Open Access Article
Open Access ArticleRegulating Co–O covalency to manipulate mechanistic transformation for enhancing activity/durability in acidic water oxidation†
Jiachen
Zhang
a,
Guangbo
Chen
 bc,
Dongmei
Sun
bc,
Dongmei
Sun
 a,
Yawen
Tang
a,
Yawen
Tang
 a,
Wei
Xing
a,
Wei
Xing
 de,
Hanjun
Sun
de,
Hanjun
Sun
 *a and
Xinliang
Feng
*a and
Xinliang
Feng
 *bf
*bf
aJiangsu Key Laboratory of New Power Batteries, Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, School of Chemistry and Materials Science, Nanjing Normal University, 210023 Nanjing, China. E-mail: hanjun.sun@njnu.edu.cn
bCenter for Advancing Electronics Dresden (CFAED) and Faculty of Chemistry and Food Chemistry, Technische Universität Dresden, 01062 Dresden, Germany. E-mail: xinliang.feng@tu-dresden.de
cKey Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, 100190 Beijing, China
dState Key Laboratory of Electroanalytic Chemistry, Jilin Province Key Laboratory of Low Carbon Chemistry Power, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 130022 Changchun, China
eSchool of Applied Chemistry and Engineering, University of Science and Technology of China, 230026 Hefei, China
fMax Planck Institute of Microstructure Physics, Halle (Saale) 06120, Germany
First published on 1st October 2024
Abstract
Developing earth-abundant electrocatalysts with high activity and durability for acidic oxygen evolution reaction is essential for H2 production, yet it remains greatly challenging. Here, guided by theoretical calculations, the challenge of overcoming the balance between catalytic activity and dynamic durability for acidic OER in Co3O4 was effectively addressed via the preferential substitution of Ru for the Co2+ (Td) site of Co3O4. In situ characterization and DFT calculations show that the enhanced Co–O covalency after the introduction of Ru SAs facilitates the generation of OH* species and mitigates the unstable structure transformation via direct O–O coupling. The designed Ru SAs-CoOx catalyst (5.16 wt% Ru) exhibits enhanced OER activity (188 mV overpotential at 10 mA cm−2) and durability, outperforming most reported Co3O4-based and Ru-based electrocatalysts in acidic media.
Introduction
Electrocatalytic water splitting is considered a promising approach for producing clean and renewable hydrogen owing to its high energy conversion efficiency and safety.1–7 To date, acidic water electrolyzers exhibit great advantages compared with conventional alkaline water electrolyzers, such as lower ohmic losses, higher voltage efficiency and faster system response.8–10 More importantly, the smaller gas crossover in acidic electrolyzers avoids the mixture of H2 and O2, which ensures higher gas purity (note S1†).11,12 The acidic oxygen evolution reaction (OER) as the key half-reaction contributes to a major energy loss in overall electrochemical water splitting, which limits the efficiency of the overall process. Compared to the alkaline OER, acidic OER exhibits inferior catalytic activity due to the following two reasons: (1) the oxidation of adsorbed hydroxyl (OH*) into O2 remains challenging in acidic media due to the scarcity of hydroxide anions (OH−), resulting in lower OH* coverage on the catalyst surface. (2) The inferior durability of earth-abundant electrocatalysts (e.g., Fe, Co, and Ni-based) in acidic electrolytes compared to that in alkaline electrolytes hinders their practical application.To achieve efficient acidic water splitting, various catalysts with noble metal iridium (Ir) or ruthenium (Ru) as active sites have been widely studied, such as pyrochlore-type material Y2Ru2O7−δ,13 bimetal oxide material (LixRuO2 and14 Cr0.6Ru0.4O2,15), and perovskite-like material (SrTi1−xIrxO3 and16 Sr2MIr(V)O6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17). However, the high cost and scarcity of noble metals limit their large-scale applications. Cobalt oxide (Co3O4) as an earth-abundant material has been theoretically predicted as a potential catalyst for the OER due to (1) the optimum binding energy with the O-intermediates, which is comparable to that of RuO2;18 (2) remarkable static durability (resting state or open-circuit potential) in acidic media owing to the relatively strong overlapping of the octahedral Co3+(Oh)–O orbital.19 However, its practical catalytic performance in acidic media is not satisfactory: (1) compared that in alkaline media with abundant OH−, the OH* coverage on the catalyst surface in acidic media is comparatively lower; (2) in the conventional adsorbate evolution mechanism (AEM) process, the multiple processes preceding the formation of the O–O bond result in frequent valence changes, which facilitate surface reconstruction and limit the durability (Fig. S1a,† step 1–3).20–22 Thereby, to enhance the activity and durability of Co3O4 for acidic OER, the local bonding environment is considered to be regulated to optimize the reaction process. With these considerations, a second adsorption site (lattice oxygen) is utilized to promote the direct O–O coupling during the OER process, namely the lattice oxygen oxidation mechanism (LOM). Direct O–O coupling not only mitigates the unstable reconstruction process before the formation of the O–O bond (Fig. S1b†), but also has the potential to surpass the theoretical limitation of activity in the conventional AEM.23–26
17). However, the high cost and scarcity of noble metals limit their large-scale applications. Cobalt oxide (Co3O4) as an earth-abundant material has been theoretically predicted as a potential catalyst for the OER due to (1) the optimum binding energy with the O-intermediates, which is comparable to that of RuO2;18 (2) remarkable static durability (resting state or open-circuit potential) in acidic media owing to the relatively strong overlapping of the octahedral Co3+(Oh)–O orbital.19 However, its practical catalytic performance in acidic media is not satisfactory: (1) compared that in alkaline media with abundant OH−, the OH* coverage on the catalyst surface in acidic media is comparatively lower; (2) in the conventional adsorbate evolution mechanism (AEM) process, the multiple processes preceding the formation of the O–O bond result in frequent valence changes, which facilitate surface reconstruction and limit the durability (Fig. S1a,† step 1–3).20–22 Thereby, to enhance the activity and durability of Co3O4 for acidic OER, the local bonding environment is considered to be regulated to optimize the reaction process. With these considerations, a second adsorption site (lattice oxygen) is utilized to promote the direct O–O coupling during the OER process, namely the lattice oxygen oxidation mechanism (LOM). Direct O–O coupling not only mitigates the unstable reconstruction process before the formation of the O–O bond (Fig. S1b†), but also has the potential to surpass the theoretical limitation of activity in the conventional AEM.23–26
In order to facilitate the reaction pathway for direct O–O coupling, the moderate orbital overlap of metal–oxygen (M–O covalency) is essential.24,27,28 Specifically, the enhanced M–O covalency results in a decreased energy gap between the metal d and O 2p band center, thereby moving the Fermi level closer to the O 2p-band center and the redox potential of the O2/H2O couple, which is thermodynamically favorable for the redox of lattice oxygen.29,30 Nevertheless, excessive covalency would cause an improved lattice oxygen migration rate compared to the vacancy filling rate during the LOM cycles, leading to structure destabilization.5,31 Therefore, we propose that moderate Co–O covalency of Co3O4 is more advantageous for enhancing durability compared to the AEM process by mitigating the unstable reconstruction process. In the present study, guided by the theoretical calculations, we design an electrocatalyst composed of Ru single atom (5.16 wt%) doped cobalt oxide (Ru SAs-CoOx) to overcome the activity/durability tradeoff for acidic OER. Our theoretical calculations suggest that (1) the introduction of heteroatom Ru could benefit the generation of OH* species, thus providing plentiful OH* species for the subsequent oxidation process;25 (2) the introduction of Ru allows the enhanced orbital overlap between the Co 3d and O 2p, thus increasing the Co–O covalency;28 (3) the doping of Ru promotes the generation of oxygen vacancies (Ov), facilitating direct O–O coupling during the OER process and the oxygen evolution from lattice oxygen.19,32,33In situ18O isotope labeling differential electrochemical mass spectrometry (DEMS) demonstrated Ru to be the switch promoting oxygen evolution from lattice oxygen. In addition, in situ Raman spectroscopy suggested that the growth of stable amorphous cobalt oxide on the electrode surface acted as the protective layer enhancing the durability. As a result, the prepared Ru SAs-CoOx catalyst delivers a low overpotential of 188 mV at a current density of 10 mA cm−2, and enhanced long-term durability in acidic media, which are superior to those of pristine Co3O4 (395 mV at 10 mA cm−2) and commercial RuO2 (312 mV at 10 mA cm−2).
Results and discussion
Theoretical prediction for the structure of the electrocatalyst
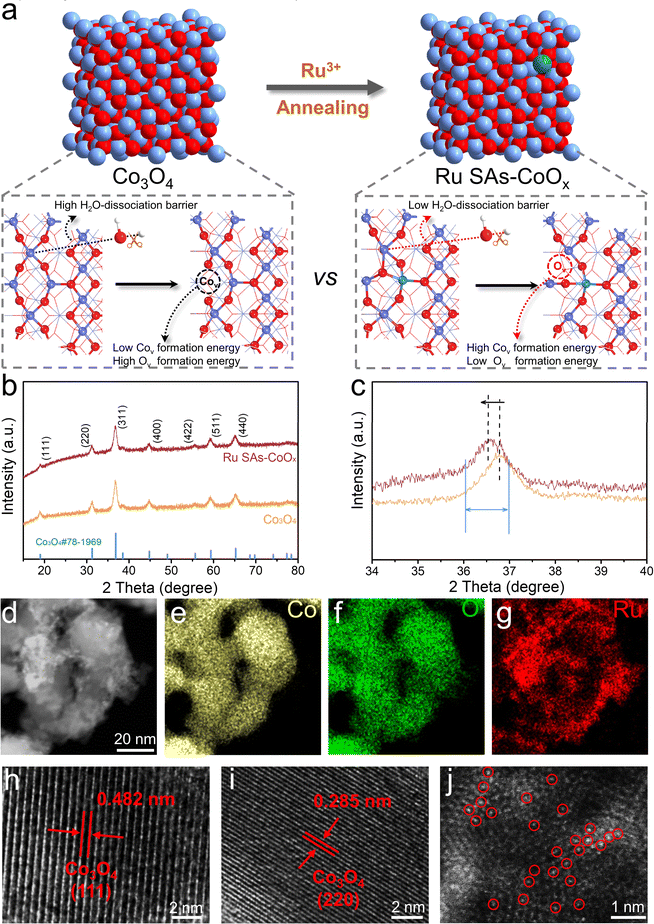
Density functional theory (DFT) calculations were first performed to investigate the participation of the lattice oxygen oxidation reaction of Ru SAs-CoOx. To ascertain the doping position of Ru, we compared the formation energy for the substitution of tetrahedral Co2+ (Td) and octahedral Co3+ (Oh) sites for a single Ru atom, which could effectively explore the influence of the doping element on the local reaction environment. As displayed in Fig. S2–S3,† replacing Co2+ (Td) with Ru leads to a lower barrier (1.194 eV) compared to that of the Co3+ (Oh) site (2.803 eV), indicating a thermodynamically preferred replacement of Ru atoms at the Co2+ (Td) site. Then, to evaluate the feasibility of the lattice oxygen oxidation reaction after Ru doping, the Co–O covalency and Ov formation energy of Co3O4 and Ru–Co3O4 are calculated, respectively. As illustrated in Fig. 1a–c and Table S1,† the substitution of divalent tetrahedral Co2+ (Td) with Ru3+ reduces the localized gap between the Co 3d and O 2p band center, accompanied by an increased orbital overlap between Co 3d and O 2p. Therefore, the antibonding states below the Fermi level displayed a higher oxygen character that results in the enhanced Co–O covalency, which would thermodynamically promote the oxidation of lattice oxygen.34,35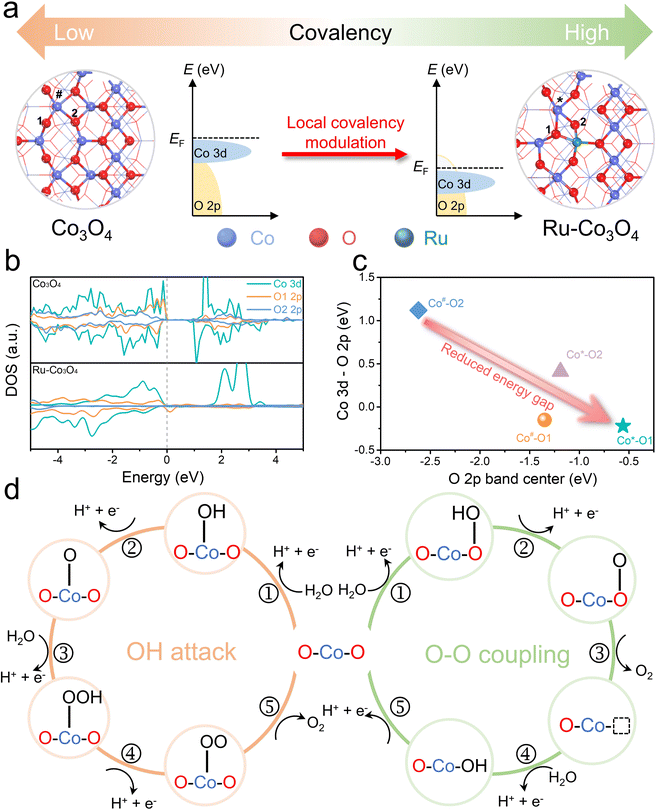 | ||
| Fig. 1 Electronic structures and OER pathways. (a) Structures and schematic energy bands of Co3O4 and Ru–Co3O4 (the corresponding structure is shown in Fig. S5a†). (b) Computed DOS of Co 3d and O 2p for Co3O4 and Ru–Co3O4. (c) Energy gap between Co 3d and O 2p versus the O 2p band center for Co3O4 and Ru–Co3O4. (d) Proposed transformation of the OER mechanism from the AEM to LOM after the doping of Ru. | ||
Besides, considering the crucial role of Ov formation in facilitating direct O–O coupling, the Ov formation energy in Co3O4 and Ru–Co3O4 is also calculated. The formation energies of four different types of Ov in Co3O4 are 1.509, 0.450, 2.863, and 2.820 eV, respectively (Fig. S4†). In the case of Ru–Co3O4, the corresponding formation energies for these four types of Ov are 0.149, 0.362, 1.676, and 1.362 eV (Fig. S5†). It is clear that the substitution of Co with Ru significantly reduces the formation energy of Ov. Therefore, the increased Co–O covalency and the lower Ov formation energy facilitate the transformation of the OER mechanism from a conventional adsorbate evolution mechanism (AEM, OH attack) to a lattice oxygen oxidation mechanism (LOM, O–O coupling) (Fig. 1d).32,33 In addition, it is worth noting that it is difficult to carry out accurate DFT studies to represent the exact surface of the electrocatalyst and the local experimental conditions. However, DFT calculations here may provide side insight for the design of electrocatalysts and lateral understanding of the structural and catalytic mechanism changes after the incorporation of Ru.
Material synthesis and characterization
Guided by the theoretical prediction results, we synthesized Ru SAs-CoOx by a cation exchange method. The detailed synthetic procedure and structural changes are illustrated in Fig. S6† and 2a. First, a cobalt precursor (Co-pre) with “flower-like” morphology (Fig. S7 and S8a–b†) was prepared via co-precipitation of Co(CH3COO)2·4H2O and polyvinylpyrrolidone (PVP) in ethylene glycol under the protection of argon at 170 °C. Herein, PVP was employed to improve the uniformity of the final architecture. Next, the prepared Co-pre was annealed at 300 °C to yield the pristine Co3O4 nanoflower (Fig. S8c–d†). Subsequently, the Co3O4 nanoflower (10 mg) was added into the ruthenium chloride (0.075 mmol) solution and stirred at 60 °C for the cation exchange reaction (5.16 wt% Ru). Finally, the Ru SAs-CoOx sample was obtained after calcination at 300 °C, which could detach the chloride ligands and improve crystallization.36,37X-ray diffraction (XRD) was employed to investigate the crystal structure of Co3O4 and Ru SAs-CoOx. As depicted in Fig. 2b–c, the diffraction peaks of Co3O4 were exhibited in the XRD pattern of Ru SAs-CoOx, and no diffraction peaks corresponding to Ru-related species could be detected, suggesting that the Ru species were highly dispersed or amorphous.38 However, the incorporation of Ru3+ with a larger ionic radius resulted in an increase in micro-strain within the Co3O4 lattice. Consequently, Ru SAs-CoOx displayed a clear XRD peak width broadening (∼0.3°), as well as a negative diffraction peak shift (∼0.2°) compared to pristine Co3O4, which confirmed that the incorporation of Ru is not limited to the surface but extends into the Co3O4 framework.38,39 Besides, the broad peak observed between 20° and 30° can be attributed to the presence of carbon residue primarily derived from ethylene glycol and Co(CH3COO)2. The morphologies of Ru SAs-CoOx were then examined using transmission electron microscopy (TEM). As shown in Fig. 2d and S9–S10,† after introducing Ru atoms, Co3O4 was transformed into the disordered nanosheet structure, where the interconnected nanostructures with certain amounts of holes could be observed. The N2 adsorption–desorption isotherms and the corresponding Brunauer–Emmett–Teller (BET) analysis showed that the surface area of Ru SAs-CoOx (155.46 m2 g−1) was higher than that of pristine Co3O4 (33.73 m2 g−1), while the average pore size of Ru SAs-CoOx (4.41 nm) was found to be smaller compared with that of pristine Co3O4 (13.27 nm) (Fig. S11†). The above-mentioned results were attributed to the “etching-recombination” that occurred during the cation exchange process, which led to an increased surface area, and exposed more sites for the electrocatalysis of the OER. In addition, the elemental mapping images indicated the uniform distribution of Ru, Co, and O atoms in the selected porous nanosheets, confirming the successful introduction of Ru after the cation exchange process (Fig. 2e–g). More structural features of Ru SAs-CoOx were then explored by high-resolution transmission electron microscopy (HRTEM). Two obvious continuous and ordered lattice fringes with lattice spacings of 0.482 and 0.285 nm were observed, which corresponded to the (111) and (220) planes of Co3O4 (Fig. 2h–i). No lattice fringes derived from Ru nanoparticles could be found. From the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images, numerous atomically dispersed bright dots (highlighted with red circles) of Ru atoms were homogeneously distributed on the surface of Ru SAs-CoOx, confirming that Ru species were anchored on Co3O4 in the form of single-atoms during the cation exchange process (Fig. 2j and S12†).40–42
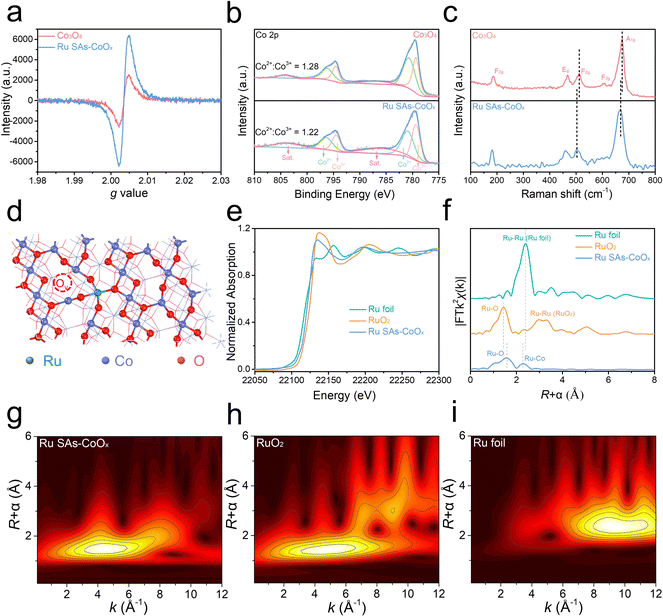
Electron spin resonance (ESR) measurements were performed to investigate the generation of Ov, and the signal corresponding to the electron trapped on Ov was detected at g = 2.003. As shown in Fig. 3a, a stronger signal intensity of Ru SAs-CoOx was exhibited relative to pristine Co3O4, demonstrating higher Ov concentration.43 To further substantiate the association between the increase in Ov concentration and Ru doping, Ru-CoOx catalysts with different Ru contents were prepared by varying the dosage of Ru3+ (i.e., 0.025 mmol, 0.05 mmol, and 0.075 mmol). The resulting products were denoted as Ru-CoOx-0.025, Ru-CoOx-0.05, and Ru-CoOx-0.075 (i.e. Ru SAs-CoOx), respectively. The results of ESR also showed that the Ov concentration increased significantly with the enhancement of Ru content (from 0 to 5.16 wt% measured by ICP-AES) (Fig. S13†).
XPS spectra were also measured to elucidate the chemical compositions and valence state of the prepared electrocatalysts (Fig. S14–S15†). For the Co 2p spectrum of Ru SAs-CoOx, two major peaks located at 779.3 and 794.7 eV were assigned to Co 2p3/2 and Co 2p1/2, which were fitted by two regions of Co2+ and Co3+. The relative atomic ratio of Co2+/Co3+ could be obtained by comparing the area of the fitted curve. As more oxygen vacancies were generated on the surface of Ru SAs-CoOx, the ratio of Co2+/Co3+ in Ru SAs-CoOx was expected to increase.44,45 However, the ratio of Co2+/Co3+ for the Ov-rich Ru SAs-CoOx (1.22) showed a slight decrease compared to that of the pristine Co3O4 (1.28) (Fig. 3b), which indicated that the Ru atoms were doped into the Co3O4 framework to maintain electrical neutrality, rather than being adsorbed on the surface. In addition, the ratio of Co2+/Co3+ for Ru SAs-CoOx (1.22) showed a slight decrease compared to that of the pristine Co3O4 (1.28), which suggested that Ru preferentially occupied Td sites (∼78%, note S2†).46,47 In the Ru 3p spectrum (Fig. S14d†), the peaks of Ru 3p3/2 and Ru 3p1/2 located at 462.60 and 484.88 eV were detected, which are positioned between those of Ru (0) and Ru (IV),48 demonstrating that the oxidation state of Ru in Ru SAs-CoOx was between 0 and +4 (Fig. S16†). Then Raman spectroscopy was conducted to reveal the structural changes in the coordination environment of Co3O4.49 As shown in Fig. 3c, five characteristic peaks corresponding to the Eg, F2g, and A1g of Co3O4 were detected. Specifically, A1g represented the octahedral (Oh) sites while F2g corresponded to the tetrahedral (Td) sites. After doping of Ru species, notable shifts in the characteristic peaks of F2g (∼11 cm−1) and A1g (∼7 cm−1) were observed, providing clear evidence of Ru substitution at both tetrahedral (Td) and octahedral (Oh) sites. The larger shift for F2g than that for A1g suggests that the Ru preferentially occupied the Td sites (Fig. 3d).49 In order to investigate the local coordination of Ru species in Ru SAs-CoOx, the Ru K-edge region X-ray absorption near edge structure (XANES) was measured, which displayed clear differences from those of Ru foil and RuO2 (Fig. 3e). Extended X-ray absorption fine structure (EXAFS) for Ru SAs-CoOx was performed to analyze the bonding forms of Ru species. The peak at 1.57 Å was associated with the back scatterings between Ru and neighboring O in Ru SAs-CoOx. Another peak at 2.29 Å could be attributed to the back scatterings between metal and metal. Furthermore, quantitative EXAFS curve-fitting analyses for Ru SAs-CoOx revealed that the average coordination number between Ru and O was 3.7 (Fig. S17 and Table S2†), which further demonstrates the preference of Ru substitution at the Td site (a 4-coordination structure) over the Oh site (6-coordination structure). Additionally, the presence of Ov may contribute to a further decrease in the average coordination number. The wavelet transform (WT) analysis of the EXAFS spectrum was performed to further confirm the formation of Ru SAs (Fig. 3g–i). Only one intensity maximum at about 4.5 Å−1 (Ru–O scattering path) was detected in the WT contour plot of Ru SAs-CoOx. The signal corresponding to RuB–O–CoB was not detected, which may be due to its weaker coordination.
Electrocatalytic performance of the OER in acidic media
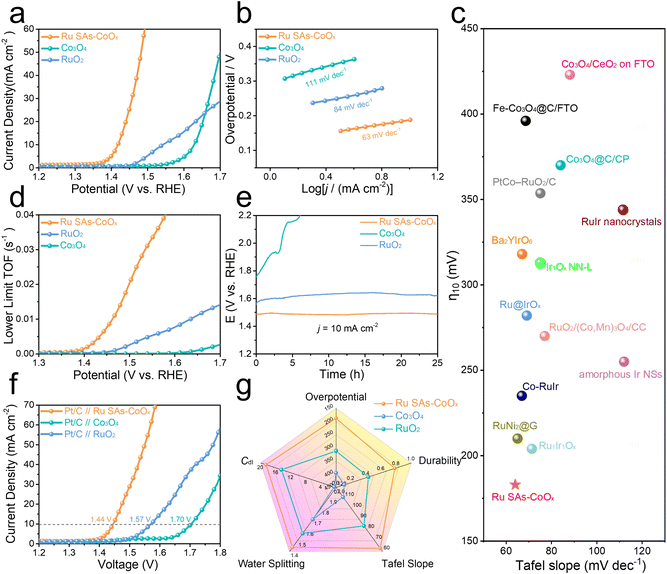
The electrocatalytic performances of the prepared catalysts were evaluated with a three-electrode system at room temperature. The iR-corrected polarization curves recorded by linear scan voltammetry (LSV) in N2-saturated 0.1 M HClO4 electrolyte were displayed. When the content of Ru reached 5.16 wt% (Fig. S18–S24 and Tables S3 and S4†) in the obtained Ru-CoOx (Ru SAs-CoOx) catalyst, it showed a minimum overpotential of 188 mV at a current density of 10 mA cm−2, which was much smaller than that of commercial RuO2 (312 mV) and pristine Co3O4 (395 mV) (Fig. 4a). Then the Tafel slopes derived from the polarization curves were used to evaluate the catalytic kinetics. As shown in Fig. 4b, the Tafel slope of Ru SAs-CoOx was calculated to be 63 mV dec−1, which was much lower than that of pristine Co3O4 (111 mV dec−1) and commercial RuO2 (84 mV dec−1), indicating the faster kinetics toward the OER.50,51 The Tafel slopes of all samples fell within the range of 60–120 mV dec−1, indicating a mixed kinetic control mechanism where the overall rate is not controlled by a single path.20,52–54 Besides, several other points may also cause the value of the Tafel slope to deviate from 60 mV dec−1 or 120 mV dec−1: (1) transfer coefficient (α) largely deviates from the commonly assumed value; (2) the coverage would vary as the electrode potential increases, leading to a corresponding alteration in the Tafel slope;1 (3) the catalytic activity may originate from multiple active sites, limiting the application of the conventional Butler–Volmer formalism. The low overpotential and Tafel slope of Ru SAs-CoOx also demonstrated its superior performance among Co3O4-based electrocatalysts, which is even well comparable with the reported Ir-based and Ru-based OER electrocatalysts in acidic media, such as Co–RuIr (235 mV at 10 mA cm−2),55 Ru@IrOx (282 mV at 10 mA cm−2),56 amorphous IrO2 (255 mV at 10 mA cm−2),57 Ba2YIrO6 (318 mV at 10 mA cm−2),58 Ir-STO (295 mV at 10 mA cm−2),59 and RuNi2@G-250 (210 mV at 10 mA cm−2)60 (Fig. 4c and Table S5†). In addition, the exchange current density (j0) of Ru SAs- CoOx (0.033 mA cm−2) could be evaluated by extrapolating the Tafel slope (Fig. S25†), which was much higher than those of pristine Co3O4 (0.004 mA cm−2) and commercial RuO2 (0.012 mA cm−2), demonstrating the fast electron transfer rate between the electrode and Ru SAs-CoOx catalyst surface.We further investigated the electrochemical surface area (ECSA) of Ru SAs-CoOx by testing the double layer capacitances (Cdl). Compared with those of pristine Co3O4 (0.5 mF cm−2) and RuO2 (14.5 mF cm−2), the larger Cdl value (18.6 mF cm−2) suggested that more electrochemical surface area was generated (Fig. S26†). Considering that the changes in morphology would impact the activity, different elements (Mn, Fe, and Ni) were introduced to explore their morphologies and catalytic activity. As shown in Fig. S27–S29,† after the introduction of other different elements, the morphologies all transformed into the nanosheet structure. However, there was no observed enhancement in the catalytic activity. This could be attributed to the fact that compared to these 3d transition metals, Ru exhibited stronger capacity for H2O dissociation, which benefited the generation of OH* species, thereby facilitating the generation of abundant OH* species and subsequently enhancing catalytic activity. Therefore, the introduction of Ru was primarily responsible for the observed enhancement in catalytic activity. Turnover frequencies (TOFs) as another important parameter could be applied to evaluate the intrinsic activity per site of the catalyst.13,44,61 Although many methods have been applied to estimate the number of active sites, accurate estimation of the TOF is still a challenge. Here, the lower limit TOF of the Ru SAs-CoOx and RuO2 electrodes was estimated by making an approximation that all metal centers in the catalysts are available for the OER.62–64 As displayed in Fig. 4d, the lower limit TOF value of Ru SAs-CoOx at 1.5 V is as high as 0.02 s−1, 7.7 times higher than that of RuO2. The faradaic efficiency of nearly 100% for the OER on Ru SAs-CoOx was evaluated by the water drainage method, indicating excellent selectivity for oxygen generation (Fig. S30†).
Durability is an important parameter to evaluate the OER performance in acidic media. Chronopotentiometry tests were conducted at a current density of 10 mA cm−2. As revealed in Fig. 4e, Ru SAs-CoOx displayed a slight potential change from 1.485 to 1.494 V after 25 hours, which was far better than that of RuO2 (1.556 V to 1.623 V, aligned with the trend of commercial RuO2 in the reported literature studies.6,9) and pristine Co3O4. Meanwhile, the chronoamperometric curves of Ru SAs-CoOx, RuO2 and pristine Co3O4 were also recorded at the same initial current density (10 mA cm−2). The results indicated that the current retention of Ru SAs-CoOx after 20 hours was 77.3%, which was superior to RuO2 (43.3%) and pristine Co3O4 (10.6%) (Fig. S31†). Meanwhile, the durability of Ru SAs-CoOx was also compared with previously reported Co3O4 electrocatalysts, which also exhibited a significantly better durability (Table S6†). After the durability test, the morphology, electronic structure, and composition of the catalysts were studied. As displayed in Fig. S32–S34,† HRTEM and EDS results confirmed that the Ru SAs-CoOx still maintained a porous structure and all the elements (Ru, Co and O) could be detected. Meanwhile, a slight positive shift was observed in Co 2p and O 1s spectra, confirming an increase in valence state at oxidative potentials. This shift can be attributed to the oxidative conditions during the OER process.65,66 The XRD pattern revealed that the Co3O4 phase of Ru SAs-CoOx was well-maintained, although the peak intensity decreased obviously. This decrease was likely due to the surface composition of Ru SAs-CoOx partially transforming into amorphous species. In addition, inductively coupled plasma mass spectrometry (ICP-MS) was used to monitor the catalyst dissolution rate during chronopotentiometry tests at 10 mA cm−2 in 0.1 M HClO4 solution. The results, as indicated in Table S7,† revealed very low rates of Ru and Co dissociation.
Owing to its excellent OER activity, an acidic electrolyzer was assembled using Ru SAs-CoOx as the anode and commercial Pt/C as the cathode. As shown in Fig. 4f, the Pt/C//Ru SAs-CoOx couple required only 1.44 V to achieve a current density of 10 mA cm−2, lower than that of the Pt/C//RuO2 couple (1.57 V at 10 mA cm−2) and Pt/C//Co3O4 couple (1.70 V at 10 mA cm−2). To sum up, compared with pristine Co3O4 and RuO2, Ru SAs-CoOx exhibited overwhelming advantages for the OER from the aspects of overpotential, Tafel slope, durability, water splitting, and Cdl (Fig. 4g).
Understanding the reaction mechanism
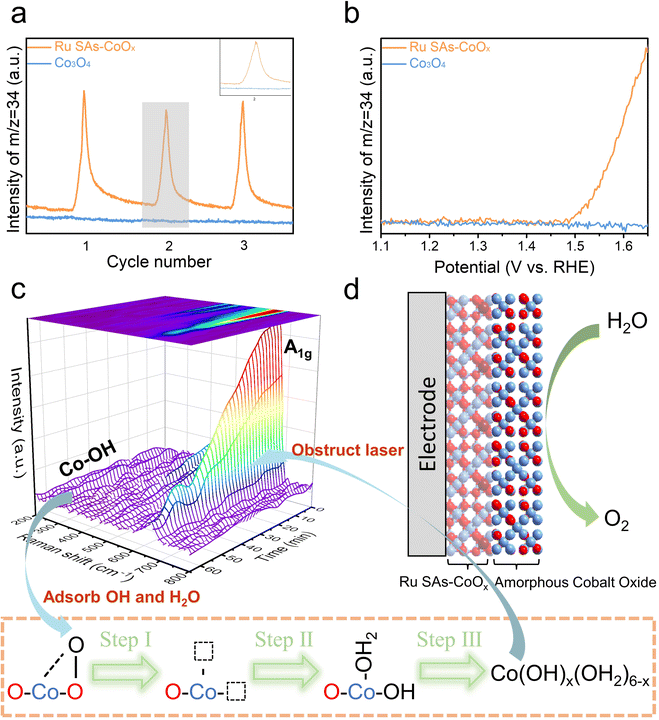
To rationalize the enhanced OER activity and durability of Ru SAs-CoOx, the reaction mechanism was explored. In situ18O isotope labeling DEMS was used to validate the participation of the LOM during the OER process (Fig. 5a and b). The signals for the mass-to-charge ratio (m/z) = 34 were detected in gas production, which included the 18O in the lattice and 16O in the water,67 confirming the successful 18O labeling and the involvement of the LOM during the OER process (Fig. S35†).34,68 In contrast, the signals of 34O2 for pristine Co3O4 were not detected, demonstrating that the low Co–O covalency and less Ov concentration of Co3O4 would inhibit the LOM process. Therefore, the DEMS measurement confirmed the doping of Ru to be the switch for the transformation of the OER mechanism from the conventional AEM to the LOM.To unveil the local environment and understand the reaction mechanisms of Ru SAs-CoOx during the OER process, in situ Raman spectroscopy was performed as well. Fig. 5c exhibits the Raman spectra of Ru SAs-CoOx recorded from 0 to 60 min at 1.5 V. Three characteristic peaks corresponding to the Eg (∼465 cm−1), F2g (∼505 cm−1), and A1g (∼660 cm−1) of Co3O4 spinel oxides were detected. With the increase in the OER time, a new signal corresponding to the chemical bonds between adsorbed OH and Co atoms in the octahedral Co–OH gradually emerged at ∼250 cm−1 (Fig. S36†),69,70 suggesting the formation of amorphous Co–OH due to the existence of interim oxygen vacancies during consecutive vacancy filling/lattice oxygen migration in the LOM cycles. Thus, these vacancies could be easily occupied by OH and H2O to form Co(OH)x(H2O)6−x, which would slowly degrade into amorphous cobalt oxide. As a result, the intensity of all the peaks decreased substantially, and the full width at half maximum (FWHM) peak shape was also broadened because the growth of amorphous cobalt oxide obstructed the focal point of the laser.19,71 Based on the above results, although the durability was maintained well during the OER process, the surface structure of Ru SAs-CoOx during the OER process has gradually transformed into an amorphous cobalt oxide layer (Fig. 5d).
DFT calculations were also carried out to shed light on the enhanced catalytic performance of Ru SAs-CoOx. The related models are constructed based on the experimental analysis. Then the Gibbs free energy of the elementary steps during the OER process is calculated, respectively. First, the traditional AEM pathway is considered for pristine Co3O4 and Ru SAs-CoOx. Since vacancy filling/lattice oxygen migration is not involved in the AEM pathway, the oxygen vacancies are pre-created in the established model (Fig. S37–S39†). As shown in Fig. S40,† for the DFT results of the AEM pathway, the Co and Ru sites display a much higherenergy barrier than that of the Co site on pristine Co3O4. This finding contradicts the observed enhanced catalyticactivity of Ru SAs-CoOx compared to that of pristine Co3O4. Then, the Gibbs free energy based on the LOM pathway was calculated. In this pathway, the oxygen vacancies are not pre-created in the established models because vacancy filling/lattice oxygen migration is involved in the LOM process. As exhibited in Fig. 6a–b and S41–S42,† the Ru site on Ru SAs-CoOx cannot be an effective adsorption/desorption site for the LOM process owing to its too strong interaction with O*. In contrast, on the Co site, the largest change in free energy (not referred to as a potential-determining step due to step 4 being a chemical process without any charge transfer) is the formation of O2 (step 4) and the corresponding free energy is 0.62 eV, significantly smaller than that of the AEM pathway without (1.04 eV)/with (1.42 eV) oxygen vacancies. It can be observed that the largest change in free energy of Ru SAs-CoOx during the OER process is the formation of O2, which is a chemical step without any charge transfer (Fig. S43–S44†). This finding was consistent with the Tafel slope analysis and suggested the presence of a mixed kinetic control mechanism.52,54 Furthermore, an advanced activity descriptor Gmax(η) was also utilized to comprehensively evaluate their activity trends. This descriptor identifies the largest free-energy span (uphill in free energy) among all intermediates within the catalytic process, considering both the electrochemical and chemical steps.72,73 As shown in Fig. S44,† when U = 1.23 V, the corresponding Gmax for the LOM process (1.24 eV) is close to (less than 0.2 eV different from each other) that of the AEM process without oxygen vacancies (1.10 eV), while being smaller than that of the AEM process with oxygen vacancies (1.96 eV). Therefore, according to these above theoretical results, we assume that the LOM mechanism is operational during the OER process on Ru SAs-CoOx. However, the actual OER process is complex, so the triggering of the LOM process does not imply the elimination of the AEM process. The enhanced catalytic activity contributed to the synergistic effect of AEM and LOM. Considering that a small portion of Ru was substituted for the Oh site of Co3O4, the free energy diagrams for the OER on the Co and Ru site of Ru SAs-CoOx (with Ru replacing Oh sites) based on the LOM were also calculated. The results showed that on the model of Ru SAs-CoOx (with Ru replacing Oh sites), both the Ru (1.13 eV) and Co sites (1.33 eV) displayed a much higher energy barrier than the Co site of Ru SAs-CoOx (with Ru replacing Td sites). Therefore, the Co and Ru site of Ru SAs-CoOx (with Ru replacing Oh sites) cannot be the effective adsorption/desorption site for the LOM process (Fig. S45–S47†).
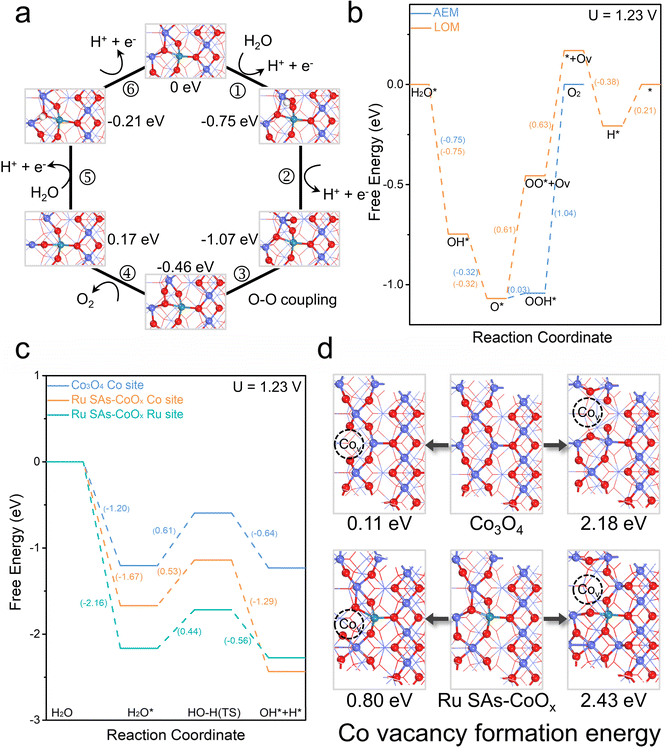 | ||
| Fig. 6 Theoretical investigations. (a) Schematic illustration of the proposed OER mechanism based on the LOM process. (b) Calculated free energy diagrams for the OER on the Co site of Ru SAs-CoOx (without oxygen vacancies) based on the AEM (corresponding to Fig. S34†) and LOM. (c) Calculated free energy diagram for H2O-adsorption and H2O-dissociation on different active sites. (d) Calculated Co vacancy formation energy on Co3O4 and Ru SAs-CoOx. | ||
In addition, considering that the OH* coverage on the catalyst surface in acidic media is comparatively low, the energy barrier for the H2O-dissociation is calculated to offer a side reflection of OH* formation and coverage.74 As shown in Fig. 6c and S48–S50,† the Co site on Ru SAs-CoOx displays a much lower energy barrier (0.53 eV) for H2O-dissociation compared to that of pristine Co3O4 (0.61 eV) as the Ru site improves the H2O-dissociation ability on the Co site nearby and provides more OH* species for the lattice oxygen oxidation. Therefore, although Ru centers cannot be the effective adsorption/desorption site for OER processes, the significance of Ru should not be disregarded. Specifically, the active sites should be derived from the “Ru–O–Co” structural unit (Co: optimal adsorption/desorption sites. Ru: improve the H2O-dissociation ability on the lattice oxygen site and provide plentiful OH* species for the subsequent oxidation process; increase the Co–O covalency and facilitate the direct O–O coupling. O: directly involved in O2 evolution).
Additionally, an acknowledged drawback that DFT computations have limitations in describing the surface structure must be pointed out. In this study, only the (311) facet of Co3O4 was selected as the model, which simplified the complex structure of the Ru SAs-CoOx electrocatalyst. In addition, the surface structure of the catalyst has changed during the OER process. However, the DFT simulations here still provide a wide understanding of why the electrocatalytic activity of Ru SAs-CoOx improved compared with that of Co3O4.
To understand the enhanced durability of the prepared Ru SAs-CoOx, the following two aspects were discussed. (1) Static durability: as displayed in Fig. 6d, the Co vacancy formation energy of Td and Oh sites at Ru SAs-CoOx (Td: 0.80 eV and Oh: 2.43 eV) is higher than that of pristine Co3O4 (Td: 0.11 eV and Oh: 2.18 eV), indicating a thermodynamically less favorable dissolution of Co from Ru SAs-CoOx. Here, the Co vacancy formation energy is calculated at 0 k in a vacuum environment. The real experimental environment involves factors such as temperature, voltage, solvent, etc., which makes it easier to produce vacancies in the actual environment. Therefore, although the DFT results indicate that Co vacancy formation energy of Ru SAs-CoOx and pristine Co3O4 is not thermodynamically favorable (formation energy > 0), it can be inferred from the trend of formation energy that the introduction of Ru enhances the static durability of Co. (2) Dynamic durability: direct O–O coupling mitigates the unstable reconstruction process before the formation of the OO bond, thereby extending the working lifetime of the electrocatalyst.
Conclusion
In this work, we reported a Ru SAs-CoOx catalyst to overcome the balance between catalytic activity and dynamic durability for acidic OER via the preferential substitution of Ru for the Co2+ (Td) site of Co3O4. Consequently, the overpotential at 10 mA cm−2 of as-prepared Co3O4 (395 mV) sharply decreases to 188 mV by doping a small amount of Ru atoms (5.16 wt%), and its long-term durability is also prolonged. Combined with in situ measurement and DFT calculation, we hypothesized that the boosted activity and durability originated from the enhanced OH* coverage and Co–O covalency of Co3O4 after the introduction of Ru, which provided more OH* species for the OER process and mitigated the unstable reconstruction process. In addition, the obtained Ru SAs-CoOx is superior to commercial RuO2 and IrO2 in price (1/3 of RuO2 and 1/15 of IrO2, respectively). We believe these findings will not only provide a guideline for the design of more efficient and stable catalysts but also pave a new avenue for the further exploration of the active site structure and catalytic mechanisms.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
J. Z., X. F., and H. S. conceived the project. J. Z. performed the experiments. G. C., D. S., Y. T. and W. X. offered help to analyze and discuss the experiment data. J. Z. wrote the manuscript. G. C., X. F., and H. S. gave helpful advice on the manuscript preparation. X. F. and H. S. co-supervised the project. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the Development Program of China (2021YFA1502700), the National Natural Science Foundation of China (22232004, 22209076 and 22279062), the National Key Research and, the Natural Science Foundation of Jiangsu Province (Grant No. BK 20220369), the European Union's Horizon 2020 research, innovation programme (GrapheneCore3: 881603), Deutsche Forschungsgemeinschaft within the Cluster of Excellence, and CRC 1415 (Grant No. 417590517). H. S is thankful for the financial support from Jiangsu Specially Appointed Professorship.References
- H. N. Nong, L. J. Falling, A. Bergmann, M. Klingenhof, H. P. Tran, C. Spöri, R. Mom, J. Timoshenko, G. Zichittella, A. Knop-Gericke, S. Piccinin, J. Pérez-Ramírez, B. R. Cuenya, R. Schlögl, P. Strasser, D. Teschner and T. E. Jones, Nature, 2020, 587, 408–413 CrossRef CAS PubMed
.
- X. Wang, S. Xi, P. Huang, Y. Du, H. Zhong, Q. Wang, A. Borgna, Y. Zhang, Z. Wang, H. Wang, Z. G. Yu, W. S. V. Lee and J. Xue, Nature, 2022, 611, 702–708 CrossRef CAS
.
- P. Zhai, M. Xia, Y. Wu, G. Zhang, J. Gao, B. Zhang, S. Cao, Y. Zhang, Z. Li, Z. Fan, C. Wang, X. Zhang, J. T. Miller, L. Sun and J. Hou, Nat. Commun., 2021, 12, 4587 CrossRef CAS PubMed
.
- D. H. Kweon, M. S. Okyay, S.-J. Kim, J.-P. Jeon, H.-J. Noh, N. Park, J. Mahmood and J.-B. Baek, Nat. Commun., 2020, 11, 1278 CrossRef CAS PubMed
.
- Y. Pan, X. Xu, Y. Zhong, L. Ge, Y. Chen, J.-P. M. Veder, D. Guan, R. O'Hayre, M. Li, G. Wang, H. Wang, W. Zhou and Z. Shao, Nat. Commun., 2020, 11, 2002 CrossRef CAS PubMed
.
- K. Wang, Y. Wang, B. Yang, Z. Li, X. Qin, Q. Zhang, L. Lei, M. Qiu, G. Wu and Y. Hou, Energy Environ. Sci., 2022, 15, 2356–2365 RSC
.
- C.-Z. Yuan, S. Wang, K. San Hui, K. Wang, J. Li, H. Gao, C. Zha, X. Zhang, D. A. Dinh, X.-L. Wu, Z. Tang, J. Wan, Z. Shao and K. N. Hui, ACS Catal., 2023, 13, 2462–2471 CrossRef CAS
.
- Y. Lin, Y. Dong, X. Wang and L. Chen, Adv. Mater., 2023, 35, 2210565 CrossRef CAS
.
- H. Jin, X. Liu, P. An, C. Tang, H. Yu, Q. Zhang, H.-J. Peng, L. Gu, Y. Zheng, T. Song, K. Davey, U. Paik, J. Dong and S.-Z. Qiao, Nat. Commun., 2023, 14, 354 CrossRef CAS
.
- M. F. Lagadec and A. Grimaud, Nat. Mater., 2020, 19, 1140–1150 CrossRef CAS
.
- L. Li, P. Wang, Q. Shao and X. Huang, Adv. Mater., 2021, 33, 2004243 CrossRef CAS
.
- Y. Wang, R. Yang, Y. Ding, B. Zhang, H. Li, B. Bai, M. Li, Y. Cui, J. Xiao and Z.-S. Wu, Nat. Commun., 2023, 14, 1412 CrossRef CAS
.
- J. Kim, P. C. Shih, K. C. Tsao, Y. T. Pan, X. Yin, C. J. Sun and H. Yang, J. Am. Chem. Soc., 2017, 139, 12076–12083 CrossRef CAS
.
- Y. Qin, T. Yu, S. Deng, X. Y. Zhou, D. Lin, Q. Zhang, Z. Jin, D. Zhang, Y. B. He, H. J. Qiu, L. He, F. Kang, K. Li and T. Y. Zhang, Nat. Commun., 2022, 13, 3784 CrossRef CAS PubMed
.
- Y. Lin, Z. Tian, L. Zhang, J. Ma, Z. Jiang, B. J. Deibert, R. Ge and L. Chen, Nat. Commun., 2019, 10, 162 CrossRef PubMed
.
- X. Liang, L. Shi, Y. Liu, H. Chen, R. Si, W. Yan, Q. Zhang, G. D. Li, L. Yang and X. Zou, Angew. Chem., Int. Ed., 2019, 58, 7631–7635 CrossRef CAS
.
- R. Zhang, N. Dubouis, M. Ben Osman, W. Yin, M. T. Sougrati, D. A. D. Corte, D. Giaume and A. Grimaud, Angew. Chem., Int. Ed., 2019, 58, 4571–4575 CrossRef CAS
.
- I. C. Man, H.-Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS
.
- K. Natarajan, E. Munirathinam and T. C. K. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 27140–27148 CrossRef CAS
.
- J. Huang, H. Sheng, R. D. Ross, J. Han, X. Wang, B. Song and S. Jin, Nat. Commun., 2021, 12, 3036 CrossRef CAS
.
- Z. Liu, G. Wang, X. Zhu, Y. Wang, Y. Zou, S. Zang and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 4736–4742 CrossRef CAS PubMed
.
- N. Wang, P. Ou, R. K. Miao, Y. Chang, Z. Wang, S.-F. Hung, J. Abed, A. Ozden, H.-Y. Chen, H.-L. Wu, J. E. Huang, D. Zhou, W. Ni, L. Fan, Y. Yan, T. Peng, D. Sinton, Y. Liu, H. Liang and E. H. Sargent, J. Am. Chem. Soc., 2023, 145, 7829–7836 CrossRef CAS
.
- F.-Y. Chen, Z.-Y. Wu, Z. Adler and H. Wang, Joule, 2021, 5, 1704–1731 CrossRef CAS
.
- X. Wang, H. Zhong, S. Xi, W. S. V. Lee and J. Xue, Adv. Mater., 2022, 34, 2107956 CrossRef CAS
.
- L. Li, P. Wang, Q. Shao and X. Huang, Adv. Mater., 2021, 33, 2004243 CrossRef CAS
.
- J. Rossmeisl, Z. W. Qu, H. Zhu, G. J. Kroes and J. K. Nørskov, J. Electroanal. Chem., 2007, 607, 83–89 CrossRef CAS
.
- A. Grimaud, O. Diaz-Morales, B. Han, W. T. Hong, Y.-L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper and Y. Shao-Horn, Nat. Chem., 2017, 9, 457–465 CrossRef CAS PubMed
.
- Z.-F. Huang, J. Song, Y. Du, S. Xi, S. Dou, J. M. V. Nsanzimana, C. Wang, Z. J. Xu and X. Wang, Nat. Energy, 2019, 4, 329–338 CrossRef CAS
.
- J. Hwang, R. R. Rao, L. Giordano, Y. Katayama, Y. Yu and Y. Shao-Horn, Science, 2017, 358, 751–756 CrossRef CAS
.
- E. Fabbri and T. J. Schmidt, ACS Catal., 2018, 8, 9765–9774 CrossRef CAS
.
- Z. Shi, J. Li, Y. Wang, S. Liu, J. Zhu, J. Yang, X. Wang, J. Ni, Z. Jiang, L. Zhang, Y. Wang, C. Liu, W. Xing and J. Ge, Nat. Commun., 2023, 14, 843 CrossRef CAS PubMed
.
- A. Grimaud, A. Demortière, M. Saubanère, W. Dachraoui, M. Duchamp, M.-L. Doublet and J.-M. Tarascon, Nat. Energy, 2016, 2, 16189 CrossRef
.
- H. Shin, H. Xiao and W. A. Goddard III, J. Am. Chem. Soc., 2018, 140, 6745–6748 CrossRef CAS PubMed
.
- A. Grimaud, O. Diaz-Morales, B. Han, W. T. Hong, Y. L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper and Y. Shao-Horn, Nat. Chem., 2017, 9, 457–465 CrossRef CAS PubMed
.
- J. B. Goodenough, Chem. Mater., 2014, 26, 820–829 CrossRef CAS
.
- J. Kim, C.-W. Roh, S. K. Sahoo, S. Yang, J. Bae, J. W. Han and H. Lee, Adv. Energy Mater., 2018, 8, 1701476 CrossRef
.
- J. Zhang, G. Chen, Q. Liu, C. Fan, D. Sun, Y. Tang, H. Sun and X. Feng, Angew. Chem., Int. Ed., 2022, 61, e202209486 CrossRef CAS PubMed
.
- Q. He, D. Tian, H. Jiang, D. Cao, S. Wei, D. Liu, P. Song, Y. Lin and L. Song, Adv. Mater., 2020, 32, e1906972 CrossRef
.
- C. Geng, D. Rathore, D. Heino, N. Zhang, I. Hamam, N. Zaker, G. A. Botton, R. Omessi, N. Phattharasupakun, T. Bond, C. Yang and J. R. Dahn, Adv. Energy Mater., 2022, 12, 2103067 CrossRef CAS
.
- M. Moses-DeBusk, M. Yoon, L. F. Allard, D. R. Mullins, Z. Wu, X. Yang, G. Veith, G. M. Stocks and C. K. Narula, J. Am. Chem. Soc., 2013, 135, 12634–12645 CrossRef CAS
.
- S. Yang, J. Kim, Y. J. Tak, A. Soon and H. Lee, Angew. Chem., Int. Ed., 2016, 55, 2058–2062 CrossRef CAS PubMed
.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634–641 CrossRef CAS
.
- J. B. Pan, B. H. Wang, J. B. Wang, H. Z. Ding, W. Zhou, X. Liu, J. R. Zhang, S. Shen, J. K. Guo, L. Chen, C. T. Au, L. L. Jiang and S. F. Yin, Angew. Chem., Int. Ed., 2021, 60, 1433–1440 CrossRef CAS
.
- Z. Xiao, Y. Wang, Y.-C. Huang, Z. Wei, C.-L. Dong, J. Ma, S. Shen, Y. Li and S. Wang, Energy Environ. Sci., 2017, 10, 2563–2569 RSC
.
- L. Xu, Q. Jiang, Z. Xiao, X. Li, J. Huo, S. Wang and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 5277–5281 CrossRef CAS
.
- B. Guo, R. Ma, Z. Li, J. Luo, M. Yang and J. Wang, Mater. Chem. Front., 2020, 4, 1390–1396 RSC
.
- W. I. Choi, S. Choi, M. Balamurugan, S. Park, K. H. Cho, H. Seo, H. Ha and K. T. Nam, ACS Omega, 2023, 8, 35034–35043 CrossRef CAS PubMed
.
- P. Li, M. Wang, X. Duan, L. Zheng, X. Cheng, Y. Zhang, Y. Kuang, Y. Li, Q. Ma, Z. Feng, W. Liu and X. Sun, Nat. Commun., 2019, 10, 1711 CrossRef PubMed
.
- J. Shan, C. Ye, S. Chen, T. Sun, Y. Jiao, L. Liu, C. Zhu, L. Song, Y. Han, M. Jaroniec, Y. Zhu, Y. Zheng and S.-Z. Qiao, J. Am. Chem. Soc., 2021, 143, 5201–5211 CrossRef CAS PubMed
.
- Z. Zhang, C. Feng, D. Wang, S. Zhou, R. Wang, S. Hu, H. Li, M. Zuo, Y. Kong, J. Bao and J. Zeng, Nat. Commun., 2022, 13, 2473 CrossRef CAS PubMed
.
- Y. Xue, J. Fang, X. Wang, Z. Xu, Y. Zhang, Q. Lv, M. Liu, W. Zhu and Z. Zhuang, Adv. Funct. Mater., 2021, 31, 2101405 CrossRef CAS
.
- E. Tsuji, A. Imanishi, K.-i. Fukui and Y. Nakato, Electrochim. Acta, 2011, 56, 2009–2016 CrossRef CAS
.
- L. A. De Faria, J. F. C. Boodts and S. Trasatti, J. Appl. Electrochem., 1996, 26, 1195–1199 CrossRef CAS
.
- J. O. M. Bockris, J. Chem. Phys., 1956, 24, 817–827 CrossRef CAS
.
- J. Shan, T. Ling, K. Davey, Y. Zheng and S.-Z. Qiao, Adv. Mater., 2019, 31, 1900510 CrossRef
.
- J. Shan, C. Guo, Y. Zhu, S. Chen, L. Song, M. Jaroniec, Y. Zheng and S.-Z. Qiao, Chem, 2019, 5, 445–459 CAS
.
- G. Wu, X. Zheng, P. Cui, H. Jiang, X. Wang, Y. Qu, W. Chen, Y. Lin, H. Li, X. Han, Y. Hu, P. Liu, Q. Zhang, J. Ge, Y. Yao, R. Sun, Y. Wu, L. Gu, X. Hong and Y. Li, Nat. Commun., 2019, 10, 4855 CrossRef PubMed
.
- O. Diaz-Morales, S. Raaijman, R. Kortlever, P. J. Kooyman, T. Wezendonk, J. Gascon, W. T. Fu and M. T. M. Koper, Nat. Commun., 2016, 7, 12363 CrossRef CAS
.
- X. Liang, L. Shi, Y. Liu, H. Chen, R. Si, W. Yan, Q. Zhang, G.-D. Li, L. Yang and X. Zou, Angew. Chem., Int. Ed., 2019, 58, 7631–7635 CrossRef CAS PubMed
.
- X. Cui, P. Ren, C. Ma, J. Zhao, R. Chen, S. Chen, N. P. Rajan, H. Li, L. Yu, Z. Tian and D. Deng, Adv. Mater., 2020, 32, 1908126 CrossRef CAS PubMed
.
- Y. L. Wu, X. Li, Y. S. Wei, Z. Fu, W. Wei, X. T. Wu, Q. L. Zhu and Q. Xu, Adv. Mater., 2021, 33, e2006965 CrossRef
.
- L. Trotochaud, J. K. Ranney, K. N. Williams and S. W. Boettcher, J. Am. Chem. Soc., 2012, 134, 17253–17261 CrossRef CAS PubMed
.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS
.
- M. B. Stevens, L. J. Enman, A. S. Batchellor, M. R. Cosby, A. E. Vise, C. D. M. Trang and S. W. Boettcher, Chem. Mater., 2017, 29, 120–140 CrossRef CAS
.
- M. Favaro, J. Yang, S. Nappini, E. Magnano, F. M. Toma, E. J. Crumlin, J. Yano and I. D. Sharp, J. Am. Chem. Soc., 2017, 139, 8960–8970 CrossRef CAS
.
- Y. Zhu, J. Wang, T. Koketsu, M. Kroschel, J. M. Chen, S. Y. Hsu, G. Henkelman, Z. Hu, P. Strasser and J. Ma, Nat. Commun., 2022, 13, 7754 CrossRef CAS PubMed
.
- Y. Wen, P. Chen, L. Wang, S. Li, Z. Wang, J. Abed, X. Mao, Y. Min, C. T. Dinh, P. D. Luna, R. Huang, L. Zhang, L. Wang, L. Wang, R. J. Nielsen, H. Li, T. Zhuang, C. Ke, O. Voznyy, Y. Hu, Y. Li, W. A. Goddard Iii, B. Zhang, H. Peng and E. H. Sargent, J. Am. Chem. Soc., 2021, 143, 6482–6490 CrossRef CAS
.
- Z. Shi, Y. Wang, J. Li, X. Wang, Y. Wang, Y. Li, W. Xu, Z. Jiang, C. Liu, W. Xing and J. Ge, Joule, 2021, 5, 2164–2176 CrossRef CAS
.
- R. Zhang, L. Pan, B. Guo, Z. F. Huang, Z. Chen, L. Wang, X. Zhang, Z. Guo, W. Xu, K. P. Loh and J. J. Zou, J. Am. Chem. Soc., 2023, 145, 2271–2281 CrossRef CAS
.
- S. R. Shieh and T. S. Duffy, Phys. Rev. B, 2002, 66, 134301 CrossRef
.
- C. Pasquini, L. D'Amario, I. Zaharieva and H. Dau, J. Chem. Phys., 2020, 152, 194202 CrossRef CAS
.
- K. S. Exner, ACS Catal., 2020, 10, 12607–12617 CrossRef CAS
.
- S. Razzaq and K. S. Exner, ACS Catal., 2023, 13, 1740–1758 CrossRef CAS
.
- J. Yin, J. Jin, M. Lu, B. Huang, H. Zhang, Y. Peng, P. Xi and C.-H. Yan, J. Am. Chem. Soc., 2020, 142, 18378–18386 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc05547k |
| This journal is © The Royal Society of Chemistry 2024 |