Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Streamlined synthetic assembly of α-chiral CAAC ligands and catalytic performance of their copper and ruthenium complexes†
Adrien
Madron du Vigné
 and
Nicolai
Cramer
and
Nicolai
Cramer
 *
*
Laboratory of Asymmetric Catalysis and Synthesis, Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), 1015 Lausanne, Switzerland. E-mail: nicolai.cramer@epfl.ch
First published on 24th July 2024
Abstract
The unique electronic and steric parameters of chiral cyclic alkyl amino carbene (CAAC) ligands render them appealing steering ligands for enantioselective transition-metal catalyzed transformations. Due to the lack of efficient synthetic strategies to access particularly attractive α-chiral CAACs assessment and exploitation of their full synthetic potential remain difficult. Herein, we report a streamlined strategy to assemble a library of diastereo- and enantiomerically pure CAAC ligands featuring the notoriously difficult to access α-quaternary stereogenic centers. A tailored Julia–Kocienski olefination reagent allows the Claisen-rearrangement to be leveraged as an expedient route to form the synthetically pivotal racemic α-chiral methallyl aldehydes. Subsequent condensation with chiral amines and further cyclization provided a library of diastereomeric mixtures of the targeted ligand precursors. The CAAC salts as well as their corresponding metal complexes are conveniently separable by standard silica gel flash chromatography closing a long-standing accessibility gap in chiral CAAC ligands with proximal α-chirality. The rapid availability of both diastereomers enables testing of the relevance and synergistic effects of two chiral centers on the ligand in catalytic applications. A broad range of metal complexes with copper, gold, rhodium and ruthenium were obtained and structurally analyzed. The catalytic performances of the corresponding chiral CAAC copper and ruthenium complexes were assessed in enantioselective conjugate borylations and asymmetric ring closing metathesis, displaying selectivities of up 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er.
5 er.
Introduction
N-Heterocyclic carbenes (NHCs) are an important ligand class for transition metals enabling a broad variety of asymmetric transformations.1 The chiral elements of NHCs have been extensively investigated and modulated providing excellent levels of enantioselectivity with a myriad of transition metal complexes.2 Introduced by Bertrand in 2005, cyclic alkyl amino carbenes (CAACs) are an intriguing class of NHC ligands.3 Compared to the classical NHC framework, one of the heteroatoms adjacent to the carbene carbon atom is switched to an sp3-hybridized quaternary carbon atom in CAACs. This change induces profound steric and electronic changes compared to typical NHCs.4,5 Notably, they exhibit enhanced sigma-donor and pi-acceptor properties.6 As a result, stronger bonds are formed with various main group elements7 and transition metals.8,9 In this respect, CAACs garnered substantial attention and underwent rapid evolution in the field.10 However, despite their great application potential, the development of chiral CAACs for asymmetric catalysis remains severely underdeveloped (Scheme 1A). Bertrand et al. reported the two first chiral CAAC complexes for asymmetric catalysis in 2019.11 The underlying ligand is based on the chiral-pool approach having one stereogenic element adjacent to the carbene carbon atom. Their selectivity and efficiency were tested in the asymmetric conjugate borylation of unsaturated esters with enantioselectivities of up to 77.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22.5 er. A library of α-chiral CAAC bearing ruthenium complexes displayed selectivities of up to 94
22.5 er. A library of α-chiral CAAC bearing ruthenium complexes displayed selectivities of up to 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er in asymmetric ring opening cross metathesis (AROCM). However, the enantiopure complexes had to be resolved by chiral preparative HPLC from the prepared racemate.12,13 Independently, we and Bertrand et al. reported in 2022 a streamlined chiral CAAC synthesis combining the benefits of functionalized methallyl aldehydes with various primary amines. The chiral primary amines used in our study resulted in CAACs having their stereogenic element at the beta position of the carbene carbon atom. The related copper CAAC catalysts provided improved selectivities of up to 89
6 er in asymmetric ring opening cross metathesis (AROCM). However, the enantiopure complexes had to be resolved by chiral preparative HPLC from the prepared racemate.12,13 Independently, we and Bertrand et al. reported in 2022 a streamlined chiral CAAC synthesis combining the benefits of functionalized methallyl aldehydes with various primary amines. The chiral primary amines used in our study resulted in CAACs having their stereogenic element at the beta position of the carbene carbon atom. The related copper CAAC catalysts provided improved selectivities of up to 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er in asymmetric conjugate borylation.14 However, the development of chiral CAACs remains substantially limited and largely restricted to these examples. This restriction primarily stems from the inconvenience and costs of resolution by chiral preparative HPLC as well as the incompatibility of many CAAC metal complexes with chiral chromatographic separation techniques. A general and synthetically attractive method to build the quaternary α-stereogenic center of CAACs suitable for a broad spectrum of transition metals is lacking. This limitation significantly impedes the development of CAACs for catalytic enantioselective transformations and represents a central challenge that needs to be addressed. In this context, methallyl aldehydes emerged as key building blocks shortening the synthesis of CAAC ligands as well as broadening the scope.14,15 However, the two main routes to access such aldehydes face significant limitations in general applicability (i.e. substrate dependent capricious phase-transfer aldehyde alkylation15,16 and transient imine alkylation17). Most importantly, both synthetic routes are based on unsymmetrically substituted tertiary aldehydes as key starting materials. Very few aldehydes are commercial and a more desirable substitution pattern requires additional and sometimes tedious preparatory steps (Scheme 1B). Recognizing this limitation, we reasoned that a Claisen rearrangement could allow straightforward access to the pivotal carbene precursors from allyl vinyl ethers.18 In turn, synthesis of a single tailored Julia–Kocienski olefination reagent would allow unsymmetrical ketones to be simply used as starting materials. This choice leverages the commercial availability of unsymmetrical ketones that span immense structural diversity (Scheme 1C).
11 er in asymmetric conjugate borylation.14 However, the development of chiral CAACs remains substantially limited and largely restricted to these examples. This restriction primarily stems from the inconvenience and costs of resolution by chiral preparative HPLC as well as the incompatibility of many CAAC metal complexes with chiral chromatographic separation techniques. A general and synthetically attractive method to build the quaternary α-stereogenic center of CAACs suitable for a broad spectrum of transition metals is lacking. This limitation significantly impedes the development of CAACs for catalytic enantioselective transformations and represents a central challenge that needs to be addressed. In this context, methallyl aldehydes emerged as key building blocks shortening the synthesis of CAAC ligands as well as broadening the scope.14,15 However, the two main routes to access such aldehydes face significant limitations in general applicability (i.e. substrate dependent capricious phase-transfer aldehyde alkylation15,16 and transient imine alkylation17). Most importantly, both synthetic routes are based on unsymmetrically substituted tertiary aldehydes as key starting materials. Very few aldehydes are commercial and a more desirable substitution pattern requires additional and sometimes tedious preparatory steps (Scheme 1B). Recognizing this limitation, we reasoned that a Claisen rearrangement could allow straightforward access to the pivotal carbene precursors from allyl vinyl ethers.18 In turn, synthesis of a single tailored Julia–Kocienski olefination reagent would allow unsymmetrical ketones to be simply used as starting materials. This choice leverages the commercial availability of unsymmetrical ketones that span immense structural diversity (Scheme 1C).
Pairing them with a selection of chiral amines directly leads to a library of carbene precursors with α- and β-stereogenic centers. The formed diastereomers can be separated by simple flash chromatography as their tetrafluoroborate salt carbene precursors or as their CAAC metal complexes. Our approach offers a substantial advantage by providing direct access to CAACs with challenging chirality of the quaternary α-carbon stereogenic centers as well as exploiting unprecedented matched/mismatched effects of multiple stereogenic centers on the CAAC ligands. The catalytic efficiency and inducible enantioselectivity of the corresponding copper and ruthenium complexes were showcased with asymmetric conjugate borylations and ARCMs as selected benchmark transformations.
Results and discussion
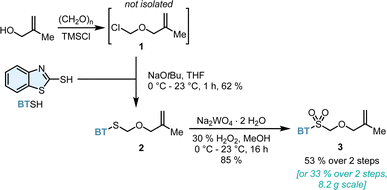
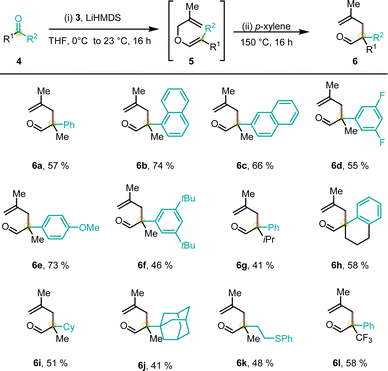
Considering the structural and synthetic challenges in accessing α-chiral CAACs, we began developing a bespoke and universal olefination reagent in order to prepare the pivotal methallyl aldehyde structural unit more efficiently (Scheme 2). In this respect, we envisioned Julia–Kocienski reagent 3 as the platform to perform the desired ketone olefination. Two single reports focused on the preparation of simple α-benzyloxy19 or α-naphthylmethyloxy20 heteroaryl sulfones and their use for vinyl ether synthesis. To the best of our knowledge, the preparation and olefination use of α-methallyloxy heteroaryl sulfones are surprisingly not yet documented. Reagent 3 was accessed conveniently from widely available and cheap starting materials. Chloromethyl methallyl ether 1 was prepared in situ from methallyl alcohol, paraformaldehyde and TMSCl and directly used. Subsequently, thioether 2 was formed from mercaptobenzothiazole and freshly prepared 1. Oxidation of sulfide 2 with aqueous hydrogen peroxide catalyzed by sodium tungstate provided the target sulfone 3 in 53% yield over two steps. The key Julia–Kocienski reagent 3 can be conveniently prepared on a multigram scale and is a stable and free-flowing white solid.To leverage the utility of 3 for the synthesis of CAAC ligands, a variety of racemic quaternary methallyl aldehydes were synthesized (Scheme 3). The olefination of a non-symmetrical ketone proceeded smoothly with deprotonated sulfone 3. The resulting crude methallyl vinyl ether 5 underwent Claisen rearrangement yielding aldehyde 6 upon refluxing in p-xylene. This approach proved to be synthetically versatile with respect to the substitution pattern of ketone 4. All methallyl vinyl ethers 5 reliably underwent [3,3]-sigmatropic rearrangement. A variety of sterically and electronically diverse aryl-substituents (phenyl (6a), 1-naphthyl (6b), 2-naphthyl (6c), 3,5-di-fluorophenyl (6d) 4-methoxyphenyl (6e) and 3,5-di-tert-butylphenyl substituted ketone (6f)) were well tolerated in the olefination–rearrangement sequence. A switch of the methyl substituent at the quaternary carbon atom by an isopropyl group or the generation of a spirocyclic tetrahydronaphthalene (THN) core provided aldehydes (6g) and (6h). Methyl ketones yielded cyclohexyl derived (6i), demanding adamantyl substituted (6j) and sulfide-functionalized aldehyde 6k. Moreover, trifluoro acetophenone was used to access aldehyde 6l in 58% yield.
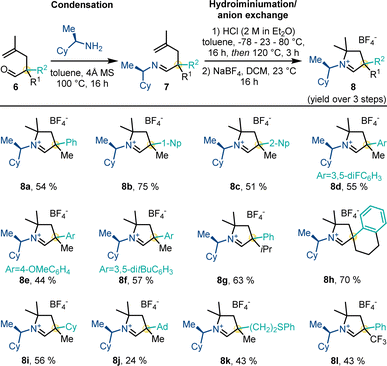
Next, aldehydes 6 were converted to their corresponding carbene precursors 8 by our previously reported procedure (Scheme 4).14 The different cyclic iminium tetrafluoroborate salts 8 were generally obtained in good to high overall yields (43–75%) as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers. The process allowed for unprecedented substitution patterns by introducing a sterically demanding adamantyl (8j) as well as an electron-withdrawing trifluoromethyl group (8l) in close proximity to the future carbene center.
1 mixture of diastereomers. The process allowed for unprecedented substitution patterns by introducing a sterically demanding adamantyl (8j) as well as an electron-withdrawing trifluoromethyl group (8l) in close proximity to the future carbene center.
We further aimed to further enhance convenience and efficiency of the route for the carbene precursor synthesis (Scheme 5). While a full one-pot procedure from 4 to 8 proved to be challenging, we established a proof-of-concept for a one-pot protocol from methallyl vinyl ether 5a. Heating 5a and (R)-cyclohexylethylamine triggered Claisen rearrangement and subsequently imine formation giving 7a. Subsequent addition of HCl and heating initiated the hydroiminiumation reaction. Anion exchange with NaBF4 provided carbene precursor 8a in 48% yield over 4 steps in a one-pot fashion.
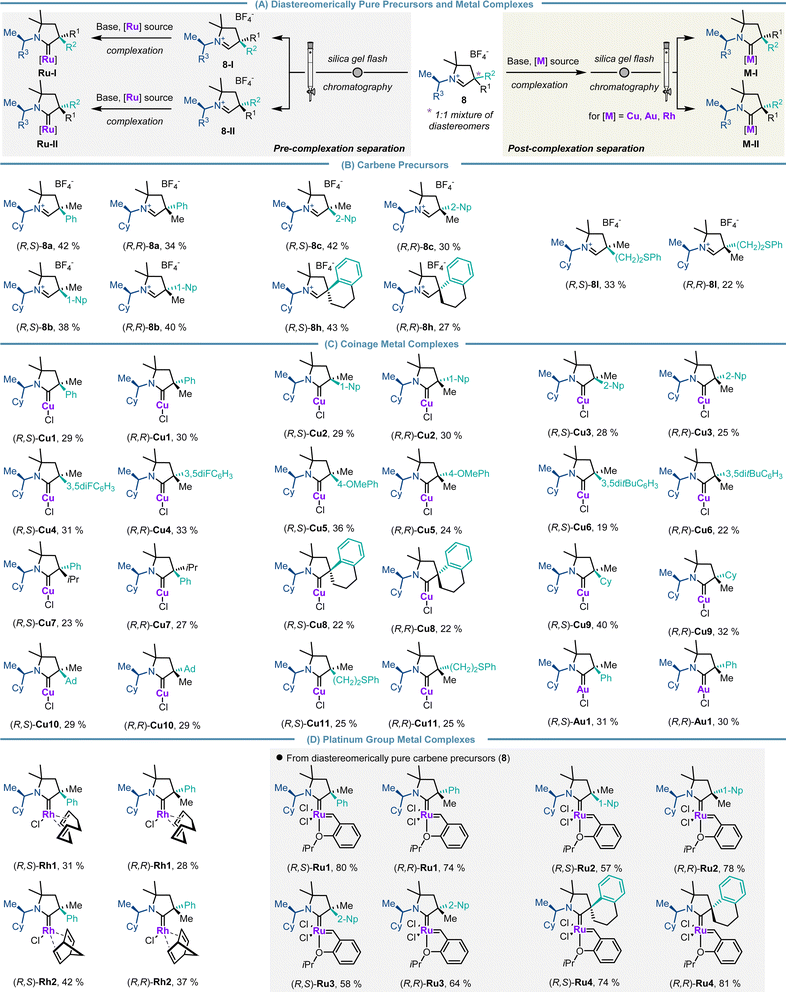
Having established a robust and reliable route to access a diverse library of CAAC precursors, we turned our focus to simple separation of the diastereomers of the parent carbene precursors as well as the related downstream CAAC transition metal complexes (Scheme 6A). The use of routine separation by silica gel flash chromatography of diastereomers instead of preparative chiral HPLC for enantiomer resolution is an appreciable simplification. Notably, diastereomerically pure iminiums 8a–8d were consistently obtained in isolated yields ranging from 27% to 43% (note: with the initial 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture of CAAC precursors the maximal yield for diastereomerically pure precursors is 50%) (Scheme 6B). Conveniently, the diastereomers of CAAC copper (Cu1–Cu11), gold (Au1) and rhodium complexes (Rh1 and Rh2) were also smoothly separated by silica gel flash chromatography. Consistently, both diastereomers were isolated with equal efficiency in yields ranging from 19% to 42% (note: with the 1
1 diastereomeric mixture of CAAC precursors the maximal yield for diastereomerically pure precursors is 50%) (Scheme 6B). Conveniently, the diastereomers of CAAC copper (Cu1–Cu11), gold (Au1) and rhodium complexes (Rh1 and Rh2) were also smoothly separated by silica gel flash chromatography. Consistently, both diastereomers were isolated with equal efficiency in yields ranging from 19% to 42% (note: with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture of CAAC precursors the maximal yield for diastereomerically pure complexes is 50%) (Scheme 6C and D). Ruthenium complexes Ru1–Ru4 were prepared utilizing the diastereomerically pure iminium salts as the separation of diastereomers at the metal complex stage was not successful for these examples (Scheme 6d).
1 diastereomeric mixture of CAAC precursors the maximal yield for diastereomerically pure complexes is 50%) (Scheme 6C and D). Ruthenium complexes Ru1–Ru4 were prepared utilizing the diastereomerically pure iminium salts as the separation of diastereomers at the metal complex stage was not successful for these examples (Scheme 6d).
The relative and absolute configurations of the CAAC ligands were determined by single-crystal X-ray diffraction analysis of a set of copper and ruthenium complexes (Fig. 1). The diastereomer of (R,S)-Cu1 adopts a pseudo-C2-symmetrical geometry placing the largest substituents in diagonally opposed quadrants. In contrast, the (R,R)-diastereomer of Cu1 displays a crowded southern hemisphere and a rather accessible northern hemisphere. With either two bulky (Cu7) or two small substituents (Cu11) at the chiral α-quaternary stereogenic center, the quadrant distinction becomes less pronounced. Contrasting the pseudo-C2-symmetrical copper complexes, ruthenium complex (R,S)-Ru3 shows a single crowded hemisphere minimizing interactions between the cyclohexyl group and both chlorides. The steric parameters were similar to those of α-gem-bis-phenyl substituted (R)-Ru0.14 In archetypical N-aryl substituted CAACs, the aromatic ring is placed in proximity to the benzylidene unit forcing the quaternary carbon atom bearing the chiral information away from the metathesis initiating part of the catalyst. Such an “inverted”-CAAC orientation21,22 resulting in a proximal chiral environment sitting above the benzylidene bond might be an exploitable structural feature for enantioselective catalysis. The suspected critical relevance of these design features with respect to the ability to induce enantioselectivity in catalytic application was tested next.
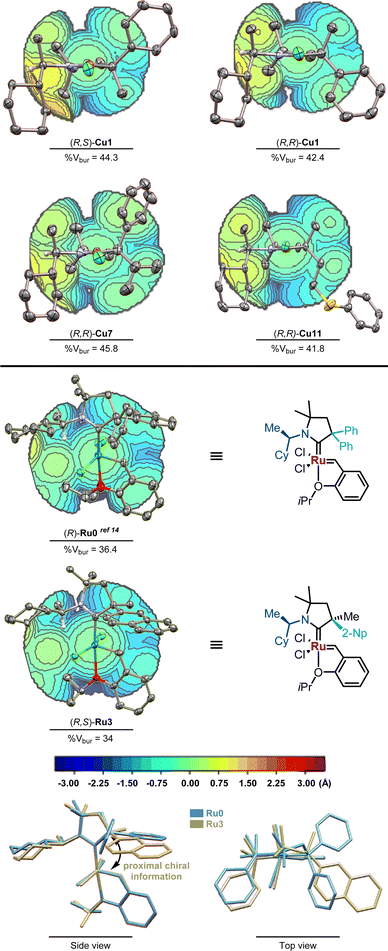 | ||
| Fig. 1 ORTEP plots (50% probability thermal ellipsoids and 30% for Cu7, Ru0 and Ru3, hydrogen atoms are omitted for clarity), topological steric maps of (R,S)-Cu1 and (R,R)-Cu1, (R,R)-Cu7 and Cu11, and (R)-Ru0 and (R,S)-Ru3 and structure overlay of (R)-Ru0 and (R,S)-Ru3. The plotted topological steric maps23 and calculated buried volumes (% VBur)24 were obtained from SambVca2.1 (ref. 25) (Bondi radii scaled by 1.17, sphere radius 3.5 Å, and mesh spacing 0.1 Å). | ||
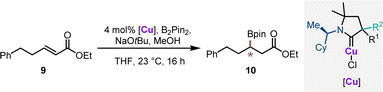
In order to evaluate the catalytic performance of the synthesized chiral CAAC ligands, the corresponding copper complexes Cu1–Cu11 were benchmarked in enantioselective conjugate borylation of α,β-unsaturated ester 9 (Table 1). The complexes were grouped as diastereomeric pairs. Notably a clear trend for the matching/mismatching pair of the stereogenic center was observed. The complexes with the (R,S)-configured CAAC complex (Cu1–Cu5 and Cu8) with an aryl/methyl substitution pattern delivered borylated ester 10 in excellent yields (86–92%) and with good enantioselectivities ranging from 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 to 91
13 to 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 er. Complexes with an alkyl/alkyl stereocenter (Cu9 and Cu10) displayed a reduced reactivity, diminished yields and lower selectivities. Reducing the size difference of the substituent of the a-stereocenter by replacing the methyl group with an isopropyl group (Cu7) caused a substantial drop in the observed enantioselectivity. The series of complexes with (R,R)-configured CAAC ligands was clearly confirmed to have mismatching stereocenters. In these cases, ester product 10 was consistently formed in substantially inferior enantioselectivities, experimentally supporting the selectivity hypothesis derived from the steric maps. To further assess catalytic activity, the two best performing catalysts Cu2 and Cu8 were tested at a reduced reaction temperature of −20 °C. Under these conditions, catalyst Cu2 provided 10 in 65% yield with an identical enantioselectivity of 89
9 er. Complexes with an alkyl/alkyl stereocenter (Cu9 and Cu10) displayed a reduced reactivity, diminished yields and lower selectivities. Reducing the size difference of the substituent of the a-stereocenter by replacing the methyl group with an isopropyl group (Cu7) caused a substantial drop in the observed enantioselectivity. The series of complexes with (R,R)-configured CAAC ligands was clearly confirmed to have mismatching stereocenters. In these cases, ester product 10 was consistently formed in substantially inferior enantioselectivities, experimentally supporting the selectivity hypothesis derived from the steric maps. To further assess catalytic activity, the two best performing catalysts Cu2 and Cu8 were tested at a reduced reaction temperature of −20 °C. Under these conditions, catalyst Cu2 provided 10 in 65% yield with an identical enantioselectivity of 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 er. Catalyst Cu8 maintained its excellent reactivity giving 10 in 95% yield with an improved selectivity of 95
11 er. Catalyst Cu8 maintained its excellent reactivity giving 10 in 95% yield with an improved selectivity of 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er.
5 er.
| Entry | [Cu] | % Yield of 10b | erc |
|---|---|---|---|
| a Reaction conditions: 0.1 mmol 9, 4 mol% [Cu], 20 mol% NaOtBu, 1.1 equiv. B2Pin2, 2.0 equiv. MeOH, 0.2 M in THF at 23 °C for 16 h. b Isolated yield. c Enantiomeric ratio determined by chiral HPLC after oxidation to the corresponding secondary alcohol. d Reaction was performed at −20 °C. | |||
| 1 | (R,S)-Cu1 | 86 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 2 | (R,R)-Cu1 | 90 | 62.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37.5 37.5 |
| 3 | (R,S)-Cu2 | 92 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 4d | (R,S)-Cu2 | 65 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 5 | (R,R)-Cu2 | 95 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
| 6 | (R,S)-Cu3 | 92 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 7 | (R,R)-Cu3 | 94 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 8 | (R,S)-Cu4 | 87 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 9 | (R,R)-Cu4 | 94 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
| 10 | (R,S)-Cu5 | 90 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 11 | (R,R)-Cu5 | 90 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
| 12 | (R,S)-Cu6 | 89 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
| 13 | (R,R)-Cu6 | 93 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| 14 | (R,R)-Cu7 | 98 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 15 | (R,S)-Cu7 | 97 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 16 | (R,S)-Cu8 | 92 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 17d | (R,S)-Cu8 | 95 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5 5
|
| 18 | (R,R)-Cu8 | 90 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
| 19 | (R,S)-Cu9 | 63 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| 20 | (R,R)-Cu9 | 78 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 21 | (R,S)-Cu10 | 75 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 22 | (R,R)-Cu10 | 99 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
| 23 | (R,S)-Cu11 | 87 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| 24 | (R,R)-Cu11 | 84 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
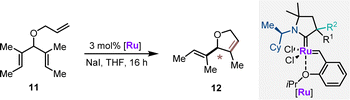
The CAAC ruthenium complexes Ru1–Ru4 and additionally (R)-Ru0 (ref. 14) were evaluated in asymmetric ring closing metathesis (ARCM) of triene 11 as the second benchmark transformation (Table 2). Ru0 provided 12 in 62% yield with an encouraging 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 er (entry 1). In all cases, the catalysts having (R,S)-diastereomeric CAAC proved to have the matched pair of stereogenic centers for this transformation (entries 2–5). (R,S)-Ru3 emerged as the best performer, providing dihydrofuran 12 in 60% yield and 92
27 er (entry 1). In all cases, the catalysts having (R,S)-diastereomeric CAAC proved to have the matched pair of stereogenic centers for this transformation (entries 2–5). (R,S)-Ru3 emerged as the best performer, providing dihydrofuran 12 in 60% yield and 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er (entry 4). (R,S)-Ru4 was not competent in this transformation and seemed prone to decomposition under the reaction conditions. In general, increasing the reaction temperature to 40 °C improved the reaction yields with a very small reduction of the enantioselectivities (entries 6–9). The series of ruthenium complexes having the (R,R)-configurations at the CAAC ligand clearly displayed mismatching characteristics (entries 10–13). The observed enantioselectivities were moderate. (R,R)-Ru3 showed the highest selectivity in this series (24
8 er (entry 4). (R,S)-Ru4 was not competent in this transformation and seemed prone to decomposition under the reaction conditions. In general, increasing the reaction temperature to 40 °C improved the reaction yields with a very small reduction of the enantioselectivities (entries 6–9). The series of ruthenium complexes having the (R,R)-configurations at the CAAC ligand clearly displayed mismatching characteristics (entries 10–13). The observed enantioselectivities were moderate. (R,R)-Ru3 showed the highest selectivity in this series (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76 er) in favor of the enantiomeric product 12. This behavior underlines the dominance of the proximal α-stereogenic center in enantioselection. Noteworthily, (R,S)-Ru2 and (R,R)-Ru2 appeared as a 1
76 er) in favor of the enantiomeric product 12. This behavior underlines the dominance of the proximal α-stereogenic center in enantioselection. Noteworthily, (R,S)-Ru2 and (R,R)-Ru2 appeared as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 and 1
0.7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rotamer mixture in 1H-NMR in CD2Cl2. The negative impact of rotamers for selectivity resonated with the observations previously reported suggesting an impaired catalytic performance due to the formation of rotamers prior to the enantio-determining step.26
1 rotamer mixture in 1H-NMR in CD2Cl2. The negative impact of rotamers for selectivity resonated with the observations previously reported suggesting an impaired catalytic performance due to the formation of rotamers prior to the enantio-determining step.26
| Entry | [Ru] | Temp. (°C) | % Yield of 12c | erd | |
|---|---|---|---|---|---|
| a Reaction conditions: 0.05 mmol 11, 3 mol% [Ru], 0.75 equiv. NaI, 0.07 M in THF for 16 h. b With 5 mol% [Ru], 0.5 M in THF. c Determined by NMR using 1,3,5-trimethoxybenzene as the internal standard. d Enantiomeric ratio was determined by chiral GC. | |||||
| Match | 1b | (R)-Ru0 | 23 | 62 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73 |
| 2 | (R,S)-Ru1 | 23 | 39 | 89.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.5 10.5 |
|
| 3 | (R,S)-Ru2 | 23 | 21 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
|
| 4 | (R,S)-Ru3 | 23 | 60 |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
|
| 5 | (R,S)-Ru4 | 23 | <1 | n.d. | |
| Match | 6 | (R,S)-Ru1 | 40 | 54 | 88.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.5 11.5 |
| 7 | (R,S)-Ru2 | 40 | 72 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
|
| 8 | (R,S)-Ru3 | 40 | 79 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
|
| 9 | (R,S)-Ru4 | 40 | <3 | n. d. | |
| Mismatch | 10 | (R,R)-Ru1 | 23 | 84 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66 |
| 11 | (R,R)-Ru2 | 23 | 27 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
|
| 12 | (R,R)-Ru3 | 23 | 80 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76 76 |
|
| 13 | (R,R)-Ru4 | 23 | 23 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 55 |
|
Conclusions
In summary, we devised a streamlined strategy to assemble a library of diastereo- and enantiomerically pure CAAC ligands featuring notoriously difficult to access α-quaternary stereogenic centers. A universal Julia–Kocienski olefination reagent allowed the Claisen-rearrangement to be leveraged as an expedient strategy forming the racemic α-chiral aldehydes as key intermediates for the CAAC synthesis. Condensation with chiral amines and subsequent cyclization provided diastereomeric mixtures of the targeted ligand precursors. These precursors, as well as their corresponding copper, gold and rhodium complexes, are conveniently separable using standard silica gel flash chromatography to diastereo- and enantiomerically pure materials. The availability of both diastereomers enabled the testing of the synergistic effects of the two chiral centers in catalytic applications. The catalytic performances of the corresponding chiral CAAC copper and ruthenium complexes were evaluated in enantioselective conjugate borylations and asymmetric ring closing metathesis, respectively. Our approach closes a gap in chiral ligand accessibility enabling the synthesis of various chiral CAAC transition metal complexes previously beyond reach. We firmly believe that the outlined streamlined and modular synthetic strategy provides substantial leverage to design further chiral CAACs able to bridge method limitations in challenging catalytic enantioselective transformations.Data availability
Experimental details and characterization data are available free of charge from the ESI† available with this article. Crystallographic data are available at CCDC (see note in ref. 27).Author contributions
A. M. d. V. and N. C. conceived and conceptualized the project. A. M. d. V. designed and performed the experiments. Analysis of all experiments was performed by A. M. d. V. and N. C. Interpretation of the results and writing and revision of the manuscript was performed by all authors. N. C. provided funding and resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the EPFL and the Swiss National Science Foundation (no. 175507). The authors thank Dr R. Scopelliti and Dr F. Fadaei-Tirani for the X-ray crystallographic analysis of compounds (R,S)- and (R,R)-Cu1, (R,R)-Cu7, (R,R)-Cu11, (R,S)-Ru3 and (R)-Ru0.Notes and references
-
(a) W. A. Herrmann, L. J. Goossen, C. Köcher and G. R. J. Artus, Angew. Chem., Int. Ed., 1996, 35, 2805–2807 CrossRef CAS
; (b) D. Enders and H. Gielen, J. Organomet. Chem., 2001, 617–618, 70–80 CrossRef CAS
; (c) Reviews: W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290–1309 CrossRef CAS
; (d) D. Janssen-Müller, C. Schlepphorst and F. Glorius, Chem. Soc. Rev., 2017, 46, 4845–4854 RSC
.
-
(a) T. J. Seiders, D. W. Ward and R. H. Grubbs, Org. Lett., 2001, 3, 3225–3228 CrossRef CAS PubMed
; (b) F. Glorius, G. Altenhoff, R. Goddard and C. Lehmann, Chem. Commun., 2002, 2, 2704–2705 RSC
; (c) Y. Sato, Y. Hinata, R. Seki, Y. Oonishi and N. Saito, Org. Lett., 2007, 9, 5597–5599 CrossRef CAS PubMed
; (d) Reviews: F. Wang, L. jun Liu, W. Wang, S. Li and M. Shi, Coord. Chem. Rev., 2012, 256, 804–853 CrossRef CAS
; (e) A. Jayaraj, A. V. Raveedran, A. T. Latha, D. Priyadarshini and P. C. A. Swamy, Coord. Chem. Rev., 2023, 478, 214922 CrossRef CAS
.
-
(a) V. Lavallo, Y. Canac, C. Präsang, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2005, 44, 5705–5709 CrossRef CAS PubMed
; (b) M. Melaimi, R. Jazzar, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10046–10068 CrossRef CAS PubMed
; (c) V. Lavallo, Y. Canac, A. DeHope, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2005, 44, 7236–7239 CrossRef CAS PubMed
; (d) S. Kumar Kushvaha, A. Mishra, H. W. Roesky and K. Chandra Mondal, Chem.–Asian J., 2022, 17, e202101301 CrossRef CAS PubMed
.
- V. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller and G. Bertrand, Angew. Chem., Int. Ed., 2006, 45, 3488–3491 CrossRef CAS PubMed
.
- M. Melaimi, M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2010, 49, 8810–8849 CrossRef CAS PubMed
.
- H. V. Huynh, Chem. Rev., 2018, 118, 9457–9492 CrossRef CAS PubMed
.
- C. Saalfrank, F. Fantuzzi, T. Kupfer, B. Ritschel, K. Hammond, I. Krummenacher, R. Bertermann, R. Wirthensohn, M. Finze, P. Schmid, V. Engel, B. Engels and H. Braunschweig, Angew. Chem., Int. Ed., 2020, 59, 19338–19343 CrossRef CAS PubMed
.
- L. Zhao and X. Zeng, Chem, 2022, 8, 2082–2113 CAS
.
- U. S. D. Paul and U. Radius, Eur. J. Inorg. Chem., 2017, 2017, 3362–3375 CrossRef CAS
.
- For CAAC structural evolution see:
(a) B. Rao, H. Tang, X. Zeng, L. L. Liu, M. Melaimi and G. Bertrand, Angew. Chem., 2015, 127, 15128–15132 CrossRef
; (b) J. Chu, D. Munz, R. Jazzar, M. Melaimi and G. Bertrand, J. Am. Chem. Soc., 2016, 138, 7884–7887 CrossRef CAS PubMed
; (c) E. Tomás-Mendivil, M. M. Hansmann, C. M. Weinstein, R. Jazzar, M. Melaimi and G. Bertrand, J. Am. Chem. Soc., 2017, 139, 7753–7756 CrossRef PubMed
; (d) C. M. Weinstein, G. P. Junor, D. R. Tolentino, R. Jazzar, M. Melaimi and G. Bertrand, J. Am. Chem. Soc., 2018, 140, 9255–9260 CrossRef CAS PubMed
.
- D. Pichon, M. Soleilhavoup, J. Morvan, G. P. Junor, T. Vives, C. Crévisy, V. Lavallo, J. M. Campagne, M. Mauduit, R. Jazzar and G. Bertrand, Chem. Sci., 2019, 10, 7807–7811 RSC
.
- J. Morvan, F. Vermersch, Z. Zhang, L. Falivene, T. Vives, V. Dorcet, T. Roisnel, C. Crévisy, L. Cavallo, N. Vanthuyne, G. Bertrand, R. Jazzar and M. Mauduit, J. Am. Chem. Soc., 2020, 142, 19895–19901 CrossRef CAS PubMed
.
- A. Del Vecchio, J. Talcik, S. Colombel-Rouen, J. Lorkowski, M. R. Serrato, T. Roisnel, N. Vanthuyne, G. Bertrand, R. Jazzar and M. Mauduit, ACS Catal., 2023, 13, 6195–6202 CrossRef CAS
.
- A. Madron du Vigné and N. Cramer, Organometallics, 2022, 41, 2731–2741 CrossRef
.
- F. Vermersch, L. Oliveira, J. Hunter, M. Soleilhavoup, R. Jazzar and G. Bertrand, J. Org. Chem., 2022, 87, 3511–3518 CrossRef CAS PubMed
.
- I. Artaud, G. Torossian and P. Viout, Tetrahedron, 1985, 41, 5031–5037 CrossRef CAS
.
- N. De Kimpe, D. De Smaele, A. Hofkens, Y. Dejaegher and B. Kesteleyn, Tetrahedron, 1997, 53, 10803–10816 CrossRef CAS
.
-
(a) L. Claisen, Ber. Dtsch. Chem. Ges., 1912, 45, 3157–3166 CrossRef CAS
; (b) M. G. Kulkarni, S. I. Davawala, A. K. Doke and D. S. Pendharkar, Synthesis, 2004, 2004, 2919–2926 CrossRef
; (c) A. M. M. Castro, Chem. Rev., 2004, 104, 2939–3002 CrossRef CAS PubMed
.
- S. Surprenant, W. Y. Chan and C. Berthelette, Org. Lett., 2003, 5, 4851–4854 CrossRef CAS PubMed
.
- M. Jeong, S. Kang, K. Torikai, S. Lee and H. Yun, Org. Prep. Proced. Int., 2021, 53, 200–205 CrossRef CAS
.
- A. Sytniczuk, A. Kajetanowicz and K. Grela, Chem Catal., 2023, 3, 100713 CrossRef CAS
.
- V. Farkas, D. Csókás, Á. Erdélyi, G. Turczel, A. Bényei, T. Nagy, S. Kéki, I. Pápai and R. Tuba, Adv. Sci., 2024, 11, 2400118 CrossRef CAS PubMed
.
- A. Poater, F. Ragone, R. Mariz, R. Dorta and L. Cavallo, Chem.–Eur. J., 2010, 16, 14348–14353 CrossRef CAS PubMed
.
- A. Poater, F. Ragone, S. Giudice, C. Costabile, R. Dorta, S. P. Nolan and L. Cavallo, Organometallics, 2008, 27, 2679–2681 CrossRef CAS
.
- L. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano and L. Cavallo, Nat. Chem., 2019, 11, 872–879 CrossRef CAS PubMed
.
- J. Morvan, F. Vermersch, Z. Zhang, T. Vives, T. Roisnel, C. Crévisy, L. Falivene, L. Cavallo, N. Vanthuyne, G. Bertrand, R. Jazzar and M. Mauduit, Organometallics, 2023, 42, 495–504 CrossRef CAS
.
- Deposition numbers 2193067 (for (R,S)-Cu1), 2193068 (for (R,R)-Cu1), 2366137 (for (R,R)-Cu7), 2366138 (for (R,R)-Cu11), 2366139 (for (R,S)-Ru3) and 2355628 (for (R)-Ru0) contain the ESI† crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2193067, 2193068, 2355628 and 2366137–2366139. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04278f |
| This journal is © The Royal Society of Chemistry 2024 |