Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A robust Fe-based heterogeneous photocatalyst for the visible-light-mediated selective reduction of an impure CO2 stream†
Topi
Ghosh‡
a,
Peng
Ren‡
 ab,
Philippe
Franck
a,
Min
Tang
c,
Aleksander
Jaworski
ab,
Philippe
Franck
a,
Min
Tang
c,
Aleksander
Jaworski
 d,
Giovanni
Barcaro
e,
Susanna
Monti
d,
Giovanni
Barcaro
e,
Susanna
Monti
 f,
Lata
Chouhan
g,
Jabor
Rabeah
f,
Lata
Chouhan
g,
Jabor
Rabeah
 h,
Alina
Skorynina
i,
Joaquin
Silvestre-Albero
h,
Alina
Skorynina
i,
Joaquin
Silvestre-Albero
 j,
Laura
Simonelli
j,
Laura
Simonelli
 i,
Anna
Rokicińska
i,
Anna
Rokicińska
 k,
Elke
Debroye
g,
Piotr
Kuśtrowski
k,
Elke
Debroye
g,
Piotr
Kuśtrowski
 k,
Sara
Bals
k,
Sara
Bals
 c and
Shoubhik
Das
c and
Shoubhik
Das
 *ab
*ab
aDepartment of Chemistry, University of Antwerp, Antwerp, Belgium. E-mail: Shoubhik.Das@uni-bayreuth.de
bDepartment of Chemistry, University of Bayreuth, Bayreuth, Germany
cEMAT and NANO Lab Center of Excellence, Department of Physics, University of Antwerp, Antwerp, Belgium
dDepartment of Materials and Environmental Chemistry, Stockholm University, Stockholm, Sweden
eCNR-IPCF, Institute for Chemical and Physical Processes, via G. Moruzzi 1, 56124 Pisa, Italy
fCNR-ICCOM, Institute of Chemistry of Organometallic Compounds, via G. Moruzzi 1, 56124 Pisa, Italy
gDepartment of Chemistry, KU Leuven, Leuven, Belgium
hLeibniz-Institut für Katalyse e. V, Albert-Einstein-Straße 29a, 18059 Rostock, Germany
iCLAESS Beamline, ALBA Synchroton, Spain
jDepartamento de Quimica Inorganica-Instituto Universitario de Materiales, Universidad de Alicante, Alicante E-03080, Spain
kFaculty of Chemistry, Jagiellonian University, Krakow, Poland
First published on 19th June 2024
Abstract
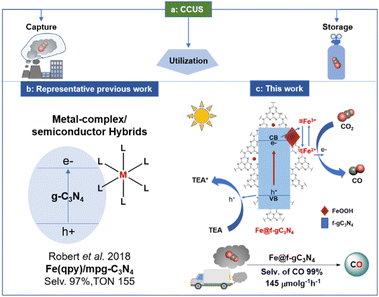
The transformation of CO2 into value-added products from an impure CO2 stream, such as flue gas or exhaust gas, directly contributes to the principle of carbon capture and utilization (CCU). Thus, we have developed a robust iron-based heterogeneous photocatalyst that can convert the exhaust gas from the car into CO with an exceptional production rate of 145 μmol g−1 h−1. We characterized this photocatalyst by PXRD, XPS, ssNMR, EXAFS, XANES, HR-TEM, and further provided mechanistic experiments, and multi-scale/level computational studies. We have reached a clear understanding of its properties and performance that indicates that this highly robust photocatalyst could be used to design an efficient visible-light-mediated reduction strategy for the transformation of impure CO2 streams into value-added products.
Introduction
The development of iron-based catalysts is attractive due to the high abundance of iron in the Earth's crust and low cost compared to the other transition metals.1–8 Furthermore, iron can adopt diverse oxidation states (from −2 to +5) and can promote single electron transfer reactions.9 These advantages triggered scientists to develop novel iron-based homo-/heterogeneous catalysts, and many of them are comparable to those of the 4d and 5d-based transition metal analogs.10–13 Parallel to the development of iron-based catalysts, the direct transformation of CO2 into value-added products has tremendous potential because CO2 is non-toxic and abundant in the atmosphere.14–27 However, in most cases, only pure CO2 streams are used as carbon sources. If impure CO2 streams such as flue gas from industries or exhaust gas from a car could be utilized, it will avoid the associated cost and energy requirement for the CO2 purification procedure. This new approach contributes to the Carbon Capture and Utilization (CCU) principle (Scheme 1a).28–39 Nonetheless, the presence of impurities such as O2, water vapor, CO, NOx, and hydrocarbons in the impure CO2 stream can be harmful to the photocatalyst and can be detrimental to the desired product formation.40–44Recently, graphitic carbon nitrides (g-C3N4) have become highly attractive as photocatalysts due to their high chemical and thermal stability, appropriate band structures, and low cost.45–51 Thus, researchers have employed g–C3N4–based hybrid photocatalysts (g-C3N4 combined with Ru,52 Co,53–56 and Fe57–59-based metal complexes) to improve the selectivity and reactivity for the reduction of CO2 into CO (Scheme 1b). Among them, Co-based transition metal complexes such as [Co(bpy)3]2+ and Co(qpy) along with g-C3N4, exhibited the selectivity of 86 and 98% (TON of CO were 3.7 and 128, with the production rate of 37 and 7.98 μmol g−1 h−1 respectively) for the formation of CO in the presence of triethanolamine (TEOA) and 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH) as sacrificial reductant.54,56 Additionally, Fe-based metal complexes such as Fe(qpy)3 and Fe(qpy)-BA exhibited the formation of CO with a production rate of 91 and 141 μmol g−1 h−1 respectively (selectivity was 97 and 95% in the presence of TEOA and TEA + BIH as sacrificial reductant).57,58 However, these hybrid systems always required expensive ligands and multistep synthetic routes (associated with the metal complexes), exhibited poor recyclability, and rarely showed reactivity toward the transformation of an impure CO2 stream.
To achieve a robust and recyclable Fe-based photocatalyst, construction of composite material could be a promising route since this could address the rapid recombination of photogenerated carriers which are the common limitations for the photocatalytic reactivity of g-C3N4.60,61 Along this direction, amorphous iron-based oxyhydroxides (FeOOH), known as promising Fenton-like catalysts, should be ideal for hybridization due to their small particle size.62–64 The coupling of amorphous FeOOH with g-C3N4 could be a sound strategy for constructing an effective recyclable photocatalyst. Considering this, we first modified the g-C3N4 moiety by introducing an aryl functionality (f-gC3N4) to improve the photocatalytic properties of g-C3N4 (ref. 49) and later, FeOOH was introduced into the moiety to fabricate a FeOOH/f-gC3N4 composite photocatalyst. This photocatalyst generated CO with a production rate of 304 μmol g−1 h−1 with a selectivity of 99%. Expediently, when exhaust gas from a car was applied, CO was formed with a production rate of 145 μmol g−1 h−1 (Scheme 1c), thanks to the enhanced interfacial electron transfer between FeOOH and f-gC3N4. To the best of our knowledge, this is the first Fe-based recyclable photocatalytic system that can be applied for the reduction of impure CO2 stream.
Results and discussion
At the beginning of this project, g-C3N4 was synthesized by calcinating dicyandiamide (DCDA) at 550 °C for 4 h (temp. increasing rate = 2.2 °C min−1) in a tube furnace under aerobic conditions (please see the detailed procedure in the ESI†).49 Followed by this method, functionalized graphitic carbon nitrides (f-gC3N4) were achieved by stirring a mixture of 9 gm of DCDA and 150 mg of 2-amino-5-trifluoromethyl benzonitrile in deionized water (45 mL) at 95 °C until it’s completely dried, resulting mixture was then grinded in an algae mortar and was calcined at 550 °C for 4 h (temp. increasing rate = 2.2 °C min−1) under aerobic conditions (please see in the ESI†). Later, Fe(NO3)3·9H2O (7.23 mg for 0.2Fe@f-gC3N4, 18 mg for 0.5Fe@f-gC3N4, 25.3 mg for 0.7Fe@f-gC3N4, and 36 mg for 1Fe@f-gC3N4) and f-gC3N4 (500 mg) were mixed in 10 mL deionized water and the reaction mixture was further stirred at 100 °C (please see in the ESI† for the detailed procedure). It should be noted that 0.2Fe@f-gC3N4, 0.5Fe@f-gC3N4, 0.7Fe@f-gC3N4 and 1Fe@f-gC3N4 denotes 0.2, 0.5, 0.7 and 1 wt% of iron loading on f-gC3N4 respectively. After the synthesis of all these photocatalysts, the Tauc plot exhibited that gC3N4, f-gC3N4, 0.5Fe@g-C3N4, 1Fe@f-gC3N4, 0.7Fe@f-gC3N4, 0.5Fe@f-gC3N4, and 0.2Fe@f-gC3N4 had the band gap of 2.64, 2.48, 2.46, 2.34, 2.47, 2.46 and 2.46 eV, respectively (Fig. S3†). Furthermore, Mott–Schottky plots of all these photocatalysts disclosed that the flat band (fb) potential of gC3N4, f-gC3N4, 0.5Fe@g-C3N4, 1Fe@f-gC3N4, 0.7Fe@f-gC3N4, 0.5Fe@f-gC3N4, and 0.2Fe@f-gC3N4 were −0.34, −0.44, −0.47, −0.52, −0.56, −0.33 and −0.38 eV vs. Normal Hydrogen Electrode (NHE). Additionally, the positive slope indicated the n-type nature of these semiconductors (Fig. S4†). The conduction band (CB) of an n-type inorganic semiconductor is commonly assumed to be ≈−0.2 V negative than the flat band potentials. Thus, the CB potentials were derived by lowering the flat band potential by 0.2 V compared to the NHE (Fig. S5†).47,65After the synthesis, photocatalytic experiments were carried out in 4 mL of CO2-saturated acetonitrile solution in the presence of a freshly distilled sacrificial electron donor (ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) triethylamine, 4
triethylamine, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
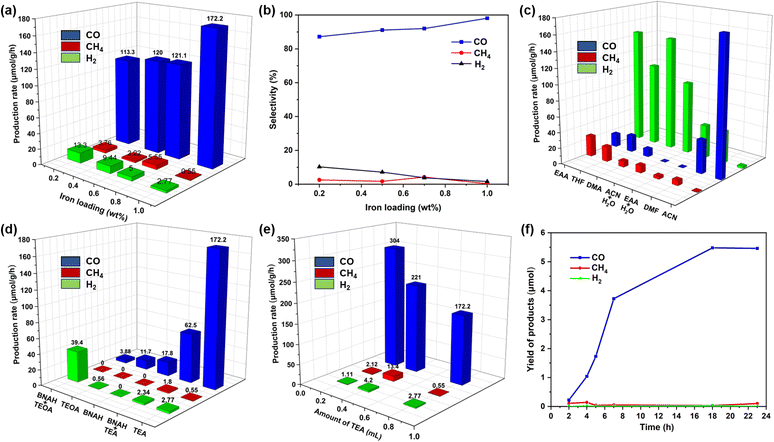
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 V/V), under the irradiation of a Kessil lamp for 18 h (λ = 427 nm, 100 mW cm−2 light intensity, Table S1†). Indeed, CO was the primary product, with a minor quantity of CH4 and H2 In this photocatalytic reaction, TEA got oxidized to form TEAH+.58 To our observation, only a trace amount of CO and H2 were obtained in the case of g-C3N4, while a moderate amount of CO with 91% selectivity was observed in the presence f-gC3N4. Furthermore, when 0.5 wt% of Fe was deposited onto both gC3N4 and f-gC3N4, the Fe@f-gC3N4 system exhibited nearly 12 times higher production rate of CO. To investigate the superior role of 0.5 wt% Fe@f-gC3N4 photocatalyst, pure Fe(NO3)3·9H2O was mixed externally with f-gC3N4 (iron content was the same as 0.5Fe@f-gC3N4). A lower quantity of CO clearly confirmed the importance of the deposition of iron onto f-gC3N4 structure. It could be due to the fact that the metal deposition enhanced the charge transfer efficiency from the conduction band of f-gC3N4 to the active metal site of Fe+n and that was ideal for the effective reduction of CO2.57 After that, we were able to further increase the catalytic reactivity through different loadings of iron (0.2–1 wt%) on f-gC3N4 (Fig. 1a and b). We observed that the production rate of CO was linearly increased up to 0.7 wt% and was drastically boosted for 1Fe@f-gC3N4 (172 μmol g−1 h−1). Indeed, iron sites are prone to adsorb CO2; therefore, with the increase of iron loadings, more electrons will be transferred to the iron sites to reduce CO2. Thus, the increased Fe-loading prolonged the lifetime of the charge carriers and enhanced the transfer of the photogenerated electrons from f-gC3N4 to the Fe+n center and then to CO2. Instead, a smaller number of electrons were transferred toward proton reduction, which in turn suppressed H2 evolution,67 and the production rate and selectivity to CO were linearly increased with the increase of iron loading.
1 V/V), under the irradiation of a Kessil lamp for 18 h (λ = 427 nm, 100 mW cm−2 light intensity, Table S1†). Indeed, CO was the primary product, with a minor quantity of CH4 and H2 In this photocatalytic reaction, TEA got oxidized to form TEAH+.58 To our observation, only a trace amount of CO and H2 were obtained in the case of g-C3N4, while a moderate amount of CO with 91% selectivity was observed in the presence f-gC3N4. Furthermore, when 0.5 wt% of Fe was deposited onto both gC3N4 and f-gC3N4, the Fe@f-gC3N4 system exhibited nearly 12 times higher production rate of CO. To investigate the superior role of 0.5 wt% Fe@f-gC3N4 photocatalyst, pure Fe(NO3)3·9H2O was mixed externally with f-gC3N4 (iron content was the same as 0.5Fe@f-gC3N4). A lower quantity of CO clearly confirmed the importance of the deposition of iron onto f-gC3N4 structure. It could be due to the fact that the metal deposition enhanced the charge transfer efficiency from the conduction band of f-gC3N4 to the active metal site of Fe+n and that was ideal for the effective reduction of CO2.57 After that, we were able to further increase the catalytic reactivity through different loadings of iron (0.2–1 wt%) on f-gC3N4 (Fig. 1a and b). We observed that the production rate of CO was linearly increased up to 0.7 wt% and was drastically boosted for 1Fe@f-gC3N4 (172 μmol g−1 h−1). Indeed, iron sites are prone to adsorb CO2; therefore, with the increase of iron loadings, more electrons will be transferred to the iron sites to reduce CO2. Thus, the increased Fe-loading prolonged the lifetime of the charge carriers and enhanced the transfer of the photogenerated electrons from f-gC3N4 to the Fe+n center and then to CO2. Instead, a smaller number of electrons were transferred toward proton reduction, which in turn suppressed H2 evolution,67 and the production rate and selectivity to CO were linearly increased with the increase of iron loading.
Then, we evaluated the importance of different solvents (CH3CN, DMF, DMA, EtOAc, THF and H2O) in the presence of 1Fe@f-gC3N4 (Fig. 1c). While all of them were favorable for this transformation, no reaction took place in pure water, and further addition of water to organic solvents such as CH3CN and EtOAc resulted in a lower evolution rate of CO. Surprisingly, in the aqueous binary solvent system, CH4 and H2 were the major products, and CO was the minor product (Fig. 1c). In fact, by adding 37% of water into CH3CN and EtOAc, the selectivity of the CO2 reduction product was changed entirely from CO to CH4 (8e−/8H+ reduction process) with a production rate of 9.30 and 3.53 μmol g−1 h−1 respectively. This could be attributed to the fact that the addition of water increased the number of available protons in the solution, which in turn took part in the CO2 reduction process to form CH4.68 Nevertheless, among all the solvents, CH3CN was the best for the photochemical reduction of CO2 to CO, with a high production rate of 172.2 μmol g−1 h−1 and an excellent selectivity of 98%. Additionally, sacrificial reductants such as triethylamine (TEA), triethanolamine (TEOA) and 1-benzyl-1,4-dihydronicotinamide (BNAH) were also investigated and a production rate of 172, 11.7, and 17.8 μmol g−1 h−1 with 98, 95, and 100% selectivity were obtained (Fig. 1d). Further investigations by using different amounts of TEA and reducing the amount of TEA to 0.2 mL resulted in an excellent production rate of 304 μmol g−1 h−1 (Fig. 1e). We argued that a higher concentration of TEA probably quenched the excited state of the photocatalyst and decreased the photocatalytic efficiency.69
Furthermore, control experiments suggested that in the absence of CO2, photocatalyst, and light, no formation of CO was observed (Fig. S8†). On the other hand, in the absence of TEA, the formation of CO was observed, but with a lower production rate of 76.6 μmol g−1 h−1. In these conditions, the kinetics of CO2 reduction exhibited a linear increase in CO production up to 18 h, and after this, the yield of CO remained constant but the production of CH4 was slightly increased. Furthermore, kinetic studies demonstrated that the evolution of CO was stable up to 23 h, which was comparable with the recently reported photocatalysts (Fig. 1f).57,70
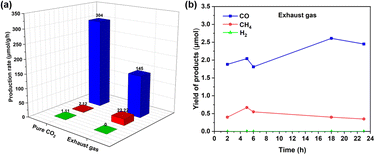
To demonstrate the application of this chemistry, the exhaust gas (containing impurities besides CO2, are shown in Fig. S7†) was directly collected from a vehicle by using gas sampling bags (Fig. S6†) and was applied directly under these reaction conditions, showing a CO production rate of 145 μmol g−1 h−1 (Fig. 2a). This decreased catalytic reactivity was due to the combined result of lower CO2 concentration in the exhaust gas as well as the presence of NOx or SOx in the exhaust gas, which can also be adsorbed on the active catalytic sites.71 In addition, the kinetic studies demonstrated the high stability of this photocatalyst in the presence of impurities such as H2, CO, CH4, O2, N2, and others, which are typically present in car exhaust gas (Fig. 2b). Furthermore, to estimate the reusability of this photocatalyst, 10 mg of the material was successfully used (under the same reaction conditions) and recycled for up to three cycles (Fig. S10†).
To achieve a deep characterization of the synthesized materials, we investigated the morphology and the structure of 1Fe@f-gC3N4 by High-Angle Annular Dark Field Scanning Transmission Electron Microscopy (HAADF-STEM). As shown in Fig. 3a, nanoparticles (NPs) were non-uniformly distributed over f-gC3N4, and EDX-mapping demonstrated that the NPs contained Fe (Fig. 3b–e). Atomic resolution HAADF-STEM images were acquired at lower (Fig. 3f) and higher (Fig. 3g) magnification. Fast Fourier transform (FFT) of the NPs marked by the red dashed rectangle in Fig. 3g provided lattice spacings of 2.690 Å, 2.580 Å, and 2.446 Å (Fig. 3h), which corresponded to FeO(OH) (130), (0−21), and (111), respectively. These results demonstrated that the supported NPs correspond to FeO(OH) species, and further investigating the edge of the f-gC3N4 support, bright dots marked by yellow dashed circles were observed, corresponding to small clusters containing Fe (Fig. 3i).
The crystal structure of prepared gC3N4, f-gC3N4, and 1Fe@f-gC3N4 were further investigated via XRD. Two distinct diffraction peaks were observed at 12.9° and 27.3°, corresponding to the (100) and (002) crystal planes of g-C3N4, related to the in-plane repeating s-triazine structural moieties and interlayer stacking of the conjugated aromatic ring (Fig. S11†).72–74 While for f-gC3N4, the peak position and the peak intensity were almost similar with gC3N4, in the presence of Fe, the intensity of diffraction peak (002 in case of 1Fe@f-gC3N4) was slightly decreased as well as a marginal shift to higher diffraction angle was observed.73,75 However, no obvious diffraction peak of Fe2O3 or FeO(OH) phase were detected in the pattern of 1Fe@f-gC3N4, due to relatively low amount of the Fe species.66,76,77 Additionally, the weakening of the intensity of (002) peak suggested a decrease in crystallinity, with a consequent increase in the number of defects. These could trap a large number of carriers, with a resulting increase in charge separation and an improvement of the photocatalytic performance.75
To verify the atomic environment of Fe in 1Fe@f-gC3N4, synchrotron X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) at Fe K-edge were performed and compared to the X-ray absorption spectra of the Fe2O3 and FeOOH reference materials. From the position of the rising edge in XANES spectra (Fig. 4a), it can be concluded that Fe in 1Fe@f-gC3N4 was predominantly in a +3 oxidation state, as confirmed by the close resemblance with Fe2O3 and FeOOH pre-edge peaks. EXAFS and Fourier transformed (FT) k3-weighted EXAFS curves were further extracted to probe the atomic iron-based local structures (Fig. 4b and c). When compared with the reference systems, 1Fe@f-gC3N4 nearly matched the peaks of FeOOH, which was especially noticeable for the shell scattering peaks of the longer-range order (2–3.5 Å), indicating Fe–Fe bonding. Concurrently, only FeOOH species could not fully describe XANES region and first shell, and a low Fe2O3 fraction below 5% cannot be excluded.
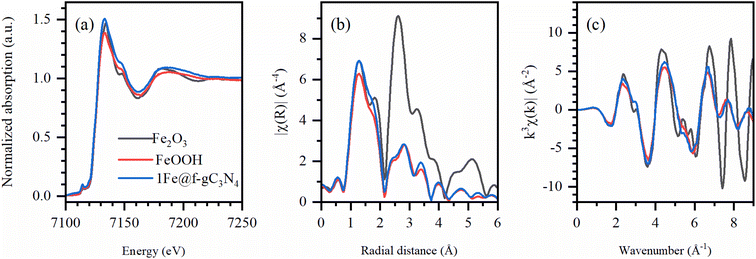 | ||
| Fig. 4 (a) Normalized XANES spectra, (b) FT-EXAFS spectra (phase uncorrected) and (c) k3-weighted EXAFS spectra at Fe K-edge for the 1Fe@f-gC3N4 and reference samples (Fe2O3 and FeOOH). | ||
Solid-state MAS NMR spectra of all NMR-active nuclei present in the material (1H, 13C, 15N, and 19F) were also collected to probe the local structure and to examine potential structural changes upon doping of the f-gC3N4 with Fen+ species (Fig. S12†). The 1H MAS, 13C CPMAS, and 15N CPMAS spectra were almost identical to NMR data we collected from the related polymeric carbon nitride catalysts and reported recently.49,78 Therefore, we concluded that the overall structure and polymerization degree in these materials were not affected to any significant extent by doping with Fen+ ions. However, in the 19F MAS NMR spectrum, the 19F NMR shift of −120 ppm was distinct from that of −105 ppm observed by us for the undoped f-gC3N4, attributed to the presence of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF2 groups (Fig. S12b†).49,79,80 Moreover, an additional weak signal at −202 ppm was detected, which had not been observed for the undoped material. The additional 19F MAS spectrum recorded using a short 0.2 s relaxation delay was collected to inspect if the appearance of the signal at −202 ppm could be related to the introduction of Fen+ ions in the material. Upon using a short relaxation delay of 0.2 s, the intensity of the signal at −202 ppm was almost unaffected, whereas the signal at −120 ppm was significantly saturated. This could be attributed to the paramagnetic relaxation enhancement of the 19F nuclei in close contact with paramagnetic Fen+ ions. The observed 19F NMR shifts of −120 and −202 ppm were most probably affected by the induced paramagnetic NMR shift interactions.81
CF2 groups (Fig. S12b†).49,79,80 Moreover, an additional weak signal at −202 ppm was detected, which had not been observed for the undoped material. The additional 19F MAS spectrum recorded using a short 0.2 s relaxation delay was collected to inspect if the appearance of the signal at −202 ppm could be related to the introduction of Fen+ ions in the material. Upon using a short relaxation delay of 0.2 s, the intensity of the signal at −202 ppm was almost unaffected, whereas the signal at −120 ppm was significantly saturated. This could be attributed to the paramagnetic relaxation enhancement of the 19F nuclei in close contact with paramagnetic Fen+ ions. The observed 19F NMR shifts of −120 and −202 ppm were most probably affected by the induced paramagnetic NMR shift interactions.81
The XPS spectra were recorded in the Fe 2p, C 1s, and N 1s regions for both fresh and used samples of 1Fe@f-gC3N4 (Fig. S13†). The dominant component of the pristine 1Fe@f-gC3N4 was the g-C3N4 phase, as confirmed by the C 1s and N 1s spectra. In the C 1s region, photoelectron emission, typical of sp2-bonded C atoms in N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, was observed at 288.1 eV, while in the N 1s region three peaks at 398.6 eV (sp2-hybridized N atoms in C–N
N, was observed at 288.1 eV, while in the N 1s region three peaks at 398.6 eV (sp2-hybridized N atoms in C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C species), 400.1 eV (©dging N atoms N-(C)3 species) and 401.2 eV (N atoms in amino groups) were found.82,83 Fen+ species also appeared on the surface of f-gC3N4. Due to the low content of this component, it was difficult to determine the chemical state of Fe unequivocally. Nevertheless, the Fe 2p3/2 peak position at 710.6 eV with an apparent multiplet splitting and a low satellite at ca. 718 eV indicated the presence of high-spin Fe3+ species. Another evidence confirming this supposition was the orbital splitting of Fe 2p3/2 and Fe 2p1/2 of 13.8 eV, which was similar to that reported previously in the literature for Fe3+.84 The elemental composition of the photocatalyst surface did not change during the process, and the used sample exhibited the same peaks in the individual XPS spectra. However, their intensity decreased due to the deposition of an additional component on the surface. Its nature was revealed by the XPS C 1s spectrum, where two new peaks were distinguished at binding energies of 284.8 eV and 285.9 eV, respectively. The former was typical for C–C/C
C species), 400.1 eV (©dging N atoms N-(C)3 species) and 401.2 eV (N atoms in amino groups) were found.82,83 Fen+ species also appeared on the surface of f-gC3N4. Due to the low content of this component, it was difficult to determine the chemical state of Fe unequivocally. Nevertheless, the Fe 2p3/2 peak position at 710.6 eV with an apparent multiplet splitting and a low satellite at ca. 718 eV indicated the presence of high-spin Fe3+ species. Another evidence confirming this supposition was the orbital splitting of Fe 2p3/2 and Fe 2p1/2 of 13.8 eV, which was similar to that reported previously in the literature for Fe3+.84 The elemental composition of the photocatalyst surface did not change during the process, and the used sample exhibited the same peaks in the individual XPS spectra. However, their intensity decreased due to the deposition of an additional component on the surface. Its nature was revealed by the XPS C 1s spectrum, where two new peaks were distinguished at binding energies of 284.8 eV and 285.9 eV, respectively. The former was typical for C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, while the latter was for the oxidized C forms, most likely C–O or C
C, while the latter was for the oxidized C forms, most likely C–O or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.85
O.85
To obtain better insights into the mechanism of the reaction, the optical properties of the photocatalysts were characterized. Through UV/vis spectroscopy (Fig. S4a†), an absorption edge at 465 nm was observed in the case of gC3N4, whereas f-gC3N4, 0.5Fe@gC3N4 and 0.5Fe@f-gC3N4 exhibited a redshift of 30 nm, 34 nm and 34 nm, respectively (corresponding to 0.16 eV, 0.18 ev and 0.18 ev respectively). However, different Fe-loading had a negligible effect on the optical spectrum. The broad and intense absorption peak of the Fe-loaded f-gC3N4 catalyst in the visible region inferred that the catalyst could absorb more photons, which was a consequent enhancement of the photocatalytic activity.86 The difference in the absorption band between gC3N4 and Fe-loaded f-gC3N4 could be due to the electrostatic interaction between Fe+n and f-gC3N4, which promoted the electron delocalization throughout the heptazine framework by Fe+n.87 This was also evident from EPR spectroscopy, which showed a broadening of the 1Fe@f-gC3N4 signal (compared to f-gC3N4 one), due to the mutual interaction between the paramagnetic species (Fig. S14†).
The photocatalysts' steady-state photoluminescence (PL) spectra (Fig. S17a†) revealed emission maxima of gC3N4 at 471 nm and of Fe-gC3N4 at 466 nm. The emission spectra of f-gC3N4 and Fe-loaded f-gC3N4 were broad and centered around 513 nm. To investigate the charge carrier generation and dynamics, we measured the PL decays of the photocatalysts at their respective emission wavelength (Fig. S17b and c†). The PL decay profiles of all samples were recorded by time-correlated photon counting (TCSPC) spectroscopy and fitted using a tri-exponential decay equation. The average PL lifetimes and the fitting parameters are given in Table S3†. According to the lifetime fitting parameters, f-gC3N4 and Fe-loaded f-gC3N4 had longer τ1 and τ2 values compared to the gC3N4 and Fe-gC3N4 catalysts. Additionally, upon increasing Fe-loading in f-gC3N4, the carrier lifetimes exhibited an increasing trend of all τ1, τ2, and τ3 values. Although there is a slight difference in the average lifetime of the photocatalysts, the iron loading in f-gC3N4 increases the average lifetime, resulting in enhanced photocatalytic activity. This result supported the above-explained enhanced photocatalytic reactivity upon increasing the iron wt% due to a more efficient transfer of photogenerated electrons from f-gC3N4 to Fe+n. Following the increase of the charge carrier lifetimes, the generated electrons were available for a longer time for the photocatalysis reaction to efficiently take place. Specifically, in 1Fe@f-gC3N4, the τ3 value, reflecting the lifetime of the free charge carriers that can diffuse over longer distances, has significantly increased.
In addition, to investigate the separation efficiency of photogenerated electron–hole pairs, we recorded the photocatalyst's transient photocurrent responses under the irradiation of a Kessil lamp (λ = 427 nm). Among all of them, 1Fe@f-gC3N4 exhibited the quickest and highest photocurrent which remained stable up to 5 cycles which indicated a more efficient charge separation and faster electron transfer rate to trigger a superior photocatalytic reactivity of the catalyst (Fig. S18b†). Furthermore, in situ EPR spectroscopy showed that the relative number of the photoexcited electrons of f-gC3N4 was higher than 1Fe@f-gC3N4 due to the facile electron transfer to Fe (Fig. S14†). Additionally, Electrochemical Impedance Spectroscopy (EIS) of gC3N4, f-gC3N4 and all the Fe-loaded gC3N4 and f-gC3N4 exhibited decreasing charge transfer resistance, demonstrating the presence of covalent linking in 1Fe@f-gC3N4 which significantly enhanced the conductivity (Fig. S18a†). The CO2 reduction was confirmed by a spin-trapping experiment with DMPO (Fig. S16†). The holes in the valence band were quenched by the sacrificial reductant (TEA) and decreased the electron–hole recombination, as it is evident from the in situ EPR investigations (Fig. S15†). In the valence band, TEA reacted with holes and subsequently formed the α-amino radical and protons.88
Further to gather detailed information about the reaction mechanism, computational calculations were done (see in the ESI†) and after ≈50 ps of RMD, the sampled structures showed that the adsorption tendency of CO2 on the metal centers was mainly due to the coordination of one of its oxygens (Fe–O distance about 2.1 Å),89 and no reaction mechanisms were observed in those conditions. Indeed, the conversion started from an activated adsorption configuration where the carbon atom was connected to the metal center, and the CO2 molecule adopted a bent arrangement (CO2˙−).90–92 Experimentally, this activation was obtained by light irradiation (λ = 427 nm), which induced a charge transfer from the catalyst to the molecule, CO2 chemisorption with the elongation of the C–O bond, bending of O–C–O angle, and finally, dissociation of CO2 on the catalyst surface into CO and O species.93,94
We could mimic this process by including, in the RMD simulations, an electric field in the plane of the melem units (x direction, −0.01 V Å−1). The effect of the external electric field is a perturbation of the atomic charges of the system and, thus, a distortion of the molecular charge distribution. The evolution of the dipole moments imitated a charge transfer from the metal center to the adsorbed CO2. This is apparent in the atomic charge distribution plots of Fig. S21,† where it is evident the charge transfer from Fe to CO2 during the first 7.5 ps of the simulation (Fig. S21† – bottom) and the change of nature of the C atom when CO2 reduces to CO (Fig. S21† – middle plot). The adsorbed CO2 in a bent geometry was negatively charged. Indeed, from the beginning of the polarized dynamics, CO2 changed from an extended to a bent conformation and remained adsorbed on the Fe atom through its carbon (Fig. S20†). The stabilized complex received a proton from the solution and formed the adsorbed *COOH species. This species was short-lived, and the OH was quickly released in solution and then protonated. In contrast, CO remained stably adsorbed on the metal center (Fig. S22†). Besides reproducing possible hydrogen exchanges between the solvent and the region around the COO–Fe complex, the mechanisms produced a water molecule that freely migrated in the solution, whereas CO remained connected to the metal.
To refine this picture, we extracted the MD snapshots describing the primary steps of the reaction mechanism, size-reduced them to three melem units with a chelated Fe atom and an adsorbed CO2 molecule (high-coordination site) or two melem units with a chelated Fe-atom, the f-substituent, and an adsorbed CO2 molecule (low-coordination site), and carried out density functional theory (DFT) calculations with Gaussian16.95 These were used to estimate minimum energy structures, charge analyses, possible reaction paths, and relative energy barriers (Fig. 5, 6 and S23†). We optimized the geometries in ACN through the integral equation formalism variant (IEFPCM) of the Polarizable Continuum Model (PCM), using the B3LYP-D3(BJ) functional with the Grimme D3 correction (Becke–Johnson parameters97), to account for the van der Waals interactions, and the 6-31(d,p) basis set for all the elements except Fe, which was described with the def2-TZVP basis set.98 Charge analysis was performed using NBO (full Natural Bond Orbital).99,100
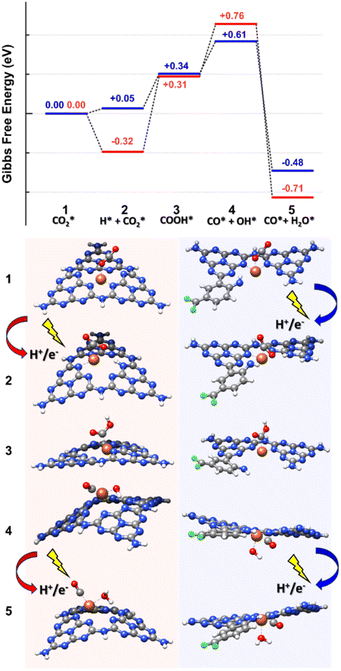 | ||
| Fig. 5 Reaction mechanism showing CO2 reduction to CO involving Fe atom in a high-coordination (red path) and a low-coordination (blue path) sites. State 1 is chosen as a reference for energy estimation, whereas the free energies of the other states (from 2 to 5) are estimated at the DFT level by employing the Norskov model.96 Color code: C gray, N blue, F green, Fe orange, O red, H white. | ||
Both high- and low-coordination sites provide a similar qualitative picture of the reaction mechanism, in agreement with the results of RMD simulations. In the absence of external stimuli, 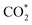 molecule interacted weakly via one of its oxygens with the catalyst metal center (Fe–O equilibrium distance is 2.04 Å, in fair agreement with the results of RMD), which acted as a Lewis acceptor (see state 1 in Fig. 5), as confirmed by the small positive charge carried by CO2 (about +0.2e) and the charge decrease on Fe after adsorption (about 1.1e, to be compared with 1.3e of a naked Fe atom – Fig. S23†). The
molecule interacted weakly via one of its oxygens with the catalyst metal center (Fe–O equilibrium distance is 2.04 Å, in fair agreement with the results of RMD), which acted as a Lewis acceptor (see state 1 in Fig. 5), as confirmed by the small positive charge carried by CO2 (about +0.2e) and the charge decrease on Fe after adsorption (about 1.1e, to be compared with 1.3e of a naked Fe atom – Fig. S23†). The 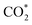 activation happened after the addition of a H+/e− couple promoted by UV irradiation: a similar bent adsorption configuration obtained by RMD (and shown in state 2 of Fig. S23†) was stabilized by a flow of negative charge to both the CO2 molecule (charge of ≈ –0.4e) and the Fe center (charge decrease from ≈+1.0e to ≈+0.7e). The proton (as in RMDs) was adsorbed on a nitrogen atom at the edge of a neighboring melem unit. CO2 negative charging is also evident in the PDOS shown in Fig. S24.†
activation happened after the addition of a H+/e− couple promoted by UV irradiation: a similar bent adsorption configuration obtained by RMD (and shown in state 2 of Fig. S23†) was stabilized by a flow of negative charge to both the CO2 molecule (charge of ≈ –0.4e) and the Fe center (charge decrease from ≈+1.0e to ≈+0.7e). The proton (as in RMDs) was adsorbed on a nitrogen atom at the edge of a neighboring melem unit. CO2 negative charging is also evident in the PDOS shown in Fig. S24.†
After proton migration to 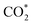 we obtained the COOH* configuration shown in state 3 (Fig. 5). This positive charge flow allows negative charge back-migration from Fe to the adsorbate (with COOH almost neutral), as shown in Fig. S23.† The elongation and weakening of the bond between the carbon and the hydroxyl group (induced by the catalyst-adsorbate charge transfer, populating the p* LUMO of CO2 (ref. 88)) can be exasperated until the breaking of the C–O bond realized the configuration shown in state 4 (Fig. 5), which presents CO and OH separately adsorbed on the Fe atom. The positive charge carried by CO was compensated by a reduced charge on Fe (relative to state 2), and the negative charge carried by OH* (about −0.2e). States 3 and 4 are at higher energy relative to state 2 (≈1.08 eV for the high-coordination site and 0.56 eV for the low-coordination). The reduced energy difference characterizing the low-coordination site suggests that this can promote CO2 reduction easier than the high-coordination site and will be further investigated in the following. The UV-vis spectra simulated for state 2 of the low-coordination site, calculated at the TD-DFT level (Fig. S25†), show a dominant peak at about 465 nm, corresponding to a charge transfer from the metal ion to the adsorbed
we obtained the COOH* configuration shown in state 3 (Fig. 5). This positive charge flow allows negative charge back-migration from Fe to the adsorbate (with COOH almost neutral), as shown in Fig. S23.† The elongation and weakening of the bond between the carbon and the hydroxyl group (induced by the catalyst-adsorbate charge transfer, populating the p* LUMO of CO2 (ref. 88)) can be exasperated until the breaking of the C–O bond realized the configuration shown in state 4 (Fig. 5), which presents CO and OH separately adsorbed on the Fe atom. The positive charge carried by CO was compensated by a reduced charge on Fe (relative to state 2), and the negative charge carried by OH* (about −0.2e). States 3 and 4 are at higher energy relative to state 2 (≈1.08 eV for the high-coordination site and 0.56 eV for the low-coordination). The reduced energy difference characterizing the low-coordination site suggests that this can promote CO2 reduction easier than the high-coordination site and will be further investigated in the following. The UV-vis spectra simulated for state 2 of the low-coordination site, calculated at the TD-DFT level (Fig. S25†), show a dominant peak at about 465 nm, corresponding to a charge transfer from the metal ion to the adsorbed 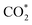 molecule, aiding the C–O bond breaking in overcoming the observed energy difference between states 2 and 4. This agrees with the experimental choice of a blue LED (λ = 427 nm) as the excitation source.
molecule, aiding the C–O bond breaking in overcoming the observed energy difference between states 2 and 4. This agrees with the experimental choice of a blue LED (λ = 427 nm) as the excitation source.
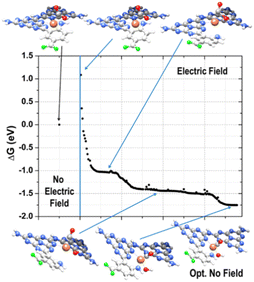
Further addition of a H+/e− couple (promoted by UV excitation) led to the configuration of state 5 (Fig. 5), which corresponded to adsorbed CO and H2O on the Fe catalyst. H2O carried a slight positive charge (about +0.2e), and CO kept its positive charge of about +0.4e. The relatively strong dipole between Fe and CO is a clear signal of a strong CO anchoring to the catalytic site, which can be disrupted if the charge flows from Fe to CO. The Gibbs free energy of this last state is lower than that of any other state investigated, confirming the thermodynamic tendency of this system to promote CO2 reduction. This sequence of events is the same as observed in RMD. We resorted to the electric field option already used for the RMD to recreate a possible dynamics mechanism at the DFT level instead of performing computationally expensive excited-state quantum chemistry simulations. Starting from the optimized complex of the low-coordination corresponding to state 2 (Fig. 5), where the CO2 molecule was adsorbed with the carbon atom on the metal ion, we emulated the first few steps of the reduction mechanism by applying an external electric field in the -x direction with a magnitude of 0.008 au.
From the examination of the free energy difference plot, it is evident that a low activation energy of ≈1.1 eV was necessary to start the process; this activation energy is reasonable when compared to the energy difference of ≈0.6 eV between states 2 and 4, estimated at the static level (Fig. 6). Then, the mechanism proceeded barrierlessly, passing through two intermediate metastable geometries (plateau regions). The first one is characterized by the appearance of the adsorbed COOH* species (state 3 in Fig. 5), whereas in the second one, the OH detached from C and became connected to the metal center (at a C–Fe–OH angle of about 103°, state 4 in Fig. 5), forming a bond with Fe (with an average Fe–O length of approximately 1.8 Å) and a hydrogen bond with the nitrogen atom of the nearby triazine ring (with an average OH–N distance of roughly 1.8 Å and a donor-H-acceptor angle of about 140°). In the final stable configuration, the hydrogen bond was lost, and the OH group moved to a farther N–O separation of about 3 Å but remained connected to the metal center. Interestingly, when simulated under the effect of the electric field, the energies of states 3 and 4 become lower than that of state 2, indicating a stabilizing effect played by the external perturbation on configurations which, when analyzed in their ground state, resulted instead higher in energy.
Conclusions
In conclusion, we have successfully demonstrated that coupling amorphous FeOOH with f-gC3N4 exhibited an efficient visible-light-mediated CO2 conversion into CO. The presence of FeOOH in functionalized gC3N4 modulated the electronic interaction between Fe species and semiconductor, making an efficient photocatalyst for CO2 reduction. This FeOOH/f-gC3N4 composite heterogeneous photocatalyst has shown the highest CO evolution rate (so far reported among Fe-based heterogeneous photocatalysts) of 304 μmol g−1 h−1 with an excellent selectivity of >99%. This earth-abundant and low-cost photocatalyst also exhibited excellent reactivity and stability for reducing car exhaust gas, which clearly depicted the strong application potential of this chemistry. We strongly believe that our photocatalytic system will open a new strategy in the field of photocatalytic CO2 reduction.Author contributions
T. G., P. R. and S. D. conceptualized the project. S. D. supervised the project. T. G and P. R. synthesized the catalysts, conducted the catalytic experiments and the related data processing, and performed materials characterization and analysis with the help of P. F., A. J., A. R., P. K., L. C. and E. D. Furthermore, A. J. collected solid-state NMR spectra and J. R. performed EPR investigations. M. T. and S. B. conducted high-resolution, high angle annular dark-field transmission electron microscope (HAADF-STEM) spectroscopy. G. B. and S. M. performed the theoretical studies. J. S.-A., A. S. and L. S. performed X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) measurement and analysis. A. C and E. D performed the steady state spectroscopy. A. R and P. K performed the X-ray photoelectron spectroscopy (XPS). The manuscript was written through the contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. D. thanks the Francqui start up grant from the University of Antwerp, Belgium for the financial support. T. G. thanks MSCA BOF postdoctoral fellowship. P. R. thanks CSC. E. D. would like to thank the KU Leuven Research Fund for financial support through STG/21/010. J. S. A. acknowledges financial support from MCIN/AEI/10.13039/501100011033 and EU “NextGeneration/PRTR (Project PCI2020-111968/3D-Photocat) and Diamond Synchrotron (Rapid access proposal SP32609). XPS measurements were carried out with the equipment purchased thanks to the financial support of the European Regional Development Fund in the framework of the Polish Innovation Economy Operational Program (contract no. POIG.02.01.00-12-023/08). The research has been supported by a grant from the Faculty of Chemistry under the Strategic Programme Excellence Initiative at Jagiellonian University.Notes and references
- I. Bauer and H. J. Knolker, Iron catalysis in organic synthesis, Chem. Rev., 2015, 115, 3170–3387 CrossRef CAS PubMed.
- V. G. Chandrashekhar, T. Senthamarai, R. G. Kadam, O. Malina, J. Kašlík, R. Zbořil, M. B. Gawande, R. V. Jagadeesh and M. Beller, Silica-supported Fe/Fe–O nanoparticles for the catalytic hydrogenation of nitriles to amines in the presence of aluminium additives, Nat. Catal., 2021, 5, 20–29 CrossRef.
- B. Singh, M. B. Gawande, A. D. Kute, R. S. Varma, P. Fornasiero, P. McNeice, R. V. Jagadeesh, M. Beller and R. Zboril, Single-Atom (Iron-Based) Catalysts: Synthesis and Applications, Chem. Rev., 2021, 121, 13620–13697 CrossRef CAS PubMed.
- A. Furstner, Iron Catalysis in Organic Synthesis: A Critical Assessment of What It Takes To Make This Base Metal a Multitasking Champion, ACS Cent. Sci., 2016, 2, 778–789 CrossRef PubMed.
- M. Neumeier, U. Chakraborty, D. Schaarschmidt, V. de la Pena O'Shea, R. Perez-Ruiz and A. Jacobi von Wangelin, Combined Photoredox and Iron Catalysis for the Cyclotrimerization of Alkynes, Angew Chem. Int. Ed. Engl., 2020, 59, 13473–13478 CrossRef CAS PubMed.
- H. T. Zhang, X. J. Su, F. Xie, R. Z. Liao and M. T. Zhang, Iron-Catalyzed Water Oxidation: O-O Bond Formation via Intramolecular Oxo-Oxo Interaction, Angew Chem. Int. Ed. Engl., 2021, 60, 12467–12474 CrossRef CAS PubMed.
- G. Jin, C. G. Werncke, Y. Escudie, S. Sabo-Etienne and S. Bontemps, Iron-Catalyzed Reduction of CO2 into Methylene: Formation of C-N, C-O, and C-C Bonds, J. Am. Chem. Soc., 2015, 137, 9563–9566 CrossRef CAS PubMed.
- E. de Smit and B. M. Weckhuysen, The renaissance of iron-based Fischer-Tropsch synthesis: on the multifaceted catalyst deactivation behaviour, Chem. Soc. Rev., 2008, 37, 2758–2781 RSC.
- S. Enthaler, K. Junge and M. Beller, Sustainable metal catalysis with iron: from rust to a rising star?, Angew Chem. Int. Ed. Engl., 2008, 47, 3317–3321 CrossRef CAS PubMed.
- W. Liu, W. Li, A. Spannenberg, K. Junge and M. Beller, Iron-catalysed regioselective hydrogenation of terminal epoxides to alcohols under mild conditions, Nat. Catal., 2019, 2, 523–528 CrossRef CAS.
- J. Gu, C. S. Hsu, L. Bai, H. M. Chen and X. Hu, Atomically dispersed Fe(3+) sites catalyze efficient CO2 electroreduction to CO, Science, 2019, 364, 1091–1094 CrossRef CAS PubMed.
- W. Liu, L. Zhang, X. Liu, X. Liu, X. Yang, S. Miao, W. Wang, A. Wang and T. Zhang, Discriminating Catalytically Active FeNx Species of Atomically Dispersed Fe-N-C Catalyst for Selective Oxidation of the C-H Bond, J. Am. Chem. Soc., 2017, 139, 10790–10798 CrossRef CAS PubMed.
- M. L. Bols, B. E. R. Snyder, H. M. Rhoda, P. Cnudde, G. Fayad, R. A. Schoonheydt, V. Van Speybroeck, E. I. Solomon and B. F. Sels, Coordination and activation of nitrous oxide by iron zeolites, Nat. Catal., 2021, 4, 332–340 CrossRef CAS.
- L. Piccirilli, B. Rabell, R. Padilla, A. Riisager, S. Das and M. Nielsen, Versatile CO2 Hydrogenation-Dehydrogenation Catalysis with a Ru-PNP/Ionic Liquid System, J. Am. Chem. Soc., 2023, 145, 5655–5663 CrossRef CAS PubMed.
- R. Cauwenbergh, V. Goyal, R. Maiti, K. Natte and S. Das, Challenges and recent advancements in the transformation of CO2 into carboxylic acids: straightforward assembly with homogeneous 3d metals, Chem. Soc. Rev., 2022, 51, 9371–9423 RSC.
- P. K. Sahoo, Y. Zhang and S. Das, CO2-Promoted Reactions: An Emerging Concept for the Synthesis of Fine Chemicals and Pharmaceuticals, ACS Catal., 2021, 11, 3414–3442 CrossRef CAS.
- R. Cauwenbergh and S. Das, Photochemical reduction of carbon dioxide to formic acid, Green Chem., 2021, 23, 2553–2574 RSC.
- Y. Zhang, T. Zhang and S. Das, Catalytic transformation of CO2 into C1 chemicals using hydrosilanes as a reducing agent, Green Chem., 2020, 22, 1800–1820 RSC.
- W. Schilling and S. Das, Transition Metal-Free Synthesis of Carbamates Using CO2 as the Carbon Source, ChemSusChem, 2020, 13, 6246–6258 CrossRef CAS PubMed.
- S. Lin, C. S. Diercks, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water, Science, 2015, 349, 1208–1213 CrossRef CAS PubMed.
- X. Li, Y. Sun, J. Xu, Y. Shao, J. Wu, X. Xu, Y. Pan, H. Ju, J. Zhu and Y. Xie, Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers, Nat. Energy, 2019, 4, 690–699 CrossRef CAS.
- H. Rao, L. C. Schmidt, J. Bonin and M. Robert, Visible-light-driven methane formation from CO2 with a molecular iron catalyst, Nature, 2017, 548, 74–77 CrossRef CAS PubMed.
- K. Kosugi, C. Akatsuka, H. Iwami, M. Kondo and S. Masaoka, Iron-Complex-Based Supramolecular Framework Catalyst for Visible-Light-Driven CO2 Reduction, J. Am. Chem. Soc., 2023, 145, 10451–10457 CrossRef CAS PubMed.
- Q. Liu, L. Wu, R. Jackstell and M. Beller, Using carbon dioxide as a building block in organic synthesis, Nat. Commun., 2015, 6, 5933 CrossRef PubMed.
- Q.-W. Song, Z.-H. Zhou and L.-N. He, Efficient, selective and sustainable catalysis of carbon dioxide, Green Chem., 2017, 19, 3707–3728 RSC.
- C. Vogt, M. Monai, E. B. Sterk, J. Palle, A. E. M. Melcherts, B. Zijlstra, E. Groeneveld, P. H. Berben, J. M. Boereboom, E. J. M. Hensen, F. Meirer, I. A. W. Filot and B. M. Weckhuysen, Understanding carbon dioxide activation and carbon-carbon coupling over nickel, Nat. Commun., 2019, 10, 5330 CrossRef PubMed.
- S. Kelly and J. A. Sullivan, CO2 Decomposition in CO2 and CO2/H2 Spark-like Plasma Discharges at Atmospheric Pressure, ChemSusChem, 2019, 12, 3785–3791 CrossRef CAS PubMed.
- Y. Qin, R. Cauwenbergh, S. Pradhan, R. Maiti, P. Frank and S. Das, Straightforward Synthesis of Functionalized γLactams using Impure CO2 Stream as the Carbon Source, Nat. Commun., 2023, 14, 7604–7612 CrossRef CAS PubMed.
- Z. Fang, Y. Wang, Y. Hu, B. Yao, Z. Ye and X. Peng, A CO2-philic ferrocene-based porous organic polymer for solar-driven CO2 conversion from flue gas, J. Mater. Chem. A, 2023, 11, 18272–18279 RSC.
- Y. Cheng, J. Hou and P. Kang, Integrated Capture and Electroreduction of Flue Gas CO2 to Formate Using Amine Functionalized SnOx Nanoparticles, ACS Energy Lett., 2021, 6, 3352–3358 CrossRef CAS.
- F. Yang, C. Liang, W. Zhou, W. Zhao, P. Li, Z. Hua, H. Yu, S. Chen, S. Deng, J. Li, Y. M. Lam and J. Wang, Oxide-Derived Bismuth as an Efficient Catalyst for Electrochemical Reduction of Flue Gas, Small, 2023, 19, 2300417–2300427 CrossRef CAS PubMed.
- T. Al-Attas, S. K. Nabil, A. S. Zeraati, H. S. Shiran, T. Alkayyali, M. Zargartalebi, T. Tran, N. N. Marei, M. A. Al Bari, H. Lin, S. Roy, P. M. Ajayan, D. Sinton, G. Shimizu and M. G. Kibria, Permselective MOF-Based Gas Diffusion Electrode for Direct Conversion of CO2 from Quasi Flue Gas, ACS Energy Lett., 2022, 8, 107–115 CrossRef.
- S.-H. Guo, X.-J. Qi, H.-M. Zhou, J. Zhou, X.-H. Wang, M. Dong, X. Zhao, C.-Y. Sun, X.-L. Wang and Z.-M. Su, A bimetallic-MOF catalyst for efficient CO2 photoreduction from simulated flue gas to value-added formate, J. Mater. Chem. A, 2020, 8, 11712–11718 RSC.
- S. Kar, M. Rahaman, V. Andrei, S. Bhattacharjee, S. Roy and E. Reisner, Integrated capture and solar-driven utilization of CO2 from flue gas and air, Joule, 2023, 7, 1496–1514 CrossRef CAS.
- M. Dong, J. Zhou, J. Zhong, H. T. Li, C. Y. Sun, Y. D. Han, J. N. Kou, Z. H. Kang, X. L. Wang and Z. M. Su, CO2 Dominated Bifunctional Catalytic Sites for Efficient Industrial Exhaust Conversion, Adv. Funct. Mater., 2021, 32, 2110136–2110144 CrossRef.
- Y. Ma, X. Yi, S. Wang, T. Li, B. Tan, C. Chen, T. Majima, E. R. Waclawik, H. Zhu and J. Wang, Selective photocatalytic CO2 reduction in aerobic environment by microporous Pd-porphyrin-based polymers coated hollow TiO2, Nat. Commun., 2022, 13, 1400–1409 CrossRef CAS PubMed.
- Z.-Z. Yang, L.-N. He, J. Gao, A.-H. Liu and B. Yu, Carbon dioxide utilization with C–N bond formation: carbon dioxide capture and subsequent conversion, Energy Environ. Sci., 2012, 5, 6602–6639 RSC.
- Z.-Z. Yang, L.-N. He, Y.-N. Zhao, B. Li and B. Yu, CO2 capture and activation by superbase/polyethylene glycol and its subsequent conversion, Energy Environ. Sci., 2011, 4, 3971–3975 RSC.
- K. Stanley, S. Kelly and J. A. Sullivan, Effect of Ni NP morphology on catalyst performance in non-thermal plasma-assisted dry reforming of methane, Appl. Catal., B, 2023, 328, 122533–122540 CrossRef CAS.
- M. Dong, J. X. Gu, C. Y. Sun, X. L. Wang and Z. M. Su, Photocatalytic reduction of low-concentration CO2 by metal-organic frameworks, Chem. Commun., 2022, 58, 10114–10126 RSC.
- T. Kajiwara, M. Fujii, M. Tsujimoto, K. Kobayashi, M. Higuchi, K. Tanaka and S. Kitagawa, Photochemical Reduction of Low Concentrations of CO2 in a Porous Coordination Polymer with a Ruthenium(II)-CO Complex, Angew Chem. Int. Ed. Engl., 2016, 55, 2697–2700 CrossRef CAS PubMed.
- D. Kim, W. Choi, H. W. Lee, S. Y. Lee, Y. Choi, D. K. Lee, W. Kim, J. Na, U. Lee, Y. J. Hwang and D. H. Won, Electrocatalytic Reduction of Low Concentrations of CO2 Gas in a Membrane Electrode Assembly Electrolyzer, ACS Energy Lett., 2021, 6, 3488–3495 CrossRef CAS.
- H. Kumagai, T. Nishikawa, H. Koizumi, T. Yatsu, G. Sahara, Y. Yamazaki, Y. Tamaki and O. Ishitani, Electrocatalytic reduction of low concentration CO2, Chem. Sci., 2019, 10, 1597–1606 RSC.
- T. Nakajima, Y. Tamaki, K. Ueno, E. Kato, T. Nishikawa, K. Ohkubo, Y. Yamazaki, T. Morimoto and O. Ishitani, Photocatalytic Reduction of Low Concentration of CO2, J. Am. Chem. Soc., 2016, 138, 13818–13821 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, A metal-free polymeric photocatalyst for hydrogen production from water under visible light, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- Y. Wang, X. Wang and M. Antonietti, Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry, Angew Chem. Int. Ed. Engl., 2012, 51, 68–89 CrossRef CAS PubMed.
- P. Ren, T. Zhang, N. Jain, H. Y. V. Ching, A. Jaworski, G. Barcaro, S. Monti, J. Silvestre-Albero, V. Celorrio, L. Chouhan, A. Rokicinska, E. Debroye, P. Kustrowski, S. Van Doorslaer, S. Van Aert, S. Bals and S. Das, An Atomically Dispersed Mn-Photocatalyst for Generating Hydrogen Peroxide from Seawater via the Water Oxidation Reaction (WOR), J. Am. Chem. Soc., 2023, 145, 16584–16596 CrossRef CAS PubMed.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, Polymer semiconductors for artificial photosynthesis: hydrogen evolution by mesoporous graphitic carbon nitride with visible light, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed.
- T. Zhang, W. Schilling, S. U. Khan, H. Y. V. Ching, C. Lu, J. Chen, A. Jaworski, G. Barcaro, S. Monti, K. De Wael, A. Slabon and S. Das, Atomic-Level Understanding for the Enhanced Generation of Hydrogen Peroxide by the Introduction of an Aryl Amino Group in Polymeric Carbon Nitrides, ACS Catal., 2021, 11, 14087–14101 CrossRef CAS.
- X. Wang, X. Chen, A. Thomas, X. Fu and M. Antonietti, Metal-Containing Carbon Nitride Compounds: A New Functional Organic-Metal Hybrid Material, Adv. Mater., 2009, 21, 1609–1612 CrossRef CAS.
- R. Cheng, H. Jin, M. B. J. Roeffaers, J. Hofkens and E. Debroye, Incorporation of Cesium Lead Halide Perovskites into g-C3N4 for Photocatalytic CO2 Reduction, ACS Omega, 2020, 5, 24495–24503 CrossRef CAS PubMed.
- R. Kuriki, M. Yamamoto, K. Higuchi, Y. Yamamoto, M. Akatsuka, D. Lu, S. Yagi, T. Yoshida, O. Ishitani and K. Maeda, Robust Binding between Carbon Nitride Nanosheets and a Binuclear Ruthenium(II) Complex Enabling Durable, Selective CO2 Reduction under Visible Light in Aqueous Solution, Angew Chem. Int. Ed. Engl., 2017, 56, 4867–4871 CrossRef CAS PubMed.
- S. R. E. Reisner, Visible-Light-Driven CO2 Reduction by Mesoporous Carbon Nitride Modified with Polymeric Cobalt Phthalocyanine, Angew Chem. Int. Ed. Engl., 2019, 58, 12180–12184 CrossRef PubMed.
- J. Lin, Z. Pan and X. Wang, Photochemical Reduction of CO2 by Graphitic Carbon Nitride Polymers, ACS Sustain. Chem. Eng., 2014, 2, 353–358 CrossRef CAS.
- Y. Zhang, M. Cao, H. Feng, D. Liu and Q. Li, Understanding and Tuning Charge Dynamics in Carbon Nitride/Cobalt(II) Complex Hybrids for Enhanced Photocatalytic CO2 Reduction, ACS Catal., 2023, 13, 11376–11388 CrossRef CAS.
- B. Ma, G. Chen, C. Fave, L. Chen, R. Kuriki, K. Maeda, O. Ishitani, T. C. Lau, J. Bonin and M. Robert, Efficient Visible-Light-Driven CO2 Reduction by a Cobalt Molecular Catalyst Covalently Linked to Mesoporous Carbon Nitride, J. Am. Chem. Soc., 2020, 142, 6188–6195 CrossRef CAS PubMed.
- C. Cometto, R. Kuriki, L. Chen, K. Maeda, T. C. Lau, O. Ishitani and M. Robert, A Carbon Nitride/Fe Quaterpyridine Catalytic System for Photostimulated CO2-to-CO Conversion with Visible Light, J. Am. Chem. Soc., 2018, 140, 7437–7440 CrossRef CAS PubMed.
- Y. Wei, L. Chen, H. Chen, L. Cai, G. Tan, Y. Qiu, Q. Xiang, G. Chen, T. C. Lau and M. Robert, Highly Efficient Photocatalytic Reduction of CO2 to CO by In Situ Formation of a Hybrid Catalytic System Based on Molecular Iron Quaterpyridine Covalently Linked to Carbon Nitride, Angew Chem. Int. Ed. Engl., 2022, 61, e202116832 CrossRef CAS PubMed.
- L. Lin, C. Hou, X. Zhang, Y. Wang, Y. Chen and T. He, Highly efficient visible-light driven photocatalytic reduction of CO2 over g-C3N4 nanosheets/tetra(4-carboxyphenyl)porphyrin iron(III) chloride heterogeneous catalysts, Appl. Catal., B, 2018, 221, 312–319 CrossRef CAS.
- Z. Jiang, W. Wan, H. Li, S. Yuan, H. Zhao and P. K. Wong, A Hierarchical Z-Scheme alpha-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction, Adv. Mater., 2018, 30, 1706108–1706116 CrossRef PubMed.
- M. Zhang, C. Lai, B. Li, F. Xu, D. Huang, S. Liu, L. Qin, Y. Fu, X. Liu, H. Yi, Y. Zhang, J. He and L. Chen, Unravelling the role of dual quantum dots cocatalyst in 0D/2D heterojunction photocatalyst for promoting photocatalytic organic pollutant degradation, Chem. Eng. J., 2020, 396, 125343–125355 CrossRef CAS.
- J. Tang, R. Xu, G. Sui, D. Guo, Z. Zhao, S. Fu, X. Yang, Y. Li and J. Li, Double-Shelled Porous g-C3N4 Nanotubes Modified with Amorphous Cu-Doped FeOOH Nanoclusters as 0D/3D Non-Homogeneous Photo-Fenton Catalysts for Effective Removal of Organic Dyes, Small, 2023, 19, e2208232 CrossRef PubMed.
- X. Qian, Y. Wu, M. Kan, M. Fang, D. Yue, J. Zeng and Y. Zhao, FeOOH quantum dots coupled g-C3N4 for visible light driving photo- Fenton degradation of organic pollutants, Appl. Catal., B, 2018, 237, 513–520 CrossRef CAS.
- M. Shi, P. Xiao, J. Lang, C. Yan and X. Yan, Porous g-C3N4 and MXene Dual-Confined FeOOH Quantum Dots for Superior Energy Storage in an Ionic Liquid, Adv. Sci., 2020, 7, 1901975 CrossRef CAS PubMed.
- B. Dong, J. Cui, Y. Gao, Y. Qi, F. Zhang and C. Li, Heterostructure of 1D Ta3N5 Nanorod/BaTaO2 N Nanoparticle Fabricated by a One-Step Ammonia Thermal Route for Remarkably Promoted Solar Hydrogen Production, Adv. Mater., 2019, 31, 1808185–1808191 CrossRef PubMed.
- D. Xiao, K. Dai, Y. Qu, Y. Yin and H. Chen, Hydrothermal synthesis of α-Fe2O3/g-C3N4 composite and its efficient photocatalytic reduction of Cr(VI) under visible light, Appl. Surf. Sci., 2015, 358, 181–187 CrossRef CAS.
- H.-K. Wu, Y.-H. Li, M.-Y. Qi, Q. Lin and Y.-J. Xu, Enhanced photocatalytic CO2 reduction with suppressing H2 evolution via Pt cocatalyst and surface SiO2 coating, Appl. Catal., B, 2020, 278, 119267–119274 CrossRef CAS.
- D. Tan, J. Zhang, J. Shi, S. Li, B. Zhang, X. Tan, F. Zhang, L. Liu, D. Shao and B. Han, Photocatalytic CO2 Transformation to CH4 by Ag/Pd Bimetals Supported on N-Doped TiO2 Nanosheet, ACS Appl. Mater. Interfaces, 2018, 10, 24516–24522 CrossRef CAS PubMed.
- J. Bonin, M. Robert and M. Routier, Selective and Efficient Photcatalytic CO2 Reduction to CO Using Visible Light and an Iron Based Homogeneous Catalyst, J. Am. Chem. Soc., 2014, 136, 16768–16771 CrossRef CAS PubMed.
- P. Huang, J. Huang, S. A. Pantovich, A. D. Carl, T. G. Fenton, C. A. Caputo, R. L. Grimm, A. I. Frenkel and G. Li, Selective CO2 Reduction Catalyzed by Single Cobalt Sites on Carbon Nitride under Visible-Light Irradiation, J. Am. Chem. Soc., 2018, 140, 16042–16047 CrossRef CAS PubMed.
- B. Xiong, J. Liu, Y. Yang, Y. Yang and Z. Hua, Effect mechanism of NO on electrocatalytic reduction of CO2 to CO over Pd@Cu bimetal catalysts, Fuel, 2022, 323, 124339–124345 CrossRef CAS.
- J. Gao, Y. Wang, S. Zhou, W. Lin and Y. Kong, A Facile One-Step Synthesis of Fe-Doped g-C3N4 Nanosheets and Their Improved Visible-Light Photocatalytic Performance, ChemCatChem, 2017, 9, 1708–1715 CrossRef CAS.
- W. Luo, W. Huang, X. Feng, Y. Huang, X. Song, H. Lin, S. Wang and G. Mailhot, The utilization of Fe-doped g-C3N4 in a heterogeneous photo-Fenton-like catalytic system: the effect of different parameters and a system mechanism investigation, RSC Adv., 2020, 10, 21876–21886 RSC.
- H. Li, C. Shan and B. Pan, Fe(III)-Doped g-C3N4 Mediated Peroxymonosulfate Activation for Selective Degradation of Phenolic Compounds via High-Valent Iron-Oxo Species, Environ. Sci. Technol., 2018, 52, 2197–2205 CrossRef CAS PubMed.
- T. Ma, Q. Shen, B. Z. J. Xue, R. Guan, X. Liu, H. Jia and B. Xu, Facile synthesis of Fe-doped g-C3N4 for enhanced visible-light photocatalytic activity, Inorg. Chem. Commun., 2019, 107, 107451–107459 CrossRef CAS.
- S. Hu, R. Jin, G. Lu, D. Liu and J. Gui, The properties and photocatalytic performance comparison of Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 composite catalysts, RSC Adv., 2014, 4, 24863–24869 RSC.
- S. Ye, L. G. Qiu, Y. P. Yuan, Y. J. Zhu, J. Xia and J. F. Zhu, Facile fabrication of magnetically separable graphitic carbon nitride photocatalysts with enhanced photocatalytic activity under visible light, J. Mater. Chem. A, 2013, 1, 3008–3015 RSC.
- Y. Zhang, S. Qin, N. Claes, W. Schilling, P. K. Sahoo, H. Y. V. Ching, A. Jaworski, F. Lemière, A. Slabon, S. Van Doorslaer, S. Bals and S. Das, Direct Solar Energy-Mediated Synthesis of Tertiary Benzylic Alcohols Using a Metal-Free Heterogeneous Photocatalyst, ACS Sustain. Chem. Eng., 2021, 10, 530–540 CrossRef.
- H. Fukaya and T. Ono, DFT-GIAO calculations of 19F NMR chemical shifts for perfluoro compounds, J. Comput. Chem., 2004, 25, 51–60 CrossRef CAS PubMed.
- E. Zhao, W. Zhang, L. Dong, R. Zbořil and Z. Chen, Photocatalytic Transfer Hydrogenation Reactions Using Water as the Proton Source, ACS Catal., 2023, 13, 7557–7567 CrossRef CAS.
- A. Jaworski and N. Hedin, Electron correlation and vibrational effects in predictions of paramagnetic NMR shifts, Phys. Chem. Chem. Phys., 2022, 24, 15230–15244 RSC.
- Y. Huang, B. Chen, J. Duan, F. Yang, T. Wang, Z. Wang, W. Yang, C. Hu, W. Luo and Y. Huang, Graphitic Carbon Nitride (g-C3N4): An Interface Enabler for Solid-State Lithium Metal Batteries, Angew. Chem., 2020, 132, 3728–3733 CrossRef.
- F. Dong, Z. Zhao, T. Xiong, Z. Ni, W. Zhang, Y. Sun and W. K. Ho, In situ construction of g-C3N4/g-C3N4 metal-free heterojunction for enhanced visible-light photocatalysis, ACS Appl. Mater. Interfaces, 2013, 5, 11392–11401 CrossRef CAS PubMed.
- C. P. Rajan, N. Abharana, S. N. Jha, D. Bhattacharyya and T. T. John, Local Structural Studies Through EXAFS and Effect of Fe2+or Fe3+ Existence in ZnO Nanoparticles, J. Phys. Chem. C, 2021, 125, 13523–13533 CrossRef CAS.
- M. C. Biesinger, Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review, Appl. Surf. Sci., 2022, 597, 153681–153691 CrossRef CAS.
- X. Xi, X. Peng, C. Xiong, D. Shi, J. Zhu, W. Wen, X. Zhang and S. Wang, Iron doped graphitic carbon nitride with peroxidase like activity for colorimetric detection of sarcosine and hydrogen peroxide, Mikrochim. Acta, 2020, 187, 383–393 CrossRef CAS PubMed.
- X. Ye, Y. Cui and X. Wang, Ferrocene-modified carbon nitride for direct oxidation of benzene to phenol with visible light, ChemSusChem, 2014, 7, 738–742 CrossRef CAS PubMed.
- A. M. Masdeu-Bultó, M. Reguero and C. Claver, Mechanistic Insights of Photocatalytic CO2 Reduction: Experimental versus Computational Studies, Eur. J. Inorg. Chem., 2022, 2022, e202100975 CrossRef.
- K. Homlamai, T. Maihom, S. Choomwattana, M. Sawangphruk and J. Limtrakul, Single-atoms supported (Fe, Co, Ni, Cu) on graphitic carbon nitride for CO2 adsorption and hydrogenation to formic acid: First-principles insights, Appl. Surf. Sci., 2020, 499, 143928–143934 CrossRef CAS.
- S. Osella and W. A. Goddard Iii, CO2 Reduction to Methane and Ethylene on a Single-Atom Catalyst: A Grand Canonical Quantum Mechanics Study, J. Am. Chem. Soc., 2023, 145, 21319–21329 CrossRef CAS PubMed.
- S. Fozia, A. Hassan, S. A. Reshi, P. Singh, G. A. Bhat, M. Dixit and M. A. Dar, Boosting CO2 Activation and Reduction by Engineering the Electronic Structure of Graphitic Carbon Nitride through Transition Metal-Free Single-Atom Functionalization, J. Phys. Chem. C, 2023, 127, 11911–11920 CrossRef CAS.
- G. Gao, Y. Jiao, E. R. Waclawik and A. Du, Single Atom (Pd/Pt) Supported on Graphitic Carbon Nitride as an Efficient Photocatalyst for Visible-Light Reduction of Carbon Dioxide, J. Am. Chem. Soc., 2016, 138, 6292–6297 CrossRef CAS PubMed.
- S. Liang, L. Huang, Y. Gao, Q. Wang and B. Liu, Electrochemical Reduction of CO2 to CO over Transition Metal/N-Doped Carbon Catalysts: The Active Sites and Reaction Mechanism, Adv. Sci., 2021, 8, e2102886 CrossRef PubMed.
- B. M. Abraham, O. Pique, M. A. Khan, F. Vines, F. Illas and J. K. Singh, Machine Learning-Driven Discovery of Key Descriptors for CO2 Activation over Two-Dimensional Transition Metal Carbides and Nitrides, ACS Appl. Mater. Interfaces, 2023, 15, 30117–30126 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gom-perts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmay-lov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lippa-rini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Ha-segawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Ada-mo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- J. K. Norskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, Origin of the overpotential for oxygen reduction at a fuel-cell cathode, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef CAS.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- C. A. Ohlin, Energetics of paramagnetic oxide clusters: the Fe(iii) oxyhydroxy Keggin ion, Phys. Chem. Chem. Phys., 2020, 22, 4043–4050 RSC.
- J. P. Foster and F. Weinhold, Natural Hybrid Orbitals, J. Am. Chem. Soc., 1980, 102, 7211–7218 CrossRef CAS.
- A. E. Reed, R. B. Weinstock and F. Weinhold, Natural population analysis, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc02773f |
| ‡ Denotes equal contributions. |
| This journal is © The Royal Society of Chemistry 2024 |