Open Access Article
Open Access ArticleA triazole-based covalent organic framework as a photocatalyst toward visible-light-driven CO2 reduction to CH4†
Sandip
Biswas
 a,
Faruk Ahamed
Rahimi
a,
Faruk Ahamed
Rahimi
 a,
R. Kamal
Saravanan
a,
Anupam
Dey
a,
R. Kamal
Saravanan
a,
Anupam
Dey
 a,
Jatin
Chauhan
a,
Devika
Surendran
a,
Sukhendu
Nath
a,
Jatin
Chauhan
a,
Devika
Surendran
a,
Sukhendu
Nath
 bc and
Tapas Kumar
Maji
bc and
Tapas Kumar
Maji
 *a
*a
aMolecular Materials Laboratory, Chemistry and Physics of Materials Unit, School of Advanced Materials (SAMat), Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur, Bangalore 560064, India. E-mail: tmaji@jncasr.ac.in; Web: https://www.jncasr.ac.in/faculty/tmaji
bRadiation and Photochemistry Division, Bhabha Atomic Research Center, Mumbai 400085, India
cHomi Bhabha National Institute, Mumbai 400094, India
First published on 4th September 2024
Abstract
Solar-light driven reduction of CO2 to CH4 is a complex process involving multiple electron and proton transfer processes with various intermediates. Therefore, achieving high CH4 activity and selectivity remains a significant challenge. Covalent organic frameworks (COFs) represent an emerging class of photoactive semiconductors with molecular level structural tunability, modular band gaps, and high charge carrier generation and transport within the network. Here, we developed a new heterocyclic triazole ring containing COF, TFPB-TRZ, through the condensation reaction between 1,3,5-tris(4-formylphenyl)benzene (TFPB) and 3,5-diamino-1,2,4-triazole (TRZ). The TFPB-TRZ COF with multiple heteroatoms shows suitable visible light absorption, high CO2 uptake capability and an appropriate band diagram for CO2 photoreduction. Photocatalysis results reveal a maximum CO2 to CH4 conversion of 2.34 mmol g−1 with a rate of 128 μmol g−1 h−1 and high selectivity (∼99%) using 1-benzyl-1,4-dihydronicotinamide (BNAH) and triethylamine (TEA) as sacrificial agents. Under similar reaction conditions in the presence of direct sunlight, the TFPB-TRZ COF displays a maximum CH4 yield of 493 μmol g−1 with a rate of 61.62 μmol g−1 h−1, suggesting the robustness and light-harvesting ability of the COF photocatalyst. A femtosecond transient absorption (TA) spectroscopy study shows fast decay of excited state absorption (ESA) in the COF compared to the TFPB building unit due to efficient electron transfer to the catalytic site in the framework. The mechanism of CO2 reduction to CH4 is studied by DFT-based theoretical calculation, which is further supported by an in situ diffuse reflectance infrared Fourier transform spectroscopic (DRIFTS) study. The DFT results reveal that the lone pair of electrons on nitrogen heteroatoms present in the triazole ring of the TRZ moiety help in the stabilization of the CO intermediate during CO2 to CH4 conversion. Overall, this work demonstrates the use of a metal-free, recyclable COF-based photocatalytic system for solar energy storage by CO2 reduction.
Introduction
Global industrialization and urbanization inevitably led to the excessive use of fossil fuels and the concomitant emission of greenhouse gases (mainly CO2) into the earth's atmosphere.1–5 Consequently, the replacement of traditional fossil fuel with renewable and clean energy has now become the utmost requirement to mitigate the energy and environmental crisis.6–8 Numerous strategies towards carbon neutralization technologies have already been developed. Among them, converting CO2 into energy fuels (such as CO, CH4, CH3OH and other C2 products) utilizing inexhaustible solar energy has been regarded as one of the finest and environmentally benign strategies for achieving the virtuous carbon cycle in nature.7–11 However, we need to develop active photocatalysts to realize selective and efficient solar fuel production based on CO2 photoreduction. A range of inorganic semiconductors, such as TiO2,12 WO3,13 Bi2WO6,14 CdS,15 ZrO2,16 In2O3,17 ZnGa2O4,18 as well as metal chalcogenides19 and other carbonaceous materials have been investigated as photocatalysts for CO2 reduction. However, most of them have drawbacks related to visible light absorption, relatively large band gap, poor mass transfer, binding of the reactant on the catalyst surface, and finally, environment friendliness.20Metal–organic frameworks (MOFs) and covalent organic frameworks (COFs) are two widely explored crystalline porous materials with modular porosity and a porous environment.21–29 The functionalities and application of these porous materials can be manipulated by design and customization of the chemical nature, size and shape of the organic building units.21–29 Suitable electronic band structures and visible light-absorption ability make them promising CO2 photoreduction catalysts.23,26 To date MOFs have been well studied in the field of photocatalysis, whereas metal-free organic porous materials like COF or conjugated microporous polymers are underexplored in the field of photocatalytic CO2 reduction.30,31 COFs have several advantages in terms of structural tunability, suitable band gap engineering, visible-light harvesting, and thermo-chemical stability.20–23 Extended conjugation in the COF structure widens the scope of visible light absorption and facile charge transfer to the catalytic site.32–45
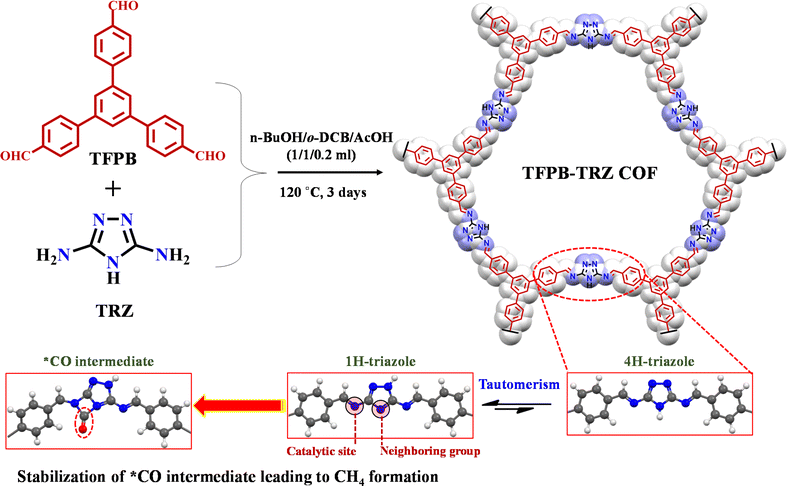
In spite of possessing the aforementioned advantages, CO2 photoreduction using a metal-free COF as a catalyst is only limited to CO (E0 = −0.53 V vs. NHE) or HCOOH (E0 = −0.61 V vs. NHE).9,40–45 However, using a COF as a photocatalyst for higher order CO2 reduction products like CH4 (E0 = −0.24 V vs. NHE), which is considered the basic fuel of modern energetics, is yet to be reported. The redox potential of the photoexcited electrons, their reaction kinetics, binding energy and stabilization of different intermediates play a significant role in achieving a higher order multielectron CO2 reduction process to CH4. During the course of the reaction, such complexities can be controlled and fine-tuned via a suitable choice of building units for the COF structure. Since stabilization of the CO intermediate is the main hurdle for a higher order multielectron CO2 reduction process, in metal-free catalysis, one way of stabilizing it is to involve the facile electron donation from a neighbouring group and satiating the π-accepting properties of CO species.8 In this context, the incorporation of a small organic building unit like triazole with multiple heteroatoms into the framework may fulfil the purpose. The lone pair of electrons on nitrogen atoms present in triazole can provide a good electron-donating site for CO intermediate stabilization. Moreover, it is well known that triazole can have two tautomers, 1-H-triazole and 4-H-triazole, in between; the former is more stable.46–48 Most importantly, the 1-H form of triazole, when present in the framework, will have a suitable arrangement of the lone pair-containing nitrogen atoms, which can stabilize the intermediate CO species (Scheme 1). Additionally, the overall electron density in the triazole component decorated with multiple heteroatoms in the COF would be high, which will further assist in multielectron CO2 reduction beyond CO.
Keeping these attributes in mind herein, we have synthesized a new COF material through the general solvothermal Schiff base condensation between 1,3,5-tris(4-formylphenyl)benzene (TFPB) and 3,5-diamino-1,2,4-triazole (TRZ) with C3–C2 symmetrical topology, denoted as TFPB-TRZ (Scheme 1). Here, the TFPB moiety helps in bringing the extended π-conjugation into the backbone. The successful formation of the COF was confirmed by various characterization methods, including powder X-ray diffraction (PXRD), FT-IR, 13C CP-MAS NMR and N2 sorption measurements. The as-synthesized TFPB-TRZ COF was successfully implemented as a metal-free heterogeneous photocatalyst for visible-light-driven CO2 reduction to CH4 with excellent yield (2.34 mmol g−1 in 18 h) and high selectivity (∼99%) in the presence of sacrificial agents. Catalytic activity is further explored under direct sunlight, where it produces ∼493 μmol g−1 of CH4 after 8 h of reaction. The electron transfer kinetics in the COF for eight electron CO2 reduction to CH4 was further supported by the transient absorption (TA) spectroscopic study.43,49–51 Crucial information on the reaction intermediates during the conversion of CO2 to CH4 has been obtained through an in situ diffuse reflectance infrared Fourier-transform (DRIFT) study along with the free energy calculation that supported the thermodynamic feasibility of the formation of different intermediates. The formation of CH4 by a COF-based metal-free photocatalyst provides a new avenue for the rational design of porous crystalline materials for multielectron photoreduction.
Results and discussion
The TFPB precursor was synthesized according to the previous report and characterized using 1H NMR spectroscopy (Fig. S1†). After several trial-and-error attempts by varying the reaction conditions (Table S1 and Fig. S2†), the TFPB-TRZ COF was synthesized solvothermally by reacting a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of TFPB and TRZ precursors using a 1
2 mixture of TFPB and TRZ precursors using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
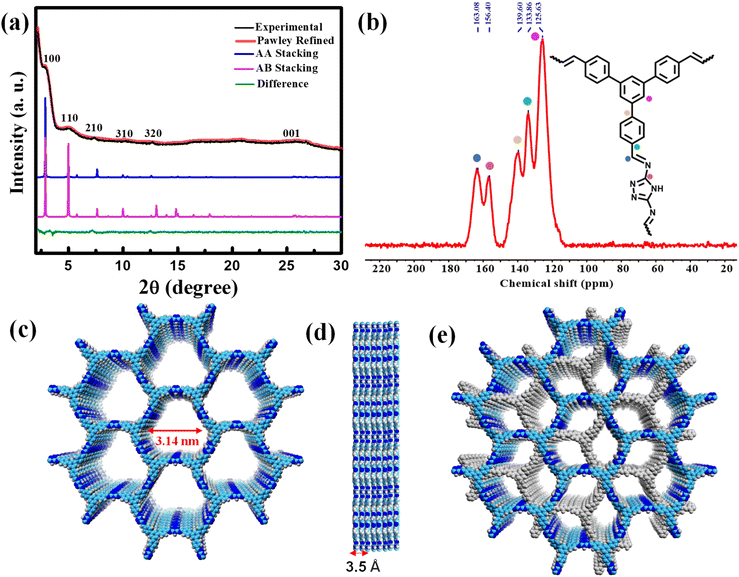
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of ortho-dichlorobenzene (o-DCB) and n-butanol in a high-precision glass tube, which was flame sealed and kept at 120 °C for three days in the presence of 6 M acetic acid (Scheme 1). The product was first characterized by a powder X-ray diffraction (PXRD) technique (Fig. 1a). The structure of the COF was modelled both in eclipsed (AA) (Fig. 1c) and staggered (AB) stacking arrangements (Fig. 1e) and compared with the experimental data. The Pawley refinement using AA stacking was found to be well-matched with the experimental data in terms of intensity ratios with an acceptable difference (Rwp = 1.75%, Rp = 1.37%; Fig. 1a). The AB stacking model did not replicate the experimentally obtained data. A typical trigonal lattice with the P31m space group was obtained with the unit cell parameters of a = b = 35.60 Å, c = 3.49 Å, α = β = 90°, γ = 120°. The PXRD pattern of the TFPB-TRZ COF showed six prominent diffraction peaks, with the most intense one at 2.88° indicating the presence of the (100) crystal plane of the TFPB-TRZ COF (Fig. 1a). Additionally, the other relatively weak diffractions at 4.9°, 7.4°, 10.3°, 12.6° and 25.9° indicated the existence of (110), (210), (310), (320) and (001) facets, respectively (Fig. 1a). The peak at 2θ = 25.9° (001 plane) was possibly due to the interlayer stacking of the COF, and distances between the individual (001) planes were 3.43 Å.
1 mixture of ortho-dichlorobenzene (o-DCB) and n-butanol in a high-precision glass tube, which was flame sealed and kept at 120 °C for three days in the presence of 6 M acetic acid (Scheme 1). The product was first characterized by a powder X-ray diffraction (PXRD) technique (Fig. 1a). The structure of the COF was modelled both in eclipsed (AA) (Fig. 1c) and staggered (AB) stacking arrangements (Fig. 1e) and compared with the experimental data. The Pawley refinement using AA stacking was found to be well-matched with the experimental data in terms of intensity ratios with an acceptable difference (Rwp = 1.75%, Rp = 1.37%; Fig. 1a). The AB stacking model did not replicate the experimentally obtained data. A typical trigonal lattice with the P31m space group was obtained with the unit cell parameters of a = b = 35.60 Å, c = 3.49 Å, α = β = 90°, γ = 120°. The PXRD pattern of the TFPB-TRZ COF showed six prominent diffraction peaks, with the most intense one at 2.88° indicating the presence of the (100) crystal plane of the TFPB-TRZ COF (Fig. 1a). Additionally, the other relatively weak diffractions at 4.9°, 7.4°, 10.3°, 12.6° and 25.9° indicated the existence of (110), (210), (310), (320) and (001) facets, respectively (Fig. 1a). The peak at 2θ = 25.9° (001 plane) was possibly due to the interlayer stacking of the COF, and distances between the individual (001) planes were 3.43 Å.
The chemical constitution of the obtained TFPB-TRZ COF was further analyzed using Fourier transform infrared (FT-IR) and 13C cross-polarization/magic angle spinning solid-state nuclear magnetic resonance (CP/MAS ssNMR) spectroscopy (Fig. S3a† and 1b). The FTIR spectrum of the TFPB-TRZ COF showed a band at 1690 cm−1 corresponding to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– stretching and also the disappearance of –N–H stretching bands of the TRZ monomer, supporting the formation of the COF by a Schiff base condensation reaction (Fig. S3a†). The peak at 163 ppm in the solid-state 13C NMR spectrum of the TFPB-TRZ COF was attributed to the –C
N– stretching and also the disappearance of –N–H stretching bands of the TRZ monomer, supporting the formation of the COF by a Schiff base condensation reaction (Fig. S3a†). The peak at 163 ppm in the solid-state 13C NMR spectrum of the TFPB-TRZ COF was attributed to the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– bonds in the COF (Fig. 1b). Signals around 120 to 140 ppm belong to the aromatic carbons (Fig. 1b). Thus, FT-IR and 13C CP-MAS NMR provided unequivocal evidence of the successful condensation of the aldehyde and amine into an imine linked structure. Thermogravimetric analysis (TGA) under nitrogen was also employed to test the thermal stability of the TFPB-TRZ COF, which showed that the as-synthesized COF retained structural stability until 300 °C (Fig. S3b†). Since no significant weight loss was observed before the onset of decomposition, it can be concluded that the framework was virtually free of trapped guest molecules (Fig. S3b†).
N– bonds in the COF (Fig. 1b). Signals around 120 to 140 ppm belong to the aromatic carbons (Fig. 1b). Thus, FT-IR and 13C CP-MAS NMR provided unequivocal evidence of the successful condensation of the aldehyde and amine into an imine linked structure. Thermogravimetric analysis (TGA) under nitrogen was also employed to test the thermal stability of the TFPB-TRZ COF, which showed that the as-synthesized COF retained structural stability until 300 °C (Fig. S3b†). Since no significant weight loss was observed before the onset of decomposition, it can be concluded that the framework was virtually free of trapped guest molecules (Fig. S3b†).
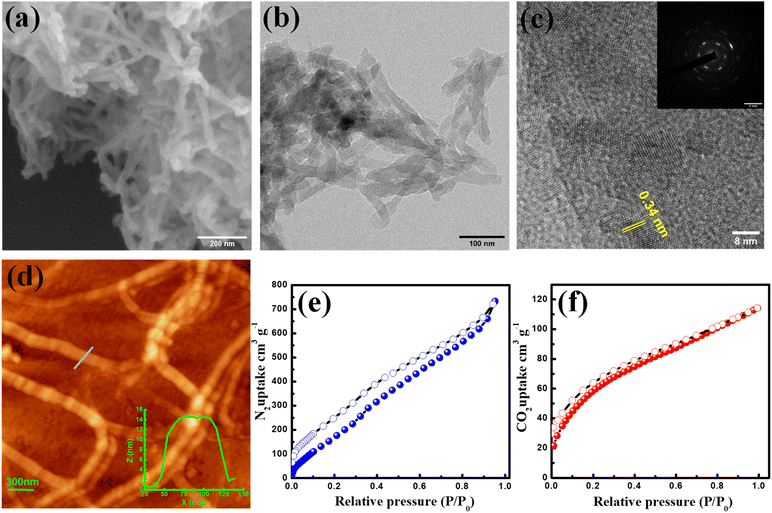
The scanning electron microscopy (SEM) image revealed the short fibrous morphology of the TFPB-TRZ COF (Fig. 2a). Homogeneous distribution of C and N throughout the entire framework was seen from the SEM elemental mapping (Fig. S4†). A transmission electron microscopy (TEM) image confirmed the fibrous morphology of the crystalline framework (Fig. 2b). The high-resolution transmission electron microscopy (HR-TEM) image revealed a lattice spacing of 3.4 Å, which can be attributed to the regular arrangement of the 2D network (Fig. 2c). The selected area electron diffraction (SAED) pattern additionally confirmed the highly crystalline nature of the COF (inset, Fig. 2c). Atomic force microscopy (AFM) imaging confirmed the fibrous morphology, and height profile analysis suggested that the height and width were within the range of 15 ± 0.5 nm and 100 ± 10 nm, respectively (Fig. 2d).
The porosity of the TFPB-TRZ COF was examined by N2 adsorption–desorption measurement at 77 K and showed a type II adsorption isotherm, unveiling the micro and mesoporous characteristics of the COF (Fig. 2e). The Brunauer–Emmett–Teller (BET) surface area of the TFPB-TRZ COF was determined to be 385 m2 g−1. The wide pore size distribution obtained from the non-linear density functional theory (NLDFT) calculation can be attributed to the inherent porosity and fibrous morphology of the COF (Fig. S5†). Furthermore, CO2 (kinetic diameter 3.3 Å) adsorption measurement was performed at 195 K for the TFPB-TRZ COF exhibiting a typical type-I profile with an uptake amount of 120 cm3 g−1 again suggesting the microporous nature of the framework (Fig. 2f).
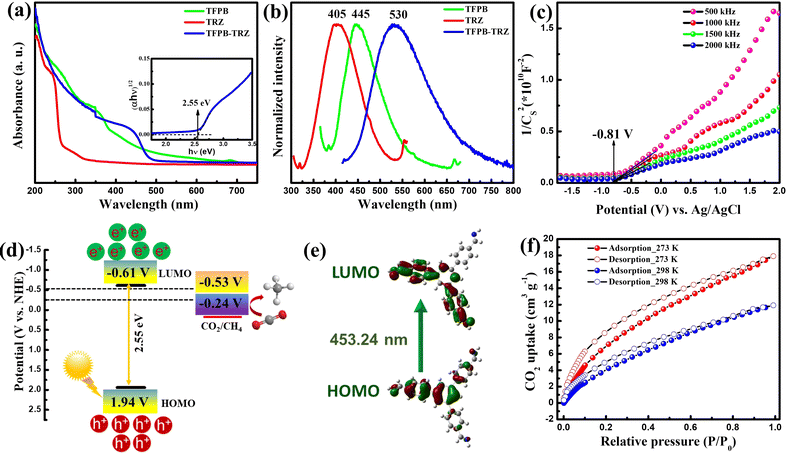
UV-vis diffuse reflectance spectroscopy (DRS) and photoluminescence (PL) spectroscopy were used to study the photophysical properties of the COF (Fig. 3a and b). The TFPB-TRZ COF exhibited a broad visible light absorption with a maximum of around 440 nm. In comparison to the TFPB-TRZ COF, the monomer TRZ showed an absorption maximum at ∼300 nm and TFPB displayed a broad absorption in the range of ∼350–500 nm (Fig. 3a). This suggested that the imine conjugated TFPB unit in the TFPB-TRZ COF is the main component for visible light absorption (Fig. 3a). To verify that, time-dependent density functional theory (TDDFT) calculation was performed on a TFPB-imine fragment, which revealed a band appearing at 453.24 nm due to the transition between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). The molecular orbital analysis further disclosed that the band at ∼440 nm of the COF appeared due to the extended conjugation through imine bonds with the TFPB core (Fig. 3e and Table S2†). The calculated energy corresponding to the HOMO–LUMO transition is 2.74 eV, which is in close agreement with the experimentally determined optical band gap of 2.55 eV obtained from the Tauc plot (inset, Fig. 3a). The solid phase PL spectrum of the TFPB-TRZ COF showed a significantly red-shifted emission (λmax = 530 nm, λex = 410 nm) compared to its corresponding monomers TFPB (λmax = 405 nm, λex = 350 nm) and TRZ (λmax = 445 nm, λex = 290 nm) which can be attributed to the extended π-conjugation in the COF skeleton (Fig. 3b).43,50,52 Time-correlated single-photon counting (TCSPC) measurement revealed a relatively shorter average lifetime of ∼0.88 ns for the TFPB-TRZ COF in comparison to the average lifetime of 1.92 ns for the TFPB building block (Fig. S6 and Table S3†).
Furthermore, Mott–Schottky (MS) measurements were conducted on the TFPB-TRZ COF at a frequency of 500, 1000, 1500, and 2000 Hz at pH 7 in a 0.2 M Na2SO4 electrolyte to determine the conduction-band (CB) position (Fig. 3c). The CB position was estimated to be −0.61 V vs. normal hydrogen electrode (NHE), which is more negative than the reduction potential of CO2 to CO/CH4 (−0.53/−0.24 V vs. NHE at pH 7) (Fig. 3c). The valence band (VB) position is 1.94 V as obtained from the band gap and CB position (Fig. 3d). A schematic representation of the band diagram towards a feasible CO2 reduction process is presented in Fig. 3d. Furthermore, volumetric CO2 adsorption measurements were conducted on the activated sample at 298 K as well as at 273 K, and the CO2 uptake amounts were found to be ∼12 and ∼18 cm3 g−1 (Fig. 3f). The isosteric heat of adsorption of the COF is about 22.5 kJ mol−1, indicating that the TFPB-TRZ COF has a good affinity toward CO2 (Fig. S7†).
The TFPB-TRZ COF was tested as the photocatalyst for the photoreduction of CO2 under different conditions under xenon lamp irradiation (800 > λ > 400 nm, 300 W xenon lamp). The photo-reduced gaseous products were analyzed and quantified by gas chromatography-mass spectrometry (GC-MS) analysis from the head-space of the reactor every two hours. At first, we explored the catalytic activity of the TFPB-TRZ COF in a pure aqueous medium without any sacrificial agent (Fig. S10a†). After 18 h of reaction, 140 μmol g−1 of CH4 and ∼10 μmol g−1 of CO were formed in pure water medium (Fig. S8, S9 and S10a†). Then, we investigated the photocatalytic activity in an acetonitrile (ACN)–water (H2O) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent system using only triethylamine (TEA) as the sacrificial electron donor where 190 μmol g−1 of CH4 and ∼20 μmol g−1 of CO were evolved after 18 h of photocatalysis (Fig. S10b†). Afterwards, the photocatalytic CO2 reduction performance of TFPB-TRZ was checked in aqueous medium using the combination of 1-benzyl-1,4-dihydronicotinamide (BNAH) and TEA as sacrificial agents (Fig. S10c†). As shown in Fig. S10c,† ∼331 μmol g−1 of CH4 and ∼15 μmol g−1 of CO were formed after 18 h of reaction. Finally, in an ACN–H2O (2
1) mixed solvent system using only triethylamine (TEA) as the sacrificial electron donor where 190 μmol g−1 of CH4 and ∼20 μmol g−1 of CO were evolved after 18 h of photocatalysis (Fig. S10b†). Afterwards, the photocatalytic CO2 reduction performance of TFPB-TRZ was checked in aqueous medium using the combination of 1-benzyl-1,4-dihydronicotinamide (BNAH) and TEA as sacrificial agents (Fig. S10c†). As shown in Fig. S10c,† ∼331 μmol g−1 of CH4 and ∼15 μmol g−1 of CO were formed after 18 h of reaction. Finally, in an ACN–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
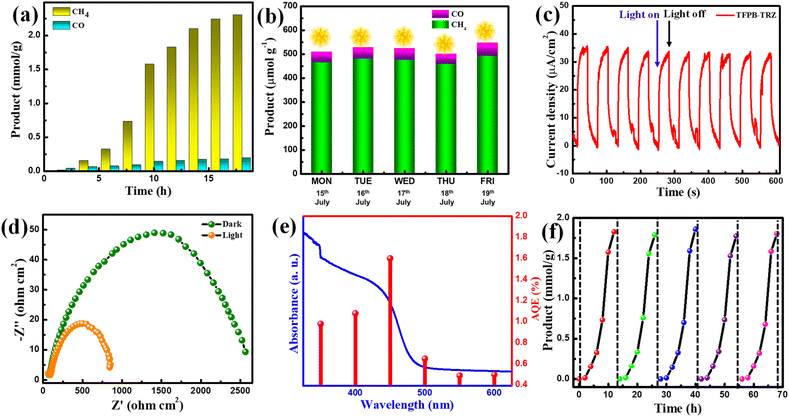
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent system using BNAH and TEA as sacrificial agents, a significant amount of CH4 (∼2304 μmol g−1) and a negligible amount of CO (∼194 μmol g−1) and H2 (∼40 μmol g−1) were produced in 18 h (Fig. 4a). However, no liquid product was formed in the course of photocatalysis, as confirmed by the 1H NMR study (Fig. S11†). The production rate and selectivity of CH4 formation were found to be 128 μmol g−1 h−1 and ∼99%, respectively. To the best of our knowledge, this is the first report on visible light-driven 8e− reduction of CO2 to CH4 by a COF (Table S4†). Interestingly, when the photocatalytic experiment was performed using only BNAH as a sacrificial agent in the mixed solvent system, the catalytic activity was not significant (CO: 7.0 μmol g−1; CH4: 50.0 μmol g−1 in 18 h) (Fig. S12†). Several other control experiments were performed in the dark, under an N2 atmosphere and without the catalyst, in which no CO2 reduction product was found, indicating the profound role of the TFPB-TRZ catalyst towards successful photocatalytic CO2 reduction (Fig. S13†). The enhanced catalytic efficiency in the presence of both BNAH and TEA can be attributed to the following reasons. First, BNAH (E0ox = 0.57 V vs. SCE) is a stronger reducing agent compared to TEA (E0ox = 0.69 V vs. SCE). As a result, BNAH is easier to oxidize than the other sacrificial electron donors, which helps in faster quenching of the hole during photocatalysis under visible light irradiation. Secondly, one-electron oxidation of BNAH induces deprotonation to yield BNA˙ which can easily dimerize to form BNA2 and its reduction power (E0ox = 0.26 V vs. SCE) is stronger than that of BNAH, enabling faster reductive quenching of the excitons.8,9,53–56 Additionally, due to the better solubility of CO2, the sacrificial agents in ACN medium propelled faster diffusion of the CO2 to the catalytic site of the COF, leading towards enhanced catalytic activity. To mimic the natural photosynthesis process, we next verified the TFPB-TRZ COF catalyzed CO2 reduction in the mixed solvent system using BNAH and TEA under direct sunlight between 9:00 am and 5:00 pm for five days from 15th to 19th July 2022 (Fig. 4b). On the sunniest day of 19th July, ∼493 μmol g−1 of CH4 production upon 8 h (activity = 61.62 μmol g−1 h−1) of sunlight irradiation was realized, suggesting the robustness and sunlight-harvesting ability of the TFPB-TRZ COF (Fig. 4b). The photocurrent response of the TFPB-TRZ COF was then conducted to understand the charge separation by light irradiation (Fig. 4c). The generation of high photocurrent (35 μA cm−2) further supports the efficient charge separation ability of the COF under light irradiation (Fig. 4c). The charge transfer ability of the TFPB-TRZ COF was further validated by an electrochemical impedance spectroscopy (EIS) study under both light and dark conditions (Fig. 4d). The semicircle radius in the Nyquist plot decreased significantly in the presence of light, which indicates much better charge transfer under light irradiated conditions in comparison to the dark, benefitting the photocatalytic activity (Fig. 4d). Apparent quantum efficiencies (AQEs) for the TFPB-TRZ photocatalyst were measured under various wavelengths and were found in parallel agreement with the HOMO–LUMO band gap of the TFPB-imine fragment (Fig. 4e). The TFPB-TRZ COF manifested a maximum AQE value of 1.6% under monochromatic light irradiation of 450 nm (see the ESI† for more details). Next, the recyclability of the TFPB-TRZ photocatalyst was evaluated for up to five consecutive cycles under a mixed solvent system in the presence of BNAH and TEA (Fig. 4f). After each catalytic cycle, the catalyst was recovered from the reaction mixture and again utilized for the next cycle under similar conditions, which showed no significant change in the yield for five consecutive cycles, hence suggesting the durability and reusability of the COF as a photocatalyst (Fig. 4f). An isotope labeling experiment was performed with 13CO2, which produced 13CH4, which unambiguously confirmed that the origin of CH4 is from the reduction of CO2 (Fig. S14†). Other than the decrease in the intensity ratios, no obvious change was seen in the PXRD pattern of the post-catalytic sample in comparison to the pristine COF, indicating the robustness of the COF structure (Fig. S15†). FT-IR and TEM analysis of the recovered sample further supports the molecular connectivity, and the structure of the COF skeleton remained intact after photocatalysis (Fig. S16†).
1) mixed solvent system using BNAH and TEA as sacrificial agents, a significant amount of CH4 (∼2304 μmol g−1) and a negligible amount of CO (∼194 μmol g−1) and H2 (∼40 μmol g−1) were produced in 18 h (Fig. 4a). However, no liquid product was formed in the course of photocatalysis, as confirmed by the 1H NMR study (Fig. S11†). The production rate and selectivity of CH4 formation were found to be 128 μmol g−1 h−1 and ∼99%, respectively. To the best of our knowledge, this is the first report on visible light-driven 8e− reduction of CO2 to CH4 by a COF (Table S4†). Interestingly, when the photocatalytic experiment was performed using only BNAH as a sacrificial agent in the mixed solvent system, the catalytic activity was not significant (CO: 7.0 μmol g−1; CH4: 50.0 μmol g−1 in 18 h) (Fig. S12†). Several other control experiments were performed in the dark, under an N2 atmosphere and without the catalyst, in which no CO2 reduction product was found, indicating the profound role of the TFPB-TRZ catalyst towards successful photocatalytic CO2 reduction (Fig. S13†). The enhanced catalytic efficiency in the presence of both BNAH and TEA can be attributed to the following reasons. First, BNAH (E0ox = 0.57 V vs. SCE) is a stronger reducing agent compared to TEA (E0ox = 0.69 V vs. SCE). As a result, BNAH is easier to oxidize than the other sacrificial electron donors, which helps in faster quenching of the hole during photocatalysis under visible light irradiation. Secondly, one-electron oxidation of BNAH induces deprotonation to yield BNA˙ which can easily dimerize to form BNA2 and its reduction power (E0ox = 0.26 V vs. SCE) is stronger than that of BNAH, enabling faster reductive quenching of the excitons.8,9,53–56 Additionally, due to the better solubility of CO2, the sacrificial agents in ACN medium propelled faster diffusion of the CO2 to the catalytic site of the COF, leading towards enhanced catalytic activity. To mimic the natural photosynthesis process, we next verified the TFPB-TRZ COF catalyzed CO2 reduction in the mixed solvent system using BNAH and TEA under direct sunlight between 9:00 am and 5:00 pm for five days from 15th to 19th July 2022 (Fig. 4b). On the sunniest day of 19th July, ∼493 μmol g−1 of CH4 production upon 8 h (activity = 61.62 μmol g−1 h−1) of sunlight irradiation was realized, suggesting the robustness and sunlight-harvesting ability of the TFPB-TRZ COF (Fig. 4b). The photocurrent response of the TFPB-TRZ COF was then conducted to understand the charge separation by light irradiation (Fig. 4c). The generation of high photocurrent (35 μA cm−2) further supports the efficient charge separation ability of the COF under light irradiation (Fig. 4c). The charge transfer ability of the TFPB-TRZ COF was further validated by an electrochemical impedance spectroscopy (EIS) study under both light and dark conditions (Fig. 4d). The semicircle radius in the Nyquist plot decreased significantly in the presence of light, which indicates much better charge transfer under light irradiated conditions in comparison to the dark, benefitting the photocatalytic activity (Fig. 4d). Apparent quantum efficiencies (AQEs) for the TFPB-TRZ photocatalyst were measured under various wavelengths and were found in parallel agreement with the HOMO–LUMO band gap of the TFPB-imine fragment (Fig. 4e). The TFPB-TRZ COF manifested a maximum AQE value of 1.6% under monochromatic light irradiation of 450 nm (see the ESI† for more details). Next, the recyclability of the TFPB-TRZ photocatalyst was evaluated for up to five consecutive cycles under a mixed solvent system in the presence of BNAH and TEA (Fig. 4f). After each catalytic cycle, the catalyst was recovered from the reaction mixture and again utilized for the next cycle under similar conditions, which showed no significant change in the yield for five consecutive cycles, hence suggesting the durability and reusability of the COF as a photocatalyst (Fig. 4f). An isotope labeling experiment was performed with 13CO2, which produced 13CH4, which unambiguously confirmed that the origin of CH4 is from the reduction of CO2 (Fig. S14†). Other than the decrease in the intensity ratios, no obvious change was seen in the PXRD pattern of the post-catalytic sample in comparison to the pristine COF, indicating the robustness of the COF structure (Fig. S15†). FT-IR and TEM analysis of the recovered sample further supports the molecular connectivity, and the structure of the COF skeleton remained intact after photocatalysis (Fig. S16†).
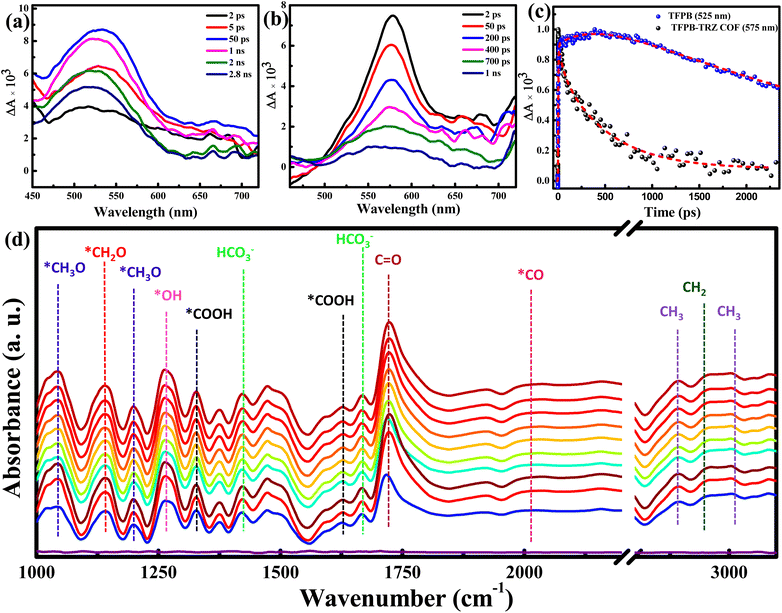
Femtosecond transient absorption (TA) experiments were performed to further confirm the electron transfer from the TFPB moiety to the catalytic site in the COF. The TA absorption spectra of TFPB in acetonitrile solution showed an excited state absorption (ESA) band peaking at ∼525 nm with a tailing broad absorption extending beyond 700 nm (Fig. 5a). The decay trace recorded at 525 nm was characterized by two growth components of 2.6 ps and 280 ps (Fig. 5c). Considering the polar nature of the solvent, the faster growth component of 2.6 ps was assigned due to the solvent relaxation process in the excited state of TFPB. Since TFPB consists of three 4-formylphenyl groups connected to the central benzene moiety through single bonds, there was a high probability that the torsional motion of the 4-formylphenyl group around the single bond could take place in its excited state. Such excited state torsional motion is well known for molecular rotors.57,58 Hence, the longer growth component of 280 ps was assigned to the excited state torsional dynamics in TFPB. Besides such a growth component, the ESA signal of TFPB showed a long decay time (>2 ns) which could not be determined accurately due to the limited temporal window of our measurements (2.5 ns). Such a long excited state decay time constant is also supported by the nanosecond decay component observed in the TRPL measurements (Fig. S6 and Table S3†). The transient absorption spectra of the TFPB-TRZ COF showed a red-shifted ESA band having maxima at ∼575 nm (Fig. 5b). The red shift of ∼50 nm in the ESA band was due to the introduction of extended π-conjugation between TFPB and TRZ in the COF.59 However, unlike TFPB, no growth in the ESA band in the TFPB-TRZ COF was observed. This is expected due to the formation of a large and closed chemical network between TFPB and TRZ. The formation of such a network completely suppressed the torsional dynamics in the excited state.51 The fitting of the ESA signal of the TFPB-TRZ COF results in the decay time constants of 55 ps (18%) and 510 ps (76%) along with a low amplitude (6%) residual signal which can have a longer decay time constant compared to our temporal window of TA setup. This residual signal is mainly responsible for the longer decay observed in the TRPL measurements of the TFPB-TRZ COF (Fig. S6 and Table S3†). The faster decay time constant observed in the TFPB-TRZ COF than in TFPB is mainly due to the electron transfer from TFPB to the catalytic site. Thus, the transient absorption data further support the faster transport of charge carriers in the TFPB-TRZ COF, resulting in higher ordered CO2 reduction products.
We have performed an in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopic study to probe reaction intermediates during photocatalytic CO2 reduction to CH4 associated with the TFPB-TRZ COF photocatalyst in a mixed solvent system using BNAH and TEA as sacrificial electron donors (Fig. 5d). Peaks appearing at 1421 and 1666 cm−1 were ascribed to the symmetric and asymmetric stretching of HCO3−, respectively.8,60 Peaks at 1325 and 1625 cm−1 corresponded to a *COOH species, which is a crucial intermediate during the photochemical conversion of CO2 to CH4 or CO.8,9,60,61 Notably, stretching vibration for the *CO intermediate was observed at 2010 cm−1. The peak observed at 1724 cm−1 is attributed to the stretching vibration of aldehyde C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.62 The presence of the *CH2O intermediate was supported by the band at 1140 cm−1.63 The observation of new IR peaks at 1043 and 1200 cm−1 was attributed to the *CH3O intermediate,60 suggesting that *CH2O and *CH3O are intermediate species of CO2 photoreduction to CH4. In addition to that, the peak at 2947 cm−1 corresponds to the asymmetric (νas) stretching vibration of –CH2.8,9,61–63 Besides, peaks at 2890 and 3000 cm−1 were ascribed to the symmetric (νs) and asymmetric (νas) stretching vibrations of the –CH3 intermediate, respectively.8,9,61–63 Hence, the detection of the key intermediates clearly suggested the progress of the photochemical 8e− reduction of CO2 to CH4.
O.62 The presence of the *CH2O intermediate was supported by the band at 1140 cm−1.63 The observation of new IR peaks at 1043 and 1200 cm−1 was attributed to the *CH3O intermediate,60 suggesting that *CH2O and *CH3O are intermediate species of CO2 photoreduction to CH4. In addition to that, the peak at 2947 cm−1 corresponds to the asymmetric (νas) stretching vibration of –CH2.8,9,61–63 Besides, peaks at 2890 and 3000 cm−1 were ascribed to the symmetric (νs) and asymmetric (νas) stretching vibrations of the –CH3 intermediate, respectively.8,9,61–63 Hence, the detection of the key intermediates clearly suggested the progress of the photochemical 8e− reduction of CO2 to CH4.
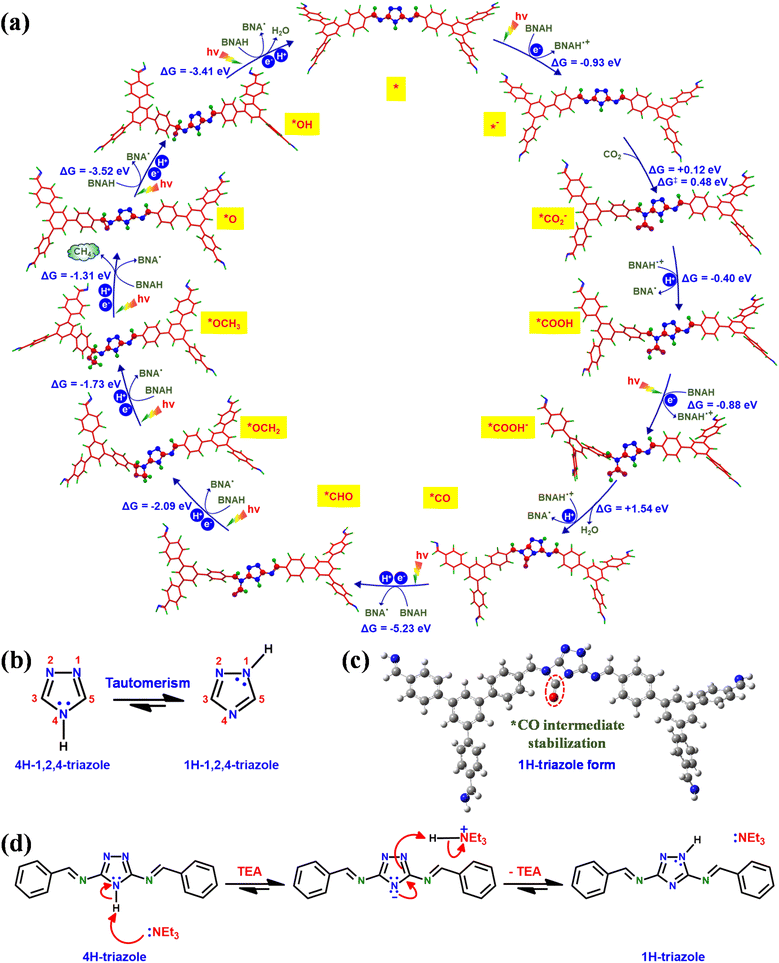
Furthermore, density functional theory (DFT) calculations were performed to understand the underlying catalytic mechanism behind the photocatalytic conversion of CO2 to CH4 on the TFPB-TRZ COF catalyst (Fig. 6). The DFT calculations were performed with the guidance of earlier literature reports and the intermediates traced from the in situ DRIFT study.8,9,52 For theoretical calculations, a small fragment of the TFPB-TRZ COF catalyst consisting of two TFPB and one TRZ unit was taken into consideration, which can be represented as TFPB-TRZ-TFPB (*). The photocatalytic process was initiated with one electron photoreduction of the initial catalyst TFPB-TRZ-TFPB (*), which formed the intermediate [TFPB-TRZ-TFPB]− (*−) (ΔG = −0.93 eV). Next, the reactant CO2 was bound on the intermediate [TFPB-TRZ-TFPB]− (*−), which was the most crucial step for CO2 reduction catalysis. In order to identify the CO2-binding site on [TFPB-TRZ-TFPB]− (*−), we performed CO2-binding energy calculation which revealed that the imine nitrogen was the most suitable active center for CO2-binding during catalysis (Fig. S17†). The CO2-binding to the intermediate [TFPB-TRZ-TFPB]− (*−) resulted in the formation of the *CO2− intermediate, which was a slightly uphill process (ΔG = +0.12 eV) with an activation barrier of 0.48 eV. In the next step of the catalytic process, *CO2− intermediate underwent protonation to generate the *COOH intermediate (ΔG = −0.40 eV), which after photoreduction afforded the *COOH− intermediate (ΔG = −0.88 eV). Next, the intermediate *COOH− after subsequent protonation and water elimination resulted in the formation of the *CO intermediate (ΔG = +1.54 eV). In metal-free CO2 reduction catalysis, the challenging aspect is to prevent the desorption of CO to achieve the higher-ordered CO2 reduction product. Reportedly, the stabilization of the *CO intermediate on the metal-free catalyst can be achieved through additional electron donation to the adsorbed *CO by an electron-rich neighboring group.8 Interestingly, DFT calculations revealed that the TFPB-TRZ COF catalyst is capable of doing that. The 1,2,4-triazole moiety present in this catalyst was reported to exist in rapid equilibrium between its two tautomeric forms, namely, 1H-triazole and 4H-triazole (Fig. 6b).46–48 In the TFPB-TRZ COF catalyst, the TRZ in its 1H-triazole form was able to stabilize the *CO intermediate by donating electrons to the CO species (Fig. 6c and S18†). The presence of the mild base TEA during the catalysis can easily trigger the interconversion between the two tautomers (Fig. 6d). This could be verified by the experimental results of photocatalysis performed in the absence of the base TEA. The catalysis performed with only the BNAH sacrificial agent displayed poorer catalytic activity than the solely used TEA (Fig. S12†), which indicated that the basicity of the sacrificial agent also plays an important role along with the reductive quenching of the exciton. In the next step of the catalytic mechanism, the intermediate *CO would undergo proton-coupled reduction to form the *CHO intermediate (ΔG = −5.23 eV) as the release of CO from the *CO intermediate was less feasible (ΔG = −3.66 eV) (Fig. S18†), which justified the high selectivity of CH4 production over CO during photocatalysis. The rapid conversion of the *CO intermediate could be easily perceived from its highly exergonic conversion steps, which is quite expected from the highly reactive *CO containing a strained four-member ring. In the following steps, successive proton-coupled reductions converted the *CHO intermediate to *OCH2 (ΔG = −2.09 eV) to *OCH3 (ΔG = −1.73 eV). Further proton-coupled reduction of the *OCH3 intermediate led to the generation of the *O intermediate64 and CH4 was produced in the process (ΔG = −1.31 eV). After proton-coupled reduction, the *O intermediate converted to the *OH intermediate (ΔG = −3.52 eV), which subsequently released water after another proton-coupled reduction to regenerate the initial catalyst [TFPB-TRZ-TFPB] (*) (ΔG = −3.41 eV). Thus, the recovered initial catalyst TFPB-TRZ-TFPB (*) again entered into the catalytic process (Fig. 6a and S18†). An important point to be noted: in the whole catalytic process, the formation of the *CO intermediate was the most uphill process (ΔG = +1.54 eV), which suggested that the rate-determining step of the photocatalytic process was the *CO formation step. Further details about the DFT calculations are presented in the ESI (Fig. S17, S18, and Tables S5–S30).†
Conclusions
In summary, we have demonstrated how photocatalytic CO2 reduction can be manipulated via a suitable selection of monomer building unit in the COF material. Here, a TFPB-TRZ COF was synthesized through the solvothermal method, employing 1,3,5-tris(4-formylphenyl)benzene and 3,5-diamino-1,2,4-triazole monomer building units. The metal-free TFPB-TRZ COF displayed CO2 to CH4 conversion with an impressive rate and high selectivity (∼99%) under visible-light irradiation. Furthermore, the TFPB-TRZ COF showed promising catalytic activity under direct sunlight irradiation, suggesting an efficient light-harvesting ability. Femtosecond transient absorption (TA) spectroscopy revealed the fast electron transfer kinetics in the COF compared to the TFPB monomer, facilitating the higher-order CO2 photoreduction. In situ DRIFT analysis indicated the real-time reaction progress of the CO2 to CH4 photoreduction process. Theoretical studies based on these observations provided mechanistic insights of 8e− reduction of CO2 to CH4 by the TFPB-TRZ COF photocatalyst. The nitrogen heteroatoms present in the triazole ring of TRZ help in the stabilization of the CO intermediate by electron donation, benefitting CO2 to CH4 conversion. In a nutshell, photocatalytic proficiency in CO2 reduction to CH4 in a COF-based system underscores the superiority and uniqueness and offers valuable perspectives for the design and synthesis of high-performance, cheap and robust metal-free photocatalysts.Data availability
All the associated data are available in the ESI.†Author contributions
S. B. and T. K. M. designed the concept of this work. S. B., K. S., A. D. and J. C. performed major experiments. S. B., F. A. R., and T. K. M. analyzed the experimental data and wrote the manuscript. F. A. R. conducted all the computational studies. A. D. and D. S. did simulation studies of PXRD patterns. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All the authors acknowledge the support and resources provided by the “PARAM Yukti Facility” under the National Supercomputing Mission, Government of India, at JNCASR, Bangalore. S. B. acknowledges the Science and Engineering Research Board (SERB), Department of Science and Technology (DST) for the National Postdoctoral fellowship (NPDF). A. D. and F. A. R. thank the CSIR (Government of India) for a fellowship. Mr Soumya Kanti Mondal, CPMU, JNCASR is gratefully acknowledged for virial fitting of CO2 adsorption isotherms at two different temperatures. T. K. M. acknowledges the Department of Science and Technology (Projects SPR/2021/000592), RAK-CAM (from UAE), SSL, ICMS, and JNCASR for financial support. The SAMat research facility and Sheikh Saqr senior fellowship are also gratefully acknowledged by T. K. M.Notes and references
- G. A. Olah, G. K. S. Prakash and A. Goeppert, Anthropogenic Chemical Carbon Cycle for a Sustainable Future, J. Am. Chem. Soc., 2011, 133, 12881–12898 CrossRef CAS PubMed.
- S. J. Davis, K. Caldeira and H. D. Matthews, Future CO2 Emissions and Climate Change from Existing Energy Infrastructure, Science, 2010, 329, 1330–1333 CrossRef CAS.
- D. M. D'Alessandro, B. Smit and J. R. Long, Carbon Dioxide Capture: Prospects for New Materials, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- A. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. Dubois, M. Dupuis, J. G. Ferry, E. Fujita, R. Hille, P. J. A. Kenis, C. A. Kerfeld, R. H. Morris, C. H. F. Peden, A. R. Portis, S. W. Ragsdale, T. B. Rauchfuss, J. N. H. Reek, L. C. Seefeldt, R. K. Thauer and G. L. Waldrop, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation, Chem. Rev., 2013, 113, 6621–6658 CrossRef CAS PubMed.
- X. Chang, T. Wang and J. Gong, CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts, Energy Environ. Sci., 2016, 9, 2177–2196 RSC.
- H. J. Zhu, M. Lu, Y. R. Wang, S. J. Yao, M. Kan, Y. H. Zhang, J. Liu, Y. Chen, S. L. Li and Y. Q. Lan, Efficient Electron Transmission in Covalent Organic Framework Nanosheets for Highly Active Electrocatalytic Carbon Dioxide Reduction, Nat. Commun., 2020, 11, 497 CrossRef CAS PubMed.
- J. Albero, Y. Peng and H. García, Photocatalytic CO2 Reduction to C2+ Products, ACS Catal., 2020, 10, 5734–5749 CrossRef CAS.
- S. Barman, A. Singh, F. A. Rahimi and T. K. Maji, Metal-Free Catalysis: A Redox-Active Donor–acceptor Conjugated Microporous Polymer for Selective Visible-light-Driven CO2 Reduction to CH4, J. Am. Chem. Soc., 2021, 143, 16284–16292 CrossRef CAS.
- S. Biswas, A. Dey, F. A. Rahimi, S. Barman and T. K. Maji, Metal-Free Highly Stable and Crystalline Covalent Organic Nanosheet for Visible-light-driven Selective Solar Fuel Production in Aqueous Medium, ACS Catal., 2023, 13, 5926–5937 CrossRef CAS.
- J. Qiao, Y. Liu, F. Hong and J. Zhang, A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-Carbon Fuels, Chem. Soc. Rev., 2014, 43, 631–675 RSC.
- C. Cometto, R. Kuriki, L. Chen, K. Maeda, T.-C. Lau, O. Ishitani and M. Robert, A carbon Nitride/Fe Quaterpyridine Catalytic System for Photostimulated CO2-to-CO Conversion with Visible Light, J. Am. Chem. Soc., 2018, 140, 7437–7440 CrossRef CAS PubMed.
- X. Chen and S. S. Mao, Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS.
- S. Chen, Y. Hu, S. Meng and X. Fu, Study on the Separation Mechanisms of Photogenerated Electrons and Holes for Composite Photocatalysts g-C3N4-WO3, Appl. Catal., B, 2014, 150–151, 564–573 CrossRef CAS.
- Y. Zhou, Y. Zhang, M. Lin, J. Long, Z. Zhang, H. Lin, J. C. Wu and X. Wang, Monolayered Bi2WO6 Nanosheets Mimicking Heterojunction Interface with Open Surfaces for Photocatalysis, Nat. Commun., 2015, 6, 8340 CrossRef.
- L. Cheng, Q. J. Xiang, Y. L. Liao and H. W. Zhang, CdS-based Photocatalysts, Energy Environ. Sci., 2018, 11, 1362–1391 RSC.
- E. García-López, G. Marcì, F. R. Pomilla, M. C. Paganini, C. Gionco, E. Giamello and L. Palmisano, ZrO2 Based Materials as Photocatalysts for 2-propanol Oxidation by Using UV and Solar Light Irradiation and Tests for CO2 Reduction, Catal. Today, 2018, 313, 100–105 CrossRef.
- H. Yang, J. Tang, Y. Luo, X. Zhan, Z. Liang, L. Jiang, H. Hou and W. Yang, MOFs-derived fusiform In2O3 mesoporous nanorods anchored with ultrafine CdZnS nanoparticles for boosting visible-Light photocatalytic hydrogen evolution, Small, 2021, 17, 2102307 CrossRef CAS PubMed.
- S. C. Yan, S. X. Ouyang, J. Gao, M. Yang, J. Y. Feng, X. X. Fan, L. J. Wan, Z. S. Li, J. H. Ye, Y. Zhou and Z. G. Zou, A Room-Temperature Reactive-template Route to Mesoporous ZnGa2O4 with Improved Photocatalytic Activity in Reduction of CO2, Angew. Chem., Int. Ed., 2010, 49, 6400–6404 CrossRef CAS.
- J. Wang, S. Lin, N. Tian, T. Ma, Y. Zhang and H. Huang, Nanostructured Metal Sulfides: Classification, Modification Strategy, and Solar-Driven CO2 Reduction Application, Adv. Funct. Mater., 2021, 31, 2008008 CrossRef CAS.
- S.-P. Qi, R.-T. Guo, Z.-X. Bi, Z.-R. Zhang, C.-F. Li and W.-G. Pan, Recent Progress of Covalent Organic Frameworks-based Materials in Photocatalytic Applications: A Review, Small, 2023, 19, 2303632 CrossRef CAS PubMed.
- X. Feng, X. S. Ding and D. L. Jiang, Covalent Organic Frameworks, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- P. J. Waller, F. Gándara and O. M. Yaghi, Chemistry of Covalent Organic Frameworks, Acc. Chem. Res., 2015, 48, 3053–3063 CrossRef CAS PubMed.
- H. Wang, H. Wang, Z. Wang, L. Tang, G. Zeng, P. Xu, M. Chen, T. Xiong, C. Zhou, X. Li, D. Huang, Y. Zhu, Z. Wang and J. Tang, Covalent Organic Framework Photocatalysts: Structures and Applications, Chem. Soc. Rev., 2020, 49, 4135–4165 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Chemistry and Applications of Metal-organic Frameworks, Science, 2013, 341, 1230444 CrossRef PubMed.
- H. C. Zhou and S. Kitagawa, Metal-organic Frameworks (MOFs), Chem. Soc. Rev., 2014, 43, 5415 RSC.
- Q. Wang and D. Astruc, State of the Art and Prospects in Metal–organic Framework (MOF)-based and MOF-derived Nanocatalysis, Chem. Rev., 2020, 120, 1438–1511 CrossRef CAS.
- H. Guo, D.-H. Si, H.-J. Zhu, Z.-A. Chen, R. Cao and Y.-B. Huang, Boosting CO2 Electroreduction over a Covalent Organic Framework in the Presence of Oxygen, Angew. Chem., Int. Ed., 2024, 63, e202319472 CrossRef CAS PubMed.
- Q. J. Wu, D. H. Si, Q. Wu, Y. L. Dong, R. Cao and Y. B. Huang, Boosting Electroreduction of CO2 over Cationic Covalent Organic Frameworks: Hydrogen Bonding Effects of Halogen Ions, Angew. Chem., Int. Ed., 2023, 62, e202215687 CrossRef CAS.
- R. Xu, D.-H. Si, S.-S. Zhao, Q.-J. Wu, X.-S. Wang, T.-F. Liu, H. Zhao, R. Cao and Y.-B. Huang, Tandem Photocatalysis of CO2 to C2H4 via a Synergistic Rhenium-(I)Bipyridine/Copper-Porphyrinic Triazine Framework, J. Am. Chem. Soc., 2023, 145, 8261–8270 CrossRef CAS PubMed.
- S. Navalon, A. Dhakshinamoorthy, M. Alvaro, B. Ferrer and H. Garcia, Metal-organic Frameworks as Photocatalysts for Solar-driven Overall Water Splitting, Chem. Rev., 2023, 123, 445–490 CrossRef CAS.
- Q. Wang, Q. Gao, A. M. Al-Enizi, A. Nafady and S. Ma, Recent Advances in MOF-based Photocatalysis: Environmental Remediation Under Visible Light, Inorg. Chem. Front., 2020, 7, 300–339 RSC.
- S. Lin, S. D. Christian, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. B. Zhao, A. R. Paris, D. Kim, P. D. Yang, O. M. Yaghi and C. J. Chang, Covalent Organic Frameworks Comprising Cobalt Porphyrins for Catalytic CO2 Reduction in Water, Science, 2015, 349, 1208–1213 CrossRef CAS.
- D. Yadav, A. Kumar, J. Y. Kim, N.-J. Park and J.-O. Baeg, Interfacially Synthesized 2D COF Thin Film Photocatalyst: Efficient Photocatalyst for Solar Formic Acid Production from CO2 and Fine Chemical Synthesis, J. Mater. Chem. A, 2021, 9, 9573–9580 RSC.
- K. Lei, D. Wang, L. Ye, M. Kou, Y. Deng, Z. Ma, L. Wang and Y. Kong, A Metal-free Donor–acceptor Covalent Organic Framework Photocatalyst for Visible-light-driven Reduction of CO2 with H2O, ChemSusChem, 2020, 13, 1725–1729 CrossRef CAS.
- J.-X. Cui, L.-J. Wang, L. Feng, B. Meng, Z.-Y. Zhou, Z.-M. Su, K. Wang and S. Liu, A Metal-free Covalent Organic Framework as a Photocatalyst for CO2 Reduction at Low CO2 Concentration in a Gas–solid system, J. Mater. Chem. A, 2021, 9, 24895–24902 RSC.
- Y. Fu, X. Zhu, L. Huang, X. Zhang, F. Zhang and W. Zhu, Azine-based Covalent Organic Frameworks as Metal-free Visible Light Photocatalysts for CO2 Reduction with H2O, Appl. Catal., B, 2018, 239, 46–51 CrossRef CAS.
- Z. Fu, X. Wang, A. M. Gardner, X. Wang, S. Y. Chong, G. Neri, A. J. Cowan, L. Liu, X. Li, A. Vogel, R. Clowes, M. Bilton, L. Chen, R. S. Sprick and A. I. Cooper, A Stable Covalent Organic Framework for Photocatalytic Carbon Dioxide Reduction, Chem. Sci., 2020, 11, 543–550 RSC.
- W. Liu, X. Li, C. Wang, H. Pan, W. Liu, K. Wang, Q. Zeng, R. Wang and J. Jiang, A Scalable General Synthetic Approach toward Ultrathin Imine-linked Two-dimensional Covalent Organic Framework Nanosheets for Photocatalytic CO2 Reduction, J. Am. Chem. Soc., 2019, 141, 17431–17440 CrossRef CAS.
- L. Peng, S. Chang, Z. Liu, Y. Fu, R. Ma, X. Lu, F. Zhang, W. Zhu, L. Kong and M. Fan, Visible-light-driven Photocatalytic CO2 Reduction Over Ketoenamine-based Covalent Organic Frameworks: Role of the Host Functional Groups, Catal. Sci. Technol., 2021, 11, 1717–1724 RSC.
- A. Dey, J. Pradhan, S. Biswas, F. A. Rahimi, K. Biswas and T. K. Maji, COF-Topological Quantum Material Nano-heterostructure for CO2 to Syngas Production under Visible Light, Angew. Chem., Int. Ed., 2024, 63, e202315596 CrossRef CAS.
- M. Lu, M. Zhang, J. Liu, Y. Chen, J.-P. Liao, M.-Y. Yang, Y.-P. Cai, S.-L. Li and Y.-Q. Lan, Covalent Organic Framework Based Functional Materials: Important Catalysts for Efficient CO2 Utilization, Angew. Chem., Int. Ed., 2022, 61, e202200003 CrossRef CAS PubMed.
- H. L. Nguyen and A. Alzamly, Covalent Organic Frameworks as Emerging Platforms for CO2 Photoreduction, ACS Catal., 2021, 11, 9809–9824 CrossRef CAS.
- S. Yang, W. Hu, X. Zhang, P. He, B. Pattengale, C. Liu, M. Cendejas, I. Hermans, X. Zhang, J. Zhang and J. Huang, 2D Covalent Organic Frameworks as Intrinsic Photocatalysts for Visible Light-driven CO2 Reduction, J. Am. Chem. Soc., 2018, 140, 14614–14618 CrossRef CAS.
- L.-j. Wang, R.-l. Wang, X. Zhang, J.-l. Mu, Z.-y. Zhou and Z.-m. Su, Improved Photoreduction of CO2 with Water by Tuning the Valence Band of Covalent Organic Frameworks, ChemSusChem, 2020, 13, 2973–2980 CrossRef CAS.
- A. Dey, F. A. Rahimi, S. Barman, A. Hazra and T. K. Maji, Metal Free 3D Donor-acceptor COF with Low Exciton Binding for Solar Fuel Production Based on CO2 Reduction, J. Mater. Chem. A, 2023, 11, 13615–13622 RSC.
- T. Sergeieva, M. Bilichenko, S. Holodnyak, Y. V. Monaykina, S. I. Okovytyy, S. I. Kovalenko, E. Voronkov and J. Leszczynski, Origin of Substituent Effect on Tautomeric Behavior of 1,2,4-triazole Derivatives: Combined Spectroscopic and Theoretical Study, J. Phys. Chem. A, 2016, 120, 10116–10122 CrossRef CAS PubMed.
- M. M. Matin, P. Matin, M. R. Rahman, T. Ben Hadda, F. A. Almalki, S. Mahmud, M. M. Ghoneim, M. Alruwaily and S. Alshehri, Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications, Front. Mol. Biosci., 2022, 9, 864286 CrossRef CAS.
- K. T. Potts, The Chemistry of 1,2,4-triazoles, Chem. Rev., 1961, 61, 87–127 CrossRef CAS.
- A. C. Jakowetz, T. F. Hinrichsen, L. Ascherl, T. Sick, M. Calik, F. Auras, D. D. Medina, R. H. Friend, A. Rao and T. Bein, Excited-State Dynamics in Fully Conjugated 2D Covalent Organic Frameworks, J. Am. Chem. Soc., 2019, 141, 11565–11571 CrossRef CAS.
- S. Yang, D. Streater, C. Fiankor, J. Zhang and J. Huang, Conjugation-and Aggregation-Directed Design of Covalent Organic Frameworks as White-Light-Emitting Diode, J. Am. Chem. Soc., 2021, 143, 1061–1068 CrossRef CAS PubMed.
- W. Helweh, N. C. Flanders, S. Wang, B. T. Phelan, P. Kim, M. J. Strauss, R. L. Li, M. S. Kelley, M. S. Kirschner and D. O. Edwards, Layered Structures of Assembled Imine-linked Macrocycles and Two-dimensional Covalent Organic Frameworks Give Rise to Prolonged Exciton Lifetimes, J. Mater. Chem. C, 2022, 10, 3015–3026 RSC.
- R. Guntermann, L. Frey, A. Biewald, A. Hartschuh, T. Clark, T. Bein and D. D. Medina, Regioisomerism in Thienothiophene-Based Covalent Organic Frameworks– A Tool for Band-Gap Engineering, J. Am. Chem. Soc., 2024, 146, 15869–15878 CrossRef CAS PubMed.
- P. Verma, F. A. Rahimi, D. Samanta, A. Kundu, J. Dasgupta and T. K. Maji, Visible-light-driven Photocatalytic CO2 Reduction to CO/CH4 Using a metal–organic “Soft” Coordination Polymer Gel, Angew. Chem., Int. Ed., 2022, 61, e202116094 CrossRef CAS PubMed.
- G. Sahara and O. Ishitani, Efficient Photocatalysts for CO2 Reduction, Inorg. Chem., 2015, 54, 5096–5104 CrossRef CAS PubMed.
- Y. Kuramochi, O. Ishitani and H. Ishida, Reaction Mechanisms of Catalytic Photochemical CO2 Reduction Using Re(I) and Ru(II) Complexes, Coord. Chem. Rev., 2018, 373, 333–356 CrossRef CAS.
- T. J. Whittemore, C. Xue, J. Huang, J. C. Gallucci and C. Turro, Single-chromophore Single-molecule Photocatalyst for the Production of Dihydrogen Using Low-energy Light, Nat. Chem., 2020, 12, 180–185 CrossRef CAS.
- P. Roy, W. R. Browne, B. L. Feringa and S. R. Meech, Ultrafast Motion in a Third Generation Photomolecular Motor, Nat. Commun., 2023, 14, 1253 CrossRef CAS PubMed.
- R. Ghosh, A. Kushwaha and D. Das, Conformational Control of Ultrafast Molecular Rotor Property: Tuning Viscosity Sensing Efficiency by Twist Angle Variation, J. Phys. Chem. B, 2017, 121, 8786–8794 CrossRef CAS.
- T. Feng, D. Streater, B. Sun, K. Duisenova, D. Wang, Y. Liu, J. Huang and J. Zhang, Tuning Photoexcited Charge Transfer in Imine-Linked Two dimensional covalent organic frameworks, J. Phys. Chem. Lett., 2022, 13, 1398–1405 CrossRef CAS PubMed.
- X. Li, Y. Sun, J. Xu, Y. Shao, J. Wu, X. Xu, Y. Pan, H. Ju, J. Zhu and Y. Xie, Selective Visible-light-driven Photocatalytic CO2 Reduction to CH4 Mediated by Atomically Thin CuIn5S8 Layers, Nat. Energy, 2019, 4, 690–699 CrossRef CAS.
- R. Zhang, H. Wang, S. Tang, C. Liu, F. Dong, H. Yue and B. Liang, Photocatalytic Oxidative Dehydrogenation of Ethane Using CO2 as a Soft Oxidant Over Pd/TiO2 Catalysts to C2H4 and Syngas, ACS Catal., 2018, 8, 9280–9286 CrossRef CAS.
- Q. Li, H. Yue, C. Liu, K. Ma, S. Zhong, B. Liang and S. Tang, A Photocatalytic Transformation Realized by Pd/TiO2 Particle Size Modulation: From Oxidative Dehydrogenation of Ethane to Direct Dehydrogenation of Ethane, Chem. Eng. J., 2020, 395, 125120 CrossRef CAS.
- F. A. Rahimi, S. Dey, P. Verma and T. K. Maji, Photocatalytic CO2 Reduction Based on a Re(I)-Integrated Conjugated Microporous Polymer: Role of a Sacrificial Electron Donor in Product Selectivity and Efficiency, ACS Catal., 2023, 13, 5969–5978 CrossRef CAS.
- D. Ren, J. Fong and B. S. Yeo, The Effects of Currents and Potentials on the Selectivities of Copper toward Carbon dioxide Electroreduction, Nat. Commun., 2018, 9, 925 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc03163f |
| This journal is © The Royal Society of Chemistry 2024 |