Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ion pair extractant selective for LiCl and LiBr†
Nam Jung Heoa,
Ju Hyun Oha,
Aimin Lib,
Kyounghoon Leec,
Qing He b,
Jonathan L. Sessler
b,
Jonathan L. Sessler *d and
Sung Kuk Kim
*d and
Sung Kuk Kim *a
*a
aDepartment of Chemistry, Research Institute of Natural Sciences, Gyeongsang National University, Jinju, 52828, Korea. E-mail: sungkukkim@gnu.ac.kr
bState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, P. R. China
cDepartment of Chemistry Education, Research Institute of Natural Sciences, Gyeongsang National University, Jinju, 52828, Korea
dDepartment of Chemistry, The University of Texas at Austin, 105 E. 24th Street-Stop A5300, Austin, Texas 78712-1224, USA. E-mail: sessler@cm.utexas.edu
First published on 12th August 2024
Abstract
Improved methods for achieving the selective extraction of lithium salts from lithium sources, including rocky ores, salt-lake brines, and end-of-life lithium-ion batteries, could help address projected increases in the demand for lithium. Here, we report an ion pair receptor (2) capable of extracting LiCl and LiBr into an organic receiving phase both from the solid state and from aqueous solutions. Ion pair receptor 2 consists of a calix[4]pyrrole framework, which acts as an anion binding site, linked to a phenanthroline cation binding motif via ether linkages. Receptor 2 binds MgBr2 and CaCl2 with high selectivity over the corresponding lithium salts in a nonpolar aprotic solvent. The preference for Mg2+ and Ca2+ salts is reversed in polar protic media, allowing receptor 2 to complex LiCl and LiBr with high selectivity and affinity in organic media containing methanol or water. The effectiveness of receptor 2 as an extractant for LiCl and LiBr under liquid–liquid extraction (LLE) conditions was found to be enhanced by the presence of other potentially competitive salts in the aqueous source phase.
Introduction
The lithium ion (Li+) supports key features of modern life; it is an essential component in lithium ion batteries (LIBs), ceramics, and lubricating greases, and is used as a pharmaceutical agent in treating depressive illness.1–6 Currently rechargeable LIBs account for approximately 80% of the end use of lithium.7 According to the U.S. Geological Survey (USGS), global consumption of lithium rose from approximately 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons in 2021 to 134
000 tons in 2021 to 134![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons in 2022.7 While the global demand for lithium is increasing, the available lithium reserves remain limited. In addition, selective separation of the lithium ion from its sources requires an energy- and labor-intensive process, and can be time-consuming.8,9 In principle, lithium may be separated from rocky ores, salt lakes, brines, and sea water.8–10 When rocky ores or clays are used as the lithium source, roasting at a high temperature (1100 °C) is followed by baking at 250 °C in acid.8 Undesired salts are then removed via several energy- and water-intensive steps that are a source of environmental concern.11–13 The ocean is the largest source of lithium; however, selective extraction of lithium from seawater is challenging because of the very low concentration of the lithium cation amounting to only 0.1–0.2 ppm while other potentially competitive ions exist at much higher concentrations.14–19 At present, salt lake brines supply the majority of the commercial lithium. However, the extraction of lithium from brine reservoirs typically requires evaporation of residual water over a period of months to years.20–26 A huge amount of water is also necessary to remove unwanted ions and contaminants. Because of the shortcomings of conventional extraction processes, efforts are being devoted increasingly to so-called direct lithium extraction (DLE) methods. In this context, the use of porous lithium sorbents and ion exchange materials has attracted attention because of their relative simplicity and potential to operate at relatively low levels of environmental stress.27,28 Unfortunately, most DLE methods developed thus far require additional processing steps to free the lithium ions from the lithium adsorbing materials.27,28 Therefore, so-called liquid–liquid extraction (LLE) and solid–liquid extraction (SLE), wherein an extractant capable of selectively complexing lithium salts is used to promote DLE, continue to attract interest. In principle, these approaches could enable the direct and selective extraction of lithium salts from mixed salt solid phases or brines. Nevertheless, it remains challenging to design and construct an extractant possessing the ability to extract selectively lithium salts due to the small size of the lithium cation and its relatively high hydration energy (ΔhydG* = −475 kJ mol−1), as well as the interference of other competing cations, such as Na+, K+, Mg2+, and Ca2+.29–31 For instance, Mg2+ not only has a similar ionic radius (72 pm for Mg2+vs. 69 pm for Li+), it also has a higher net charge, and is typically present at ≥8× the concentration of Li+ in most salt lake brines.23,29 The likely presence of Mg2+ and other potential interferants underscores the challenge associated with designing Li+-selective extractants. One way to meet this challenge could involve the use of ion pair receptors. Here we report a new phenanthroline-strapped calix[4]pyrrole (2) that acts as a selective receptor for LiCl and LiBr in polar media and which promotes lithium salt extraction under LLE conditions.
000 tons in 2022.7 While the global demand for lithium is increasing, the available lithium reserves remain limited. In addition, selective separation of the lithium ion from its sources requires an energy- and labor-intensive process, and can be time-consuming.8,9 In principle, lithium may be separated from rocky ores, salt lakes, brines, and sea water.8–10 When rocky ores or clays are used as the lithium source, roasting at a high temperature (1100 °C) is followed by baking at 250 °C in acid.8 Undesired salts are then removed via several energy- and water-intensive steps that are a source of environmental concern.11–13 The ocean is the largest source of lithium; however, selective extraction of lithium from seawater is challenging because of the very low concentration of the lithium cation amounting to only 0.1–0.2 ppm while other potentially competitive ions exist at much higher concentrations.14–19 At present, salt lake brines supply the majority of the commercial lithium. However, the extraction of lithium from brine reservoirs typically requires evaporation of residual water over a period of months to years.20–26 A huge amount of water is also necessary to remove unwanted ions and contaminants. Because of the shortcomings of conventional extraction processes, efforts are being devoted increasingly to so-called direct lithium extraction (DLE) methods. In this context, the use of porous lithium sorbents and ion exchange materials has attracted attention because of their relative simplicity and potential to operate at relatively low levels of environmental stress.27,28 Unfortunately, most DLE methods developed thus far require additional processing steps to free the lithium ions from the lithium adsorbing materials.27,28 Therefore, so-called liquid–liquid extraction (LLE) and solid–liquid extraction (SLE), wherein an extractant capable of selectively complexing lithium salts is used to promote DLE, continue to attract interest. In principle, these approaches could enable the direct and selective extraction of lithium salts from mixed salt solid phases or brines. Nevertheless, it remains challenging to design and construct an extractant possessing the ability to extract selectively lithium salts due to the small size of the lithium cation and its relatively high hydration energy (ΔhydG* = −475 kJ mol−1), as well as the interference of other competing cations, such as Na+, K+, Mg2+, and Ca2+.29–31 For instance, Mg2+ not only has a similar ionic radius (72 pm for Mg2+vs. 69 pm for Li+), it also has a higher net charge, and is typically present at ≥8× the concentration of Li+ in most salt lake brines.23,29 The likely presence of Mg2+ and other potential interferants underscores the challenge associated with designing Li+-selective extractants. One way to meet this challenge could involve the use of ion pair receptors. Here we report a new phenanthroline-strapped calix[4]pyrrole (2) that acts as a selective receptor for LiCl and LiBr in polar media and which promotes lithium salt extraction under LLE conditions.
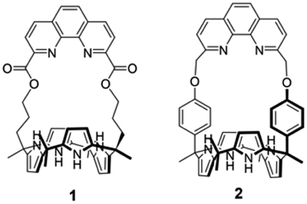
Ion pair receptors are systems with an ability to complex concurrently both a cation and an anion. Appropriately designed ion pair receptors display enhanced selectivity and affinity for target ions relative to cation or anion receptors, systems that bind either a cation or an anion, but not both.32–36 Although numerous ion pair receptors capable of binding various alkali metal salts have been reported to date, only a very small number of receptors were found to bind lithium salts with sufficient affinity and selectivity to permit effective SLE or LLE of Li+ salts.37–39 Unfortunately, even in the most favorable cases the actual lithium extraction efficiencies proved very low. For instance, we reported ion pair receptors based on calix[4]pyrroles strapped with pyridine-fused multi-aromatic rings containing a methoxybenzene group. These heteroditopic ion pair receptors proved capable of extracting LiCl or LiNO3 from the solid state into nitrobenzene-d5 or dichloromethane-d2 under solid–liquid extraction conditions provided the salts were present in excess.37,39 The phenanthroline-strapped calix[4]pyrrole (1) having ester likers was found to extract LiCl into chloroform from an aqueous solution containing near-saturated quantities of LiCl, but not at lower source phase concentrations.39 In the case of receptor 1, the presence of the electron-withdrawing ester groups directly linked to the phenanthroline cation binding site was thought to reduce the inherent Li+ affinity accounting for the limits on its extraction ability. As inferred from a solid state X-ray crystal structure and solution phase 1H NMR spectral studies, the ester carbonyl oxygen atoms present in receptor 1 do not participate in complexation with lithium.39 We thus considered it likely that an analogue of 1 that incorporated oxygen donor sites within the tethering subunits that link the phenanthroline strap to the calix[4]pyrrole core would prove more effective as an ion pair receptor for lithium salts, such as LiCl and LiBr. With such considerations in mind, we designed a new ion pair receptor (2) wherein the ester groups present in 1 are replaced by phenoxy ether linkages (Fig. 1). The relatively rigid nature of the phenoxy linkers was also expected to provide for increased structural preorganization within the receptor framework leading to improved LiCl and LiBr recognition. As detailed below, receptor 2 binds LiCl and LiBr with high selectivity and with an affinity (Ka > 105 M−1 for LiCl in 10% methanol in chloroform-d) that exceeds that of receptor 1 (Ka = 180 ± 7), as well as all other lithium ion receptors of which we are aware. Receptor 2 also acts as an effective LLE extractant promoting the transfer of LiCl and LiBr from aqueous solutions into dichloromethane or nitrobenzene receiving phases.
Results and discussion
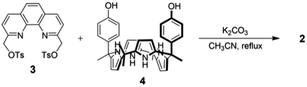
We previously reported that the ion pair receptor 1, a phenanthroline-strapped calix[4]pyrrole with ester linkers, was able to extract LiCl from LiCl-saturated aqueous solutions into a chloroform layer.39 However, receptor 1 failed to extract LiCl from aqueous solutions containing relatively low concentrations of LiCl. Moreover, receptor 1 was not examined for its LiCl selectivity in the presence of MgX2, CaX2, NaX, or KX (X = Cl and Br), which coexist in most lithium sources, under LLE conditions.39 As noted above, receptor 2, wherein relatively rigid phenoxy groups serve to link the phenanthroline cation and calix[4]pyrrole anion recognition subunits, was prepared in an effort to address these shortcomings.The synthesis of receptor 2 is summarized in Scheme 1. Briefly, phenanthroline ditosylate 3 and cis-bisphenolic calix[4]pyrrole 4 were prepared following known literature procedures (Scheme 1).40,41 Reaction of compound 3 with calix[4]pyrrole 4 in the presence of K2CO3 as a base in acetonitrile gave the desired ion pair receptor 2 in 11% yield. Receptor 2 was characterized by means of 1H and 13C NMR spectroscopies and high resolution QTOF (quadrupole time of flight) mass spectrometry, as well as a single crystal X-ray diffraction analysis of its LiBr complex.
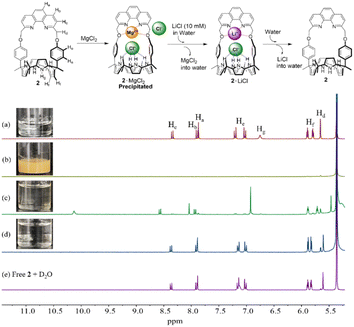
Initially, we examined the ability of receptor 2 to bind various alkali and alkaline earth metal chloride salts, including LiCl, NaCl, KCl, MgCl2, and CaCl2 in CH3OH/CDCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) using 1H NMR spectroscopy. This specific solvent system was chosen with consideration of the solubility of both the receptor and the test salts in mind. Upon exposure of receptor 2 to excess LiCl (ca. 100 equiv.), noticeable chemical shift changes were observed in the proton signals of both the calix[4]pyrrole subunit and the phenanthroline group, findings leading us to conclude that receptor 2 forms an ion pair complex with LiCl (Fig. S1 and S2, see the ESI† for details). By contrast, no chemical shift movements were seen in the presence of NaCl and KCl, which was taken as evidence for receptor 2 being incapable of complexing NaCl and KCl. In contrast, in the presence of MgCl2 (ca. 100 equiv.), roughly 16% of receptor 2 forms a complex with MgCl2 under these conditions (CH3OH/CDCl3; 1
9, v/v) using 1H NMR spectroscopy. This specific solvent system was chosen with consideration of the solubility of both the receptor and the test salts in mind. Upon exposure of receptor 2 to excess LiCl (ca. 100 equiv.), noticeable chemical shift changes were observed in the proton signals of both the calix[4]pyrrole subunit and the phenanthroline group, findings leading us to conclude that receptor 2 forms an ion pair complex with LiCl (Fig. S1 and S2, see the ESI† for details). By contrast, no chemical shift movements were seen in the presence of NaCl and KCl, which was taken as evidence for receptor 2 being incapable of complexing NaCl and KCl. In contrast, in the presence of MgCl2 (ca. 100 equiv.), roughly 16% of receptor 2 forms a complex with MgCl2 under these conditions (CH3OH/CDCl3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v), which leads us to suggest that the affinity of receptor 2 for MgCl2 is very low (Ka < 5 M−1) (Fig. S1†).42,43 In analogy to what was seen with LiCl, Mg2+ is presumed to be bound to the phenanthroline unit while one of two Cl− anions is bound to the calix[4]pyrrole moiety via hydrogen bonds (Fig. S1 and S2; see the ESI† for details). Distinct chemical shift changes were also observed in the 1H NMR spectrum when receptor 2 was treated with CaCl2 in CH3OH/CDCl3 (1
9, v/v), which leads us to suggest that the affinity of receptor 2 for MgCl2 is very low (Ka < 5 M−1) (Fig. S1†).42,43 In analogy to what was seen with LiCl, Mg2+ is presumed to be bound to the phenanthroline unit while one of two Cl− anions is bound to the calix[4]pyrrole moiety via hydrogen bonds (Fig. S1 and S2; see the ESI† for details). Distinct chemical shift changes were also observed in the 1H NMR spectrum when receptor 2 was treated with CaCl2 in CH3OH/CDCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) (Fig. S1†). These 1H NMR spectral changes are consistent with the Ca2+ cation only, and not the Cl− anion, being complexed within receptor 2 (Fig. S2†). A follow up 1H NMR spectral titration with CaCl2 provided further support for this binding mode (Fig. S3; see the ESI† for details). This non-ion pair binding mode stands in contrast to what was seen in the cases of LiCl and MgCl2 (Fig. S2†). Taken in concert, these findings are thought to reflect a cation recognition site that is near-optimal for Li+ cation complexation, somewhat small for fully effective Na+ and K+ recognition, and essentially able to accommodate only Ca2+ without Cl− being co-bound.
9, v/v) (Fig. S1†). These 1H NMR spectral changes are consistent with the Ca2+ cation only, and not the Cl− anion, being complexed within receptor 2 (Fig. S2†). A follow up 1H NMR spectral titration with CaCl2 provided further support for this binding mode (Fig. S3; see the ESI† for details). This non-ion pair binding mode stands in contrast to what was seen in the cases of LiCl and MgCl2 (Fig. S2†). Taken in concert, these findings are thought to reflect a cation recognition site that is near-optimal for Li+ cation complexation, somewhat small for fully effective Na+ and K+ recognition, and essentially able to accommodate only Ca2+ without Cl− being co-bound.
We also investigated the capability of receptor 2 to complex the bromide salts of alkali and alkaline earth metal cations, including LiBr, NaBr, KBr, MgBr2 and CaBr2, in 10% methanol in chloroform-d. In contrast to what was seen with the corresponding chloride salts, upon exposure of receptor 2 to the respective bromide salts, only LiBr gave rise to chemical shift movements attributable to ion pair complexation (Fig. S4†). This finding is taken as evidence that receptor 2 is capable of binding LiBr selectively over other test bromide salts. We also examined the binding selectivity of receptor 2 for other lithium halide salts, i.e., LiF, LiCl, LiBr, and LiI, in CH3OH/CDCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v). Addition of LiCl and LiBr produced chemical shift changes for the signals of receptor 2 in the 1H NMR spectra ascribable to co-binding of the lithium cations and the halide anions while LiF caused no appreciable chemical shift changes (Fig. S5†). When receptor 2 was exposed to LiI, only the lithium cation was bound to the phenanthroline moiety without the iodide anion being co-complexed by the calix[4]pyrrole subunit (Fig. S5; see the ESI† for details).
9, v/v). Addition of LiCl and LiBr produced chemical shift changes for the signals of receptor 2 in the 1H NMR spectra ascribable to co-binding of the lithium cations and the halide anions while LiF caused no appreciable chemical shift changes (Fig. S5†). When receptor 2 was exposed to LiI, only the lithium cation was bound to the phenanthroline moiety without the iodide anion being co-complexed by the calix[4]pyrrole subunit (Fig. S5; see the ESI† for details).
To quantify the ability of receptor 2 to bind cations and anions as well as various test ion pairs, we performed 1H NMR spectroscopic titrations in CH3OH/CDCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v). For instance, upon the titration of receptor 2 with LiCl, the resulting 1H NMR spectral changes support the conclusion receptor 2 binds LiCl quantitatively with a 1
9, v/v). For instance, upon the titration of receptor 2 with LiCl, the resulting 1H NMR spectral changes support the conclusion receptor 2 binds LiCl quantitatively with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry via a binding-release equilibrium that is slow on the NMR time scale (Fig. S6; see the ESI† for details). The association constant for LiCl approximated from this titration experiment was found to be Ka > 105 M−1. This value is at least three orders of magnitude larger than what was found in the case of receptor 1 (Table 1 and Fig. S7†).42
1 binding stoichiometry via a binding-release equilibrium that is slow on the NMR time scale (Fig. S6; see the ESI† for details). The association constant for LiCl approximated from this titration experiment was found to be Ka > 105 M−1. This value is at least three orders of magnitude larger than what was found in the case of receptor 1 (Table 1 and Fig. S7†).42
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v)
9, v/v)
| Hostb | Guest | K a (M−1) |
|---|---|---|
| a Values were obtained from 1H NMR spectroscopic titrations of 2. b Unless otherwise indicated, the anions and lithium cations were used in the forms of their respective tetrabutylammonium and perchlorate salts. c The Ka value was approximated using BindFit v0.5 available from https://app.supramolecular.org/bindfit. | ||
| 2 | Cl− | No binding |
| 2 | Br− | No binding |
| 2 | Li+ | <5c |
| 1 | LiCl | 180 ± 7c |
| 2 | LiCl | >105 |
| 1 | LiBr | 24 ± 1c |
| 2 | LiBr | 63 ± 3c |
| 2 + Li+ | Cl− | >105 |
| 2 + Li+ | Br− | 148 ± 5c |
| 2 + Cl− | Li+ | >105 |
| 2 + Br− | Li+ | 95 ± 3c |
Receptor 2 was also found to bind LiBr, albeit with low affinity relative to LiCl and via an equilibrium process that is fast on the NMR timescale (Fig. S8, see the ESI† for details). The corresponding LiBr association constant was calculated to be Ka = 63 ± 3 M−1.43 The relatively low affinity for LiBr is presumably because the Br− anion is too large to be co-complexed with the Li+ cation effectively within the receptor cavity. However, this association constant for LiBr is 2.6× larger than that of receptor 1 (Table 1 and Fig. S9†). The association constants for MgCl2 and CaCl2 were likewise determined to be Ka < 5 M−1 for both salts in 10% CH3OH in CDCl3 (Fig. S1 and S3†).43 Again, this finding is ascribed to a mismatch with the binding cavity present in receptor 2 as well as to larger solvation energies of the divalent cations (vide infra).
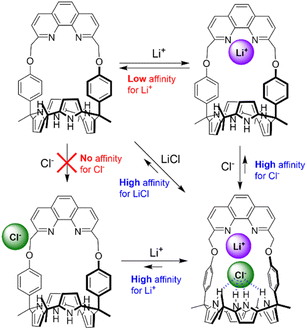
We also investigated the interactions of receptor 2 with Cl−, Br−, and Li+ with non-coordinating counter ions in 10% CH3OH in CDCl3. When receptor 2 was treated with excess Cl− and Br− (as their TBA+ (tetrabutylammonium) salts), no appreciable chemical shift changes were observed in the 1H NMR spectrum, a finding taken as evidence that receptor 2 fails to bind these halide anion salts in this protic solvent system (Fig. S10 and S11†). In contrast, in the presence of excess Li+ (as its ClO4− (perchlorate anion) salt), the CH proton signal (Ha) of the phenanthroline subunit of receptor 2 underwent a slight downfield shift (Δδ = 0.04 ppm). Although modest, this change is thought to reflect Li+ cation complexation by the phenanthroline group (Fig. S10†). The association constant of receptor 2 for LiClO4 was calculated from this 1H NMR spectral titration to be <5 M−1 (Fig. S12†).43 On the basis of these studies, we conclude that receptor 2 binds the LiCl and LiBr ion pairs far more effectively than the individual ions, Cl−, and Br−, and Li+, when the latter were tested using a non-coordinating counter ion. These findings are rationalized in terms of the ion pair complexes with LiCl and LiBr being stabilized by electrostatic attractions between the co-bound anion and cation.
Consistent with the above conclusion, the ability of receptor 2 to bind the Cl− and Br− anions was markedly improved in the presence of the Li+ cation and vice versa (Fig. 2). For instance, when receptor 2 was titrated with Cl− in the presence of Li+ in 10% CH3OH in CDCl3, a new set of proton signals attributable to the LiCl complex of receptor 2 emerged with saturation being achieved upon the addition of 1.0 equiv. of Cl− (Fig. S13†). The association constant of receptor 2 for Cl− in the presence of Li+ was found to be >105 M−1 on the basis of this 1H NMR spectral titration experiment.42 Receptor 2 was also found to bind the Br− anion in the presence of the Li+ cation (ca. 50 equiv.) with an association constant of Ka = 148 ± 5 M−1 (Fig. S14†).43 This value is considerably larger than what was seen in its absence (no affinity for Br−; vide supra). We thus conclude that co-complexation of Cl− and Br− with Li+ significantly enhances the affinity of 2 for these two halide anions, which are otherwise incapable of binding to the receptor (Fig. 2).
The affinity of receptor 2 for Li+ was also found to be significantly enhanced in the presence of Cl− and Br−. On the basis of the 1H NMR spectral titration of receptor 2 with LiClO4 in the presence of TBACl (5.0 equiv.), the association constant of receptor 2 for Li+ was estimated to be >105 M−1 (Fig. S15; see the ESI† for details), a value that is higher than that measured in the absence of Cl− by >100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold (Table 1).42 In contrast, the affinity of receptor 2 for Li+ was enhanced in the presence of Br− by only approx. 20-fold (Fig. S16† and Table 1). We thus conclude that cooperativity between the cation and anion binding sites within receptor 2 plays a crucial role in regulating the ion binding affinity of the receptor for appropriately chosen lithium halide ion pairs, with the effect being particularly dramatic in the case of LiCl, but also noteworthy for LiBr.
000-fold (Table 1).42 In contrast, the affinity of receptor 2 for Li+ was enhanced in the presence of Br− by only approx. 20-fold (Fig. S16† and Table 1). We thus conclude that cooperativity between the cation and anion binding sites within receptor 2 plays a crucial role in regulating the ion binding affinity of the receptor for appropriately chosen lithium halide ion pairs, with the effect being particularly dramatic in the case of LiCl, but also noteworthy for LiBr.
Further evidence that receptor 2 forms an ion pair complex with LiBr came from a single crystal X-ray diffraction analysis. Single crystals of the complex 2·LiBr appropriate for an X-ray diffraction analysis were grown by allowing a mixture of CHCl3 and CH3OH containing 2 and an excess LiBr to evaporate slowly. The resulting crystal structure revealed that the Br− anion is bound to the calix[4]pyrrole moiety via hydrogen bonds with N–H⋯Br− distances of 2.50–2.51 Å while the Li+ cation is coordinated not only to the phenanthroline nitrogen atoms but also to the ether oxygen atoms with N⋯Li+ distances of 1.91 Å and 2.34 Å and O⋯Li+ distances of 2.627 Å and 3.269 Å, respectively (Fig. 3). The Li+ cation is further stabilized by the coordination of two methanol molecules. The Li+ cation was also found to interact directly with the co-bound Br− anion at a distance of 4.02 Å.
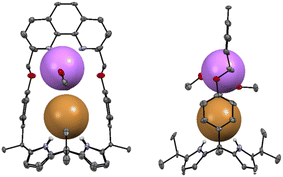 | ||
| Fig. 3 Two different views of the X-ray crystal structure of the 2·LiBr. Thermal ellipsoids are scaled to the 50% probability level. Most hydrogen atoms are omitted for clarity. | ||
We also tested receptor 2 for its ability to solubilize and extract the solid LiCl and LiBr into dichloromethane-d2 (CD2Cl2). When receptor 2 along with LiCl or LiBr (ca. 100 equiv.) was subjected to sonication for 1 hour in CD2Cl2, distinct 1H NMR spectral changes attributable to the formation of ion pair complexes 2·LiCl and 2·LiBr were seen (Fig. S17†). These ion pair complexes proved soluble in CD2Cl2 enabling receptor 2 to extract LiCl and LiBr into this organic phase under solid–liquid extraction conditions. When an equimolar ratio of LiCl and LiBr (ca. 100 equiv. each) was used, receptor 2 was found to complex both LiCl and LiBr at a nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in CD2Cl2. This proved true in spite of the greater affinity displayed for LiCl over LiBr in CH3OH/CDCl3; 1/9, v/v (Table 1). This seeming disparity is rationalized in terms of the relatively small lattice energy of LiBr in CD2Cl2. On the other hand, release of the lithium cation from 2·LiCl could be triggered in CD2Cl2via the addition of the fluoride anion (as its tetrabutylammonium (TBA+) salt). This treatment enables recovery of Li+ in the form of its insoluble LiF salt by filtration (Fig. S18†). The process could be followed by 1H NMR spectroscopy (Fig. S18; see the ESI† for details).
1 ratio in CD2Cl2. This proved true in spite of the greater affinity displayed for LiCl over LiBr in CH3OH/CDCl3; 1/9, v/v (Table 1). This seeming disparity is rationalized in terms of the relatively small lattice energy of LiBr in CD2Cl2. On the other hand, release of the lithium cation from 2·LiCl could be triggered in CD2Cl2via the addition of the fluoride anion (as its tetrabutylammonium (TBA+) salt). This treatment enables recovery of Li+ in the form of its insoluble LiF salt by filtration (Fig. S18†). The process could be followed by 1H NMR spectroscopy (Fig. S18; see the ESI† for details).
The selectivity of 2 for the chloride and bromide salts of alkali and alkaline earth cations was also evaluated under SLE conditions using dichloromethane-d2 as the receiving phase. Receptor 2 is soluble in this solvent, whereas the test salts are insoluble. In this aprotic solvent, receptor 2 exhibited different binding behavior as compared to 10% CH3OH in CDCl3. Upon treatment of receptor 2 with approx. 500 equiv. each of LiCl, NaCl, KCl, MgCl2, and CaCl2 in CD2Cl2 (separate studies), only LiCl induced chemical shift changes in the spectrum that could be readily interpreted in terms of LiCl complexation (Fig. S19†). In contrast, NaCl, KCl, and CaCl2 gave rise to no appreciable chemical shift changes in the 1H NMR spectrum of 2. Meanwhile, treatment with MgCl2 produced an orange precipitate (Fig. S19†). This latter finding is interpreted in terms of receptor 2 forming an ion pair complex with MgCl2 that is insoluble in CD2Cl2. We next monitored the changes in the 1H NMR spectrum of 2 when treated with a mixture of LiCl, NaCl, KCl, MgCl2, and CaCl2 salts (ca. 100 equiv. each) in CD2Cl2. Under these conditions, an orange precipitate was formed with no discernable proton signals being seen in the 1H NMR spectrum of the residual CD2Cl2 layer. Given the analogy to what was seen when receptor 2 was treated with MgCl2 alone, we interpret these findings in terms of receptor 2 capturing MgCl2 in preference to the other chloride salts with this nominal selectivity being driven in part by solubility considerations (Fig. S20†).
In case of the corresponding bromide salts, receptor 2 was found to extract LiBr and MgBr2 in the solid state into CD2Cl2 but via different apparent binding modes. For instance, when exposed to LiBr in CD2Cl2, receptor 2 exhibited 1H NMR spectral changes consistent with the formation of 2·LiBr where the Li+ and Br− are bound to the phenanthroline nitrogen atoms and the calix[4]pyrrole NH protons, respectively (Fig. S20†). In contrast, exposure of 2 to MgBr2 led the signal assignable to the calix[4]pyrrole NH protons to undergo a relatively small downfield shift (Δδ = 0.26 ppm for MgBr2vs. Δδ = 3.31 ppm for LiBr, respectively) while the proton signals of the phenanthroline and the phenoxy CH hydrogens were seen to undergo noticeable downfield shifts (Fig. S20†). These 1H NMR spectral changes provide support for the notion that only the Mg2+ cation is bound to the receptor with the two Br− counter anions being located outside the receptor cavity. In contrast, treatment of receptor 2 with CaBr2 led to formation of a white solid with no observable proton signals appearing in the 1H NMR spectrum of 2. Again, this finding is consistent with the formation of a strong, insoluble complex with CaBr2 (Fig. S20†). A similar phenomenon took place when receptor 2 in CD2Cl2 was treated with an equimolar mixture of LiBr, NaBr, KBr, MgBr2, and CaBr2 (ca. 100 equiv. each) (Fig. S20†). We thus conclude that receptor 2 complexes CaBr2 with high selectivity among the various test bromide salts.
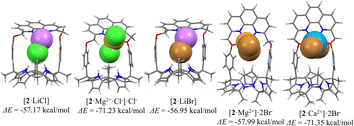
The high selectivity of receptor 2 for MgCl2 over LiCl and for CaBr2 over LiBr and MgBr2 observed in CD2Cl2 was rationalized in terms of the associated binding energies and geometries of the resulting complexes obtained via density functional theory (DFT) calculations carried out in the gas phase at the X3LYP/6-31g* level (Fig. 4; see the ESI† for details). For instance, the complexation energies of receptor 2 for LiCl and MgCl2 were computed to be −57.17 kcal mol−1 and −71.23 kcal mol−1, respectively. In case of the optimized structure of the MgCl2 complex, one of two chloride anions was bound to the calix[4]pyrrole subunit of receptor 2 interacting directly with the magnesium cation coordinated by the phenanthroline nitrogen atoms as well as one ether oxygen atom (Fig. 4; cf. Fig. S1 and S2†). In contrast, the other chloride anion was located outside the receptor cavity forming a contact ion pair with the magnesium cation. The complexation energy of receptor 2 for MgCl2 proved larger than that for LiCl by −14.06 and could account for the selective binding of the receptor for MgCl2 over LiCl; however, solubility considerations are ignored in this analysis. The stabilization energies of receptor 2 upon complexing various bromide salts were similarly calculated to be −56.95 kcal mol−1 for LiBr, −57.99 kcal mol−1 for MgBr2, and −71.35 kcal mol−1 for CaBr2, respectively (Fig. 5). These computed values could account for the selectivity seen for CaBr2 over LiBr or MgBr2; however, as above, effects such as solvation and solubility are not considered.
The selectivity of receptor 2 seen in 10% CH3OH in CDCl3 is considered to reflect solvation effects. Based on their respective hydration energies (ΔhydG* = −1830 kJ mol−1 for Mg2+, ΔhydG* = −1505 kJ mol−1 for Ca2+, and ΔhydG* = −475 kJ mol−1 for Li+), Mg2+ and Ca2+ are presumed to be more strongly solvated by methanol molecules than Li+.29 This strong solvation is thought to reduce the binding interactions with receptor 2 leading to the observed selective binding of LiCl and LiBr in CH3OH/CDCl3 (1/9, v/v). Support for this presumption came from a 1H NMR spectroscopic analysis. For instance, when receptor 2 was treated with an equimolar mixture of LiCl and MgCl2 (ca. 100 equiv. each) in CD2Cl2, orange solids corresponding to 2·MgCl2 precipitated out with no proton signals of the receptor appearing in the 1H NMR spectrum of the residual solvent phase (Fig. S21†). Adding methanol (10% by volume relative to CD2Cl2) to the sample caused the precipitates to dissolve and produced a spectrum analogous to that of 2·LiCl (Fig. S21†). The reversal in selectivity seen for receptor 2 from MgCl2 to LiCl upon moving to a more polar medium is rationalized in terms of MgCl2 being more strongly solvated than LiCl by methanol. Similarly, the apparent high selectivity of receptor 2 for CaBr2 over LiBr in CD2Cl2 was also reversed when methanol (5% by volume) was added to a CD2Cl2 solution containing precipitated 2·CaBr2 (Fig. S22†).
The solvation effects on the receptor selectivity were further supported by two-phase liquid–liquid extraction experiments using CD2Cl2 as the organic receiving phase and an aqueous D2O solution as the lithium salt source. For instance, when CD2Cl2 solutions containing precipitates of the respective 2·MgCl2 and 2·CaBr2 complexes were contacted with aqueous solutions of LiCl (10 M) and LiBr (13 M), respectively, complete dissolution ensued (Fig. 5 and S23†). The 1H NMR spectra of the organic phases exhibited proton signals consistent with those of the LiCl and LiBr complexes, respectively (Fig. 5 and S23†). These findings lead us to suggest that MgCl2 and CaBr2 are released from receptor 2 in the organic phase into the aqueous phases while LiCl and LiBr initially present in the aqueous phase form complexes with receptor 2 that are soluble in the organic phase. This permits the selective extraction of these two lithium salts from an aqueous source phase into a CD2Cl2 receiving phase. When the resulting organic phases containing the respective LiCl and LiBr complexes of receptor 2 were further contacted with ion-free D2O, release of LiCl and LiBr into the aqueous D2O phase occurs. This produces receptor 2 in its ion-free form (Fig. 5 and S23†).
In order to obtain further insights into the ability of receptor 2 to extract the above metal chloride and bromide salts from an aqueous phase, we carried out liquid–liquid extraction experiments using CD2Cl2/D2O. For instance, when an CD2Cl2 layer containing receptor 2 (3 mM) was contacted with aqueous solution layers containing excess LiCl, NaCl, KCl, MgCl2, and CaCl2, respectively, only in the case of LiCl were chemical shift changes seen in the 1H NMR spectrum of the organic layer consistent with effective extraction (Fig. S24†). The resulting 1H NMR spectrum was almost identical to that seen upon complexation of receptor 2 with LiCl (cf. Fig. S19†). This finding was taken as evidence that receptor 2 is able to extract LiCl with high selectivity from an aqueous source phase into a CD2Cl2 organic layer. Upon exposure of a CD2Cl2 solution of receptor 2 (3 mM) to aqueous solutions containing various respective bromide salts, as above, only the lithium salt (LiBr) induced chemical shift changes attributable to LiBr complex formation (Fig. S25†).
To evaluate further the extraction capacity of receptor 2 for LiCl and LiBr, we determined the percentages of the receptor loaded with LiCl and LiBr in the CD2Cl2 organic phase after contacting with aqueous solutions containing different concentrations of LiCl and LiBr, respectively. When receptor 2 was contacted with an aqueous D2O solution containing 5 M of LiCl, two sets of proton signals were visible in the 1H NMR spectrum of the organic phase (Fig. S26†). Based on integrations, ca. 24% of the receptor was presumed to participate in the LiCl extraction (Fig. S26†). In contrast, the LiCl loading percentage of the receptor from an aqueous solution containing 10 M of LiCl was calculated to be ca. ≈ 100% (Fig. S26†). Further evidence for the ability of receptor 2 to extract LiCl came from a high resolution ESI mass spectrometric analysis. A major peak at m/z = 795.4105, a value corresponding to [M + Li]+, was seen (Fig. S27†). On the other hand, the corresponding receptor loading levels for LiBr were estimated to be ca. (70 ± 10)% and ca. 100% when a CD2Cl2 solution containing receptor 2 (3 mM) was contacted with aqueous solutions containing 10 M and 15 M concentrations of LiBr, respectively (Fig. S28†). These findings stand in sharp contrast to what was seen with receptor 1 that fails to extract LiCl or LiBr under the same LLE conditions (Fig. S29 and S30†). Both the LiCl and LiBr complexes of receptor 2 could be separated off and washed with D2O to release the bound salts into an aqueous D2O layer (Fig. 5 and S23†) while freeing up receptor 2 for possible reuse.
The extraction efficiency of receptor 2 for LiCl and LiBr could be improved by employing relatively polar nitrobenzene-d5 as the organic receiving phase instead of CD2Cl2. For instance, after a nitrobenzene-d5 solution containing receptor 2 was contacted with an aqueous source phase containing an excess of LiCl, NaCl, KCl, MgCl2, and CaCl2, it was found that 100% of the receptor in the organic phase existed in the form of the LiCl complex (Fig. S31†). In the case of NaCl and KCl, less than 30% of the receptor was loaded with these salts in the organic phase while no evidence for MgCl2 and CaCl2 extraction by receptor 2 was found (Fig. S31†). In contrast, when a nitrobenzene-d5 organic layer of receptor 2 (3 mM) was contacted with an aqueous layer containing all five test chloride anion salts (LiCl, NaCl, KCl, MgCl2, and CaCl2) at a concentration of ≈5.0 M each, the resulting 1H NMR spectrum of the nitrobenzene layer was consistent with that recorded after receptor 2 was treated with LiCl only (Fig. S31†). This finding was taken as evidence that receptor 2 is capable of extracting LiCl with high selectivity from a mixed salt aqueous solution. In analogy to what was seen in the case of the metal chloride salts, receptor 2 was found to extract LiBr selectively from an aqueous solution over other test bromide salts. For instance, upon subjecting the nitrobenzene-d5 layer containing receptor 2 to contact with a D2O layer containing an excess amount of LiBr, NaBr, KBr, MgBr2, and CaBr2, respectively, only LiBr gave rise to a 1H NMR spectrum of the organic layer consistent with formation of an ion pair complex (Fig. S32†).
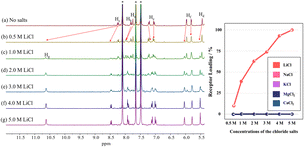
We also examined the capacity of receptor 2 to extract LiCl and LiBr into nitrobenzene-d5 from an aqueous D2O solution containing various concentrations of LiCl and LiBr, respectively. For instance, when a nitrobenzene-d5 phase containing receptor 2 (3 mM) was contacted with aqueous D2O layers containing 0.5–5.0 M of LiCl, the resulting 1H NMR spectra of the nitrobenzene-d5 phase exhibited two distinguishable sets of proton signals that could be assigned to the ion-free form and the LiCl complex of receptor 2, respectively. The LiCl loading percentages of the receptor in the organic layer were determined to be <10%, 39%, 63%, 74%, 93%, and 100% when the nitrobenzene-d5 phases containing the receptor were contacted with D2O solutions containing 0.5 M, 1.0 M, 2.0 M, 3.0 M, 4.0 M, and 5.0 M of LiCl, respectively (Fig. 6). The LiCl loading levels for receptor 2 were compared to those achieved by receptor 1. For instance, the LiCl loading percentage of receptor 1 from a 10 M LiCl aqueous solution was 15%.39 In the case of LiBr, when nitrobenzene-d5 solutions of receptor 2 were treated with D2O aqueous solutions containing LiBr at concentrations of 3.0 M, 5.0 M, and 8.0 M, respectively, 21%, 53%, and 100% of the receptor molecules formed a LiBr complex within the organic phase (Fig. S33†).
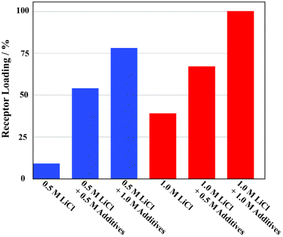
The ability of receptor 2 to extract LiCl selectively was evaluated under liquid–liquid extraction conditions using aqueous solutions containing other potentially competitive chloride salts including NaCl, KCl, MgCl2, and CaCl2. In the case of the individual salts, receptor 2 failed to extract chloride salts other than LiCl under otherwise identical LLE conditions when aqueous solutions containing 5.0 M of the metal cation chloride anion salts in question were tested (Fig. 6). This selectivity was retained when mixtures of salts were tested. However, unexpectedly, the extraction efficiency of the receptor for LiCl was boosted in the presence of the other test chloride salts. For instance, the proton integration ratios corresponding to the 2·LiCl complex in the 1H NMR spectra of the nitrobenzene-d5 phases increased as the concentration of the other chloride salts increased (Fig. 7 and S34†). By way of a specific example, when a nitrobenzene-d5 phase containing receptor 2 (3 mM) was contacted with an aqueous solution containing 0.5 M of LiCl in the absence of the other salts, <10% of the receptor was loaded with LiCl in the organic layer (Fig. 7 and S34†). By contrast, the LiCl loading percentages of receptor 2 were enhanced up to 54% and 78% when the aqueous LiCl source phase (0.5 M) consisted of a mixture of NaCl, KCl, MgCl2, and CaCl2 at concentrations of 0.5 M each and 1.0 M each, respectively. This loading increased to nearly 100% when each of the salts, including LiCl, was present at 1.0 M (Fig. 7 and S32†). Similar results were observed in liquid–liquid extraction studies involving dichloromethane-d2 as the receiving phase. For instance, upon contacting an organic dichloromethane-d2 phase containing receptor 2 with an aqueous D2O phase containing 5.0 M LiCl, 24% of the receptor in the organic phase was loaded with LiCl. In the presence of excess NaCl and NaBr along with 5.0 M LiCl in the aqueous phase, the LiCl loading percentages improved to 36% and 42%, respectively (Fig. S35 and S36†). These findings, which appear analogous to a classic salting-out effect, lead us to suggest that the ion extraction efficiency of other LLE extractants could be improved by employing aqueous source phases containing non-competing salts that are intrinsically insoluble in the organic receiving phase.
Conclusions
The new ion pair receptor 2 reported here acts as a selective extractant for LiCl and LiBr under solid–liquid and liquid–liquid extraction conditions. Ion pair receptor 2 is composed of a phenanthroline unit of a cation binding site connected via ether linkages to a calix[4]pyrrole anion binding site. In dichloromethane-d2, receptor 2 proved able to complex LiCl, LiBr, MgCl2, MgBr2, and CaBr2. When treated with the salts in question (i.e., LiX, NaX, KX, MgX2, and CaX2 where X = Cl or Br), receptor 2 forms insoluble complexes selectively with MgCl2 and CaBr2. This operational selectivity is reversed in more polar protic solvents, such as water or methanol. For instance, upon the in situ addition of methanol to CD2Cl2 solutions containing the presumed MgCl2 and CaBr2 complexes of receptor 2 along with the other chloride and bromide salts, the bound MgCl2 and CaBr2 are released from the receptor giving rise to the corresponding LiCl and LiBr complexes. This finding is ascribed to the Mg2+ and Ca2+ cations being strongly solvated by polar protic media as compared to the Li+ cation. In a moderately polar medium consisting of 10% CH3OH in CDCl3, receptor 2 binds LiCl and LiBr ion with significantly higher affinity and selectivity than it does other competitive chloride and bromide salts. Receptor 2 was also found to able to extract LiCl and LiBr efficiently into an organic phase from aqueous sources under two-phase liquid–liquid extraction conditions. This efficiency could be further boosted by adding other chloride salts, including NaCl, KCl, MgCl2, and CaCl2 to the aqueous source phase. We thus suggest that receptor 2 may have a role to play in lithium cation separation scenarios.Data availability
All data, including synthetic details, 1H NMR spectroscopic analyses, DFT calculations, and a single crystal X-ray diffraction analysis of 2·LiBr (CCDC 2356046), are available in the ESI.† All data are included either in the main text, the ESI,† or (X-ray work only) uploaded with the Cambridge Crystallographic Data Centre.Author contributions
SKK and JLS conceived and supervised the project. NJH synthesized the compounds. NJH and JHO performed ion binding studies using 1H NMR spectroscopy. NJH and KL carried out the X-ray diffraction analysis. AL and QH carried out DFT calculations. SKK and JLS wrote the manuscript. All authors contributed to the editing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korea Government (MIST) (NRF-2022R1A4A1021817 to S. K. K.). The work in Austin was supported by the Department of Energy Office of Basic Energy Sciences (grant no. DE-SC0024393 to J. L. S.). Further support from the Robert A. Welch Foundation (F-0018 to J. L. S.) is also acknowledged.Notes and references
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- R. Oruch, M. A. Elderbi, H. A. Khattab, I. F. Pryme and A. Lund, Eur. J. Pharmacol., 2014, 740, 464–473 CrossRef CAS PubMed.
- E. C. Evarts, Nature, 2015, 526, S93–S95 CrossRef CAS PubMed.
- G. Martin, L. Rentsch, M. Höck and M. Bertau, Energy Storage Mater., 2017, 6, 171–179 CrossRef.
- L. Kavanagh, J. Keohane, G. G. Cabellos, A. Lloyd and J. Cleary, Resources, 2018, 7, 57 CrossRef.
- S. K. Sarai, H. M. Madurui and S. Lippmann, Innov. Clin. Neurosci., 2018, 15, 30–32 Search PubMed.
- U.S. Geological Survey, Mineral Commodity Summaries 2022, U.S. Geological Survey, Reston, VA, 2022 Search PubMed.
- B. Swain, Sep. Purif. Technol., 2017, 172, 388–403 CrossRef CAS.
- A. Z. Haddad, L. Hackl, B. Akuzum, G. Pohlman, J.-F. Magnan and R. Kostecki, Nature, 2023, 616, 245–248 CrossRef CAS PubMed.
- P. Meshram, B. D. Pandey and T. R. Mankhand, Hydrometallurgy, 2014, 150, 192–208 CrossRef CAS.
- N. Vieceli, C. A. Nogueira, M. F. C. Pereira, A. P. S. Dias, F. O. Durao, C. Guimaraes and F. Margarido, Miner. Eng., 2017, 102, 1–14 CrossRef CAS.
- D. Yelatontsev and A. Mukhachev, Hydrometallurgy, 2021, 201, 105578 CrossRef CAS.
- T. Gao, N. Fan, W. Chen and T. Dai, Chin. Geol., 2023, 6, 137–153 CAS.
- S. Nishihama, K. Onishi and K. Yoshizuka, Solvent Extr. Ion Exch., 2011, 29, 421–431 CrossRef CAS.
- P. Loganathan, G. Naidu and S. Vigneswaran, Environ. Sci.: Water Res. Technol., 2017, 3, 37–53 RSC.
- S. Yang, F. Zhang, H. Ding, P. He and H. Zhou, Joule, 2018, 2, 1648–1651 CrossRef.
- G. R. Harvianto, S.-H. Kim and C.-S. Ju, Rare Met., 2016, 35, 948–953 CrossRef CAS.
- C. Liu, Y. Li, D. Lin, P.-C. Hsu, B. Liu, G. Yan, T. Wu, Y. Cui and S. Chu, Joule, 2020, 4, 1459–1469 CrossRef CAS.
- X. He, S. Kaur and R. Kostecki, Joule, 2020, 4, 1357–1368 CrossRef CAS.
- V. Flexer, C. F. Baspineiro and C. I. Galli, Sci. Total Environ., 2018, 639, 1188–1204 CrossRef CAS PubMed.
- G. Liu, Z. Zhao and A. Ghahreman, Hydrometallurgy, 2019, 187, 81–100 CrossRef CAS.
- P. Xu, J. Hong, X. Qian, Z. Xu, H. Xia, X. Tao, Z. Xu and Q.-Q. Ni, J. Mater. Sci., 2021, 56, 16–63 CrossRef CAS.
- Y. Sun, Q. Wang, Y. Wang, R. Yun and X. Xiang, Sep. Purif. Technol., 2021, 256, 117807 CrossRef CAS.
- X. Zhao, M. Feng, Y. Jiao, Y. Zhang, Y. Wang and Z. Sha, Desalination, 2020, 481, 114360 CrossRef CAS.
- A. Khalil, S. Mohammed, R. Hashaikeh and N. Hilal, Desalination, 2022, 528, 115611 CrossRef CAS.
- J. Ying, Y. Lin, Y. Zhang and J. Yu, ACS ES&T Water, 2023, 3, 1720–1739 Search PubMed.
- W. T. Stringfellow and P. F. Dobson, Energies, 2021, 14, 6805 CrossRef CAS.
- M. L. Vera, W. R. Torres, C. I. Galli, A. Chagnes and V. Flexer, Nat. Rev. Earth Environ., 2023, 4, 149–165 CrossRef CAS.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1991, 87, 2995–2999 RSC.
- P. Xu, J. Hong, X. Qian, Z. Xu, H. Xia, X. Tao, Z. Xu and Q.-Q. Ni, J. Mater. Sci., 2021, 56, 16–63 CrossRef CAS.
- J. Zhang, N. Tanjedrew, M. Wenzel, P. Royola, H. Du, S. Kiatisevi, L. F. Lindoy and J. J. Weigand, Angew. Chem., Int. Ed., 2023, 62, e202216011 CrossRef CAS PubMed.
- S. K. Kim and J. L. Sessler, Chem. Soc. Rev., 2010, 39, 3784–3809 RSC.
- S. K. Kim and J. L. Sessler, Acc. Chem. Res., 2014, 47, 2525–2536 CrossRef CAS PubMed.
- Q. He, G. I. Vargas-Zuniga, S. H. Kim, S. K. Kim and J. L. Sessler, Chem. Rev., 2019, 119, 9753–9835 CrossRef CAS PubMed.
- A. J. McConnell and P. D. Beer, Angew. Chem., Int. Ed., 2012, 51, 5052–5061 CrossRef CAS PubMed.
- A. J. McConnell, A. Docker and P. D. Beer, ChemPlusChem, 2020, 85, 1824–1841 CrossRef CAS PubMed.
- Q. He, Z. Zhang, J. T. Brewster, V. M. Lynch, S. K. Kim and J. L. Sessler, J. Am. Chem. Soc., 2016, 138, 9779–9782 CrossRef CAS PubMed.
- K. I. Hong, H. Kim, Y. Kim, M. G. Choi and W. D. Jang, Chem. Commun., 2020, 56, 10541–10544 RSC.
- Q. He, N. J. Williams, J. H. Oh, V. M. Lynch, S. K. Kim, B. A. Moyer and J. L. Sessler, Angew. Chem., Int. Ed., 2018, 57, 11924–11928 CrossRef CAS PubMed.
- K. Wang, C.-C. Yee and H. Y. Au-Yeung, Chem. Sci., 2016, 7, 2787–2792 RSC.
- J. Yoo, I.-W. Park, T.-Y. Kim and C.-H. Lee, Bull. Korean Chem. Soc., 2010, 31, 630–634 CrossRef CAS.
- K. A. Connors, Binding Constants: The Measurement of Molecular Complex Stability, Wiley-Interscience, New York, 1987 Search PubMed.
- Association constants (Ka) were evaluated using BindFit v0.5, available from, https://app.supramolecular.org/bindfit/ Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2356046. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc03760j |
| This journal is © The Royal Society of Chemistry 2024 |