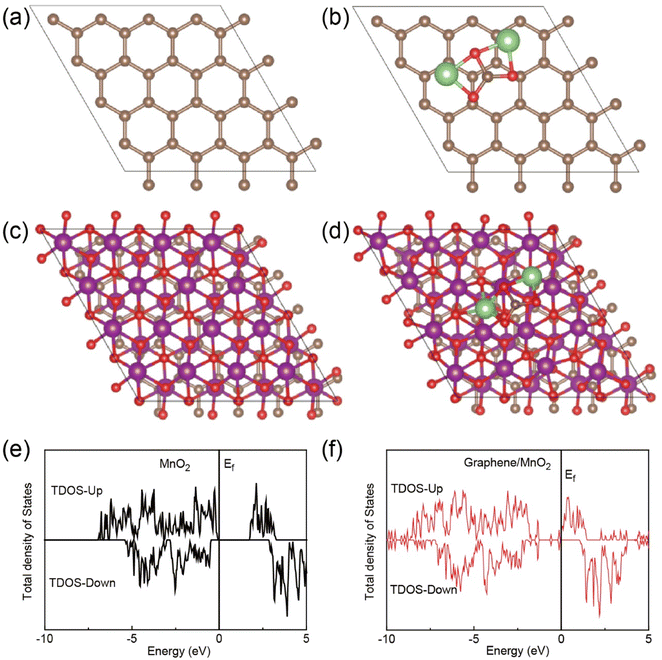
Density functional theory (DFT) calculations have been conducted to reveal the relationship between the structure and performance. The configurations of graphene and graphene/MnO2 heterostructures are shown in Fig. 5a and b, respectively. The capability of Li2CO3 adsorption was also evaluated. The binding energies with Li2CO3 are −0.15 eV for the graphene layer (Fig. 5c) and −2.65 eV for the heterostructure (Fig. 5d), indicating the strong interaction between Li2CO3 and the heterostructure. Moreover, pure MnO2 possesses a band gap, and the heterostructure demonstrates no band gap in reverse, indicating improved electrical conductivity according to the density of state results (Fig. 5e and f).
Conclusions
In summary, we successfully designed a K0.5MnO2/CNT composite as a cathode for Li–CO2 batteries. Benefiting from the excellent catalytic activity of K0.5MnO2/CNT, the as-assembled Li–CO2 battery achieves a low overpotential of 1.05 V at a current density of 100 mA g−1. Meanwhile, the K0.5MnO2/CNT cathode can be stably operated over 100 cycles in the voltage window of 2.72–3.92 V with a high average energy efficiency (87.95%). In addition, the K0.5MnO2/CNT cathode still displays a low overpotential of 1.47 V even though the current density is increased to 1.0 A g−1, indicating its superior rate performance for CO2 reduction and evolution reactions. This work affords an outstanding strategy for designing cheap and efficient catalysts, and the K0.5MnO2/CNT cathode promises to be a promising candidate for further commercial application in Li–CO2 batteries.Data availability
Data will be made available on request.Author contributions
Y. L., F.-J. L. and Y. Q. proposed the concept and supervised the work; J-W. W. and J. C. designed the experiments and wrote the paper; Z. H. and S-L. C. contributed to the discussion and provided suggestions. X-Y. C. helped to analyze the data. All authors have discussed the results, drafted the manuscript and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. Wu and J. Chen contributed equally to this work. This work was supported by the National Natural Science Foundation of China (grant no. U1904187), Natural Science Foundation of Henan for Excellent Young Scholars (no. 202300410223), and the Innovative Research Team of High-Level Local Universities in Shanghai.Notes and references
- Y. Yang, S. Louisia, S. Yu, J. Jin, I. Roh, C. Chen, M. V. Fonseca Guzman, J. Feijóo, P.-C. Chen, H. Wang, C. J. Pollock, X. Huang, Y.-T. Shao, C. Wang, D. A. Muller, H. D. Abruña and P. Yang, Nature, 2023, 614, 262–269 CrossRef CAS PubMed.
- M. T. Dunstan, F. Donat, A. H. Bork, C. P. Grey and C. R. Müller, Chem. Rev., 2021, 121, 12681–12745 CrossRef CAS PubMed.
- S. Ren, D. Joulié, D. Salvatore, K. Torbensen, M. Wang, M. Robert and C. P. Berlinguette, Science, 2019, 365, 367–369 CrossRef CAS PubMed.
- L. Fan, H. Shen, D. Ji, Y. Xing, L. Tao, Q. Sun and S. Guo, Adv. Mater., 2022, 34, e2204134 CrossRef PubMed.
- K. Zhang, J. Li, W. Zhai, C. Li, Z. Zhu, X. Kang, M. Liao, L. Ye, T. Kong, C. Wang, Y. Zhao, P. Chen, Y. Gao, B. Wang and H. Peng, Angew. Chem., Int. Ed., 2022, 61, 2201718 Search PubMed.
- B. Chen, D. Wang, J. Tan, Y. Liu, M. Jiao, B. Liu, N. Zhao, X. Zou, G. Zhou and H.-M. Cheng, J. Am. Chem. Soc., 2022, 144, 3106–3116 CrossRef CAS PubMed.
- X. Sun, X. Mu, W. Zheng, L. Wang, S. Yang, C. Sheng, H. Pan, W. Li, C.-H. Li, P. He and H. Zhou, Nat. Commun., 2023, 14, 536 CrossRef CAS PubMed.
- Z. Hu, Y. Xie, D. Yu, Q. Liu, L. Zhou, K. Zhang, P. Li, F. Hu, L. Li, S. Chou and S. Peng, ACS Nano, 2021, 15, 8407–8417 CrossRef CAS PubMed.
- F. Ye, L. Gong, Y. Long, S. N. Talapaneni, L. Zhang, Y. Xiao, D. Liu, C. Hu and L. Dai, Adv. Energy Mater., 2021, 11, 2101390 CrossRef CAS.
- J. Chen, X.-Y. Chen, Y. Liu, Y. Qiao, S.-Y. Guan, L. Li and S.-L. Chou, Energy Environ. Sci., 2023, 16, 792–829 RSC.
- J. Zou, G. Liang, F. Zhang, S. Zhang, K. Davey and Z. Guo, Adv. Mater., 2023, 35, 2210671 CrossRef CAS PubMed.
- Q. Deng, Y. Yang, S. Qu, W. Wang, Y. Zhang, X. Ma, W. Yan and Y. Zhang, Energy Storage Mater., 2021, 42, 484–492 CrossRef.
- F. Wang, Y. Li, X. Xia, W. Cai, Q. Chen and M. Chen, Adv. Energy Mater., 2021, 11, 2100667 CrossRef CAS.
- Z. Cheng, Y. Fang, Y. Yang, H. Zhang, Z. Fan, J. Zhang, S. Xiang, B. Chen and Z. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202311480 CrossRef CAS PubMed.
- J. Lin, J. Ding, H. Wang, X. Yang, X. Zheng, Z. Huang, W. Song, J. Ding, X. Han and W. Hu, Adv. Mater., 2022, 32, 2200559 CrossRef PubMed.
- Y. Xu, H. Gong, L. Song, Y. Kong, C. Jiang, H. Xue, P. Li, X. Huang, J. He and T. Wang, Mater. Today Energy, 2022, 1, 100967 CrossRef.
- C. Guo, F. Zhang, X. Han, L. Zhang, Q. Hou, L. Gong, J. Wang, Z. Xia, J. Hao and K. Xie, Adv. Mater., 2023, 35, 2302325 CrossRef CAS PubMed.
- H. Xue, H. Gong, X. Lu, B. Gao, T. Wang, J. He, Y. Yamauchi, T. Sasaki and R. Ma, Adv. Energy Mater., 2021, 11, 2101630 CrossRef CAS.
- Y. Xing, Y. Yang, D. Li, M. Luo, N. Chen, Y. Ye, J. Qian, L. Li, D. Yang, F. Wu, R. Chen and S. Guo, Adv. Mater., 2018, 30, 1803124 CrossRef PubMed.
- Y. Qiao, S. Xu, Y. Liu, J. Dai, H. Xie, Y. Yao, X. Mu, C. Chen, D. J. Kline, E. M. Hitz, B. Liu, J. Song, P. He, M. R. Zachariah and L. Hu, Energy Environ. Sci., 2019, 12, 1100–1107 RSC.
- Y. Ding, Y. Li and Z.-S. Wu, Battery Energy, 2023, 2, 20220014 CrossRef CAS.
- S. Li, Y. Dong, J. Zhou, Y. Liu, J. Wang, X. Gao, Y. Han, P. Qi and B. Wang, Energy Environ. Sci., 2018, 11, 1318–1325 RSC.
- B. Ge, Y. Sun, J. Guo, X. Yan, C. Fernandez and Q. Peng, Small, 2019, 15, 1902220 CrossRef PubMed.
- Y. Mao, C. Tang, Z. Tang, J. Xie, Z. Chen, J. Tu, G. Cao and X. Zhao, Energy Storage Mater., 2019, 18, 405–413 CrossRef.
- Q. Liu, Z. Hu, L. Li, W. Li, C. Zou, H. Jin, S. Wang and S.-L. Chou, ACS Appl. Mater. Interfaces, 2021, 13, 16585–16593 CrossRef CAS PubMed.
- X. Chen, J. Chen, Y. Qiao, Y. Gao, S. Fan, Y. Liu, L. Li, Y. Liu and S. Chou, Chem. Sci., 2024, 15, 2473–2479 RSC.
- X. Chen, J. Chen, Y. Liu, Y. Liu, Y. Gao, S. Fan, X. He, X. Liu, C. Shen, Y. Jiang, L. Li, Y. Qiao and S. Chou, ACS Appl. Mater. Interfaces, 2023, 15, 28106–28115 CrossRef CAS PubMed.
- V. B. R. Boppana, S. Yusuf, G. S. Hutchings and F. Jiao, Adv. Funct. Mater., 2013, 23, 878–884 CrossRef CAS.
- W. Lv, J. Meng, Y. Li, W. Yang, Y. Tian, X. Lyu, C. Duan, X. Ma and Y. Wu, Nano Energy, 2022, 98, 107274 CrossRef CAS.
- Y. Liu, W. Zong, H. Zhou, D. Wang, R. Cao, J. Zhan, L. Liu and B. W.-L. Jang, Catal. Sci. Technol., 2018, 8, 5344–5358 RSC.
- J. Liu, Y. Zhang, L. Zhang, F. Xie, A. Vasileff and S.-Z. Qiao, Adv. Mater., 2019, 31, 1901261 CrossRef PubMed.
- X. Li, J. Zhang, G. Qi, J. Cheng and B. Wang, Energy Storage Mater., 2021, 35, 148–156 CrossRef.
- K. Lv, D. Wan, R. Pan, W. Suo and Y. Zhu, Carbon Neutralization, 2022, 1, 189–197 CrossRef.
- Y. Liu, Y. Qiao, X. Lou, X. Zhang, W. Zhang and Y. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 14564–14571 CrossRef CAS PubMed.
- D. Banerjee and H. W. Nesbitt, Geochim. Cosmochim. Acta, 1999, 63, 3025–3038 CrossRef CAS.
- Y. Liu, Y. Qiao, W. Zhang, H. Wang, K. Chen, H. Zhu, Z. Li and Y. Huang, J. Mater. Chem. A, 2015, 3, 7780–7785 RSC.
- Z. Zhuo, K. Dai, R. Qiao, R. Wang, J. Wu, Y. Liu, J. Peng, L. Chen, Y.-d. Chuang, F. Pan, Z.-x. Shen, G. Liu, H. Li, T. P. Devereaux and W. Yang, Joule, 2021, 5, 975–997 CrossRef CAS.
- M. Chigane and M. Ishikawa, J. Electrochem. Soc., 2000, 147, 2246 CrossRef CAS.
- J. Chen, K. Zou, P. Ding, J. Deng, C. Zha, Y. Hu, X. Zhao, J. Wu, J. Fan and Y. Li, Adv. Mater., 2019, 31, e1805484 CrossRef PubMed.
- P. Tan, Z. H. Wei, W. Shyy, T. S. Zhao and X. B. Zhu, Energy Environ. Sci., 2016, 9, 1783–1793 RSC.
- S. Yang, P. He and H. Zhou, Energy Environ. Sci., 2016, 9, 1650–1654 RSC.
- J. Zhou, X. Li, C. Yang, Y. Li, K. Guo, J. Cheng, D. Yuan, C. Song, J. Lu and B. Wang, Adv. Mater., 2019, 31, 1804439 CrossRef PubMed.
- Y. Li, J. Zhou, T. Zhang, T. Wang, X. Li, Y. Jia, J. Cheng, Q. Guan, E. Liu, H. Peng and B. Wang, Adv. Funct. Mater., 2019, 29, 1808117 CrossRef.
- T. Jian, W. Ma, C. Xu, H. Liu and J. Wang, eScience, 2023, 3, 100114 CrossRef.
- Z. Wang, B. Liu, X. Yang, C. Zhao, P. Dong, X. Li, Y. Zhang, K. Doyle-Davis, X. Zeng, Y. Zhang and X. Sun, Adv. Funct. Mater., 2023, 33, 2213931 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc01799d |
| ‡ J. Wu and. J. Chen contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |