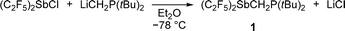Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A geminal antimony(III)/phosphorus(III) frustrated Lewis pair†
Jonas
Krieft
,
Pia C.
Trapp
,
Yury V.
Vishnevskiy
 ,
Beate
Neumann
,
Hans-Georg
Stammler
,
Jan-Hendrik
Lamm
and
Norbert W.
Mitzel
,
Beate
Neumann
,
Hans-Georg
Stammler
,
Jan-Hendrik
Lamm
and
Norbert W.
Mitzel
 *
*
Chair of Inorganic and Structural Chemistry, Center for Molecular Materials CM2, Faculty of Chemistry, Bielefeld University, Universitätsstraße 25, Bielefeld 33615, Germany. E-mail: mitzel@uni-bielefeld.de
First published on 1st July 2024
Abstract
The geminal Lewis pair (F5C2)2SbCH2P(tBu)2 (1) was prepared by reacting (F5C2)2SbCl with LiCH2P(tBu)2. Despite its extremely electronegative pentafluoroethyl substituents, the neutral 1 exhibits a relatively soft acidic antimony function according to the HSAB concept (hard–soft acid–base). These properties lead to a reversibility in the binding of CS2 to 1, as observed by VT-NMR spectroscopy, while no reaction with CO2 is observed. The reaction behaviour towards heterocumulenes and the specific interaction situation in the CS2 adduct were analysed by quantum chemical calculations. The FLP-type reactivity of 1 has also been demonstrated by reaction with a variety of small molecules (SO2, PhNCO, PhNCS, (MePh2P)AuCl). The reactions of 1 with PhNCO and PhNCS led to different types of cyclic addition products: PhNCO adds with its N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and PhNCS adds preferentially with its C
C bond and PhNCS adds preferentially with its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond. The reaction of 1 with (MePh2P)AuCl gave an adduct {[(F5C2)2SbCH2(tBu)2P]2Au}+ with a clamp-like structure binding a chloride anion by its two antimony atoms in chelate mode. Compound 1 and its adducts have been characterised by X-ray diffraction experiments, multinuclear NMR spectroscopy, elemental analyses and computational calculations (DFT, QTAIM, IQA).
S bond. The reaction of 1 with (MePh2P)AuCl gave an adduct {[(F5C2)2SbCH2(tBu)2P]2Au}+ with a clamp-like structure binding a chloride anion by its two antimony atoms in chelate mode. Compound 1 and its adducts have been characterised by X-ray diffraction experiments, multinuclear NMR spectroscopy, elemental analyses and computational calculations (DFT, QTAIM, IQA).
Introduction
Since the pioneering work of Stephan and Erker in the field of Frustrated Lewis Pairs (FLP), this part of modern main group chemistry has developed rapidly and in many directions.1 Steric shielding and ring strain can prevent the formation of a stable adduct between Lewis acid and Lewis base sites and thus the neutralisation of the two functions within one molecule. The unused reaction potential can now be used to activate various small molecules or to catalyse reactions.2–5 The diversity of combinations of Lewis acids and bases in FLP systems continues to grow, but the “typical” combinations of Lewis acids of the third main group (B, Al) and Lewis bases of the fifth main group (N, P) dominate.6,7 The use of pnictogens in the base functions is established, but the elements of this main group can also have interesting Lewis acidic properties, making them very interesting and variable building blocks.8 At least since Olah's work on the so-called magic acid, antimony compounds have become an indispensable part of Lewis acid chemistry.9,10 Several contributions of Gabbaï and co-workers have shown that Lewis acidic stibonium ions and various stiboranes are not only able to activate C–F bonds,11 but also to trap halide ions12 and to act as ligands for transition metal complexes.13 Antimony has a special position in this respect due to its most pronounced Lewis acidity within the group.14 Despite Sb(III) atoms have a free pair of electrons, their Lewis acidity can be reinforced by introducing perfluorinated substituents.10,15 In this way, a distinct σ-hole can be induced on the formally Lewis basic Sb(III) atom. The acidity of this σ-hole depends on the electron-withdrawing properties of the spatially opposite substituent, making it an interesting and flexible building block for the synthesis of functionalised Lewis acids and FLP systems.16We have recently reported a bidentate and a tetradentate Sb(III)-based poly-Lewis acid capable of chelate-binding halide ions, dimethyl chalcogenides and nitrogen heterocycles by pnictogen bonding.17,18 We have also exploited the special properties of the Sb(C2F5)2 moiety to develop the new neutral geminal FLP presented here. In terms of Pearson's HSAB concept,19 the relatively soft Lewis acid Sb(III) of this unit should be favourable for reversible reactions. Similarly, we have previously introduced the Sn/P-FLP (F5C2)3SnCH2P(tBu)2, which reversibly binds CO2 while forming a stable adduct with the softer CS2. (F5C2)3SnCH2P(tBu)2 also reacts with a variety of small molecules and stabilises highly reactive species, including the elusive sulphur monoxide.5,20,21
Results and discussion
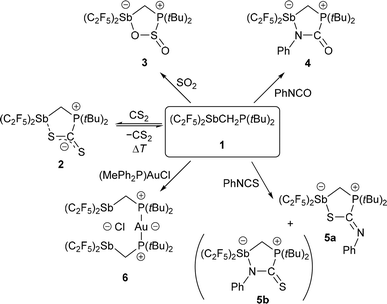
Starting from (F5C2)2SbCl,17 the intramolecular FLP (F5C2)2SbCH2P(tBu)2 (1) was prepared by reaction with LiCH2P(tBu)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 in a nucleophilic substitution (Scheme 1).
22 in a nucleophilic substitution (Scheme 1).
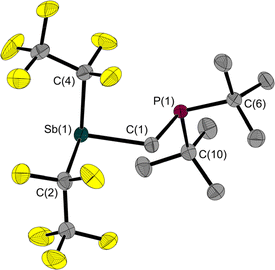
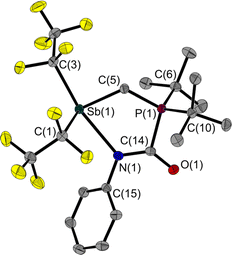
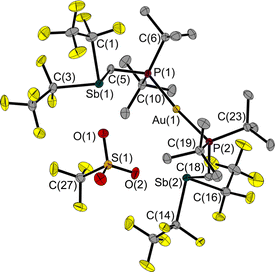
Since 1 is a liquid under normal conditions, a single crystal suitable for X-ray diffraction was grown by in situ crystallisation at 283.9 K on the diffractometer. After the formation of a tiny seed crystal, the sample was cooled to 100 K. The molecular structure (Fig. 1) shows a distance between the Sb and P atoms in 1 of 3.306(1) Å, much longer than the sum of the covalent radii indicating that, at most, only a weakly stabilising interaction exists.23 The Sb⋯P distance is between geminal atoms and thus by its nature less than the sum of the van der Waals radii. Therefore, the Sb–C–P angle is more telling about a possible attractive Sb⋯P interaction. At 110.6(1)° it is smaller than the corresponding Sn–C–P angle in the Sn/P-FLP mentioned above with 113.9(1)°.5 However, the Sb–C–P angle of the rigid methylene backbone is too obtuse for the formation of an intramolecular Lewis acid/base adduct, as was also observed in all (F5C2)nECH2P(tBu)2 FLPs we have presented so far.3–5 The Sb atom is trigonal-pyramidal coordinated, as is common for trisubstituted pnictogen atoms in the oxidation state +III. Expectedly, the angles including the Sb position are between 91.3(1) and 95.8(1)°, i.e. close to 90°.24
The 31P NMR resonance of 1 at 15.5 ppm is in the typical range for methylene-bridged Lewis acid/P(tBu)2 systems (e.g. δ(31P) (F5C2)3SnCH2P(tBu)2 17.2 ppm,5 (F5C2)3SiCH2P(tBu)2 18.5 ppm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Also typical for these systems are the 13C{1H} NMR resonances of 1 for the methylene carbon atoms at 9.0 ppm and the 19F NMR resonances of the pentafluoroethyl groups at −82.7 and −110.5/−111.1 ppm.4,5
4). Also typical for these systems are the 13C{1H} NMR resonances of 1 for the methylene carbon atoms at 9.0 ppm and the 19F NMR resonances of the pentafluoroethyl groups at −82.7 and −110.5/−111.1 ppm.4,5
We performed Lewis acidity tests by the Gutmann–Beckett method25 with OPEt3 and the modified method for soft Lewis acids with SePMe3 presented by Lichtenberg.26 After addition of OPEt3 to 1, we did not see any variation in the chemical shifts of 1 and OPEt3. We assume that the antimony-oxygen interaction is too unfavourable. With the softer Lewis base SePMe3, we observed a selenium transfer from SePMe3 to 1 to give (F5C2)2SbCH2(Se)P(tBu)2 and PMe3.
Since these experimental Lewis acidity tests did not give a conclusive answer, we calculated the fluorine ion affinity (FIA) of 1 using a method by Greb et al. with an FIA of 278 kJ mol−1 it is well comparable to AsCl3 (276 kJ mol−1) and is below, for example, SbF3 (290 kJ mol−1), SbCl3 (309 kJ mol−1) and Sb(C2F5)3 (315 kJ mol−1).15
The reaction of FLP 1 with CO2 gave no detectable adduct. In contrast, the reaction with CS2 resulted in a temperature-dependent equilibrium, as observed by VT-NMR spectroscopy (Fig. 2). At room temperature, there is an equilibrium between the adduct 2 and the free FLP 1 plus free CS2 in approximately equal proportions. After cooling the solution to 233 K, the adduct is dominant in the solution and only 10% of the free FLP remains unbound. Cooling the solution shifts the resonance of 1 (δ(31P) at 298 K: 15.6 ppm) towards high field, while the multiplet of 2 (δ(31P) at 298 K: 32.5 ppm) is low-field shifted.
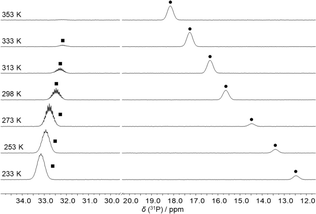 | ||
| Fig. 2 31P NMR spectra of a sample of a mixture of 1 and CS2 at different temperatures. The peaks of the FLP 1 (●) and of the CS2 adduct 2 (■) are labelled. | ||
This experimentally observed behaviour is confirmed by the results of Density Functional Theory (DFT) calculations (composite method r2SCAN-3c).27 For the reaction of 1 with CS2 to give the adduct 2 at room temperature, the change in free enthalpy is predicted to be very small: 4 kJ mol−1.
The calculation predicts that the reaction is exergonic at 233 K (ΔG233![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = −9 kJ mol−1). In contrast, when considering the conversion of 1 with CO2, clearly positive values are calculated for both temperatures, 298 K (ΔG298
K = −9 kJ mol−1). In contrast, when considering the conversion of 1 with CO2, clearly positive values are calculated for both temperatures, 298 K (ΔG298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 23 kJ mol−1) and 233 K (ΔG233
K = 23 kJ mol−1) and 233 K (ΔG233![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 11 kJ mol−1). Even when the pressure is increased from 1 to 10 atm, the values for the two temperatures remain positive, although slightly lower (ΔG298
K = 11 kJ mol−1). Even when the pressure is increased from 1 to 10 atm, the values for the two temperatures remain positive, although slightly lower (ΔG298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 17 kJ mol−1; ΔG233
K = 17 kJ mol−1; ΔG233![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 7 kJ mol−1). This theoretical prediction supports the experimental finding that 1 does not react with CO2 to form a corresponding adduct.
K = 7 kJ mol−1). This theoretical prediction supports the experimental finding that 1 does not react with CO2 to form a corresponding adduct.
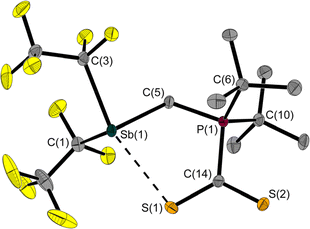
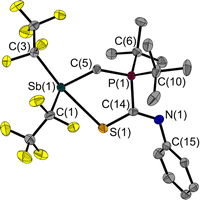
The room temperature labile deep red crystals of 2 have been examined by X-ray diffraction. Unlike CS2 adducts of comparable FLPs,5,7 the structure of 2 is not that of a typical five-membered heterocycle (Fig. 3).
The Sb⋯S distance of 2.964(1) Å is intermediate between the sum of the van der Waals radii (ΣrvdW(Sb,S) = 3.86 Å)28,29 and the sum of the covalent radii (Σrcovalent(Sb,S) = 2.45 Å) with a tendency towards the latter. The attractive Sb⋯S interaction leads to a quasi-five-membered ring. The S(1)–Sb(1)–C(3) angle of 160.8(1)° identifies the Sb⋯S interaction as a weak pnictogen bond with a deviation from 180° expected for a σ-hole-type interaction. The P–C–Sb angle in 2 at 115.2(1)° is larger than in 1 at 110.6(1)°.
Another even weaker (intermolecular) interaction is between Sb(1) and S(2)’ (symmetry: 1 − x, 1 − y, 1 − z; Fig. S41†). The distance of 3.640(1) Å is slightly below the sum of the van der Waals radii (ΣrvdW(Sb,S) = 3.86 Å).28,29 This interaction presumably also contributes to the non-formation of a five-membered ring.
In order to better describe the interaction of the two heteroatoms Sb(1) and S(1), quantum chemical calculations were carried out. A Quantum Theory of Atoms in Molecules (QTAIM, PBE0/def2-TZVPP)30 analysis gives a bond path for the Sb(1)⋯S(1) interaction with a not-so-small value for the charge density at the bond critical point (BCP) of 0.27e Å−3 compared to the value of the Sb–C bond of 0.67e Å−3 and other similar systems like Me2Sb–SMe (ρBCP (Sb–S) 0.61e Å−3) or the adduct of the anthracene based, (F5C2)2Sb–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– substituted poly-Lewis acid with SMe2 (ρBCP (Sb⋯S) 0.11/0.16e Å−3).17
C– substituted poly-Lewis acid with SMe2 (ρBCP (Sb⋯S) 0.11/0.16e Å−3).17
This confirms the classification as half covalent, which is supported by the distance criterion. The corresponding Laplacian ∇2ρBCP(Sb–S) has a small value of 0.96e Å−5.
Based on the results of the QTAIM30 and IQA (Interacting Quantum Molecules)31 analyses (Tables 1, S2,†Fig. 4), the interaction can be described as weakly stabilising, polar and partially covalent. For classification purposes we calculated reference systems, which are listed in the ESI.†
| Compound | Atom pair A–B | ρ BCP | ∇2ρBCP |
|---|---|---|---|
| 2 | Sb(1)–S(1) | 0.27 | 0.96 |
| Sb(1)–C(5) | 0.67 | 1.69 | |
| S(1)–C(14) | 1.55 | −9.64 | |
| 6 | Au(1)–P(1) | 0.73 | 1.66 |
| Au(1)–P(2) | 0.72 | 1.66 | |
| Au(1)–Cl(1) | 0.22 | 2.19 | |
| Sb(1)–Cl(1) | 0.19 | 1.20 | |
| Sb(2)–Cl(1) | 0.18 | 1.13 |
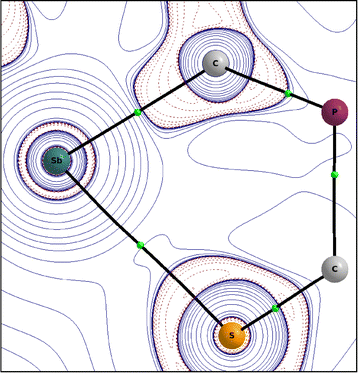 | ||
| Fig. 4 Contour plot of the Laplacian ∇2ρ (positive isovalues are printed in full blue and negative ones as dashed red lines) of the electron density in the C(5)–Sb(1)–S(1) plane of 2. | ||
The interaction energy EABint of the atom pair Sb–S with −8.20 × 10−2 a.u., lies between those of Li–F (EABint = −3.27 × 10−1 a.u.) and Xe–Xe (EABint = −5.78 × 10−3 a.u.) as reference values for typical ionic and typical dispersion interactions. As with the interaction in the dixenon molecule, the majority of the Sb–S interaction energy (84%) is due to electron exchange and correlation effects (see ESI† for more details).
In order to be able to make a statement about the influence of the Lewis acid on the reactivity of the phosphorus Lewis base towards CS2 and the formation of a corresponding adduct, additional DFT calculations27 were carried out. Due to the presence of the Lewis acid site in 1, the reaction 1 + CS2 → 1·CS2 (ΔH298K = −53 kJ mol−1) is significantly more exothermic than a comparable reaction of a phosphane of similar constitution around phosphorus, namely di-tert-butylmethylphosphane, with CS2 (ΔH298 K = −13 kJ mol−1).
Although both reactions are endergonic, the reaction with di-tert-butylmethylphosphane, i.e. without the influence of a Lewis acid, is significantly more endergonic (ΔG298 K = 36 kJ mol−1). The theoretical values thus indicate that the Sb⋯S interaction, although weak, supports the adduct formation.
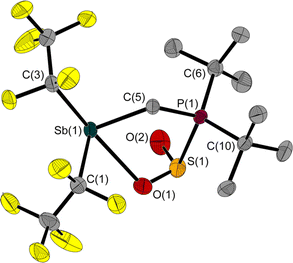
The reaction of 1 with SO2 gives the adduct 3 with a five-membered heterocycle and an exocyclic S–O bond (Fig. 5). The angles around the sulphur atom add up to 309.7(3)°, describing distortion of the trigonal pyramidal coordination environment. The Sb atom is bisphenoidally surrounded with a C(3)–Sb(1)–O(1) angle of 158.5(1)° and a C(5)–Sb(1)–C(1) angle of 95.5(1)°.
Similar to 2, the Sb(1)–C(5)–P(1) angle of 114.9(1)° in 3 is wider than in 1. The 31P NMR chemical shift of 3 is 39.8 ppm, which is in a typical range for P atoms with a similar substitution pattern (e.g.: δ(31P) (F5C2)3SnCH2P(tBu)2·SO2: 47.4 ppm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5).
5).
Surprisingly, the addition of phenyl isocyanate to 1 does not proceed via the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond but via the C
O bond but via the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. This results in a five-membered heterocycle with exocyclic C
N bond. This results in a five-membered heterocycle with exocyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–Cipso bonds (Fig. 6).
O and N–Cipso bonds (Fig. 6).
However, in the light of the HSAB concept, this behaviour is to be expected: comparing the O and N atoms of the phenyl isocyanate, the latter is the softer one and should therefore be preferred to interact with the soft Sb atom. In 4, the Sb(1)–C(5)–P(1) angle is also widened at 113.6(1)° compared to the free FLP 1. In addition to the product signal set, the dissolved NMR sample contains a small proportion of the two reactants. IR spectroscopy was used to compare the isolated product as a solid and dissolved in CCl4 with a solution of phenyl isocyanate in CCl4. A band characteristic of phenyl isocyanate was detected in both solutions, but not in the solid sample. The isolated product 4 seems to decompose to a small extent into its reactants by dissolution.
In contrast to the addition of phenyl isocyanate described above, 1 reacts only to a small extent with phenyl isothiocyanate at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N but mainly at the C
N but mainly at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bond (Fig. 7). The main product 5a has a five-membered heterocycle, but in this case with an exocyclic C
S double bond (Fig. 7). The main product 5a has a five-membered heterocycle, but in this case with an exocyclic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-Ph unit. This is consistent with the predictions of the HSAB concept. Compared to the other adducts presented here, the largest Sb(1)–C(5)–P(1) angle is found in 5a with 119.3(1)°. The Sb(1)–S(1) bond with 2.881(1) Å is shorter than that in the CS2 adduct 2, and thus shorter than the sum of the van der Waals radii (ΣrvdW(Sb–S) = 3.86 Å).28,29 Again, the Sb atom is bisphenoidally surrounded, with the C(3)–Sb(1)–S(1) angle of 164.7(1)° in the same range as in the previously discussed adducts.
N-Ph unit. This is consistent with the predictions of the HSAB concept. Compared to the other adducts presented here, the largest Sb(1)–C(5)–P(1) angle is found in 5a with 119.3(1)°. The Sb(1)–S(1) bond with 2.881(1) Å is shorter than that in the CS2 adduct 2, and thus shorter than the sum of the van der Waals radii (ΣrvdW(Sb–S) = 3.86 Å).28,29 Again, the Sb atom is bisphenoidally surrounded, with the C(3)–Sb(1)–S(1) angle of 164.7(1)° in the same range as in the previously discussed adducts.
Quantum chemical calculations (DFT)27 on the different types of adduct formation behaviour are in agreement with the experimental results: the formation of the addition product to phenyl isocyanate 4 at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond is not significantly favoured in terms of energy (4: ΔG = −10 kJ mol−1) compared to the addition at the C
N bond is not significantly favoured in terms of energy (4: ΔG = −10 kJ mol−1) compared to the addition at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (ΔG = −6 kJ mol−1). The same is true for the addition to phenyl isothiocyanate (5a: ΔG = −16 kJ mol−1; C
O bond (ΔG = −6 kJ mol−1). The same is true for the addition to phenyl isothiocyanate (5a: ΔG = −16 kJ mol−1; C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N addition product 5b: ΔG = −11 kJ mol−1), so it is not surprising that addition to both sites is experimentally observed and not exclusively adduct 5a is formed (Scheme 2). In the NMR spectra of the isolated solid of this reaction, there is a second set of signals with only slightly different chemical shifts, representing about a quarter of the mixture. The recorded data allow the following considerations: The connectivity of 5a and the related tin compound, the adduct (F5C2)3SnCH2P(tBu)2·PhNCS, is analogous; consequently, there is a clear similarity in the chemical shifts of the respective isothiocyanate carbon atom and the ipso carbon atoms (Table 2). In contrast, 5b shows larger deviations from the data of (F5C2)3SnCH2P(tBu)2·PhNCS.
N addition product 5b: ΔG = −11 kJ mol−1), so it is not surprising that addition to both sites is experimentally observed and not exclusively adduct 5a is formed (Scheme 2). In the NMR spectra of the isolated solid of this reaction, there is a second set of signals with only slightly different chemical shifts, representing about a quarter of the mixture. The recorded data allow the following considerations: The connectivity of 5a and the related tin compound, the adduct (F5C2)3SnCH2P(tBu)2·PhNCS, is analogous; consequently, there is a clear similarity in the chemical shifts of the respective isothiocyanate carbon atom and the ipso carbon atoms (Table 2). In contrast, 5b shows larger deviations from the data of (F5C2)3SnCH2P(tBu)2·PhNCS.
| Compound | δ(13C) PhNCE | δ(13C) Cipso |
|---|---|---|
| 1·PhNCS (5a) | 168.7 | 151.0 |
| 1·PhNCS (5b) | 178.2 | 147.3 |
| 1·PhNCO (4) | 160.1 | 144.6 |
| (F5C2)3SnCH2P(tBu)2·PhNCS | 164.3 | 150.0 |
| (F5C2)3SnCH2P(tBu)2·PhNCO | 150.0 | 146.4 |
We also analysed the adducts of phenyl isocyanate 4 and phenyl isothiocyanate 5a/5b using two-dimensional NMR techniques (15N 1H HMBC).
The spectra contained signals of the free phenyl isocyanate or phenyl isothiocyanate, respectively, which were used as additional references for these samples. For both samples we observed only one other cross peak. These were very different from the chemical shifts of the reactants (δ(15N) of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150.3 ppm, δ(15N) of PhNCO: 48.4 ppm; δ(15N) of 5a: 345.2 ppm, δ(15N) of PhNCS: 107.7 ppm) and also from each other; for 5b we would expect a less significant deviation compared to 4. Probably due to too low a concentration and a possible different relaxation behaviour, we could not detect a cross peak attributable to 5b.
150.3 ppm, δ(15N) of PhNCO: 48.4 ppm; δ(15N) of 5a: 345.2 ppm, δ(15N) of PhNCS: 107.7 ppm) and also from each other; for 5b we would expect a less significant deviation compared to 4. Probably due to too low a concentration and a possible different relaxation behaviour, we could not detect a cross peak attributable to 5b.
An attempt to assign the IR spectroscopic data of the adducts by using the results of quantum chemical calculations failed due to the lack of characteristic vibrational bands; a more precise identification of the constitutional isomers 5a and 5b is therefore not possible.
We note that a reference system for FLP 1 without Lewis acid function, namely MePtBu2, shows no reactions with the substrates SO2 and PhNCO, whereas it forms equilibria of adducts and precursors with CS2 and PhNCS. The fact that all adducts of 1 are more stable than those of MePtBu2 demonstrates that the antimony function in 1 (i.e. its FLP nature) is crucial for adduct formation with SO2 and PhNCO and highly supportive for CS2 and PhNCS.
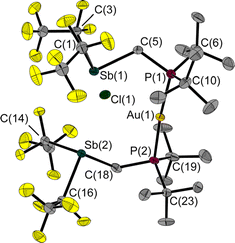
In a reaction of 1 with the phosphane-gold chloride (MePh2P)AuCl, two molecules of the free FLP reacted with one molecule of the gold compound, the MePPh2 being displaced by the phosphane function of the FLP (Fig. 8). An almost linear coordinated gold atom is obtained; the P(1)–Au(1)–P(2) angle is 174.6(1)°, similar to other gold(I) complexes with two phosphane ligands.32,33 The distance between the gold and chlorine atom (2.939(1) Å) is greater than the sum of the corresponding covalence radii of 2.23 Å,23 but well below the sum of the van der Waals radii of 3.41 Å,29 indicating an attractive interaction between these two atoms. A distance shorter than the sum of the van der Waals radii is also found between the chlorine and the two antimony atoms (Cl(1)–Sb(1): 2.966(1) Å, Cl(1)–Sb(2): 2.981(1) Å, ΣrvdW(Sb–Cl) = 3.81 Å).28,29 The Sb–C–P angles are 118.6(1)° and 117.8(1)°, which are wider than in the reactant 1. For molecule 6, we also performed QTAIM and IQA analyses (PBE0/def2-TZVPP) to describe the interaction of selected atom pairs (Tables 1 and S3†).30,31 Based on the calculations, the Au(1)–P(1/2) bonds are typically polarised bonds with a strong covalent character. In contrast the Au(1)–Cl(1) interaction can be considered as a weakly polarised bond with covalent character and a stabilisation energy about three times lower than that for Au(1)–P(1/2).
The interactions between the two antimony atoms and the chlorine atom are strongly stabilising, mainly ionic and have a stabilisation energy similar to that of the Au–P bonds. The calculations show that the P⋯Cl interactions are purely ionic and are even twice as strong as the Au(1)–Cl(1) interaction, despite the absence of bond critical points and bond paths. Also noteworthy is the presence of other low electron density bond paths for Cl⋯F and Cl⋯H (see ESI† for more details). The pronounced clamp-like structure is thus maintained not only in the solid state, but also likely to exist in the free molecules as predicted by quantum chemical optimisations. This seems to be due to the stabilising interactions between the FLP clamp and the chlorine atom.
To see if this structural motif was also present without the chloride anion, we reacted compound 6 with silver triflate (AgOTf). By replacing the chloride anion with triflate, compound 7 was obtained as a colourless solid.
Its structure in the solid state shows no clamp-like shape (Fig. 9); the torsion angle (Sb–P–P–Sb) over the P axis is 106° wider than in 6. The P(1)–Au(1)–P(2) angle of 6 (174.6(1)°) is almost identical to that of 7 (173.9(1)°).
The distance between the gold atom and the nearest oxygen atom of the triflate is 3.521(2) Å and thus exceeds the sum of the van der Waals radii (ΣrvdW(Au–O) = 3.18 Å).29 In turn, the distances between the antimony atoms and the nearest oxygen atoms of the anion fall below (O(1)–Sb(1): 2.647(2) Å, O(2)–Sb(2): 2.808(2) Å, ΣrvdW(Sb–O) = 3.58 Å).27,28 The 31P NMR chemical shifts of the two gold adducts of this work (δ(31P) 6: 74.0 ppm, 7: 78.3 ppm) and the Sn FLP adduct (δ(31P) (F5C2)3SnCH2P(tBu)2·AuCl(PPh3) 74.4 ppm)5 are in a similar range.
Conclusions
We present here the neutral pre-organised Sb/P-Lewis pair (F5C2)2SbCH2P(tBu)2 (1) capable of forming the corresponding 1,2-addition products with various substrates, including CS2, SO2 and PhNCS, and the 2,3-addition product with PhNCO. The relatively soft acidic Sb(III)-Lewis function allows reversible binding of CS2, whereas an adduct formation with CO2 was not observed under similar conditions; an evaluation of the energy contributions to both reactions by quantum chemical calculations explains this experimental finding: the difference in free enthalpy for the formation of the CS2 adduct at 298 K is 19 kJ mol−1 less than for the formation of the analogous CO2 adduct and therefore its formation is much more favourable. These results are consistent with qualitative predictions from the HSAB concept. QTAIM and IQA analyses found a bond path with a bond critical point for the Sb–S interaction in the distorted five-membered ring adduct (F5C2)2SbCH2P(tBu)2·CS2 and predicted its stabilisation energy to be −8.20 × 10−2 a.u.For the adduct formation of the FLP with phenyl isocyanate and phenyl isothiocyanate, we found a preference for the adduct favoured by the HSAB concept, although the energetic difference between the different addition products is not significant. In the case of phenyl isothiocyanate both possible adducts are formed.
During adduct formation with (MePh2P)AuCl, the phosphorus base of the gold moiety is displaced by a second FLP molecule, resulting in a stabilising clamp-like structure. Replacing the chloride anion with the larger triflate ion twists the FLP arms by 106° – another proof of the soft acid properties of 1.
Data availability
The data published in this contribution are available as ESI, submitted with the manuscript. Crystallographic data have been deposited with the Cambridge Crystal Structure Database (CCDC).†Author contributions
J. Krieft: investigation, methodology, validation, visualization, writing (original draft), P. C. Trapp: investigation (DFT), Y. V. Vishnevskiy: investigation (QTAIM, IQA), B. Neumann: investigation (SCXRD), H.-G. Stammler: investigation (SCXRD), J.-H. Lamm: investigation (SCXRD), N. W. Mitzel: funding, acquisition, project administration, supervision, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Marco Wiβbrock and Dr Andreas Mix for recording NMR spectra and Barbara Teichner for performing elemental analyses. This work was supported by Deutsche Forschungsgemeinschaft (grant MI 477/44-1, project number 461833739 and grant VI713/1-3, project no. 243500032). We furthermore thank the Regional Computing Center of the University of Cologne (RRZK) for providing computing time and support on the DFG-funded (Funding number: INST 216/512/1FUGG) HPC system CHEOPS.References
- (a) D. W. Stephan, Science, 2016, 354, aaf7229 CrossRef PubMed; (b) D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2015, 54, 6400 CrossRef CAS PubMed; (c) D. W. Stephan, Dalton Trans., 2009, 17, 3129 RSC.
- (a) G. C. Welch, R. R. San Juan, J. D. Masuda and D. W. Stephan, Science, 2006, 314, 1124 CrossRef CAS PubMed; (b) L. Keweloh, H. Klöcker, E.-U. Würthwein and W. Uhl, Angew. Chem., Int. Ed., 2016, 55, 3212 CrossRef CAS PubMed; (c) A. F. G. Maier, S. Tussing, T. Schneider, U. Flörke, Z.-W. Qu, S. Grimme and J. Paradies, Angew. Chem., Int. Ed., 2016, 55, 12219 CrossRef CAS PubMed; (d) C. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2009, 48, 6643 CrossRef PubMed; (e) E. Otten, R. C. Neu and D. W. Stephan, J. Am. Chem. Soc., 2009, 131, 9918 CrossRef CAS PubMed.
- T. A. Kinder, R. Pior, S. Blomeyer, B. Neumann, H.-G. Stammler and N. W. Mitzel, Chem.–Eur. J., 2019, 25, 5899 CrossRef CAS PubMed.
- B. Waerder, M. Pieper, L. A. Körte, T. A. Kinder, A. Mix, B. Neumann, H.-G. Stammler and N. W. Mitzel, Angew. Chem., Int. Ed., 2015, 54, 13416 CrossRef CAS PubMed.
- P. Holtkamp, F. Friedrich, E. Stratmann, A. Mix, B. Neumann, H.-G. Stammler and N. W. Mitzel, Angew. Chem., Int. Ed., 2019, 58, 5114 CrossRef CAS PubMed.
- (a) D. Zhu, Z.-W. Qu and D. W. Stephan, Dalton Trans., 2020, 49, 901 RSC; (b) E. A. Ilin, V. O. Smirnov, A. D. Volodin, A. A. Korlyukov and A. D. Dilman, Chem. Commun., 2020, 56, 7140 RSC; (c) L. Wickemeyer, N. Aders, A. Mix, B. Neumann, H.-G. Stammler, J. J. Cabrera-Trujillo, I. Fernández and N. W. Mitzel, Chem. Sci., 2022, 13, 8088 RSC; (d) C. Appelt, H. Westenberg, F. Bertini, A. W. Ehlers, J. C. Slootweg, K. Lammertsma and W. Uhl, Angew. Chem., Int. Ed., 2011, 50, 3925 CrossRef CAS PubMed; (e) C. Appelt, J. C. Slootweg, K. Lammertsma and W. Uhl, Angew. Chem., Int. Ed., 2013, 52, 4256 CrossRef CAS PubMed; (f) P. Federmann, T. Bosse, S. Wolff, B. Cula, C. Herwig and C. Limberg, Chem. Commun., 2022, 58, 1345 RSC.
- K. Samigullin, I. Georg, M. Bolte, H.-W. Lerner and M. Wagner, Chem.–Eur. J., 2016, 22, 3478 CrossRef CAS PubMed.
- M. J. Gallagher, D. P. Graddon and A. R. Sheikh, Thermochim. Acta, 1978, 27, 269 CrossRef CAS.
- (a) G. A. Olah and R. H. Schlosberg, J. Am. Chem. Soc., 1968, 90, 2726 CrossRef CAS; (b) G. A. Olah, G. Klopman and R. H. Schlosberg, J. Am. Chem. Soc., 1969, 91, 3261 CrossRef CAS; (c) G. A. Olah, J. Org. Chem., 2005, 70, 2413 CrossRef CAS PubMed; (d) L. Greb, Chem.–Eur. J., 2018, 24, 17881 CrossRef CAS PubMed; (e) D. Tofan and F. P. Gabbaï, Chem. Sci., 2016, 7, 6768 RSC; (f) C.-H. Chen and F. P. Gabbaï, Dalton Trans., 2018, 47, 12075 RSC.
- M. Yang, D. Tofan, C.-H. Chen, K. M. Jack and F. P. Gabbaï, Angew. Chem., Int. Ed., 2018, 57, 13868 CrossRef CAS PubMed.
- B. Pan and F. P. Gabbaï, J. Am. Chem. Soc., 2014, 136, 9564 CrossRef CAS PubMed.
- (a) C.-H. Chen and F. P. Gabbaï, Angew. Chem., Int. Ed., 2017, 56, 1799 CrossRef CAS PubMed; (b) G. Park and F. P. Gabbaï, Chem. Sci., 2020, 11, 10107 RSC; (c) Di You, B. Zhou, M. Hirai and F. P. Gabbaï, Org. Biomol. Chem., 2021, 19, 4949 RSC; (d) V. M. Gonzalez, G. Park, M. Yang and F. P. Gabbaï, Dalton Trans., 2021, 50, 17897 RSC.
- (a) J. S. Jones and F. P. Gabbaï, Acc. Chem. Res., 2016, 49, 857 CrossRef CAS PubMed; (b) D. You, H. Yang, S. Sen and F. P. Gabbaï, J. Am. Chem. Soc., 2018, 140, 9644 CrossRef CAS PubMed; (c) R. R. Rodrigues and F. P. Gabbaï, Molecules, 2021, 26, 1985 CrossRef CAS PubMed; (d) J. E. Smith, H. Yang and F. P. Gabbaï, Organometallics, 2021, 40, 3886 CrossRef CAS.
- D. Sharma, S. Balasubramaniam, S. Kumar, E. D. Jemmis and A. Venugopal, Chem. Commun., 2021, 57, 8889 RSC.
- (a) S. Benz, A. I. Poblador-Bahamonde, N. Low-Ders and S. Matile, Angew. Chem., Int. Ed., 2018, 57, 5408 CrossRef CAS PubMed; (b) P. Erdmann, J. Leitner, J. Schwarz and L. Greb, ChemPhysChem, 2020, 21, 987 CrossRef CAS PubMed.
- (a) M. Yang, M. Hirai and F. P. Gabbaï, Dalton Trans., 2019, 48, 6685 RSC; (b) S. L. Benjamin, W. Levason, G. Reid and R. P. Warr, Organometallics, 2012, 31, 1025 CrossRef CAS; (c) K. Ohkata, S. Takemoto, M. Ohnishi and K. Akiba, Tetrahedron Lett., 1989, 30, 4841 CrossRef CAS; (d) C. J. Carmalt, A. H. Cowley, R. D. Culp, R. A. Jones, S. Kamepalli and N. C. Norman, Inorg. Chem., 1997, 36, 2770 CrossRef CAS PubMed; (e) A. M. Christianson and F. P. Gabbaï, Organometallics, 2017, 36, 3013 CrossRef CAS; (f) J. Qiu, B. Song, X. Li and A. F. Cozzolino, Phys. Chem. Chem. Phys., 2017, 20, 46 RSC; (g) M. Á. García-Monforte, M. Baya, D. Joven-Sancho, I. Ara, A. Martín and B. Menjón, J. Organomet. Chem., 2019, 897, 185 CrossRef.
- J. L. Beckmann, J. Krieft, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler and N. W. Mitzel, Angew. Chem., Int. Ed., 2023, 62, e202310439 CrossRef CAS PubMed.
- J. L. Beckmann, J. Krieft, Y. V. Vishnevskiy, B. Neumann, H.-G. Stammler and N. W. Mitzel, Chem. Sci., 2023, 14, 13551 RSC.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533 CrossRef CAS.
- P. Holtkamp, D. Poier, B. Neumann, H.-G. Stammler and N. W. Mitzel, Chem.–Eur. J., 2021, 27, 3793 CrossRef CAS PubMed.
- (a) P. Holtkamp, J. Schwabedissen, B. Neumann, H.-G. Stammler, I. V. Koptyug, V. V. Zhivonitko and N. W. Mitzel, Chem.–Eur. J., 2020, 26, 17381 CrossRef CAS PubMed; (b) P. Holtkamp, T. Glodde, D. Poier, B. Neumann, H.-G. Stammler and N. W. Mitzel, Angew. Chem., Int. Ed., 2020, 59, 17388 CrossRef CAS PubMed.
- F. Eisenträger, A. Göthlich, I. Gruber, H. Heiss, C. A. Kiener, C. Krüger, J. U. Notheis, F. Rominger, G. Scherhag and M. Schultz, New J. Chem., 2003, 27, 540 RSC.
- P. Pyykkö and M. Atsumi, Chemistry, 2009, 15, 186 CrossRef PubMed.
- R. J. Gillespie and R. S. Nyholm, Q. Rev. Chem. Soc., 1957, 61, 339 RSC.
- (a) M. A. Beckett, G. C. Strickland, J. R. Holland and K. S. Varma, Polymer, 1996, 37, 4629–4631 CrossRef CAS; (b) U. Mayer, V. Gutmann and W. Gerger, Monatsh. Chem., 1975, 106, 1235–1257 CrossRef CAS.
- J. Ramler and C. Lichtenberg, Chem.–Eur. J., 2020, 26, 10250–10258 CrossRef CAS PubMed.
- (a) S. Grimme, A. Hansen, S. Ehlert and J.-M. Mewes, J. Chem. Phys., 2021, 154, 064103 CrossRef CAS PubMed; (b) J. W. Furness, A. D. Kaplan, J. Ning, J. P. Perdew and J. Sun, J. Phys. Chem. Lett., 2020, 11, 8208 CrossRef CAS PubMed; (c) J. W. Furness, A. D. Kaplan, J. Ning, J. P. Perdew and J. Sun, J. Phys. Chem. Lett., 2020, 11, 9248 CrossRef CAS PubMed; (d) S. Ehlert, U. Huniar, J. Ning, J. W. Furness, J. Sun, A. D. Kaplan, J. P. Perdew and J. G. Brandenburg, J. Chem. Phys., 2021, 154, 061101 CrossRef CAS PubMed; (e) H. Kruse and S. Grimme, J. Chem. Phys., 2012, 136, 154101 CrossRef PubMed; (f) B. Metz, H. Stoll and M. Dolg, J. Chem. Phys., 2000, 113, 2563 CrossRef CAS; (g) G. Herzberg, Molecular Spectra and Molecular Structure. II. Infrared and Raman Spectra of Polyatomic Molecules, Van Nostrand Comp., Toronto, 1945 Search PubMed.
- M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. A, 2009, 113, 5806 CrossRef CAS PubMed.
- A. Bondi, J. Phys. Chem., 1964, 68, 441 CrossRef CAS.
- (a) R. F. W. Bader, Atoms in Molecules - A Quantum Theory, Oxford University Press, Oxford, 1990 CrossRef; (b) C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158 CrossRef CAS; (c) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC; (d) M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Revision C.01, Gaussian Inc. Wallingford CT, 2016 Search PubMed.
- M. A. Blanco, A. Martín Pendás and E. Francisco, J. Chem. Theory Comput., 2005, 1, 1096 CrossRef CAS PubMed.
- M. Barrow, H. B. Buergi, D. K. Johnson and L. M. Venanzi, J. Am. Chem. Soc., 1976, 98, 2356 CrossRef CAS.
- A.-M. Larsonneur, R. Turpin, P. Castan and G. Bernardinelli, Inorg. Chim. Acta, 1994, 227, 85 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2350371–2350377. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc02785j |
| This journal is © The Royal Society of Chemistry 2024 |