Open Access Article
Open Access ArticleN2H4Zn(HC3N3O3): exceptionally strong second harmonic generation and ultra-long phosphorescence†
Can
Yang‡
a,
Yuwei
Kang‡
a,
Xuefei
Wang
a,
Jie
Gou
a,
Yi
Xiong
 a,
Zece
Zhu
a,
Zece
Zhu
 a,
Ling
Chen
a,
Ling
Chen
 *b and
Qi
Wu
*b and
Qi
Wu
 *a
*a
aState /Key Laboratory of New Textile Materials and Advanced Processing Technologies, Wuhan Textile University, Wuhan 430200, China. E-mail: wuqi2011@whu.edu.cn
bBeijing Key Laboratory of Energy Conversion and Storage Materials, College of Chemistry, Beijing Normal University, Beijing 100875, China. E-mail: chenl@bnu.edu.cn
First published on 30th August 2024
Abstract
The discovery and designed synthesis of multifunctional materials is a leading pursuit in materials science. Herein, we report a novel hydro-isocyanurate, N2H4Zn(HC3N3O3), which combines strong second harmonic generation (SHG) and ultra-long room-temperature phosphorescence (RTP). The SHG intensity is the highest within the cyanurate system (13 × KDP), and RTP lifetime extends up to 448 ms, accompanied by a long-lasting afterglow visible to the naked eye for 1.2 s, surpassing most of the current metal–organic complexes. This advancement holds promise for the development of multifunctional optoelectronic devices, particularly leveraging second-harmonic generation (SHG) processes.
Introduction
The field of nonlinear optical (NLO) materials has been at the forefront of materials science, driving innovation in frequency conversion technologies such as second harmonic generation (SHG), which find wide applications in everyday life and industry.1–5 On the other hand, materials that exhibit long-lived room-temperature phosphorescence (RTP) have been a great challenge. The rarity of materials that can stably maintain ultra-long RTP alongside strong SHG properties is primarily attributed to the complex interplay required between structural asymmetry for SHG activity and the delicate balance of energy states necessary for long-lived phosphorescence.6–11 This dual functionality is highly desirable for applications ranging from secure optical communication to precise bio-imaging.12–15Semi-organic NLO crystals emerge as a promising class of materials that combine the processability and tunability of organic components with the structural stability of their inorganic counterparts.16–27 Herein, we report a novel semi-organic crystal, N2H4Zn(HC3N3O3), exhibiting exceptionally strong SHG and ultra-long RTP. The design rationale was to exploit the synergistic combination of a SHG-active (HC3N3O3)2− building unit with the coordination tendency of Zn2+ and the unique neutral N2H4 ligand. This assembly not only results in a non-centrosymmetric (NCS) structure conducive to SHG activity but also facilitates efficient exciton transfer and intersystem crossing (ISC), essential for RTP. The SHG of N2H4Zn(HC3N3O3) is the strongest among that of metal cyanurates to date, surpassing that of KDP by a factor of 13, and it has an RTP lifetime of 448 ms, which is exceptionally long for metal complexes. Moreover, the material exhibits a visibly bright blue and green afterglow for up to 1.2 s. N2H4Zn(HC3N3O3) also shows a large bandgap of 5.38 eV, a thermal stability up to 250 °C, insensitivity to air, robust resistance to water as well as a notable birefringence (Δn) of 0.29 @ 546 nm, which further underscore its practicality. Finally, theoretical calculations and analyses are conducted to understand the observed phenomena, offering insights into the future design synthesis of multifunctional materials.
Results and discussion
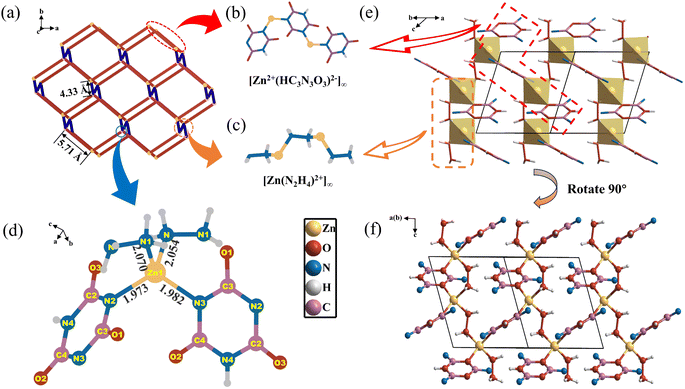
Block-like single crystals of N2H4Zn(HC3N3O3), measuring up to 5 × 2 × 1 mm3, were successfully synthesized using a hydrothermal stoichiometric reaction of CH6N4·HNO3, ZnBr2, C3H3N3O3, and H2O (Fig. S1†). The purity of the product was confirmed by using the fully assigned experimental powder X-ray diffraction (PXRD) data, which agree well with the single-crystal X-ray diffraction (SC-XRD) refinement results (Fig. S2†).N2H4Zn(HC3N3O3) crystallizes in the monoclinic polar Cc space group (Tables S1–S4†). The CCDC number is 2331157. The structure of N2H4Zn(HC3N3O3) is a novel one, in which the Zn-centered tetrahedra are connected in a diamond-like topological three-dimensional (3D) framework (Fig. 1a) via the infinite anionic chains of ([Zn2+(HC3N3O3)2−]∞) (Fig. 1b) extending along the c-axis that are interwoven with the infinite cationic chains of ([Zn(N2H4)]∞2+) (Fig. 1c), together with hydrogen bonds (Table S5†). Within this network, the Zn–Zn distance (bridged by two (HC3N3O3)2− rings) is 5.71 Å or 4.33 Å (bridged by two [N2H4] molecules) (Fig. 1c). Each Zn1 atom is tetrahedrally coordinated to two distinct (HC3N3O3)2− 6-MRs (Zn1–N1/3 bond lengths range from 1.973 Å to 1.982 Å) and two [N2H4] molecules (Zn1–N4/5 bond lengths are in the range of 2.054 Å to 2.070 Å), resulting in the formation of the highly polarized [Zn(HC3N3O3)2(N2H4)2] tetrahedron (Fig. 1d). The Zn–N bond coordinated to the neutral hydrazine groups is slightly longer than that to the (HC3N3O3)2− ring, which is mainly due to the mutual electrostatic attraction between Zn2+ and (HC3N3O3)2− ( and S3†). Considering the bond valence sum (BVS) calculation results (Table S6†) and the coordination environment of the Zn atom, the H atoms are added to N2 by geometry optimization, giving a hydro-isocyanuric anionic group, (HC3N3O3)2−. Within the (HC3N3O3)2− group, the C–N bond lengths span a range from 1.357(4) to 1.374(4) Å. In contrast, the C–O bond varies in a slightly broader range, extending from 1.225(4) to 1.247(5) Å. Furthermore, the N–N bond within the [N2H4] molecule is 1.457(4) Å. These bond distances agree well with those reported.28,29
In this structure, two differently oriented π-conjugated delocalized (HC3N3O3)2− groups are present, and the dihedral angle between the two (HC3N3O3)2− groups is 40.9° (Fig. S4†), while the intersection angle between two [N2H4] molecules is 38° (Fig. S5†). The [Zn(HC3N3O3)2(N2H4)2] tetrahedron is highly polarized and constructs the diamond-like 3D network (Fig. 1e and f). Due to the absence of an inversion center in the polar Cc space group, all the polarized [Zn(HC3N3O3)2(N2H4)2] groups align uniformly facilitating significant anisotropy and effective superposition of 2nd nonlinear susceptibility.
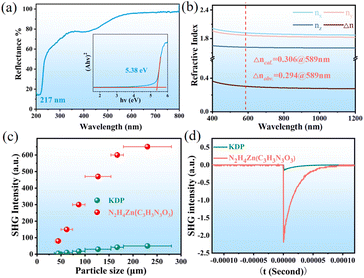
The optical properties of N2H4Zn(HC3N3O3) were measured through infrared (IR), ultraviolet-visible (UV-vis) and photoluminescence (PL) spectroscopies. The IR stretching vibrations between N–H nitrogen and N–H⋯O at 2700–3500 and 792 cm−1 are observed. The vibrations at 1400–1700 cm−1 are attributed to (HC3N3O3)2−, and those at 1157 and 1374 cm−1 are attributed to C–O and C–N, respectively. These are the typical spectral features for cyanurate and isocyanurate derivatives (Fig. S6†). The UV-vis spectrum reveals a cutoff edge at 217 nm (Eg = 5.38 eV), the shortest in the hydro-isocyanurate family (Fig. 2a).
The birefringence (Δn) properties of N2H4Zn(HC3N3O3) were measured on an as-obtained crystal with a thickness of 4.71 μm by using a polarized optical microscope fitted with a Berek compensator, illuminated with a 589 nm monochromatic green light source. The extinction process, facilitated by optical compensation, yielded a retardation value of −1387.3 nm, giving a Δnobv. of 0.294 (Fig. S7†). The Δncal. = 0.306 @ 589 nm in principle agrees well with this observation (Fig. 2b). The Δnobv. is comparable with those of other cyanurates and isocyanurates, such as Pb2Cd(HC3N3O3)2(OH)2 (0.291@800 nm), LiRbHC3N3O3·2H2O (0.259@532 nm) and NaRb0.84Cs0.16HC3N3O3·2H2O (0.238@532 nm) (Table S7†).29–34 The large Δn of the title compound N2H4Zn(HC3N3O3) may arise mainly from the strong anisotropy and π–π conjugation interactions of the (HC3N3O3)2− rings.
The particle-size-dependent SHG responses were measured by the Kurtz–Perry powder method utilizing a 1064 nm fundamental laser with KDP as a reference. The SHG responses of polycrystalline N2H4Zn(HC3N3O3) samples intensify with increasing particle size, reaching a plateau at approximately 180 μm, indicating a phase matching behavior (Fig. 2c). The maximal SHG response is 13 times that of KDP, the strongest intensity among cyanurates (Fig. 2d and Table S8†).35–40
The thermal stability and water and air resistance were studied. Unlike many isocyanurates, N2H4Zn(HC3N3O3) possesses an anhydrous 3D network structure and is thermally stable until 250 °C without undergoing decomposition (Fig. S8†). Moreover, after a six-month exposure to ambient air and humidity, the crystals remained unfazed, showing no signs of degradation or moisture absorption. As shown in Fig. 3, the color and weight of N2H4Zn(HC3N3O3) crystals remained unchanged after immersion in water for 10 days, and their the XRD patterns show no change before and after the immersion (Fig. S2†). Such a water treatment does not influence the green SHG light (532 nm) emission (Fig. 3).
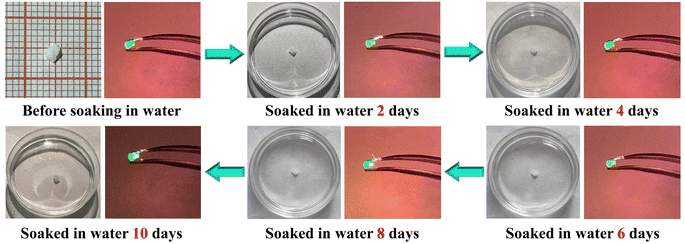 | ||
| Fig. 3 Water resistance test of the as-synthesized N2H4Zn(HC3N3O3) crystal (the crystal emits green light at 532 nm when irradiated by using a 1064 nm laser). | ||
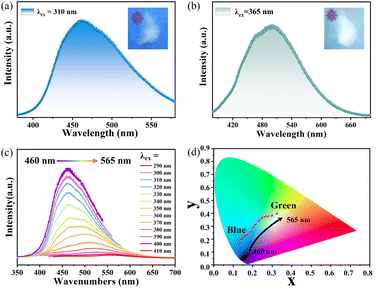
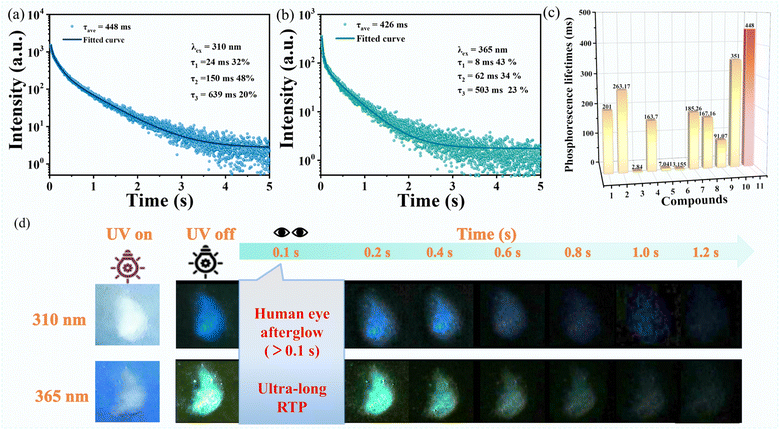
The room-temperature photoluminescence properties were investigated. The fluorescence displays a broad emission band peaking at approximately 360 nm, corresponding to an excitation wavelength of 310 nm (Fig. S9†). Subsequent phosphorescence spectroscopy (Fig. 4c) indicated a dynamic red-shift in the emission peak position as the excitation wavelength was incrementally varied from 290 to 410 nm. This shift was observed to transition from around 460 nm to a range between 460 and 565 nm, marking emission adaptability. Fig. 4 depicts the dual-color emission capability; with an excitation wavelength of 310 nm, the emission peak is situated at around 460 nm indicative of blue light, while at 365 nm, the peak red-shifts to approximately 500 nm, corresponding to green light. This bioluminescence is corroborated by the 1931 International Commission on Radiation (CIE) chromaticity diagram (Fig. 4d). The naked-eye can distinctly perceive a persistent blue and green phosphorescent glow for about 1.2 s post-irradiation (Fig. 5d). The luminescence decay curves (Fig. 5a and b) further elucidate the multi-exponential decay behavior. The phosphorescence lifetimes are measured to be 448 ms and 428 ms for excitation wavelengths of 310 nm and 365 nm, respectively, with an average lifetime exceeding 400 ms. This longevity is superior to most organic–Zn metal complexes (Table S9† and Fig. 5c).41–47 Simultaneously, the temperature-dependent luminescence measurements have elucidated that the phosphorescence intensity of the sample escalates incrementally as the experimental temperature diminishes, corroborating the typical characteristics of phosphorescence phenomena (Fig. S10†).
To elucidate the relationship between the structure and properties of N2H4Zn(HC3N3O3), first-principles calculations were conducted using the CASTEP software package.48 The electronic band structure indicates that the valence band maximum (VBM) is positioned at the A point, and the conduction band minimum (CBM) is located at the M point, signifying a direct band gap of 4.60 eV (Fig. S11†). Given the tendency for the band gap to be underestimated due to the discontinuity in the exchange-correlation energy functional, a scissor operator of 0.78 eV was employed for subsequent optical property calculations. The total and partial densities of states (DOS) reveal that the band gap is predominantly dictated by the interactions between C-2p and O-2p orbitals, as well as the C-2p and N-2p orbitals within the [HC3N3O3]2− anionic group. The band associated with Zn-4s, 3d, and 3p states, and the N-2p state within the [N2H4] group, are primarily located in the lower energy region ranging from −10 to −5 eV and contributes minimally around the Fermi level (Fig. S12†). The electron distribution curves of the VBM and CBM (Fig. 6a and d) suggest that the VBM is mainly composed of O-n states, while the CBM is mainly derived from the (HC3N3O3)2−-π* states. Thus, the organic component (HC3N3O3)2− contributes to both the CBM and VBM by promoting n–π* leaps, which, according to the EI-Sayed rule, can effectively promote inter-system crossing (ISC) in favor of ultra-long room-temperature phosphorescence, which is consistent with the proposed organic emission mechanism.
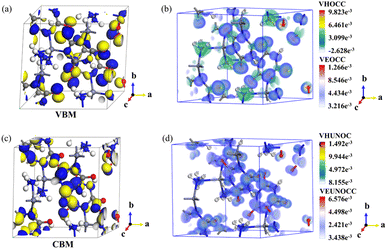 | ||
| Fig. 6 The electron distribution profiles of N2H4Zn(HC3N3O3): the (a) VBM and (c) CBM. SHG weight factor analysis of the d11 component of the N2H4Zn(HC3N3O3) crystal (b) and (d). | ||
Previous studies reveal that the N-2p orbitals in cyanuric 6-MRs can experience a down-shift in their band structure when the bridging N atoms coordinate with H or Zn atoms, leading to an enlarged band gap for Zn(H2C3N3O3)2·3H2O.28 In the case of the title N2H4Zn(HC3N3O3) compound, one of the bridging N atoms forms a N–H bond, while the other two bridge N atoms coordinate with two distinct Zn atoms to form Zn–N bonds. This arrangement effectively diminishes the N-2p electronic states near the Fermi level, leading to a band gap of 5.38 eV.
The refractive index calculations revealed a distinct nz – ny < ny – nx relationship, characterizing N2H4Zn(HC3N3O3) as a negative biaxial crystal. The calculated birefringence of 0.306@589 nm is in close agreement with the Δnobv. = 0.294@589 nm. The real-space atom-cutting calculations indicate that the (HC3N3O3)2− anionic groups contribute primarily to the optical anisotropy with a substantial 0.340@589 nm. Meanwhile, Zn2+ and the [N2H4] molecule have non-negligible but small contributions of 0.079 and 0.049@589 nm, respectively (Table S10†).
Utilizing the Kleinman approximation for point group m, N2H4Zn(HC3N3O3) has a set of five non-zero SHG tensors that were calculated to be d12 = −7.6815 pm V−1, d13 = −1.652 pm V−1, d25 = −1.153 pm V−1, d36 = 0.12 pm V−1, and d11 = 8.017 pm V−1. The dominant d11 tensor is about 20-fold enhanced over the d36 of KDP. The SHG-weighted electron density analysis focusing on the d11 tensor was conducted to quantify the contributions from each structure building unit (Fig. 6). The SHG weight of the d11 component in the N2H4Zn(HC3N3O3) crystal (Fig. 6b and d) demonstrates that the virtual electron and virtual hole states are primarily driven by the (HC3N3O3)2− ring, with Zn2+ and [N2H4] contributing to a lesser extent.
The unoccupied molecular orbitals are attributed to the (HC3N3O3)2− ring and the nitrogen atoms in the [N2H4] molecule. This indicates that the SHG predominantly stems from the (HC3N3O3)2− rings, with non-negligible contributions from the Zn2+ and [N2H4] molecules, which contribute 79.60%, 10.50%, and 9.90% (Table S10†), respectively. This finding underscores the effective enhancement of the SHG responses in N2H4Zn(HC3N3O3) through the synergistic action of multiple chromophores.
Conclusions
In summary, a novel hydro-isocyanurate N2H4Zn(HC3N3O3) was discovered. This material exhibits an SHG response that is 13 × KDP, representing the strongest among cyanurates. The unique topological diamond-like framework not only achieves a short absorption edge at 217 nm and a large birefringence of Δnobv. = 0.294@589 nm, but also facilitates an exceptionally high thermal stability, and strong water resistance. Moreover, such a topological diamond-like framework also displays tunable multicolor room-temperature phosphorescence and a long RTP lifetime of 448 ms, accompanied by a long-lasting afterglow visible to the naked eye for 1.2 s, which is the longest among SHG-based metal–organic complexes, highlighting the material's potential for diverse applications in optoelectronics and beyond.Data availability
Data available in the ESI† include the experimental section and additional tables and figures.Author contributions
Can Yang and Yuwei Kang synthesized and characterized the title compound, and completed the writing of this manuscript. Qi Wu and Ling Chen provided experimental ideas and revised the manuscript. Xuefei Wang and Jie Gou participated in the data analysis and discussion of the article. Yi Xiong provided assistance in the characterization of compounds. Zece Zhu provided a discussion of the testing and data analysis of phosphorescence. The manuscript was written through contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 22275052). The authors would like to thank Chensheng Lin, Jun Zhang, Yuchao Li and Xingxing Jiang for their help in the theoretical calculations.Notes and references
- P. Becker, Adv. Mater., 1998, 10, 979–992 CrossRef CAS.
- D. Cyranoski, Nature, 2009, 457, 953–956 CrossRef CAS PubMed.
- D. F. Eaton, Science, 1991, 253, 281–287 CrossRef CAS PubMed.
- N. Savage, Nat. Photonics, 2007, 1, 83–85 CrossRef CAS.
- R. Ikuta, Y. Kusaka, T. Kitano, H. Kato, T. Yamamoto, M. Koashi and N. Imoto, Nat. Commun., 2011, 2, 537 CrossRef.
- C. Wu, T. Wu, X. Jiang, Z. Wang, H. Sha, L. Lin, Z. Lin, Z. Huang, X. Long and M. G. Humphrey, J. Am. Chem. Soc., 2021, 143, 4138–4142 CrossRef CAS.
- H. Liu, H. Wu, Z. Hu, J. Wang, Y. Wu and H. Yu, J. Am. Chem. Soc., 2023, 145, 12691–12700 CrossRef CAS PubMed.
- Y. Yang, Y. Xiao, B. Li, Y.-G. Chen, P. Guo, B. Zhang and X.-M. Zhang, J. Am. Chem. Soc., 2023, 145, 22577–22583 CrossRef CAS PubMed.
- S. Fang, H. Li, Y. Xie, H. Li, Y. Wang and Y. Shi, Small, 2021, 17, 2103831 CrossRef CAS.
- Z. Zhu, L. Zeng, W. Li, W. Xu and D. Tian, ACS Sustainable Chem. Eng., 2022, 10, 16752–16759 CrossRef CAS.
- Z. Qi, B. Zhou and D. Yan, Mater. Chem. Front., 2023, 7, 3475–3493 RSC.
- X. Kang and M. Zhu, Chem. Soc. Rev., 2019, 48, 2422–2457 RSC.
- L. Chen, L. Yu, Y. Liu, H. Xu, W. Li, F. Wang, J. Zhu, K. Yi, L. Ma and H. Xiao, ACS Sens., 2023, 8, 3104–3115 CrossRef CAS PubMed.
- Z. Zhu, L. Zeng, W. Li, D. Tian and W. Xu, ACS Sustainable Chem. Eng., 2021, 9, 17420–17426 CrossRef CAS.
- S. Xu, R. Chen, C. Zheng and W. Huang, Adv. Mater., 2016, 28, 9920–9940 CrossRef CAS PubMed.
- J. Lu, X. Liu, M. Zhao, X. B. Deng and L. M. Wu, J. Am. Chem. Soc., 2021, 143, 3647–3654 CrossRef CAS PubMed.
- F. Ge, B.-H. Li, P. Cheng, G. Li, Z. Ren, J. Xu and X.-H. Bu, Angew. Chem., Int. Ed., 2022, 61, e202115024 CrossRef CAS PubMed.
- D. Lin, M. Luo, C. Lin, F. Xu and N. Ye, J. Am. Chem. Soc., 2019, 141, 3390–3394 CrossRef CAS.
- Y. Li, W. Huang, Y. Zhou, X. Song, J. Zheng, H. Wang, Y. Song, M. Li, J. Luo and S. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202215145 CrossRef CAS.
- L. Liu, Z. Bai, L. Hu, D. Wei, Z. Lin and L. Zhang, J. Mater. Chem. C, 2021, 9, 7452–7457 RSC.
- Y. Liu, Y. P. Gong, S. Geng, M. L. Feng, D. Manidaki, Z. Deng, C. C. Stoumpos, P. Canepa, Z. Xiao and W. X. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208875 CrossRef CAS.
- X. Meng, X. Zhang, Q. Liu, Z. Zhou, X. Jiang, Y. Wang, Z. Lin and M. Xia, Angew. Chem., 2023, 135, e202214848 CrossRef.
- Y. Kang, C. Yang, J. Gou, Y. Zhu, Q. Zhu, W. Xu and Q. Wu, Angew. Chem., 2024, 136, e202402086 CrossRef.
- K. Wan, B. Tian, Y. Zhai, Y. Liu, H. Wang, S. Liu, S. Li, W. Ye, Z. An and C. Li, Nat. Commun., 2022, 13, 5508 CrossRef CAS.
- H. Liu, Y. Gao, J. Cao, T. Li, Y. Wen, Y. Ge, L. Zhang, G. Pan, T. Zhou and B. Yang, Mater. Chem. Front., 2018, 2, 1853–1858 RSC.
- J. Yu, H. Ma, W. Huang, Z. Liang, K. Zhou, A. Lv, X.-G. Li and Z. He, JACS Au, 2021, 1, 1694–1699 CrossRef CAS PubMed.
- H. Ma, Q. Peng, Z. An, W. Huang and Z. Shuai, J. Am. Chem. Soc., 2018, 141, 1010–1015 CrossRef.
- D. Wang, X. Zhang, F. Liang, Z. Hu and Y. Wu, Dalton Trans., 2021, 50, 5617–5623 RSC.
- D. Lin, M. Luo, C. Lin, F. Xu and N. Ye, J. Am. Chem. Soc., 2019, 141, 3390–3394 CrossRef CAS PubMed.
- J. Lu, Y.-K. Lian, L. Xiong, Q.-R. Wu, M. Zhao, K.-X. Shi, L. Chen and L.-M. Wu, J. Am. Chem. Soc., 2019, 141, 16151–16159 CrossRef CAS.
- N. Wang, F. Liang, Y. Yang, S. Zhang and Z. Lin, Dalton Trans., 2019, 48, 2271–2274 RSC.
- X. Meng, F. Liang, K. Kang, J. Tang, T. Zeng, Z. Lin and M. Xia, Inorg. Chem., 2019, 58, 11289–11293 CrossRef CAS.
- X. Meng, K. Kang, F. Liang, J. Tang, Z. Lin, W. Yin and M. Xia, Dalton Trans., 2020, 49, 1370–1374 RSC.
- X. Meng, K. Kang, F. Liang, J. Tang, W. Yin, Z. Lin and M. Xia, Inorg. Chem. Front., 2020, 7, 3674–3686 RSC.
- M. Xia, M. Zhou, F. Liang, X. Meng, J. Yao, Z. Lin and R. Li, Inorg. Chem., 2018, 57, 32–36 CrossRef CAS PubMed.
- X. Meng, X. Zhang, Q. Liu, Z. Zhou, X. Jiang, Y. Wang, Z. Lin and M. Xia, Angew. Chem., 2023, 135, e202214848 CrossRef.
- Y. Song, D. Lin, M. Luo, C. Lin, Q. Chen and N. Ye, Inorg. Chem. Front., 2020, 7, 150–156 RSC.
- Y. Chen, C. Hu, Z. Fang and J. Mao, Inorg. Chem. Front., 2021, 8, 3547–3555 RSC.
- M. Aibibula, L. Wang and S. Huang, ACS Omega, 2019, 4, 22197–22202 CrossRef CAS.
- X. Meng, F. Liang, J. Tang, K. Kang, Q. Huang, W. Yin, Z. Lin and M. Xia, Eur. J. Inorg. Chem., 2019, 2019, 2791–2795 CrossRef CAS.
- F.-Y. Cao, H.-H. Liu, Y. Mu, Z.-Z. Xue, J.-H. Li and G.-M. Wang, J. Phys. Chem. Lett., 2022, 13, 6975–6980 CrossRef CAS PubMed.
- Z. Luo, Y. Liu, Y. Liu, C. Li, Y. Li, Q. Li, Y. Wei, L. Zhang, B. Xu and X. Chang, Adv. Mater., 2022, 34, 2200607 CrossRef CAS.
- H. Liu, W. Ye, Y. Mu, H. Ma, A. Lv, S. Han, H. Shi, J. Li, Z. An and G. Wang, Adv. Mater., 2022, 34, 2107612 CrossRef CAS.
- D. Li, X. Yang and D. Yan, ACS Appl. Mater. Interfaces, 2018, 10, 34377–34384 CrossRef CAS PubMed.
- Y. J. Ma, X. Fang, G. Xiao and D. Yan, Angew. Chem., Int. Ed., 2022, 61, e202114100 CrossRef CAS.
- Y. Wang, C. Wang, M. Sun, P. Zhao, X. Fang, J. Zhang, Y. Guo and G. Zhao, Adv. Opt. Mater., 2024, 12, 2301843 CrossRef CAS.
- H. Zhang, Y. Yan, G. Qiao and J. Li, Inorg. Chem. Commun., 2019, 104, 119–123 CrossRef CAS.
- S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. I. Probert, K. Refson, M. C. Payne and Z. Krist, Cryst. Mater., 2005, 220, 567–570 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2331157. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc04476b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |