Open Access Article
Open Access ArticleAn efficient all-visible light-activated photoswitch based on diarylethenes and CdS quantum dots†
Kezhou
Chen
ab,
Jiayi
Liu
ab,
Joakim
Andréasson
 c,
Bo
Albinsson
c,
Bo
Albinsson
 *c,
Tiegen
Liu
*ab and
Lili
Hou
*c,
Tiegen
Liu
*ab and
Lili
Hou
 *abc
*abc
aSchool of Precision Instrument and Opto-Electronics Engineering, Tianjin University, Tianjin 300072, China. E-mail: lilihou@tju.edu.cn
bKey Laboratory of Opto-electronics Information Technology (Tianjin University), Tianjin 300072, China
cDepartment of Chemistry and Chemical Engineering, Chalmers University of Technology, Gothenburg 412 96, Sweden
First published on 13th November 2024
Abstract
All-visible light-activated diarylethene (DAE) photoswitches are highly attractive for applications in smart photoresponsive materials. The photocyclization of DAE via the low-lying excited triplet state through triplet energy transfer (TET) from a sensitizer has been proven to be an effective approach for the realization of this scheme. However, the TET process is sensitive to oxygen and typically requires more than one sensitizer per photoswitch to facilitate sensitized photocyclization. Herein, we present a bi-component system comprising carboxylic acid-functionalized DAEs and CdS quantum dots (QDs) to achieve all-visible light-activated photoswitching. Due to the large surface area-to-volume ratio of CdS QDs and surface anchored DAEs, one CdS QD can activate at least 18 DAE molecules in the solution without oxygen exclusion. The efficiency of photocyclization of DAEs under visible light irradiation through energy transfer from CdS QDs is nearly comparable to that of direct UV light irradiation. Moreover, our strategy is adaptable for solid-state applications in the presence of air, enabling reversible writing and erasing of color and patterns by adjusting irradiation wavelengths in the visible region.
Introduction
Photoresponsive materials, possessing the ability to react or respond to light stimuli, have been widely applied in various fields such as biological imaging, smart devices, and energy storage.1–3 Among these materials, photochromic molecules, also referred to as molecular photoswitches, have attracted extensive research interest due to their capability of reversibly converting between two states with distinct physical and chemical properties upon irradiation at different light wavelengths.4–7 Diarylethene (DAE) is one of the most commonly used photoswitches, whose ring-open and ring-closed isomers can be reversibly and repeatedly interconverted into each other upon alternating irradiation with UV and visible light.8,9 DAEs exhibit excellent properties, such as high coloration/decoloration contrast and thermal stability, making them ideal photoresponsive materials for sensing, bioimaging, and memory applications.10–14 Photoluminescence switching materials composed of quantum dots (QDs) and DAE have been widely researched.15–17 However, most DAE photoswitches require UV light to trigger the closing reaction, which can result in the formation of by-products during photoisomerization and thus limit the photostability.18 Moreover, UV light has a very poor penetration depth and causes cell damage, which presents obstacles in future biological applications. Therefore, it is highly desired to develop all-visible light-activated DAEs.19One approach to achieving this goal is through a triplet energy transfer (TET) reaction from a sensitizer, by which the photocyclization of DAEs (conversion from the ring-open to the closed isomer) occurs in the triplet excited state (T1).20 The lower energy of T1 compared to the singlet excited state (S1) enables the photocyclization of DAEs using longer excitation wavelengths, and the absence of by-product formation in the T1 state can improve the photostability of DAEs.21–26 Typical metal complexes (Ru, Re, Os, Pt, etc.) and organic triplet sensitizers (biacetyl, perylene, xanthone, etc.) have been used to activate different DAE derivatives upon visible light irradiation through intersystem crossing (ISC) and subsequent TET to DAE.26–32 The strategies to introduce a triplet sensitizer can involve direct mixing or covalent bonding. As the commonly used sensitizers feature a large singlet–triplet energy gap (ΔEST) and weak light absorption in the visible region (low molar absorption coefficients), a large excess (up to 250-fold) of the organic sensitizer is required to induce efficient photoswitching via direct mixing.21 Zhang and co-workers introduced thermally activated delayed fluorescence (TADF) materials, 1,2-bis(carbazol-9-yl)-4,5-dicyanobenzene (2CzPN) and 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN), as sensitizers to achieve efficient visible-light driven isomerization of DAEs.21 The small ΔEST of TADF molecules allows for reducing the ratio between the sensitizer and DAE molecules to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Recently, our group and others introduced colloidal QDs, specifically CdS and perovskite QDs, as sensitizers to achieve all-visible light-activated DAEs.33,34 The amount of sensitizing QDs required can be significantly reduced due to the narrow ΔEST, high molar absorption coefficients, and large surface area-to-volume ratio of QDs. However, a mediator is required to relay the TET from QDs to DAEs. It should be noted that previously, when considering the ratio between the molecular mediator and DAE, a minimum ratio of 3
1. Recently, our group and others introduced colloidal QDs, specifically CdS and perovskite QDs, as sensitizers to achieve all-visible light-activated DAEs.33,34 The amount of sensitizing QDs required can be significantly reduced due to the narrow ΔEST, high molar absorption coefficients, and large surface area-to-volume ratio of QDs. However, a mediator is required to relay the TET from QDs to DAEs. It should be noted that previously, when considering the ratio between the molecular mediator and DAE, a minimum ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was required. Covalent linkage between organic molecules and DAEs is also a significant breakthrough in all-visible light-activated DAEs.31,35–40 By extending the π-conjugation or linking sensitizers, visible light was able to drive the photo-induced ring-closing process of DAE. However, this approach involves complicated synthesis procedures, which reduce the overall appeal. Moreover, most of these triplet sensitized approaches are sensitive to the presence of oxygen due to the inherent characteristics of the long-lived triplet states involved.
1 was required. Covalent linkage between organic molecules and DAEs is also a significant breakthrough in all-visible light-activated DAEs.31,35–40 By extending the π-conjugation or linking sensitizers, visible light was able to drive the photo-induced ring-closing process of DAE. However, this approach involves complicated synthesis procedures, which reduce the overall appeal. Moreover, most of these triplet sensitized approaches are sensitive to the presence of oxygen due to the inherent characteristics of the long-lived triplet states involved.
Herein, we have developed an efficient all-visible light-activated photoswitchable system composed of CdS QDs and carboxylated DAEs (DAE 1), as shown in Scheme 1. In the solution, DAE 1 can dynamically anchor to the surface of CdS QDs through coordination bonding of its carboxylic acid functional groups during collisions as proved by Fourier-transform Infrared (FTIR) spectra (details in the ESI†), resulting in a photoswitchable system where a single CdS QD can efficiently activate at least 18 DAE molecules upon visible light irradiation. Importantly, our all-visible light activated photoswitchable system can be operated under air equilibrated conditions in the presence of oxygen. Moreover, when casting CdS QDs and DAE 1 on paper in the solid state, different patterns can be written and erased using light at 405 nm and 515 nm as external stimuli.
 | ||
| Scheme 1 Schematic illustration of all-visible light activated modulation of the CdS QDs and carboxylated DAE 1 in the open and closed forms. | ||
Results and discussion
Photochromic behaviour
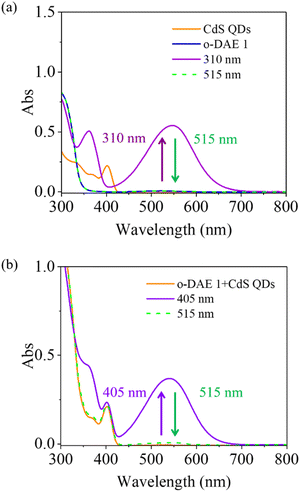
CdS QDs and DAE 1 were synthesized according to previous reports.16,41,42 The Transmission Electron Microscope (TEM) image of CdS QDs reveals a uniform spherical morphology with an average diameter of 3.5–4 nm (Fig. S1a†). The crystallinity of CdS QDs has been investigated by conventional X-ray diffraction (XRD) (see Fig. S1b†). The diffraction peaks of 2θ appearing at ∼26.57° and 43.74° correspond to the (111) and (220) crystal planes of CdS QDs,41 consistent with previously reported CdS QDs, which indicates that CdS QDs have been successfully prepared by the method used. The individual absorption spectra of CdS QDs and DAE 1 in toluene are shown in Fig. 1a. 310 nm (4.5 mW for 16 s) light exposure of the open form of DAE 1 (o-DAE 1, red spectrum) triggers photocyclization to the closed isomer (c-DAE 1, blue spectrum), as evidenced by the appearance of the absorption band in the visible region. Subsequent irradiation with 515 nm light (50 mW for 9 min) recovers the initial absorption spectrum as a result of reverse photoisomerization from c-DAE 1 to o-DAE 1. The first absorption peak of CdS QDs was deliberately chosen to be located within the minimal absorption region of both c-DAE 1 and o-DAE 1 (here at 405 nm). To confirm the reversible all-visible light photoswitching of our designed system, a mixed solution of CdS QDs (0.5 μM) and DAE 1 (50 μM), yielding a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, was initially irradiated at 405 nm followed by exposure to light at 515 nm. The resulting changes in the absorption spectra are shown in Fig. 1b. Upon 405 nm light irradiation, the appearance of the absorption band centered at 540 nm is a clear testament to the photocyclization from o-DAE 1 to c-DAE 1. Since o-DAE 1 exhibits negligible absorption at 405 nm, direct photocyclization by this light can be excluded as shown in Fig. S2.† The observed photocyclization results from light absorption by CdS QDs and subsequent TET to o-DAE 1, which sensitizes the isomerization to c-DAE 1. Further irradiation of the mixed solution with 515 nm light recovers the initial state to 100%. To investigate the reversibility of the mixed solution, the absorbance at 548 nm of the mixed solution is monitored over five irradiation cycles of alternating 405 nm and 515 nm light (Fig. S7a†). For comparison, the sample of only DAE 1 over five irradiation cycles of alternating 310 nm and 515 nm was measured. Although some fatigue still appears after more than three cycles, it shows improvement compared to under UV light irradiation. We also compare the long-term irradiation stability; two samples were irradiated at 310 nm (only o-DAE 1) and 405 nm (CdS QDs + o-DAE 1), and the changes in absorbance at 548 nm over 210 s were monitored (Fig. S7b†). It shows that the absorbance of o-DAE 1 decreased to 87% of its maximum value, while that of the mixed solution only reduced 2% over 210 s, demonstrating the enhanced long-term irradiation stability of our approach (Fig. S7b†).
100, was initially irradiated at 405 nm followed by exposure to light at 515 nm. The resulting changes in the absorption spectra are shown in Fig. 1b. Upon 405 nm light irradiation, the appearance of the absorption band centered at 540 nm is a clear testament to the photocyclization from o-DAE 1 to c-DAE 1. Since o-DAE 1 exhibits negligible absorption at 405 nm, direct photocyclization by this light can be excluded as shown in Fig. S2.† The observed photocyclization results from light absorption by CdS QDs and subsequent TET to o-DAE 1, which sensitizes the isomerization to c-DAE 1. Further irradiation of the mixed solution with 515 nm light recovers the initial state to 100%. To investigate the reversibility of the mixed solution, the absorbance at 548 nm of the mixed solution is monitored over five irradiation cycles of alternating 405 nm and 515 nm light (Fig. S7a†). For comparison, the sample of only DAE 1 over five irradiation cycles of alternating 310 nm and 515 nm was measured. Although some fatigue still appears after more than three cycles, it shows improvement compared to under UV light irradiation. We also compare the long-term irradiation stability; two samples were irradiated at 310 nm (only o-DAE 1) and 405 nm (CdS QDs + o-DAE 1), and the changes in absorbance at 548 nm over 210 s were monitored (Fig. S7b†). It shows that the absorbance of o-DAE 1 decreased to 87% of its maximum value, while that of the mixed solution only reduced 2% over 210 s, demonstrating the enhanced long-term irradiation stability of our approach (Fig. S7b†).
The reversible process demonstrates that a single CdS QD can achieve reversible photoswitching of multiple DAE 1 molecules upon exposure to visible light at different wavelengths. It is worth emphasizing that our measurements were conducted under normal atmospheric conditions without excluding oxygen, which simplifies sample preparation and measurements and facilitates further applications.
To gain comprehensive insight into the efficiency of our all-visible light driven system, we investigated four samples with varying molar ratios between CdS QDs and DAE 1. The concentration of CdS QDs remained constant at 0.5 μM, whereas the relative ratio of DAE 1 to CdS QDs was set to 20, 50, 100, and 200. The main photoswitching properties, including the photostationary state (PSS) and photoisomerization quantum yields (QYs), are summarized in Table 1. For ease of comparison, the photochemical properties of also DAE 1 alone are included in Table 1. Upon direct photocyclization of DAE 1 using UV light, the PSS consists of 90% c-DAE 1 and 10% o-DAE 1.16 When CdS QDs were introduced with 20 equivalents of DAE 1 together with irradiation at 405 nm, an identical conversion rate of 90% c-DAE 1 and 10% o-DAE 1 was observed at the PSS. It should be noted that one CdS QD can sensitize the photocyclization of 18 DAE 1 molecules using this relative ratio. Although the fraction of photoconverted o-DAE 1 to yield c-DAE 1 gradually decreases as the relative amount of DAE 1 in the mixture increases, the absolute number of formed c-DAE 1 molecules per CdS QD is increased. In the case of CdS QDs and DAE 1 at a concentration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, a 44% conversion indicates that one CdS QD can isomerize 88 DAE 1 molecules to the closed form (Table 1). The increased concentration of DAE 1 in the mixture implies a larger number of molecules actively engaging in dynamic anchoring to the surface of QDs, allowing a single CdS QD to sensitize more DAEs. At the same time, as a larger fraction of DAE 1 molecules remain free in solution, the overall fraction of DAE 1 bound to CdS QDs is reduced. Without efficient TET to this fraction, these molecules are more prone to photocycloreversion to yield o-DAE 1 by direct absorption of the 405 nm light. To underscore the merit of our simple direct mixing approach, a CdS QD-DAE sample was prepared using the ligand exchange method (details in the ESI†). Although the ligand exchange sample also undergoes all-visible light-activated photoswitching as shown in Fig. S3,† the sensitized DAE 1 molecule per QD in this approach is 9, significantly lower than those achieved through the method of direct mixing.
200, a 44% conversion indicates that one CdS QD can isomerize 88 DAE 1 molecules to the closed form (Table 1). The increased concentration of DAE 1 in the mixture implies a larger number of molecules actively engaging in dynamic anchoring to the surface of QDs, allowing a single CdS QD to sensitize more DAEs. At the same time, as a larger fraction of DAE 1 molecules remain free in solution, the overall fraction of DAE 1 bound to CdS QDs is reduced. Without efficient TET to this fraction, these molecules are more prone to photocycloreversion to yield o-DAE 1 by direct absorption of the 405 nm light. To underscore the merit of our simple direct mixing approach, a CdS QD-DAE sample was prepared using the ligand exchange method (details in the ESI†). Although the ligand exchange sample also undergoes all-visible light-activated photoswitching as shown in Fig. S3,† the sensitized DAE 1 molecule per QD in this approach is 9, significantly lower than those achieved through the method of direct mixing.
CdS QDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DAE 1 DAE 1 |
PSS | n | Φ c–o | Φ o–c |
|---|---|---|---|---|
| a Irradiation at 405 nm. b Irradiation at 310 nm. c Irradiation at 515 nm. d The number of c-DAE 1 formed per CdS QD. e Photocycloreversion QYs. f Photocyclization QYs. | ||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
90%a | 18 | 0.014c | 0.20a |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
75%a | 37 | 0.011c | 0.29a |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
61%a | 61 | 0.012c | 0.30a |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
44%a | 88 | 0.014c | 0.34a |
| DAE 1 only | 90%a | 0.012c | 0.40b | |
In order to quantify the efficiency of visible light control, the photoisomerization QYs of DAE 1 mixed with CdS QDs under 405 nm and 515 nm were determined by using ferrioxalate and Aberchrome 670 actinometers according to previous reports43–46 (see the ESI† for more details). When the concentration of DAE 1 in the CdS QD solution increased, the photocyclization QYs of DAE 1 (Φo–c) under 405 nm light irradiation improved from 20% (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to 34% (molar ratio 1
20) to 34% (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), approaching that of DAE 1 alone under direct exposure to 310 nm light (40%). The enhanced Φo–c is ascribed to the increased TET efficiency as more DAE molecules dynamically attach to the CdS QDs (vide infra and Table S1†). However, the energy transfer efficiency no longer improves once DAE 1 saturates the surface coverage. When the molar ratio of CdS QD/DAE reaches 1
200), approaching that of DAE 1 alone under direct exposure to 310 nm light (40%). The enhanced Φo–c is ascribed to the increased TET efficiency as more DAE molecules dynamically attach to the CdS QDs (vide infra and Table S1†). However, the energy transfer efficiency no longer improves once DAE 1 saturates the surface coverage. When the molar ratio of CdS QD/DAE reaches 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, the lifetime and energy transfer efficiency are identical to those at a 1
400, the lifetime and energy transfer efficiency are identical to those at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 ratio (Table S1†). We also investigated the photocycloreversion QYs of DAE 1 (Φc–o) upon 515 nm light irradiation, and it was found that the presence of CdS QDs had no impact on the efficiency of the back conversion from the ring-closed to the ring-open form of DAE 1. This underscores another advantage of the direct mixing approach: it does not affect the ring-opening reaction, which typically is substantially suppressed in previously reported examples relying on covalent attachment and conjugation extension.39,47,48
200 ratio (Table S1†). We also investigated the photocycloreversion QYs of DAE 1 (Φc–o) upon 515 nm light irradiation, and it was found that the presence of CdS QDs had no impact on the efficiency of the back conversion from the ring-closed to the ring-open form of DAE 1. This underscores another advantage of the direct mixing approach: it does not affect the ring-opening reaction, which typically is substantially suppressed in previously reported examples relying on covalent attachment and conjugation extension.39,47,48
Mechanistic considerations
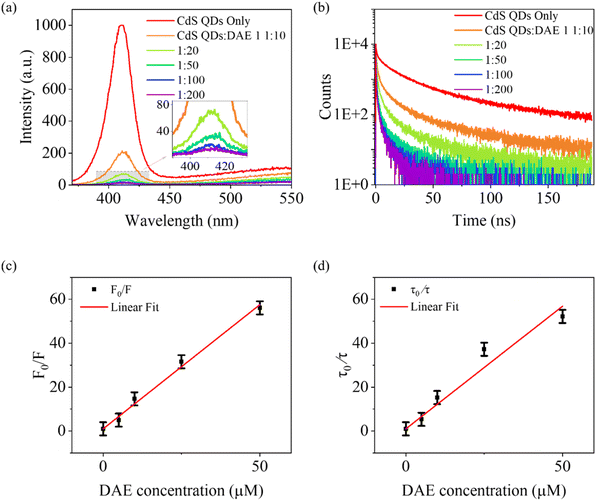
To gain deeper mechanistic insights into the system, steady-state and time resolved photoluminescence (PL) measurements were performed. The emitting state of semiconductor QDs involves exchange splitting between excited singlets and triplets,49 facilitating the direct population of molecular triplets via energy transfer upon excitation of the QDs.50 This energy transfer can be evidenced by the observed PL quenching in the QDs.51 CdS QDs exhibit an emission band with the PL maximum at 425 nm (red curve in Fig. 2a). The PL decay of CdS QDs is multi-exponential with an amplitude-weighted average lifetime of 13.4 ns, as shown by the red curve in Fig. 2b (fitting procedures are described in Fig. S4†). Introducing DAE 1 into the CdS QD solution leads to significant quenching of both the PL intensity and lifetime as shown in Fig. 2. Remarkably, strong PL quenching occurred even at a molar ratio as low as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 between CdS QDs and DAE 1, resulting in a significant reduction in the PL intensity and a shortening of the PL lifetime to 0.9 ns (Fig. S4 and Table S1†). The efficiency of the energy transfer (ΦET) from CdS QDs to DAE 1 can be estimated to be 93% from both PL intensity quenching and PL lifetime quenching. ΦET was further enhanced and approached unity when the concentration of DAE 1 successively increased. This highly efficient energy transfer from CdS QDs to DAE 1 is key to achieving well-performing visible-light photoswitching in our design. The Stern–Volmer plots of PL intensity and lifetime are shown in Fig. 2c and d and fitted to eqn S11.† The fitting resulted in a high quenching rate constant kq = 8.3 × 1013 M−1 s−1, which is two orders of magnitudes higher than the expected diffusion-controlled rate constant kdiff = 1.1 × 1011 M−1 s−1 in toluene.28 The high quenching rate constant here is almost as high as that previously reported for CdSe QDs conjugated to organic dyes, kq ∼ 5 × 1014 M−1 s−1, where efficient energy transfer occurs between adsorbed dye molecules and the excited CdSe QDs.52 In our case, the high quenching rate constant results from the close proximity between the core of CdS QDs and DAE 1 molecules, facilitated by the carboxylic acid functional group of DAE 1 anchoring to the surface of the CdS QDs. This close proximity, along with the short PL lifetime of CdS QDs (13.4 ns compared to the previously used sensitizer's microsecond and millisecond triplet lifetimes), also explains the insensitivity to oxygen.
20 between CdS QDs and DAE 1, resulting in a significant reduction in the PL intensity and a shortening of the PL lifetime to 0.9 ns (Fig. S4 and Table S1†). The efficiency of the energy transfer (ΦET) from CdS QDs to DAE 1 can be estimated to be 93% from both PL intensity quenching and PL lifetime quenching. ΦET was further enhanced and approached unity when the concentration of DAE 1 successively increased. This highly efficient energy transfer from CdS QDs to DAE 1 is key to achieving well-performing visible-light photoswitching in our design. The Stern–Volmer plots of PL intensity and lifetime are shown in Fig. 2c and d and fitted to eqn S11.† The fitting resulted in a high quenching rate constant kq = 8.3 × 1013 M−1 s−1, which is two orders of magnitudes higher than the expected diffusion-controlled rate constant kdiff = 1.1 × 1011 M−1 s−1 in toluene.28 The high quenching rate constant here is almost as high as that previously reported for CdSe QDs conjugated to organic dyes, kq ∼ 5 × 1014 M−1 s−1, where efficient energy transfer occurs between adsorbed dye molecules and the excited CdSe QDs.52 In our case, the high quenching rate constant results from the close proximity between the core of CdS QDs and DAE 1 molecules, facilitated by the carboxylic acid functional group of DAE 1 anchoring to the surface of the CdS QDs. This close proximity, along with the short PL lifetime of CdS QDs (13.4 ns compared to the previously used sensitizer's microsecond and millisecond triplet lifetimes), also explains the insensitivity to oxygen.
Solid-state manipulation
The efficient and oxygen-insensitive all-visible photoswitchable system inspired us to further explore the solid-state performance because of the need for deep penetration irradiation in photomodulation devices such as transistors. A solid sample was prepared by depositing CdS QDs (5 μM) and DAE 1 (1 mM) solution onto a 10 mm × 10 mm filter paper (see the ESI† for details on the sample preparation). This solid sample allowed to reversibly write and erase different patterns through alternating irradiation with 405 nm and 515 nm light, as shown in Fig. 3. Initially, the paper appeared white due to the weak absorption of CdS QDs and o-DAE 1 in the visible region. When uniformly illuminated with 405 nm light, the entire paper turned a pink-purple hue as a result of the absorption of c-DAE 1 in the visible region. The color of the paper can be completely faded when exposed to 515 nm light. By employing photo-masks, the patterns of ‘TJU’ and the Tianjin University logo were also written and erased consecutively by simply altering between the two visible wavelengths. This solid-state sample also shows good reversibility over five irradiation cycles of alternating 405 and 515 nm light (Fig. S6†), indicating that the material can be repeatedly used for information encryption. Moreover, this solid-state approach offers good oxygen and fatigue resistance, as it can still undergo conversion upon 405 nm irradiation even after 5 days of exposure to the ambient environment (see Fig. S5†).Conclusions
In conclusion, we have demonstrated an efficient all-visible light-activated photoswitchable system comprising CdS QDs and carboxylated DAE (DAE 1) through a simple non-covalent mixing approach. Upon 405 nm irradiation, DAE 1 undergoes photocyclization via triplet energy transfer from CdS QDs, with PSS and photochemical QYs comparable to those observed under direct UV light irradiation. Due to the high surface area-to-volume ratio and the efficient nature of the light absorption and subsequent TET from CdS QDs to the DAEs, a single CdS QD can sensitize at least 18 DAE 1 molecules to undergo all-visible light switching, surpassing the performance of previous organic sensitizers and covalent designs. Importantly, the all-visible light switching process of this system is not affected by the presence of oxygen, thanks to the close proximity of the QDs and the DAEs, the short PL lifetime, and the efficient energy transfer process. When adapted to the solid-state, it allows for the reversible writing and erasing of colour patterns within the same sample, even when exposed to the ambient atmosphere. These findings hold the potential to develop all-visible light-activated photoresponsive materials towards smart optoelectronic devices and imaging.Data availability
All the relevant data are provided as part of the ESI.†Author contributions
L. H. conceived the concept and supervised the research. L. H. and K. C. designed the project. L. H. and K. C. carried out material synthesis. K. C. and J. L. performed material characterization tests. K. Z., L. H., and B. A. wrote the paper. T. L. provided advice on the research. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. H. acknowledges support from the National Key Research and Development Project of China (2023YFB3609300) and National Natural Science Foundation of China (62205240 and 12274320), and L. H. and B. A. acknowledge support from the Swedish Foundation for International Cooperation in Research and Higher Education (STINT IB2023-9189). We thank Dr Shiming Li for providing DAE.Notes and references
- S. Kundu, A. Chowdhury, S. Nandi, K. Bhattacharyya and A. Patra, Chem. Sci., 2021, 12, 5874–5882 RSC.
- J. Zhang, Y. Fu, H. H. Han, Y. Zang, J. Li, X. P. He, B. L. Feringa and H. Tian, Nat. Commun., 2017, 8, 987 CrossRef PubMed.
- H. B. Cheng, S. Zhang, E. Bai, X. Cao, J. Wang, J. Qi, J. Liu, J. Zhao, L. Zhang and J. Yoon, Adv. Mater., 2022, 34, e2108289 CrossRef PubMed.
- Z. Zhang, W. Wang, M. O'Hagan, J. Dai, J. Zhang and H. Tian, Angew. Chem., Int. Ed., 2022, 61, e202205758 CrossRef CAS PubMed.
- N. Hosono, M. Yoshikawa, H. Furukawa, K. Totani, K. Yamada, T. Watanabe and K. Horie, Macromolecules, 2013, 46, 1017–1026 CrossRef CAS.
- A. Goulet-Hanssens, T. C. Corkery, A. Priimagi and C. J. Barrett, J. Mater. Chem. C, 2014, 2, 7505–7512 RSC.
- J. Gemen, J. R. Church, T. P. Ruoko, N. Durandin, M. J. Bialek, M. Weissenfels, M. Feller, M. Kazes, M. Odaybat and V. A. Borin, et al. , Science, 2023, 381, 1357–1363 CrossRef CAS PubMed.
- J. Zhang and H. Tian, Adv. Opt. Mater., 2018, 6, 1701278 CrossRef.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- M. Irie, T. Fukaminato, T. Sasaki, N. Tamai and T. Kawai, Nature, 2002, 420, 759–760 CrossRef CAS PubMed.
- V. Lemieux and N. R. Branda, Org. Lett., 2005, 7, 2969–2972 CrossRef CAS PubMed.
- U. Al-Atar, R. Fernandes, B. Johnsen, D. Baillie and N. R. Branda, J. Am. Chem. Soc., 2009, 131, 15966–15967 CrossRef CAS PubMed.
- K. Uchida, M. Saito, A. Murakami, S. Nakamura and M. Irie, ChemPhysChem, 2003, 4, 1124–1127 CrossRef CAS PubMed.
- G. Naren, W. Larsson, C. Benitez-Martin, S. Li, E. Perez-Inestrosa, B. Albinsson and J. Andreasson, Chem. Sci., 2021, 12, 7073–7078 RSC.
- A. A. Scherbovich, S. A. Maskevich, P. V. Karpach, G. T. Vasilyuk, V. I. Stsiapura, O. V. Venidiktova, A. O. Ayt, V. A. Barachevsky, A. A. Khuzin and A. R. Tuktarov, et al. , J. Phys. Chem. C, 2020, 124, 27064–27070 CrossRef CAS.
- L. Hou, R. Ringstrom, A. B. Maurer, M. Abrahamsson, J. Andreasson and B. Albinsson, J. Am. Chem. Soc., 2022, 144, 17758–17762 CrossRef CAS PubMed.
- S. A. Diaz, F. Gillanders, E. A. Jares-Erijman and T. M. Jovin, Nat. Commun., 2015, 6, 6036 CrossRef CAS PubMed.
- D. Mendive-Tapia, A. Perrier, M. J. Bearpark, M. A. Robb, B. Lasorne and D. Jacquemin, Phys. Chem. Chem. Phys., 2014, 16, 18463–18471 RSC.
- D. Bleger and S. Hecht, Angew. Chem., Int. Ed., 2015, 54, 11338–11349 CrossRef CAS PubMed.
- Z. Xu, T. Jin, Y. Huang, K. Mulla, F. A. Evangelista, E. Egap and T. Lian, Chem. Sci., 2019, 10, 6120–6124 RSC.
- Z. Zhang, J. Zhang, B. Wu, X. Li, Y. Chen, J. Huang, L. Zhu and H. Tian, Adv. Opt. Mater., 2018, 6, 1700847 CrossRef.
- J. Ma, X. Cui, F. Wang, X. Wu, J. Zhao and X. Li, J. Org. Chem., 2014, 79, 10855–10866 CrossRef CAS PubMed.
- V. W. Yam, C. C. Ko and N. Zhu, J. Am. Chem. Soc., 2004, 126, 12734–12735 CrossRef CAS PubMed.
- Z. Zhang, W. Wang, P. Jin, J. Xue, L. Sun, J. Huang, J. Zhang and H. Tian, Nat. Commun., 2019, 10, 4232 CrossRef PubMed.
- Z. Li, C. He, Z. Lu, P. Li and Y.-P. Zhu, Dyes Pigm., 2020, 182, 108623 CrossRef CAS.
- M. Herder, B. M. Schmidt, L. Grubert, M. Patzel, J. Schwarz and S. Hecht, J. Am. Chem. Soc., 2015, 137, 2738–2747 CrossRef CAS PubMed.
- R. Murata, T. Yago and M. Wakasa, J. Phys. Chem. A, 2015, 119, 11138–11145 CrossRef CAS PubMed.
- M. Montalti and S. L. Murov, Handbook of photochemistry, CRC/Taylor & Francis, 2006 Search PubMed.
- R. Murata, T. Yago and M. Wakasa, Bull. Chem. Soc. Jpn., 2011, 84, 1336–1338 CrossRef CAS.
- M. T. Indelli, S. Carli, M. Ghirotti, C. Chiorboli, M. Ravaglia, M. Garavelli and F. Scandola, J. Am. Chem. Soc., 2008, 130, 7286–7299 CrossRef CAS PubMed.
- S. Fredrich, R. Gostl, M. Herder, L. Grubert and S. Hecht, Angew. Chem., Int. Ed., 2016, 55, 1208 CrossRef CAS PubMed.
- W. Wang, W. Yang, Z. Zhang, J. Dai, Y. Xu and J. Zhang, Chem. Sci., 2024, 15, 5539–5547 RSC.
- M. Liu, P. Xia, G. Zhao, C. Nie, K. Gao, S. He, L. Wang and K. Wu, Angew. Chem., Int. Ed., 2022, 61, e202208241 CrossRef CAS PubMed.
- L. Hou, W. Larsson, S. Hecht, J. Andréasson and B. Albinsson, J. Mater. Chem. C, 2022, 10, 15833–15842 RSC.
- K. Taruno, I. Ikariko, T. Taniguchi, S. Kim and T. Fukaminato, J. Phys. Chem. B, 2024, 128, 273–279 CrossRef CAS PubMed.
- P. Hong, J. Liu, K. X. Qin, R. Tian, L. Y. Peng, Y. S. Su, Z. Gan, X. X. Yu, L. Ye and M. Q. Zhu, et al. , Angew. Chem., Int. Ed., 2023, 63, e202316706 CrossRef PubMed.
- T. Fukaminato, T. Doi, M. Tanaka and M. Irie, J. Phys. Chem. C, 2009, 113, 11623–11627 CrossRef CAS.
- R. T. Jukes, V. Adamo, F. Hartl, P. Belser and L. De Cola, Inorg. Chem., 2004, 43, 2779–2792 CrossRef CAS PubMed.
- T. Fukaminato, T. Hirose, T. Doi, M. Hazama, K. Matsuda and M. Irie, J. Am. Chem. Soc., 2014, 136, 17145–17154 CrossRef CAS PubMed.
- S. Qiu, A. T. Frawley, K. G. Leslie and H. L. Anderson, Chem. Sci., 2023, 14, 9123–9135 RSC.
- Z. Li, Y. Ji, R. Xie, S. Y. Grisham and X. Peng, J. Am. Chem. Soc., 2011, 133, 17248–17256 CrossRef CAS PubMed.
- W. W. Yu and X. Peng, Angew. Chem., Int. Ed., 2002, 41, 2368–2371 CrossRef CAS PubMed.
- F. Gillanders, L. Giordano, S. A. Diaz, T. M. Jovin and E. A. Jares-Erijman, Photochem. Photobiol. Sci., 2014, 13, 603–612 CrossRef CAS PubMed.
- A. P. Glaze, H. G. Heller and J. Whittall, J. Chem. Soc., Perkin Trans. 2, 1992, 591–594 RSC.
- K. Shibata, K. Muto, S. Kobatake and M. Irie, J. Phys. Chem. A, 2001, 106, 209–214 CrossRef.
- T. Sumi, Y. Takagi, A. Yagi, M. Morimoto and M. Irie, Chem. Commun., 2014, 50, 3928–3930 RSC.
- A. Osuka, D. Fujikane, H. Shinmori, S. Kobatake and M. Irie, J. Org. Chem., 2001, 66, 3913–3923 CrossRef CAS PubMed.
- F. Hu, M. Cao, X. Ma, S. H. Liu and J. Yin, J. Org. Chem., 2015, 80, 7830–7835 CrossRef CAS PubMed.
- M. Nirmal and L. Brus, Acc. Chem. Res., 1998, 32, 407–414 CrossRef.
- C. Mongin, S. Garakyaraghi, N. Razgoniaeva, M. Zamkov and F. N. Castellano, Science, 2016, 351, 369–372 CrossRef CAS PubMed.
- L. Hou, A. Olesund, S. Thurakkal, X. Zhang and B. Albinsson, Adv. Funct. Mater., 2021, 31, 2106198 CrossRef CAS.
- A. M. Funston, J. J. Jasieniak and P. Mulvaney, Adv. Mater., 2008, 20, 4274–4280 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06110a |
| This journal is © The Royal Society of Chemistry 2024 |