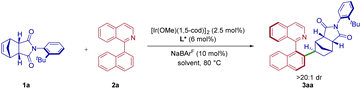Open Access Article
Open Access ArticleAccess to distal biaxial atropisomers by iridium catalyzed asymmetric C–H alkylation†
Xueqing
Hu‡
a,
Yunxu
Zhao‡
a,
Tong
He
b,
Caoyue
Niu
a,
Feipeng
Liu
a,
Wei
Jia
a,
Yi
Mu
a,
Xingwei
Li
 *b and
Zi-Qiang
Rong
*b and
Zi-Qiang
Rong
 *a
*a
aFrontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE), Northwestern Polytechnical University (NPU), Xi'an 710072, China. E-mail: iamzqrong@nwpu.edu.cn
bSchool of Chemistry and Chemical Engineering, Shaanxi Normal University (SNNU), Xi'an 710119, China. E-mail: lixw@snnu.edu.cn
First published on 22nd July 2024
Abstract
Distal biaxial atropisomers are typical structures in chiral catalysts and ligands and offer a wide variety of applications in biology and materials technology, but the development of efficient synthesis of these valuable scaffolds is still in great demand. Herein, we describe a highly efficient iridium catalyzed asymmetric C–H alkylation reaction that provides a range of new distal biaxial atropisomers with excellent yields (up to 99%) and stereoselectivity (up to 99% ee and essentially one isomer). Based on this unprecedented strategy, a polycyclic skeleton with five successive chiral centers as well as C–C and C–N (or N–N) two distal chiral axes was created successfully in mild circumstances. In addition, the optically pure products bearing fluorophores show circular polarized luminescence (CPL) properties, being potential candidate materials for CPL applications.
Introduction
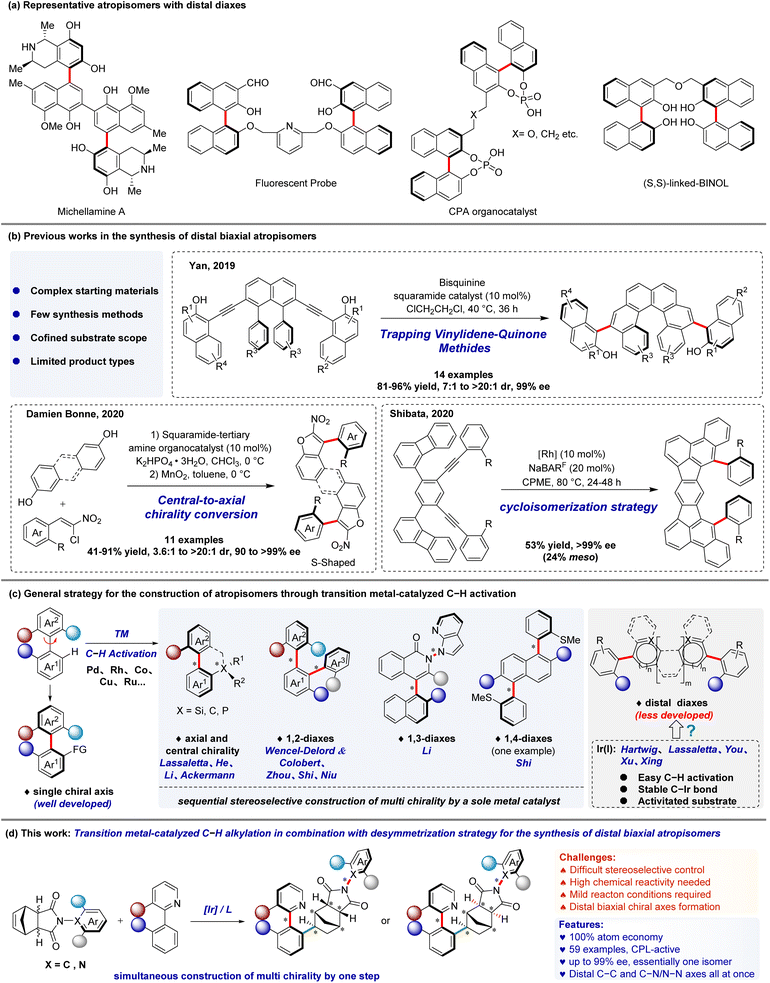
Atropisomers are common structural motifs in natural products,1 chiral catalysts,2 and functional materials.3 Due to their significant importance, a lot of investigations have been conducted on the enantioselective synthesis of these compounds, especially the biaryls, which are the most representative atropisomers with a single chiral axis.4 But it is noteworthy that atropisomers containing chiral diaxes, particularly when the position of chiral axes is at quite a distance,5 also demonstrate unique features and applications in the disciplines of drug development,6 materials research,7 catalysis8 and ligand design9 (Scheme 1a). Nevertheless, due to the spatial interaction of various chiral axes, distal biaxial atropisomers have more challenges in diastereoselective and enantioselective control. Up to now, only a few examples of the efficient asymmetric construction of distal biaxial atropisomers have been reported. In 2019, Yan and co-workers have demonstrated a highly diastereo- and enantio-selective organocatalytic strategy for the synthesis of molecules featuring helicenes and two distal stereogenic axes10via the corresponding VQM intermediate (Scheme 1b).11 In 2020, Bonne and co-workers reported a bidirectional enantioselective synthesis of bis-benzofuran atropisomeric oligoarenes containing two distal C–C stereogenic axes, which were obtained by a two-fold central-to-axial chirality conversion upon oxidative aromatization (Scheme 1b).12 In the same year, Shibata and co-workers reported a cycloisomerization strategy closely related to (2 + 2 + 2) cycloaddition for the enantioselective synthesis of unichiral and distal biaxial chiral polycyclic hydrocarbons (PAHs) (Scheme 1b).13 Despite the effectiveness of the reported reaction modes, there are still plenty of challenges to overcome in the development of new methods for the synthesis of distal-axes chiral molecules.In recent decades, transition metal-catalyzed C–H activation reactions involving the (dynamic) kinetic resolution or desymmetrization process for the construction of atropisomers have attracted great prominence as a potent tactic.14 Compared with traditional coupling reactions, this approach exhibits good atom economy, high synthetic efficiency, and significantly widens the range of substrates. The utilization of C–H activation for the synthesis of uniaxial chiral compounds has so far produced impressive results,15 but the synthesis of multiaxial chirality still presents a significant difficulty, and little progress has been made until recently. Lassaletta,16 He,17 Ackermann18 and our group19 successfully accomplished the molecules with excellent axial and central chirality; Wencel-Delord and Colobert,20 Zhou,21 Niu22 and Shi23 respectively succeeded in the formation of enantiopure vicinal 1,2-diaxes compounds; and most recently, our group24 reported highly atroposelective construction of 1,3-diaxes compounds via C–H activation; in addition, the synthesis of chiral 1,4-diaxes compound utilizing two fold C–H activation strategy was reported by Shi and co-workers25 (Scheme 1c). These works mainly focus on transition metals such as Pd, Rh, Co, Cu, Ru, etc., using sequential control to complete the construction of multiple chiral axes or chiral centers. However, this approach makes it more challenging to simultaneously control the enantioselectivity and diastereoselectivity of the products, particularly when the multiple chiral axes are distally distributed. Consequently, there is an urgent need to find solutions to these problems.
Recently, as a potent tool for the activation of C–H bonds,26 iridium(I) complexes have garnered a lot of interest in the realm of the enantioselective synthesis of atropisomers. The synthesis of axially chiral scaffolds is made possible by the reaction of the stable C–Ir bond with activated substrate, which guarantees a smooth reaction and leads to a more effective response. Notably, some elegant works have been reported by Hartwig,27 Lassaletta,16,28 Xu,29 You,30 and Xing.31 Despite the significant progress in this research area, however, these reports focus on the construction of atropisomers featuring one stereogenic axis, atroposelective synthesis of distal biaxial atropisomers via C–H activation remains underexplored. This probably due to (1) the great difficulty in assembling multiple distal stereogenic axes with a high degree of enantioselective control and (2) the high steric requirements of the substrates needed to stop the rotation of multiple distal stereogenic axes reducing their chemical reactivity. Recently, our interest was drawn to the ability of N-aryl maleimide derivative as a Diels–Alder adduct from cyclopentadiene and maleimide with a prochiral C–N axis,32 which can desymmetrically build a C–N axis chiral scaffold. Inspired by this, we wondered if a new distal biaxial chiral molecule with both C–C and C–N (or N–N) axes could be generated with a N-aryl maleimide derivative and heterobiaryl as precursor materials using an iridium(I) catalyzed C–H activation in combination with desymmetrization strategy (Scheme 1d). Herein, we describe the details of this study towards distal biaxial chiral compounds by iridium catalyzed asymmetric C–H alkylation.
Results and discussion
Reaction optimization
To explore the proposed construction of new distal biaxial atropisomers, preliminary studies were carried out using N-aryl 5-norbornene-endo-cis-2,3-dicarboximides 1a and naphthylisoquinoline 2a as the model substrates. The reaction parameters, including catalyst, ligand, solvent, and temperature, were carefully optimized (see the ESI† for more details). First, ligands L1–L6, which were previously used in the asymmetric C–H functionalization reactions, were investigated, and proven to be a significant influence on this transformation (Table 1, entries 1–6). Although none of the screening ligands gave satisfactory results, the attempt of L2 gave 3aa with excellent enantioselectivity albeit with low yield (Table 1, entry 2). To further improve the yield, we evaluated the influence of other critical reaction parameters, such as the solvent (Table 1, entries 7–13). We found that toluene was the best choice for this iridium catalyzed asymmetric C–H alkylation (Table 1, entry 11). After considerable optimization (Table 1), a combination of [Ir(OMe)(1,5-cod)]2 (2.5 mol%), L2 (6 mol%) and 10 mol% NaBArF (ArF = 3,5-CF3-C6H3) in toluene at 80 °C for 24 h afforded the best results, giving rise to distal biaxial atropisomer 3aa in quantitative yield with 97% ee and high diastereoselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
| Entry | Ligand | Solvent | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Reaction condition: 1a (0.05 mmol), 2a (0.06 mmol), [Ir(OMe)(1,5-cod)]2 (2.5 mol%), L (10 mol%), NaBArF (10 mol%) in solvent (1.0 mL) at 80 °C under N2 for 24 h. b Yield was detected by 1H NMR using 1,1,2,2-tetrachloroethane as the internal standard. c Determined by chiral high performance liquid chromatography (HPLC) analysis. DCE: 1,2-dichloroethane. MTBE: methyl tert-butyl ether. | ||||
| 1 | L1 | 1,4-Dioxane | 15 | 89 |
| 2 | L2 | 1,4-Dioxane | 11 | 96 |
| 3 | L3 | 1,4-Dioxane | 9 | 73 |
| 4 | L4 | 1,4-Dioxane | >99 | 36 |
| 5 | L5 | 1,4-Dioxane | 71 | 18 |
| 6 | L6 | 1,4-Dioxane | 26 | 22 |
| 7 | L2 | Xylene | 98 | 97 |
| 8 | L2 | DCE | 76 | 96 |
| 9 | L2 | MTBE | >99 | 96 |
| 10 | L2 | DCM | 72 | 95 |
| 11 | L2 | Toluene | >99 | 97 |
| 12 | L2 | THF | 37 | 95 |
| 13 | L2 | Ph–F | >99 | 96 |
|
||||
Reaction scope
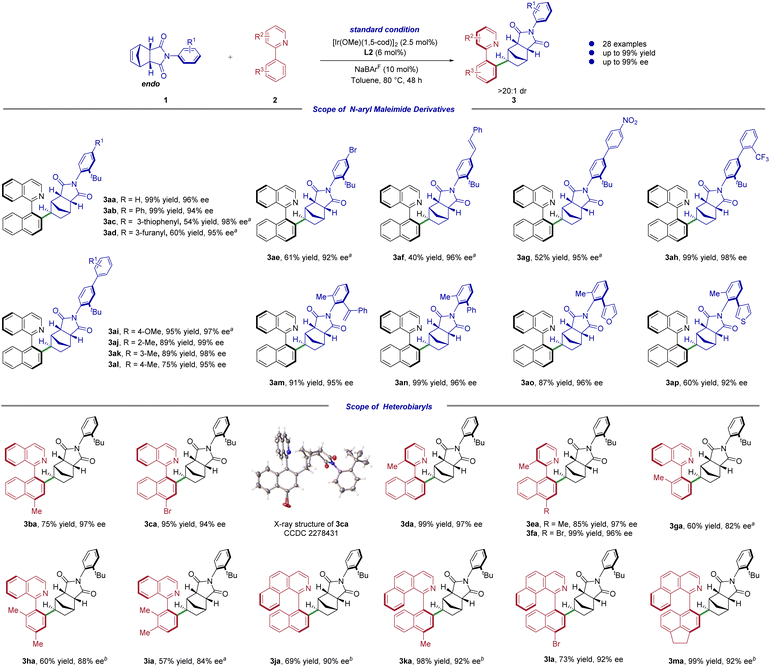
With the optimal conditions in hand, we subsequently examined the iridium catalyzed asymmetric C–H alkylation reactions of a series of N-aryl maleimide derivatives 1 with various heterobiaryls 2 by using the desymmetrization strategy (Scheme 2). To our delight, the reaction took place without a hitch under the usual reaction circumstances, though it took a little longer time to react. A range of substituents on the phenyl ring of N-aryl maleimide derivatives were well tolerated, resulting in the desired products in excellent yields and enantioselectivities. No matter whether the benzene ring is charged with an electron-donating group or an electron-withdrawing group, the product could be obtained in good reactivity with high enantioselectivity (Scheme 2, 3aa–3al). However, when the substituents are functional groups like furan, thiophene, bromo, alkenyl, and nitro, the yields of the products are significantly decreased and the reactions need to be heated up to 110 °C to produce moderate yields (Scheme 2, 3ac–3ag, 40–61% yields, 92–98% ee). Notably, when the strong electron-withdrawing trifluoromethyl group is attached to the benzene ring, high yield and ee value can be obtained (Scheme 2, 3ah, 99% yield, 98% ee). To evaluate the remote control of distal axial chirality, the substrates scope of bulky ortho-substituents on the N-aryl groups was also tested. Delightfully, when the N-aryl ortho-substituents were replaced from tert-butyl to other bulky site blocking groups like phenyl, heteroaryl and α-phenylethenyl, the reactions still proceeded with moderate to good yields and excellent enantioselectivities (Scheme 2, 3am–3ap, 60–99% yields, 92–96% ee).We next investigated the scope of the heterobiaryl substrates. As shown in Scheme 2, an array of heterobiaryls was well tolerated, and the desired distal biaxial chiral products 3ba–3ma were isolated in good yields (up to 99%) with excellent enantioselective control (up to 97% ee). However, substrates with a methyl group on the ortho position of phenyl ring showed relatively low reaction efficiency (3ga–3ia). Products 3ga–3ia were obtained in only moderate yields even at higher temperature or longer reaction time. Satisfactorily, this procedure could successfully be extended to aryl benzo[h]isoquinolines15c,33 with methyl, bromo group on the naphthalene ring, delivering the corresponding products 3ja–3ma in moderate to excellent yields and enantioselectivities (69–99% yields, 90–92% ee). The relative and absolute configuration of 3ca was determined by single crystal X-ray analysis, and those of other products were assigned by analogy.
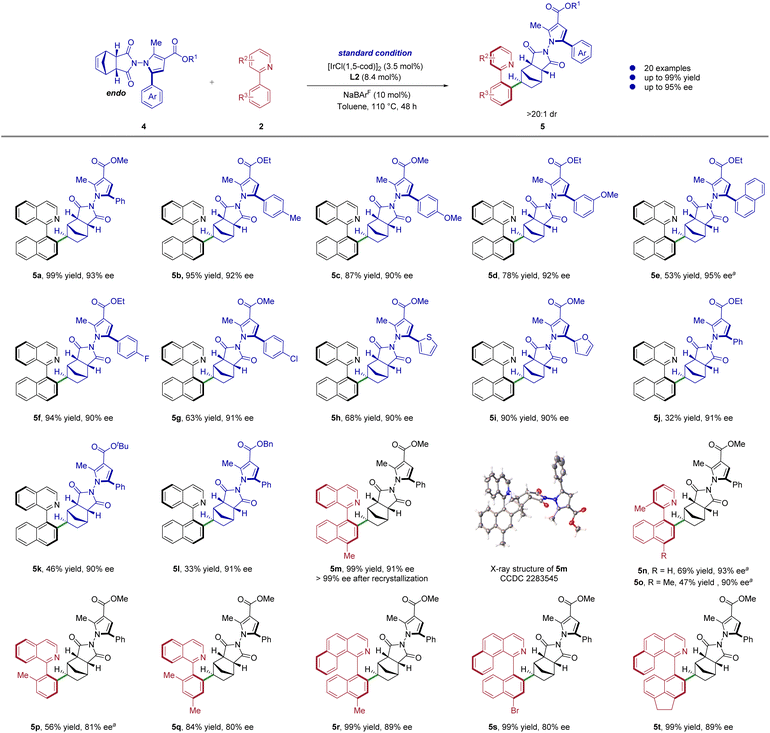
Following that, the formation of C–C and N–N atropisomers was investigated to show the wide application of this asymmetric catalytic system even more (Scheme 3). Prochiral substrates with a N–N axis have been effectively synthesized based on related studies.34 To our delight, under the optimum reaction conditions, the N–N prochiral pyrrole substrates were applicable with this reaction, affording the corresponding products with adequate efficiency and great enantioselectivity (5a–5t). The C5-substituents on the pyrrole ring were first examined. Pleasingly, the reactions of various aryl-substituted pyrroles proceeded smoothly to give the desired products in moderate to excellent yields and enantioselectivities (5a–5g, 53–99% yields, 90–95% ee). The substrate with heterocycles on the pyrrole fragment can also adapt to this reaction system (5h–5i, 68–90% yields, 90% ee). Moreover, the substrates bearing an ethyl, tert-butyl, benzyl substituted ester group at the 3-position of pyrrole were well compatible in this reaction, although there has been a decrease in reactivity (5j–5l, 32–46% yields, 90–91% ee). In addition, the N–N prochiral pyrrole substrates can also react with different types of heterobiaryls to deliver the expected products in moderate to excellent yields and enantioselectivities (5m–5t, 47–99% yields, 80–93% ee), greatly expanding the range of substrates. The absolute configuration of product 5m was determined by single-crystal X-ray diffraction analysis, and those of other products were assigned by analogy.
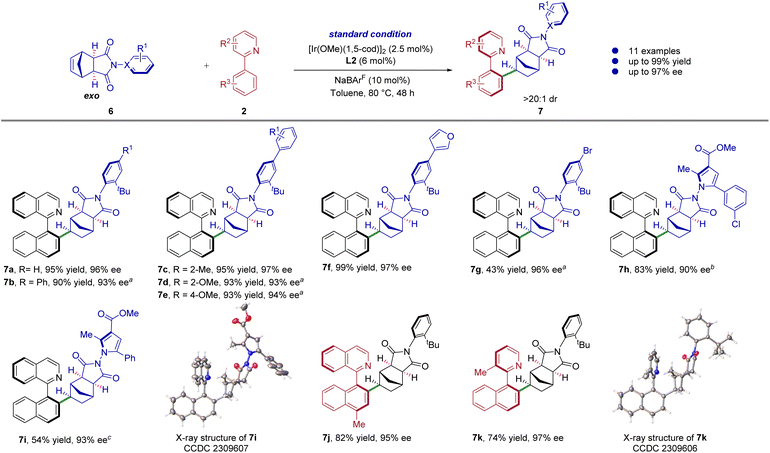
Furthermore, we tested the reactivity of the more challenging exo substrate in this scenario. Compared with endo substrates, exo substrates formed polychiral control centers that were more distant and difficult to react during the catalytic reaction, which greatly affected the yields and enantioselectivities of the products. Remarkably, the matching compounds were successfully obtained from the attempts conducted under regular conditions (Scheme 4). Various C–N axes derivatives of N-aryl maleimide successfully reacted with heterobiaryls 2, accommodating a range of electron-donating and electron-withdrawing groups, resulting in good enantioselectivities and yields (7a–7g, 43–99% yields, 93–97% ee). Surprisingly, the exo pre-chiral substrates possessing the N–N axis, were also compatible with the reaction, as demonstrated by two representative examples (7h–7i, 54–83% yields, 90–93% ee). Furthermore, the study revealed that different types of heterodiaryl derivatives could react with the exo substrates (7j–7k, 74–82% yields, 95–97% ee). The absolute configuration of products 7i and 7k was determined through single-crystal X-ray diffraction analysis, while the configurations of other products were assigned by analogy.
Gram scale reaction and synthetic transformations
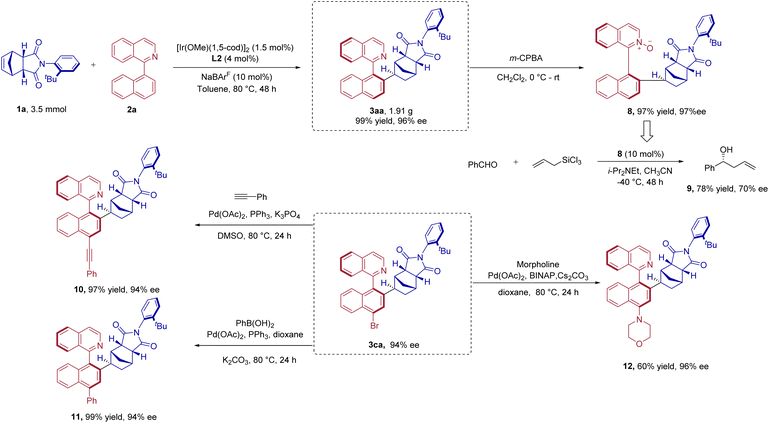
In order to evaluate the scalability of the current method, a gram scale reaction of 1a with 2a was conducted. To our delight, with the catalyst loading reduced to 1.5 mol% based on the ligand used, the desired product 3aa was isolated in 99% yield (1.91 g) and 96% ee (Scheme 5). Several transformations were then carried out to demonstrate the synthetic utility of the products obtained above. First, 3aa could be efficiently oxidized to N-oxide 8 in 97% yield and 97% ee, without losing its enantioselectivity (Scheme 5). Additionally, it was discovered that chiral N-oxide 8 was a potential catalyst for the asymmetric allylation of aromatic aldehyde with allyltrichlorosilane, and product 9 was produced in 78% yield with 70% ee (Scheme 5).35 Second, 3ca was converted into a variety of functionalized derivatives 10, 11, and 12 through several easy-to-run coupling reactions (Scheme 5).Photophysical properties of products
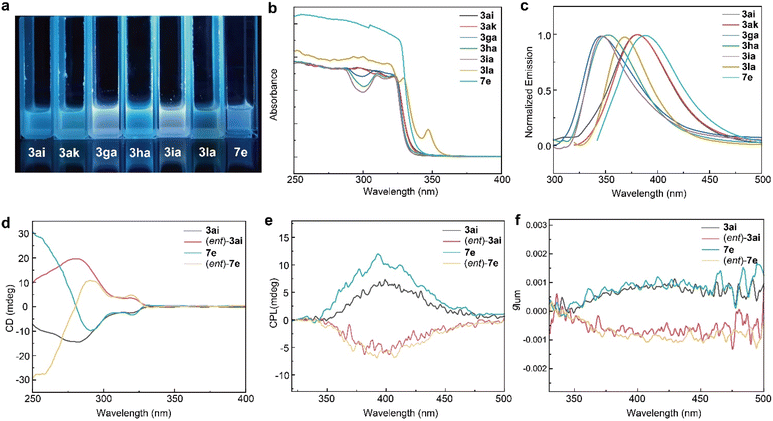
We further investigated the photophysical properties of several selected products to illustrate their perspectives as chiral functional materials. Under UV light irradiation (365 nm), all solutions revealed luminescence phenomena (Fig. 1a). First, the UV/vis spectra of 3ai, 3ak, 3ga, 3ha, 3ia, 3la and 7e in CH2Cl2 (10−3 M) were obtained. All absorption spectra exhibited structured absorption bands, and their absorption maxima were observed at similar wavelengths (308–327 nm, Fig. 1b). Next, the electronic emission spectra (Fig. 1c) of these compounds in CH2Cl2 (10−3 M) were acquired. These compounds display similar fluorescent spectra (344–388 nm) when excited. A remarkable red-shift was observed in 3ai, 3ak, 3la and 7e for both the absorption and emission maxima, maybe due to the extended π-conjugated system. Then, the chiroptical properties of the distal biaxial atropisomers 3ai, 7e and their enantiomers were examined using circular dichroism (CD) and circular polarized luminescence (CPL) spectroscopies. The CD spectra of 7e and (ent)-7e was a mirror image and displayed clear Cotton effects at around 290 nm and 319 nm (Fig. 1d). To our delight, 3ai, 7e and their enantiomers in CH2Cl2 solution are CPL-active, displaying clear mirror images at 400 nm (Fig. 1e). The luminescence dissymmetry factor (glum) was 1.1 × 10−3 for 7e and −1.0 × 10−3 for (ent)-7e respectively, measured at emission maxima (Fig. 1f).Conclusions
In conclusion, we developed an efficient and direct Ir(I)-catalyzed asymmetric C–H alkylation procedure to synthesize a unique distal biaxial chiral molecule with high yield and excellent enantioselectivity through the desymmetrization strategy. The various transformations of the products greatly expanded the diversity of the related distal biaxial atropisomers.According to tests, the resulting series of compounds have good optical properties to be employed as potential chiral organic light-emitting materials in the future. This method also provides a unique approach for creating brand-new distal multi-axial atropisomers.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its ESI†].Author contributions
X. Hu and Y. Zhao developed and conducted the reactions. T. He and W. Jia guided the characterization of photophysical properties. C. Niu, F. Liu and Y. Mu prepared some starting materials. X. Li and Z.-Q. Rong directed the project and wrote the manuscript with input from all authors. All authors analysed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the generous financial support from the National Natural Science Foundation of China (21801206), Shaanxi Fundamental Science Research Project for Chemistry & Biology (22JHQ002), the Program for Young Talents of Shaanxi Province (5113190023) and Natural Science Basic Research Program of Shaanxi (2024JC-ZDXM-08).Notes and references
- (a) J. Clayden, W. J. Moran, P. J. Edwards and S. R. LaPlante, Angew. Chem., Int. Ed., 2009, 48, 6398 CrossRef CAS PubMed; (b) G. Bringmann, T. Gulder, T. A. M. Gulder and M. Breuning, Chem. Rev., 2011, 111, 563 CrossRef CAS PubMed; (c) J. E. Smyth, N. M. Butler and P. A. Keller, Nat. Prod. Rep., 2015, 32, 1562 RSC; (d) B. K. Lombe, D. Feineis and G. Bringmann, Nat. Prod. Rep., 2019, 36, 1513 RSC.
- (a) R. Noyori and H. Takaya, Acc. Chem. Res., 1990, 23, 345 CrossRef CAS; (b) Y. Chen, S. Yekta and A. K. Yudin, Chem. Rev., 2003, 103, 3155 CrossRef CAS PubMed; (c) M. Terada, Chem. Commun., 2008, 35, 4097 RSC; (d) J. F. Teichert and B. L. Feringa, Angew. Chem., Int. Ed., 2010, 49, 2486 CrossRef CAS PubMed; (e) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047 CrossRef CAS PubMed; (f) T. Akiyama and K. Mori, Chem. Rev., 2015, 115, 9277 CrossRef CAS PubMed; (g) W. Fu and W. Tang, ACS Catal., 2016, 6, 4814 CrossRef CAS; (h) Q. Yue, B. Liu, G. Liao and B.-F. Shi, ACS Catal., 2022, 12, 9359 CrossRef CAS.
- (a) L. Pu, Acc. Chem. Res., 2012, 45, 150 CrossRef CAS PubMed; (b) D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331 RSC.
- (a) G. Bringmann, A. J. P. Mortimer, P. A. Keller, M. J. Gresser, J. Garner and M. Breuning, Angew. Chem., Int. Ed., 2005, 44, 5384 CrossRef CAS PubMed; (b) O. Baudoin, Eur. J. Org Chem., 2005, 2005, 4223 CrossRef; (c) G. Bringmann, T. Gulder, T. A. M. Gulder and M. Breuning, Chem. Rev., 2011, 111, 563 CrossRef CAS PubMed; (d) J. Wencel-Delord, A. Panossian, F. R. Leroux and F. Colobert, Chem. Soc. Rev., 2015, 44, 3418 RSC; (e) E. Kumarasamy, R. Raghunathan, M. P. Sibi and J. Sivaguru, Chem. Rev., 2015, 115, 11239 CrossRef CAS PubMed; (f) Y. N. Ma, S. X. Li and S. D. Yang, Acc. Chem. Res., 2017, 50, 1480 CrossRef CAS PubMed; (g) C. G. Newton, S.-G. Wang, C. C. Oliveira and N. Cramer, Chem. Rev., 2017, 117, 8908 CrossRef CAS PubMed; (h) B. Zilate, A. Castrogiovanni and C. Sparr, ACS Catal., 2018, 8, 2981 CrossRef CAS; (i) G. Liao, T. Zhou, Q.-J. Yao and B.-F. Shi, Chem. Commun., 2019, 55, 8514 RSC; (j) A. J. Metrano and S. J. Miller, Acc. Chem. Res., 2019, 52, 199 CrossRef CAS PubMed; (k) G. Liao, T. Zhang, Z.-K. Lin and B.-F. Shi, Angew. Chem., Int. Ed., 2020, 59, 19773 CrossRef CAS PubMed; (l) T.-Z. Li, S.-J. Liu, W. Tan and F. Shi, Chem.–Eur. J., 2020, 26, 15779 CrossRef CAS PubMed; (m) J. K. Cheng, S.-H. Xiang, S. Li, L. Ye and B. Tan, Chem. Rev., 2021, 121, 4805 CrossRef CAS PubMed; (n) Y.-J. Wu, G. Liao and B.-F. Shi, Green Synth. Catal., 2022, 3, 117 CrossRef; (o) G.-J. Mei, W. L. Koay, C.-Y. Guan and Y. Lu, Chem, 2022, 8, 1855 CrossRef CAS; (p) X.-F. Bai, Y.-M. Cui, J. Cao and L.-W. Xu, Acc. Chem. Res., 2022, 55, 2545 CrossRef CAS PubMed; (q) J. K. Cheng, S.-H. Xiang and B. Tan, Acc. Chem. Res., 2022, 55, 2920 CrossRef CAS PubMed.
- (a) X. Bao, J. Rodriguez and D. Bonne, Angew. Chem., Int. Ed., 2020, 59, 12623 CrossRef CAS PubMed; (b) H.-H. Zhang, T.-Z. Li, S.-J. Liu and F. Shi, Angew. Chem., Int. Ed., 2023, e202311053 Search PubMed.
- (a) B. K. Lombe, D. Feineis and G. Bringmann, Nat. Prod. Rep., 2019, 36, 1513 RSC; (b) S. Fayez, J. Li, D. Feineis, L. A. Assi, M. Kaiser, R. Brun, M. A. Anany, H. Wajant and G. Bringmann, J. Nat. Prod., 2019, 82, 3033 CrossRef CAS PubMed.
- (a) Y. L. Wu, F. Ferroni, S. Pieraccini, W. B. Schweizer, B. B. Frank, G. P. Spada and F. Diederich, Org. Biomol. Chem., 2012, 10, 8016 RSC; (b) Y. Y. Zhu, X. D. Wu, S.-X. Gu and L. Pu, J. Am. Chem. Soc., 2019, 141, 175 CrossRef CAS PubMed; (c) K. Takaishi, K. Iwachido, R. Takehana, M. Uchiyama and T. Ema, J. Am. Chem. Soc., 2019, 141, 6185 CrossRef CAS PubMed; (d) D.-W. Zhang, M. Li and C.-F. Chen, Chem. Soc. Rev., 2020, 49, 1331 RSC.
- R. Mitra and J. Niemeyer, ChemCatChem, 2018, 10, 1221 CrossRef CAS.
- M. Shibasaki and S. Matsunaga, Chem. Soc. Rev., 2006, 35, 269 RSC.
- S. Jia, S. Li, Y. Liu, W. Qin and H. Yan, Angew. Chem., Int. Ed., 2019, 58, 18496 CrossRef CAS PubMed.
- W. Qin, Y. Liu and H. Yan, Acc. Chem. Res., 2022, 55, 2780 CrossRef CAS PubMed.
- X. Bao, J. Rodriguez and D. Bonne, Chem. Sci., 2020, 11, 403 RSC.
- H. Takano, N. Shiozawa, Y. Imai, K. S. Kanyiva and T. Shibata, J. Am. Chem. Soc., 2020, 142, 4714 CrossRef CAS PubMed.
- (a) T. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu and J.-Q. Yu, Science, 2018, 359, eaao4798 CrossRef PubMed; (b) T. K. Achar, S. Maiti, S. Jana and D. Maiti, ACS Catal., 2020, 10, 13748 CrossRef CAS; (c) C.-X. Liu, W.-W. Zhang, S.-Y. Yin, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2021, 143, 14025 CrossRef CAS PubMed; (d) X. Yu, Z.-Z. Zhang, J.-L. Niu and B.-F. Shi, Org. Chem. Front., 2022, 9, 1458 RSC; (e) M. Li and J. Wang, Synthesis, 2022, 54, 4734 CrossRef CAS; (f) S. Choppin and J. Wencel-Delord, Acc. Chem. Res., 2023, 56, 189 CrossRef CAS PubMed; (g) R. Giri, B.-F. Shi, K. M. Engle, N. Maugel and J.-Q. Yu, Chem. Soc. Rev., 2009, 38, 3242 RSC; (h) G. Liao, T. Zhou, Q.-J. Yao and B.-F. Shi, Chem. Commun., 2019, 55, 8514 RSC.
- (a) C. He, M. Hou, Z. Zhu and Z. Gu, ACS Catal., 2017, 7, 5316 CrossRef CAS; (b) H. Li, X. Yan, J. Zhang, W. Guo, J. Jiang and J. Wang, Angew. Chem., Int. Ed., 2019, 58, 6732 CrossRef CAS PubMed; (c) Q. Wang, Z.-J. Cai, C.-X. Liu, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2019, 141, 9504 CrossRef CAS PubMed; (d) L. Jin, Q.-J. Yao, P.-P. Xie, Y. Li, B.-B. Zhan, Y.-Q. Han, X. Hong and B.-F. Shi, Chem, 2020, 6, 497 CrossRef CAS; (e) Z. Li, X. Wang, Y.-M. Cui, J.-H. Ma, L.-L. Fang, L.-L. Han, Q. Yang, Z. Xu and L.-W. Xu, Chem.–Eur. J., 2021, 27, 4336 CrossRef CAS PubMed; (f) Q. Xu, H. Zhang, F.-B. Ge, X.-M. Wang, P. Zhang, C.-J. Lu and R.-R. Liu, Org. Lett., 2022, 24, 3138 CrossRef CAS PubMed; (g) J. Zhang, J. Fan, Y. Wu, Z. Guo, J. Wu and M. Xie, Org. Lett., 2022, 24, 5143 CrossRef CAS PubMed; (h) P. Hu, B. Liu, F. Wang, R. Mi, X.-X. Li and X. Li, ACS Catal., 2022, 12, 13884 CrossRef CAS; (i) T. Uchikura, S. Kato, Y. Makino, M. J. Fujikawa, M. Yamanaka and T. Akiyama, J. Am. Chem. Soc., 2023, 145, 15906 CrossRef CAS PubMed; (j) L.-C. Xu, J. Frey, X. Hou, S.-Q. Zhang, Y.-Y. Li, J. C. A. Oliveira, S.-W. Li, L. Ackermann and X. Hong, Nat. Synth., 2023, 2, 321 CrossRef; (k) J.-J. Li, J.-H. Zhao, H.-C. Shen, K. Wu, X. Kuang, P. Wang and J.-Q. Yu, Chem, 2023, 9, 1452 CrossRef CAS.
- A. Romero-Arenas, V. Hornillos, J. Iglesias-Sigüenza, R. Fernández, J. López-Serrano, A. Ros and J. M. Lassaletta, J. Am. Chem. Soc., 2020, 142, 2628 CrossRef CAS PubMed.
- Y. Guo, M.-M. Liu, X. Zhu, L. Zhu and C. He, Angew. Chem., Int. Ed., 2021, 60, 13887 CrossRef CAS PubMed.
- (a) Y. Li, Y.-C. Liou, J. C. A. Oliveira and L. Ackermann, Angew. Chem., Int. Ed., 2022, 61, e202212595 CrossRef CAS PubMed; (b) T. von Münchow, S. Dana, Y. Xu, B. Yuan and L. Ackermann, Science, 2023, 379, 1036 CrossRef PubMed; (c) Z. J. Zhang, S. W. Li, J. C. A. Oliveira, Y. Li, X. Chen, S. Q. Zhang, L. C. Xu, T. Rogge, X. Hong and L. Ackermann, Nat. Commun., 2023, 14, 3149 CrossRef CAS PubMed.
- (a) F. Wang, J. Jing, Y. Zhao, X. Zhu, X.-P. Zhang, L. Zhao, P. Hu, W.-Q. Deng and X. Li, Angew. Chem., Int. Ed., 2021, 60, 16628 CrossRef CAS PubMed; (b) J. Wang, H. Chen, L. Kong, F. Wang, Y. Lan and X. Li, ACS Catal., 2021, 11, 9151 CrossRef CAS; (c) R. Mi, Z. Ding, S. Yu, R. H. Crabtree and X. Li, J. Am. Chem. Soc., 2023, 145, 8150 CrossRef CAS PubMed.
- (a) Q. Dherbassy, J.-P. Djukic, J. Wencel-Delord and F. Colobert, Angew. Chem., Int. Ed., 2018, 57, 4668 CrossRef CAS PubMed; (b) Q. Dherbassy, J. Wencel-Delord and F. Colobert, Tetrahedron, 2018, 74, 6205 CrossRef CAS.
- (a) Q. Gao, C. Wu, S. Deng, L. Li, Z.-S. Liu, Y. Hua, J. Ye, C. Liu, H.-G. Cheng, H. Cong, Y. Jiao and Q. Zhou, J. Am. Chem. Soc., 2021, 143, 7253 CrossRef CAS PubMed; (b) Z.-S. Liu, P.-P. Xie, Y. Hua, C. Wu, Y. Ma, J. Chen, H.-G. Cheng, X. Hong and Q. Zhou, Chem, 2021, 7, 1917 CrossRef CAS.
- X.-J. Si, D. Yang, M.-C. Sun, D. Wei, M.-P. Song and J.-L. Niu, Nat. Synth., 2022, 1, 709 CrossRef.
- B.-J. Wang, G.-X. Xu, Z.-W. Huang, X. Wu, X. Hong, Q.-J. Yao and B.-F. Shi, Angew. Chem., Int. Ed., 2022, 61, e202208912 CrossRef CAS PubMed.
- Y. Wang, X. Zhu, D. Pan, J. Jing, F. Wang, R. Mi, G. Huang and X. Li, Nat. Commun., 2023, 14, 4661 CrossRef CAS PubMed.
- G. Liao, T. Zhang, L. Jin, B.-J. Wang, C.-K. Xu, Y. Lan, Y. Zhao and B.-F. Shi, Angew. Chem., Int. Ed., 2022, 61, e202115221 CrossRef CAS PubMed.
- (a) S. Pan and T. Shibata, ACS Catal., 2013, 3, 704 CrossRef CAS; (b) Ł. Woźniak, J.-F. Tan, Q.-H. Nguyen, A. M. du Vigné, V. Smal, Y.-X. Cao and N. Cramer, Chem. Rev., 2020, 120, 10516 CrossRef PubMed; (c) D. F. Fernández, J. L. Mascareñas and F. López, Chem. Soc. Rev., 2020, 49, 7378 RSC.
- C. S. Sevov and J. F. Hartwig, J. Am. Chem. Soc., 2013, 135, 2116 CrossRef CAS PubMed.
- P. Vázquez-Domínguez, A. Romero-Arenas, R. Fernández, J. M. Lassaletta and A. Ros, ACS Catal., 2023, 13, 42 CrossRef.
- H. Zhao, L. Chen, C. Xia and S. Xu, Asian J. Org. Chem., 2023, 12, e202200695 CrossRef CAS.
- (a) Q. Zhou, S.-Y. Yin, D.-S. Zheng, W.-W. Zhang, S.-Z. Zhang, Q. Gu and S.-L. You, Synlett, 2023, 34, 1442 CrossRef CAS; (b) S.-Y. Yin, Q. Zhou, C.-X. Liu, Q. Gu and S.-L. You, Angew. Chem., Int. Ed., 2023, 62, e202305067 CrossRef CAS PubMed.
- (a) M. Xiong, F. Chen, Y. Shu, X. Wu, J. Tang, F. Yang and D. Xing, Org. Lett., 2023, 25, 5703 CrossRef CAS PubMed; (b) M. Xiong, Z. Yan, S.-C. Chen, J. Tang, F. Yang and D. Xing, ACS Catal., 2024, 14, 7243 CrossRef CAS.
- (a) W.-L. Duan, Y. Imazaki, R. Shintani and T. Hayashi, Tetrahedron, 2007, 63, 8529 CrossRef CAS; (b) N. Di Iorio, P. Righi, A. Mazzanti, M. Mancinelli, A. Ciogli and G. Bencivenni, J. Am. Chem. Soc., 2014, 136, 10250 CrossRef CAS PubMed; (c) J. Zhang, Y. Zhang, L. Lin, Q. Yao, X. Liu and X. Feng, Chem. Commun., 2015, 51, 10554 RSC; (d) F. Eudier, P. Righi, A. Mazzanti, A. Ciogli and G. Bencivenni, Org. Lett., 2015, 17, 1728 CrossRef CAS PubMed; (e) L. Zhang, S.-H. Xiang, J. J. Wang, J. Xiao, J.-Q. Wang and B. Tan, Nat. Commun., 2019, 10, 566 CrossRef PubMed; (f) X.-W. Gu, Y.-L. Sun, J.-L. Xie, X.-B. Wang, Z. Xu, G.-W. Yin, L. Li, K.-F. Yang and L.-W. Xu, Nat. Commun., 2020, 11, 2904 CrossRef CAS PubMed; (g) S. Barik, S. Shee, S. Das, R. G. Gonnade, G. Jindal, S. Mukherjee and A. T. Biju, Angew. Chem., Int. Ed., 2021, 60, 12264 CrossRef CAS PubMed; (h) F. Sung, T. Wang, G.-J. Cheng and X. Fang, ACS Catal., 2021, 11, 7578 CrossRef; (i) J. Wang and X. Li, Sci. China: Chem., 2023, 66, 2046 CrossRef CAS.
- (a) J. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2014, 53, 13244 CrossRef CAS PubMed; (b) J. Zheng, W.-J. Cui, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2016, 138, 5242 CrossRef CAS PubMed; (c) S.-J. Wang, X. Wang, X. Xin, S. Zhang, H. Yang, M. W. Wong and S. Lu, Nat. Commun., 2024, 15, 518 CrossRef CAS PubMed.
- (a) G.-J. Mei, J. J. Wong, W. Zheng, A. A. Nangia, K. N. Houk and Y. Lu, Chem, 2021, 7, 2743 CrossRef CAS; (b) X.-M. Wang, P. Zhang, Q. Xu, C.-Q. Guo, D.-B. Zhang, C.-J. Lu and R.-R. Liu, J. Am. Chem. Soc., 2021, 143, 15005 CrossRef CAS PubMed; (c) Y. Gao, L.-Y. Wang, T. Zhang, B.-M. Yang and Y. Zhao, Angew. Chem., Int. Ed., 2022, 61, e202200371 CrossRef CAS PubMed; (d) K.-W. Chen, Z.-H. Chen, S. Yang, S.-F. Wu, Y.-C. Zhang and F. Shi, Angew. Chem., Int. Ed., 2022, 61, e202116829 CrossRef CAS PubMed; (e) P. Zhang, Q. Xu, X.-M. Wang, J. Feng, C.-J. Lu, Y. Li and R.-R. Liu, Angew. Chem., Int. Ed., 2022, 61, e202212101 CrossRef CAS PubMed; (f) G. Centonze, C. Portolani, P. Righi and G. Bencivenni, Angew. Chem., Int. Ed., 2023, 62, e202303966 CrossRef PubMed; (g) T.-Y. Song, R. Li, L. Huang, S. K. Jia and G. J. Mei, Chin. J. Org. Chem., 2023, 43, 1977 CrossRef CAS; (h) Z.-H. Chen, T.-Z. Li, N.-Y. Wang, X.-F. Ma, S.-F. Ni, Y.-C. Zhang and F. Shi, Angew. Chem., Int. Ed., 2023, 62, e202300419 CrossRef CAS PubMed.
- Q. Wang, Z.-J. Cai, C.-X. Liu, Q. Gu and S.-L. You, J. Am. Chem. Soc., 2019, 141, 9504 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2278431, 2283545, 2309607 and 2309606. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc01837k |
| ‡ The authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |