Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diastereoselective hydride transfer enables a synthesis of chiral 1,5-carboxamido-trifluoromethylcarbinols†
Roberto
Tinelli
ab,
Manuel
Schupp
 ac,
Immo
Klose
a,
Saad
Shaaban
a,
Boris
Maryasin
ac,
Immo
Klose
a,
Saad
Shaaban
a,
Boris
Maryasin
 ad,
Leticia
González
ad,
Leticia
González
 ad and
Nuno
Maulide
ad and
Nuno
Maulide
 *ac
*ac
aInstitute of Organic Chemistry, University of Vienna, Währinger Straße 38, 1090 Vienna, Austria. E-mail: nuno.maulide@univie.ac.at
bVienna Doctoral School in Chemistry, University of Vienna, Währinger Straße 42, 1090 Vienna, Austria
cCeMM – Research Center for Molecular Medicine of the Austrian Academy of Sciences, Lazarettgasse 14, AKH BT 25.3, 1090 Vienna, Austria
dInstitute of Theoretical Chemistry, University of Vienna, Währinger Straße 17, 1090 Vienna, Austria
First published on 9th September 2024
Abstract
The deployment of fluorinated functional groups has become a widespread tool in medicinal chemistry due to the impact of fluorine on lipophilicity and metabolic stability. Among these compounds, enantiopure secondary trifluoromethylcarbinols are recurrent features in bioactive compounds. Herein, we present a diastereoselective redox-neutral process allowing the stereospecific synthesis of 1,5-carboxamido-trifluoromethylcarbinols through the formal reduction of a trifluoromethylketone into a trifluoromethylcarbinol. A combined experimental and computational investigation unveiled a network of interconnected equilibria leading to a key hydride transfer event.
Introduction
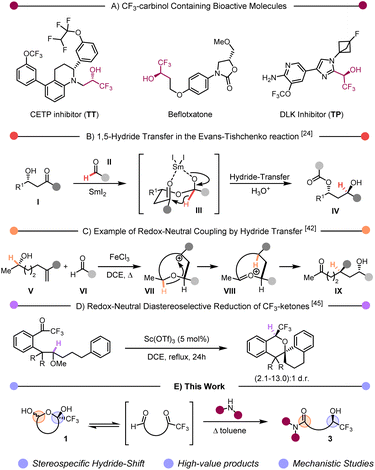
The introduction of fluorinated functional groups to organic compounds has become a recurrent tool in medicinal chemistry, given both the pronounced influence of fluorine on lipophilicity and metabolic stability, and the resulting boost of physicochemical and biological properties. Accordingly, a growing proportion of newly approved drugs contain at least a fluorine atom.1–6 Among the (by now) many fluorinated moieties commonly employed, enantiopure secondary trifluoromethylcarbinols are recurrent features in bioactive compounds, such as the cholesteryl ester transfer protein (CETP) inhibitor TT, the monoamine oxidase A inhibitor befloxatone or the dual leucine zipper kinase (DLK) inhibitor TP (Scheme 1A).7–10 Although not ideally suited for the synthesis of the trifluoromethylcarbinol scaffold in the latter compounds, several methods for the synthesis of such motifs have recently been developed involving direct reduction of CF3-ketones,11–17 or nucleophilic addition to trifluoroacetaldehyde (or its hemiacetal).18–21 In particular, the former methods require the use of stoichiometric amounts of bulky boranes,11,13 or, when resorting to catalytic protocols, high pressures of H2 to tackle the poor reactivity of CF3-ketones.14–17 In contrast, the latter methods encounter challenges regarding functional group tolerance and limited diastereomeric ratios.Therefore, and despite these advances, alternative mild methods allowing the synthesis of densely substituted secondary trifluoromethylcarbinols with high levels of diastereo- and enantiomeric excess are still in demand.
In recent years, so-called “redox-neutral” synthetic methods have emerged22,23 as atom-economical processes that effect changes to molecular connectivity while simultaneously adjusting the redox state of two functional groups.24–26 Although recently somewhat re-profiled, the field effectively includes classical transformations such as the venerable Tishchenko (and Evans–Tishchenko) reaction (Scheme 1B).27 In its most commonly deployed variant, a β-ketoalcohol (I) and an aldehyde (II) associate into a hemiketal intermediate (III). In the presence of SmI2, III can undergo a 1,5-hydride transfer delivering IV in stereoselective fashion.28–48 Previously, our group has deployed hydride transfers as devices to accomplish reductive couplings that proceed by cationic mechanisms. In a notable example, stereocontrolled alkene–aldehyde coupling could be achieved with unique selectivity by engendering a cyclic carbocation reactive intermediate VIII. The subsequent hydride transfer event forms oxocarbenium VIII, which ultimately undergoes hydrolysis to the observed coupling product IX (Scheme 1C).49–51 Recent work by Mori demonstrated the possibility of using CF3-ketones as hydride acceptors to create trifluoromethyl-ethers in a diastereoselective fashion (Scheme 1D).52–54 In our continued efforts to further extend and harness the synthetic possibilities offered by redox neutral processes, we herein report a diastereoselective hydride transfer process allowing the stereospecific synthesis of 1,5-carboxamido-trifluoromethylcarbinols 3 from cyclic hemiacetals 1 (Scheme 1E).
Results and discussion
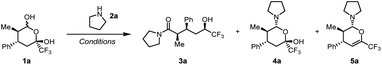
During our research on inverse shuttle catalysis, we serendipitously discovered that, in the presence of a secondary amine, 1 was thermally converted to 3 in a metal-free process.55 Indeed, initial experiments showed that organocatalytic Hetero-Diels–Alder adduct561a reacts with pyrrolidine at room temperature in toluene to deliver hemiaminal 4a in quantitative yield (Table 1, entry 1).| Entry | Eq. 2a | Additive | Solvent | T (°C) | Yield 3a | Yield 4a | Yield 5a |
|---|---|---|---|---|---|---|---|
a
1a (0.1 mmol, 0.04 M) 24 h.
b BF3·OEt2 10%.
c Sc(OTf)3 10%.
d MS 3 A.
e
1a (0.15 mmol, 0.04 M), 48 h.
f d.r. > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, ee1a = 94%, ee3a = 90%. 1, ee1a = 94%, ee3a = 90%.
|
|||||||
| 1a | 1.1 | — | Toluene | 25 | — | 100 | — |
| 2a | 1.1 | — | Toluene | 70 | 60% | 21% | — |
| 3a | 1.1 | — | Toluene | 100 | 82% | 8% | — |
| 4a | 1.1 | — | DMSO | 100 | — | — | — |
| 5a | 1.1 | — | DCE | 80 | 22% | 28% | — |
| 6a,b | 1.1 | BF3·OEt2 | Toluene | 100 | — | — | 17% |
| 7a,c | 1.1 | Sc(OTf)3 | Toluene | 100 | 30% | — | — |
| 8a,d | 1.1 | 3 Å MS | Toluene | 100 | 53% | 27% | — |
| 9e,f | 2.0 | — | Toluene | 100 | 89% | — | — |
Conversely, heating the mixture led to formation of the trifluoromethyl carbinol product 3a in good yield, accompanied by elimination and formation of byproduct 5a. Importantly, hemiaminal 4a was completely consumed (entry 2). Screening of different solvents showed a negative impact of increasing solvent polarity (entries 4–5), and the addition of Lewis acid catalysts (entries 6–7) or drying agents (entry 8) were found to also not have a beneficial effect on the reaction outcome. As detailed in the ESI,† the reaction revealed a good stability towards variation of concentration, reaction time and stoichiometry of pyrrolidine (see ESI, Section 3† for a full table of optimisation), and we ultimately determined that addition of 2 equivalents of amine allowed isolation of 3a in an optimised yield of 89% (entry 9). Notably, 3a is formed as a single diastereomer in 90% enantiomeric excess.
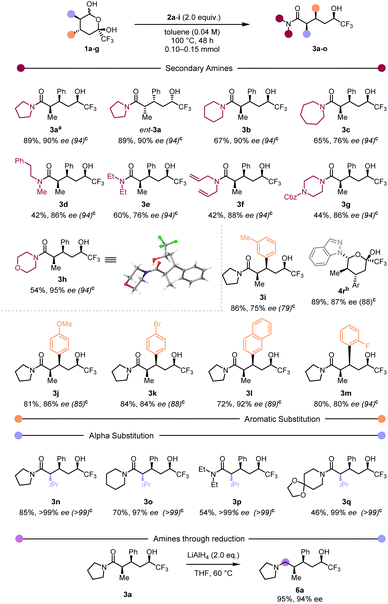
With optimised conditions in hand we assessed the scope of this redox-neutral process (Scheme 2).
Employing hemiketal ent-1a (Scheme 2) resulted in product ent-3a with the same diastereo- and enantioselectivity as its antipode. Focusing on the amine component, we were pleased to observe that cyclic amines generally led to the desired products in very good yields (3b and 3c). However, a slight decrease in yield was observed when acyclic (3d–3f) or heterocyclic (3g and 3h) amines were employed. Single crystal X-ray analysis of 3h and 3k (the latter not shown in Scheme 2) provided unambiguous assignment of the absolute configuration of our products.57 Variation in the arene substituent revealed that both electron-rich and electron-poor aryls afforded the products in high yield (3i–3m). Replacing the α-methyl group with a more hindered iso-propyl group (3n–3q) led to good yields and excellent enantioselectivities. Worthy of note, employing indazole as the amine component led to the formation of a hemiaminal 4r that failed to afford the trifluoromethylcarbinol product. We believe this could be a consequence of the reduced availability of the indazole nitrogen lone pair. Representative limitations of the reported method can be found in the ESI† (primary amines, highly-hindered secondary amines, ketones, esters). In addition, reduction of the carboxamide delivered aminoalcohol 6a in excellent yield and ee value.
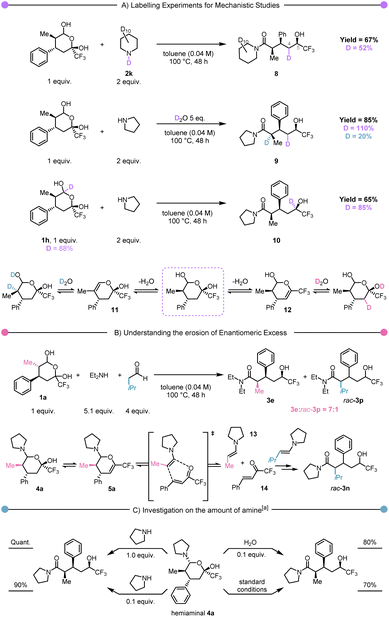
From the outset, we were interested in elucidating the mechanism of this atom-economical rearrangement. To explore the plausibility of a hydride originating from the amine component, we conducted the reaction in the presence of fully deuterated piperidine (Scheme 3A, first experiment). Interestingly, while this experiment demonstrated the absence of deuterium at the carbinol stereocentre (C-5) in 8, it unexpectedly revealed its presence on C-4. This intriguing observation suggested the existence of an elimination/rehydration equilibrium (cf.12, Scheme 3A, bottom). To validate this hypothesis, we performed the reaction in the presence of 5 equiv. of D2O, which resulted in deuterium labelling at C-4 and at C-2 in 9 (Scheme 3A, second experiment). In contrast to previous examples in the literature, this equilibrium did not lead to any epimerisation.58 Notably, the use of deuterated hemiacetal 1h led to a complete transfer of deuterium from the hemiacetal carbon to the CF3-alcohol carbon in 10, strongly supporting an internal hydride-transfer mechanism (Scheme 3A, third experiment).
Subsequently, our focus shifted towards unravelling the loss in enantiomeric excess reported for 3c and 3e. In particular (cf. equilibria shown in Scheme 3B), we speculated about the possibility of intermediate 5a undergoing retro Hetero-Diels–Alder to return to enamine 13 and trifluoromethyl enone 14. In the absence of a chiral catalyst, the equilibrium between 5a and 13–14 must lead to the racemisation of the product (Scheme 3B). To demonstrate this hypothesis, we envisioned capture by an alternative enamine in a secondary, parallel Hetero-Diels–Alder process. Therefore, we performed the rearrangement of 1a with diethylamine in the presence of isovaleraldehyde under thermal conditions. The observation of a mixture of products 3e and rac-3p in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Scheme 3B) was a striking observation. Firstly, it unambiguously demonstrates the possibility of incorporation of an extraneous aldehyde, which supports the cycloreversion/cycloaddition hypothesis articulated earlier.
1 ratio (Scheme 3B) was a striking observation. Firstly, it unambiguously demonstrates the possibility of incorporation of an extraneous aldehyde, which supports the cycloreversion/cycloaddition hypothesis articulated earlier.
Secondly, the ratio between 3e and rac-3p suggests that the retro Hetero-Diels–Alder pathway, though operative, is not prevalent. Finally, we became curious about the possible role of hemiaminal 4a. Accordingly, it was isolated and exposed to different conditions (Scheme 3C). These experiments showed that once the hemiaminal intermediate is formed, the reaction proceeds quantitatively by the addition of an additional equivalent of pyrrolidine. The use of reduced (catalytic) amounts of pyrrolidine or water also allowed the formation of the product, albeit with a small drop in yield. We then performed computational studies at the density functional theory (DFT: B3LYP-D3(BJ)/def2-TZVP//B3LYP-D3(BJ)/def2-SVP,59–65 see ESI† for details) level to shed light on the reaction mechanism. The in silico computed process is shown in Scheme 4A, and Scheme 4B presents the computed Gibbs free energy profile. The hemiaminal B (4a) can intramolecularly ring-open to the acyclic form C. Notably, this step B → C is endergonic (ΔG(B → C) = 7.6 kcal mol−1) and, accordingly, has a relatively high kinetic barrier of 28.5 kcal mol−1. This computational result is in good agreement with the experimentally applied elevated temperature of 100 °C. The intermediate C undergoes a concerted combination of hydride and proton transfers, ultimately leading to the experimentally observed product D (3a). Interestingly, two diastereoisomeric pathways are possible for the step C→D, while only one diastereomer, depicted in Scheme 4B as D_R, was observed experimentally. Thermodynamically, the structures D_S and D_R are very similar (ΔG(C → D_S) = −29.1 kcal mol−1 and ΔG(C → D_R) = −28.2 kcal mol−1). However, we see a significant difference in the kinetic barriers favoring the formation of the experimentally observed product D_R. Indeed, ΔG‡(C → D_S) = 18.8 kcal mol−1 and ΔG‡(C → D_R) = 12.2 kcal mol−1. This barrier gap can be explained via analysis of the transition state structures shown on the right part of Scheme 4B. The transition state structures are bicyclic with interconnected six-membered rings. Both rings adopt a favourable chair conformation in the energetically lower transition state structure TSCD_R. However, in the case of the energetically disfavoured transition state TSCD_S, one of the rings adopts in a boat-like conformation, destabilising the entire structure. The described mechanistic pathway allows the formation of the product D in the absence of water. However, our experimental data shows that adding water to the system can benefit the reaction. In order to understand the influence of water, we have performed further calculations with an explicitly added singular water molecule. As shown in Scheme 4B (left), the initially formed reactant complex B′ is 4.6 kcal mol−1 higher as compared to the starting point B due to the entropic penalty paid for the involved water molecule. Remarkably, the subsequent transition state TSB′C is better stabilised than the transition state TSBC: ΔG‡(B → C, viaTSBC) = 28.5 kcal mol−1 and ΔG‡(B → C, viaTSB′C) = 26.8 kcal mol−1. This is in complete agreement with the experimentally observed reaction acceleration in the presence of water. Both transition states, TSBC and TSB′C, lead to the same intermediate C, but the reaction mechanism is dramatically different. Transition state TSB′C is an energetically favorable six-membered cycle in which water assists the ring opening event. Instead of the hydroxy group shift within TSBC, the OH bond is cleaved in the transition state TSB′C, and the proton exchange with the water molecule allows the C–O bond to break. We also considered a four-membered ring alternative to the TSB′C without water assistance, but the calculations strictly deny the possibility of this event, highlighting the crucial role of water.
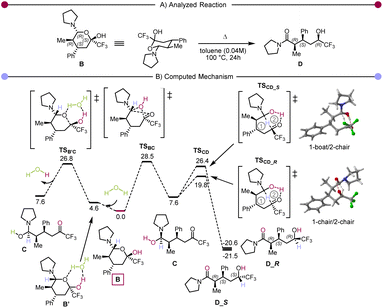 | ||
| Scheme 4 (A) The computationally analysed system. (B) Computed Gibbs free energy reaction profile (ΔG373, toluene). The hemiaminal B is used as a reference (0.0 kcal mol−1). | ||
Conclusion
In conclusion, we have developed a new redox-neutral process enabling the stereospecific synthesis of multisubstituted 1,5-carboxamido trifluoromethylcarbinols from cyclic hemiketals. The mechanism of the process was studied in depth, revealing a network of interconnected equilibria that productively lead to the final product and showcasing the influence of water on the overall process. Extensive DFT calculations provided an elegant model to rationalise the observed diastereoselectivity. Notably, in this method, a redox-neutral event formally reduces a trifluoromethylketone into a trifluoromethylcarbinol, thus obviating the need for strongly nucleophilic (organometallic) reagents or high-pressured hydrogenations. We believe that this work further emphasises the growing importance of hydride transfer processes in mediating formal reductive transformations under mild conditions.Data availability
A data availability statement (DAS) is submitted alongside the article.Author contributions
The work was conceptualised by N. M. The experiments were performed by R. T., M. S. and I. K. B. M. performed the DFT calculations. The manuscript was written through contributions of all authors. S. S. and N. M. were involved in manuscript editing, finalizing and overall supervision of the project. N. M. and L. G. secured funding and supervised the entire work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded in whole or in part by the Austrian Science Fund (FWF) [10.55776/P37182]. We thank Dr D. Kaiser for helpful discussions and suggestions and Dr S. Woolford for proofreading the ESI.† We are grateful to the Centre for X-Ray Structure Analysis (U. Vienna) for crystal-structure determination and E. Macoratti (U. Vienna) for HPLC analysis. Calculations were performed at the Vienna Scientific Cluster (VSC).Notes and references
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. Del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed.
- D. C. Lankin, N. S. Chandrakumar, S. N. Rao, D. P. Spangler and J. P. Snyder, J. Am. Chem. Soc., 1993, 115, 3356–3357 CrossRef CAS.
- B. K. Park, N. R. Kitteringham and P. M. O'Neill, Annu. Rev. Pharmacol. Toxicol., 2001, 41, 443–470 CrossRef CAS PubMed.
- M. B. van Niel, I. Collins, M. S. Beer, H. B. Broughton, S. K. Cheng, S. C. Goodacre, A. Heald, K. L. Locker, A. M. MacLeod, D. Morrison, C. R. Moyes, D. O'Connor, A. Pike, M. Rowley, M. G. Russell, B. Sohal, J. A. Stanton, S. Thomas, H. Verrier, A. P. Watt and J. L. Castro, J. Med. Chem., 1999, 42, 2087–2104 CrossRef PubMed.
- S. Mesch, D. Walter, A. Laux-Biehlmann, D. Basting, S. Flanagan, H. Miyatake Ondozabal, S. Bäurle, C. Pearson, J. Jenkins, P. Elves, S. Hess, A.-M. Coelho, A. Rotgeri, U. Bothe, S. Nawaz, T. M. Zollner and A. Steinmeyer, J. Med. Chem., 2023, 66, 1583–1600 CrossRef CAS PubMed.
- K. Le, M. J. Soth, J. B. Cross, G. Liu, W. J. Ray, J. Ma, S. G. Goodwani, P. J. Acton, V. Buggia-Prevot, O. Akkermans, J. Barker, M. L. Conner, Y. Jiang, Z. Liu, P. McEwan, J. Warner-Schmidt, A. Xu, M. Zebisch, C. J. Heijnen, B. Abrahams and P. Jones, J. Med. Chem., 2023, 66, 9954–9971 CrossRef CAS PubMed.
- O. Curet, G. Damoiseau, N. Aubin, N. Sontag, V. Rovei and F. X. Jarreau, J. Pharmacol. Exp. Ther., 1996, 277, 253–264 CAS.
- T. A. Rano, E. Sieber-McMaster, P. D. Pelton, M. Yang, K. T. Demarest and G.-H. Kuo, Bioorg. Med. Chem. Lett., 2009, 19, 2456–2460 CrossRef CAS PubMed.
- P. D. O'Shea, C.-Y. Chen, D. Gauvreau, F. Gosselin, G. Hughes, C. Nadeau and R. P. Volante, J. Org. Chem., 2009, 74, 1605–1610 CrossRef PubMed.
- R. Noyori, I. Tomino, Y. Tanimoto and M. Nishizawa, J. Am. Chem. Soc., 1984, 106, 6709–6716 CrossRef CAS.
- H. C. Brown and G. G. Pai, J. Org. Chem., 1985, 50, 1384–1394 CrossRef CAS.
- Y. Kuroki, Y. Sakamaki and K. Iseki, Org. Lett., 2001, 3, 457–459 CrossRef CAS PubMed.
- N. Sayo, S. Akutagawa, T. Saito, R. Noyori, H. Kumobayashi and H. Takaya, EP Pat., 295109 A1, 1988.
- T. Sturm, W. Weissensteiner and F. Spindler, Adv. Synth. Catal., 2003, 345, 160 CrossRef CAS.
- Y. Kuroki, D. Asada and K. Iseki, Tetrahedron Lett., 2000, 41, 9853 CrossRef CAS.
- K. Mikami, T. Yajima, M. Terada, E. Kato and M. Maruta, Tetrahedron: Asymmetry, 1994, 5, 1087–1090 CrossRef CAS.
- Y. Makino, K. Iseki, K. Fujii, S. Oishi, T. Hirano and Y. Kobayashi, Tetrahedron Lett., 1995, 36, 6527–6530 CrossRef CAS.
- H. Torii, M. Nakadai, K. Ishihara, S. Saito and H. Yamamoto, Angew. Chem., Int. Ed., 2004, 43, 1983–1986 CrossRef CAS PubMed.
- F. Zhang, Y. Peng, S. Liao and Y. Gong, Tetrahedron, 2007, 63, 4636–4641 CrossRef CAS.
- N. Z. Burns, P. S. Baran and R. W. Hoffmann, Angew. Chem., Int. Ed., 2009, 48, 2854–2867 CrossRef CAS PubMed.
- P. A. Wender, M. P. Croatt and B. Witulski, Tetrahedron, 2006, 62, 7505–7511 CrossRef CAS.
- J. M. Richter, Y. Ishihara, T. Masuda, B. W. Whitefield, T. Llamas, A. Pohjakallio and P. S. Baran, J. Am. Chem. Soc., 2008, 130, 17938–17954 CrossRef CAS PubMed.
- N. Bai, X. Wang, Z. Wang, F. Liu and Z.-Q. Rong, Org. Chem. Front., 2022, 9, 5942–5948 RSC.
- Q. Feng, Q. Wang and J. Zhu, Science, 2023, 379, 1363–1368 CrossRef CAS PubMed.
- D. A. Evans and A. H. Hoveyda, J. Am. Chem. Soc., 1990, 112, 6447–6449 CrossRef CAS.
- Hydride transfer typically occurs from the α-position of an electron-donating group to the α-position of an electron-withdrawing group at a 5 atoms distance. For examples and reviews, see.26–38 For examples where transfer occurs across more or less than 5 atoms, see39–45..
- I. D. Jurberg, B. Peng, E. Wöstefeld, M. Wasserloos and N. Maulide, Angew. Chem., Int. Ed., 2012, 51, 1950–1953 CrossRef CAS PubMed.
- S. J. Pastine and D. Sames, Org. Lett., 2005, 7, 5429–5431 CrossRef CAS PubMed.
- N. Maulide, S. Shaaban and B. Peng, Synlett, 2013, 24, 1722–1724 CrossRef.
- K. M. McQuaid and D. Sames, J. Am. Chem. Soc., 2009, 131, 402–403 CrossRef CAS PubMed.
- I. D. Jurberg, Y. Odabachian and F. Gagosz, J. Am. Chem. Soc., 2010, 132, 3543–3552 CrossRef CAS PubMed.
- B. Bolte and F. Gagosz, J. Am. Chem. Soc., 2011, 133, 7696–7699 CrossRef CAS PubMed.
- D. Shikanai, H. Murase, T. Hata and H. Urabe, J. Am. Chem. Soc., 2009, 131, 3166–3167 CrossRef CAS PubMed.
- W. Cao, X. Liu, W. Wang, L. Lin and X. Feng, Org. Lett., 2011, 13, 600–603 CrossRef CAS PubMed.
- Y. K. Kang, S. M. Kim and D. Y. Kim, J. Am. Chem. Soc., 2010, 132, 11847–11849 CrossRef CAS PubMed.
- Y. Dong, F. Hu, H. Wu, F.-W. Guo, L. Wang, F.-Y. Du and S.-S. Li, Org. Lett., 2024, 26, 332–337 CrossRef CAS.
- Z.-W. Jiao, S.-Y. Zhang, C. He, Y.-Q. Tu, S.-H. Wang, F.-M. Zhang, Y.-Q. Zhang and H. Li, Angew. Chem., Int. Ed., 2012, 51, 8811–8815 CrossRef CAS PubMed.
- M. Shang, J. Z. Chan, M. Cao, Y. Chang, Q. Wang, B. Cook, S. Torker and M. Wasa, J. Am. Chem. Soc., 2018, 140, 10593–10601 CrossRef CAS PubMed.
- B. Peng and N. Maulide, Chem.–Eur. J., 2013, 19, 13274–13287 CrossRef CAS PubMed.
- K. Chen, J. M. Richter and P. S. Baran, J. Am. Chem. Soc., 2008, 130, 7247–7249 CrossRef CAS PubMed.
- M. C. Haibach and D. Seidel, Angew. Chem., Int. Ed., 2014, 53, 5010–5036 CrossRef CAS PubMed.
- F. Hu, X. Li, Z. Ding, L. Wang, C. Ge, L. Xu and S.-S. Li, ACS Catal., 2022, 12, 943–952 CrossRef CAS.
- S.-S. Li, X. Lv, D. Ren, C.-L. Shao, Q. Liu and J. Xiao, Chem. Sci., 2018, 9, 8253–8259 RSC.
- A. M. Gómez, F. Lobo, D. Pérez de las Vacas, S. Valverde and J. C. López, Chem. Commun., 2010, 46, 6159–6161 RSC.
- P. Pigeon, A. Mamouni, J. Sikoraiova, S. Marchalin and B. Decroix, Tetrahedron, 2001, 57, 4939–4943 CrossRef CAS.
- D. T. H. Chou, X. Huang, R. J. Batchelor, F. W. B. Einstein and A. J. Bennet, J. Org. Chem., 1998, 63, 575–581 CrossRef CAS.
- J. Li, A. Preinfalk and N. Maulide, J. Am. Chem. Soc., 2019, 141, 143–147 CrossRef CAS.
- J. Li, A. Preinfalk and N. Maulide, Angew. Chem., Int. Ed., 2019, 58, 5887–5890 CrossRef CAS PubMed.
- D. Kaiser, V. Tona, C. R. Gonçalves, S. Shaaban, A. Oppedisano and N. Maulide, Angew. Chem., Int. Ed., 2019, 58, 14639–14643 CrossRef CAS PubMed.
- R. Tamura, E. Kitamura, R. Tsutsumi, M. Yamanaka, T. Akiyama and K. Mori, Org. Lett., 2019, 21, 2383–2387 CrossRef CAS.
- K. Yokoo and K. Mori, Chem. Commun., 2018, 54, 6927–6930 RSC.
- D. Hoshino and K. Mori, Org. Biomol. Chem., 2020, 18, 6602–6606 RSC.
- I. Klose, G. Di Mauro, D. Kaldre and N. Maulide, Nat. Chem., 2022, 14, 1306–1310 CrossRef CAS PubMed.
- Y. Zhao, X. Wang and J. Liu, Synlett, 2008, 7, 1017–1020 Search PubMed.
- Deposition numbers 2335899 (for 3h), 2335900 (for 3k), 2351935 for (4r) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service..
- Y. Kang, W. Chen, M. Breugst and D. Seidel, J. Org. Chem., 2015, 80, 9628–9640 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200–1211 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc05049e |
| This journal is © The Royal Society of Chemistry 2024 |