Open Access Article
Open Access ArticleEnantioselective dearomative formal (3+3) cycloadditions of bicyclobutanes with aromatic azomethine imines: access to fused 2,3-diazabicyclo[3.1.1]heptanes†
Xue-Chun
Yang‡
 a,
Feng
Wu‡
a,
Feng
Wu‡
 a,
Wen-Biao
Wu‡
a,
Wen-Biao
Wu‡
 a,
Xu
Zhang
a,
Xu
Zhang
 b and
Jian-Jun
Feng
b and
Jian-Jun
Feng
 *a
*a
aState Key Laboratory of Chemo/Biosensing and Chemometrics, Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan 410082, P. R. China. E-mail: jianjunfeng@hnu.edu.cn
bSchool of Chemistry & Chemical Engineering, Yangzhou University, Yangzhou, 225002, P. R. China
First published on 8th November 2024
Abstract
Although cycloadditions of bicyclobutanes (BCBs) have emerged as a reliable approach for producing bicyclo[n.1.1]alkanes such as azabicyclo[3.1.1]heptanes (aza-BCHeps), serving as saturated bioisosteres of arenes, the catalytic asymmetric variant remains underdeveloped and presents challenges. Herein, we developed several Lewis acid-catalyzed systems for the challenging dearomative (3+3) cycloaddition of BCBs and aromatic azomethine imines. This resulted in fused 2,3-diazabicyclo[3.1.1]heptanes, introducing a novel chemical space for the caged hydrocarbons. Moreover, an asymmetric Lewis acid catalysis strategy was devised for the (3+3) cycloadditions of BCBs and N-iminoisoquinolinium ylides, forming chiral diaza-BCHeps with up to 99% yield and 97% ee. This study showcases a unique instance of asymmetric (3+3) cycloaddition facilitated by the creation of a chiral environment via the activation of BCBs.
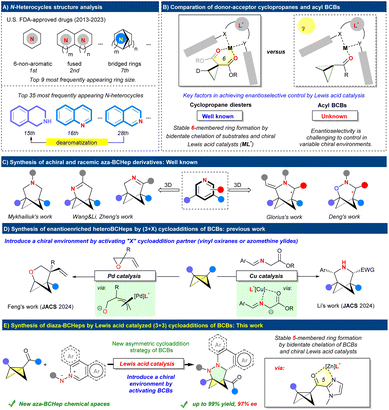
Introduction
Nitrogen heterocycles are ubiquitously present in nature and represent crucial structural elements in pharmaceuticals.1 Njardarson's analysis of FDA-approved small-molecule drugs from 2013 to 2023 shows that 82% of the newly approved small-molecule drugs in this period contain a nitrogen heterocycle. Six-membered non-aromatic N-heterocycles are the most prevalent, followed by fused N-heterocycles, in the approved new drugs.1b Besides fused N-heterocycles, bridged bicyclic nitrogen heterocycles like diazabicyclo[3.2.1]octane and tropane derivatives belong to the top 35 most frequently appearing N-heterocyclic compounds.1 Therefore, developing atom-economical and particularly catalytic asymmetric strategies to construct the aforementioned N-heterocyclic scaffolds is crucial for diverse applications, including drug discovery (Scheme 1A).Aromatic azomethine imines are versatile and easily synthesized compounds employed as substrates in dipolar (3+3) cycloadditions to produce valuable hydroisoquinoline and related six-membered N-heterocycle frameworks.2 These structures serve as core components in numerous natural products and pharmaceuticals.3 In 2008, the Charette group pioneered the Lewis acid-catalyzed non-asymmetric (3+3) cycloadditions of 1,1-cyclopropane diesters with N-iminoquinolinium and N-iminoisoquinolinium ylides.4a Tang's group later successfully developed the asymmetric version of the aforementioned (3+3) reaction.4b A crucial element contributing to their success was the utilization of a carefully designed trisoxazoline (TOX)-nickel catalyst to create a stable chiral environment by coordinating with two chelating, electron-withdrawing groups on cyclopropanes (Scheme 1B).5 Until now, there have been five literature reports on asymmetric (3+3) cycloadditions with aromatic azomethine imines.4b,6 However, these reports are mainly focused on constructing fused six-membered N-heterocycles. Cycloaddition reactions using these versatile 1,3-dipoles to form bridged bicyclic nitrogen heterocycles, especially enantioenriched bridged bicyclic cycloadducts, have not been reported yet.
Recently, bicyclo[3.1.1]heptanes (BCHeps) and aza-BCHeps have attracted increasing attention from chemists and medicinal chemists for serving as bioisosteres of meta-substituted benzenes and pyridines, respectively.7 Significantly, Mykhailiuk's studies show that heteroatom (N- or O-) incorporated analogs of caged hydrocarbons (bicyclo[2.1.1]hexanes (BCHs)8 and BCHeps) often demonstrate enhanced water solubility, improved metabolic stability, and reduced lipophilicity.7d,9 In this context, the (3+3) cycloadditions of bicyclobutanes (BCBs)10 have emerged as a vital synthetic platform for building BCHep and hetero-BCHep skeletons, owing to the pioneering scientific contributions of Molander,11 Waser,12 Li,13 Wang,14 Zheng,15 Deng,16 Glorius17 and our group.18 Several strategies, including photocycloaddition,11,12 pyridine-boryl radical catalysis,13,14 Ti-catalyzed radical-relay process,15 Lewis acid catalysis,16,18 and silver-promoted tandem (3+3)/(3+2)/retro-(3+2) cycloaddition,17 have been developed for efficient (3+3) cycloadditions of BCBs (Scheme 1C). Despite significant progress, the access to enantioenriched (hetero)BCHeps through catalytic asymmetric cycloadditions of BCBs remains limited and presents a persistent challenge. Very recently, our group developed the first asymmetric (3+3) cycloadditions of BCBs with vinyl oxiranes using palladium catalysis.19 The Li group pioneered the synthesis of enantioenriched 3-aza-BCHeps through copper-catalyzed cycloadditions of BCBs with azomethine ylides.20 The strategies employed by both groups focus exclusively on creating a stable chiral environment by activating the “X” component in the (3 + X) reaction of BCBs (Scheme 1D).
Based on our interest in BCB chemistry,21 we envisioned that the asymmetric (3+3) cycloaddition of BCBs with aromatic azomethine imines might serve as a new approach to enantiomerically enriched fused 2,3-diazabicyclo[3.1.1]heptanes (N,N-BCHeps) through a chiral Lewis acid catalyst via activating the BCBs (Scheme 1E).22 However, this hypothesis encountered significant challenges. (a) For instance, although Deng's group has successfully established the non-asymmetric (3+3) cycloadditions of BCBs with nitrones,16 the analogous reaction with aromatic azomethine imines remains unexplored because an energy barrier exists in the cycloaddition process, requiring dearomatization with aromatic azomethine imines.23 (b) Achieving enantioselective control in the cycloaddition of BCBs with a single electron-withdrawing group, as opposed to donor–acceptor cyclopropanes containing two chelating, electron-withdrawing groups,4 poses greater challenges in establishing chiral environments by forming a chiral catalyst–substrate complex with varying conformations. (c) Another key challenge in the Lewis acid catalyzed asymmetric (3+3) version is identifying optimal chiral catalysts that can overcome the strong racemic background reaction, thereby enabling the simultaneous construction of two congested quaternary centers and a chiral aza-trisubstituted carbon center with excellent ee values.24
Results and discussion
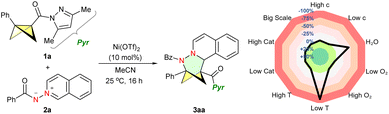
Our study commences by exploring the non-asymmetric cycloadditions of Glorius's BCB 1a (ref. 25) with isoquinoline azomethine imine 2a. After screening of various reaction parameters, we found that the desired (3+3) reaction occurred with Ni(OTf)2 as the catalyst in acetonitrile at room temperature (conditions A); the N,N-BCHep 3aa was obtained in almost quantitative yield (Table 1, entry 1). Control experiments revealed that the solvent had substantial effect on the yield but no improvement over acetonitrile was seen (entries 2–6). Although other metal Lewis acids can produce the desired product with reduced yield (entries 7–10), main-group Lewis acids like BF3 did not yield 3aa (entry 11). Control experiments demonstrated that the reaction did not occur at 80 °C without the presence of the Lewis acid catalyst (entry 12). Condition-based sensitivity screening was performed,26 revealing that low temperature inhibited the reaction. The reaction showed moderate sensitivity to moisture, with concentration, scale, catalyst loading, high temperature, and O2 level having no significant impact on the reaction (Table 1).| Entry | Variation | Yieldb (%) | |
|---|---|---|---|
| a Conditions A: 1a (1.0 equiv.), 2a (1.2 equiv.), Ni(OTf)2 (10 mol%) in CH3CN (0.05 M) at room temperature for 16 h. b NMR yield with CH2Br2 as an internal standard. c Performed in CH2Cl2. d Run at 80 °C. | |||
| 1 | None | >99 | |
| 2 | Toluene instead of MeCN | 25 | |
| 3 | EtOAc instead of MeCN | 23 | |
| 4 | 1,4-Dioxane instead of MeCN | 22 | |
| 5 | DCE instead of MeCN | 63 | |
| 6 | CH2Cl2 instead of MeCN | 35 | |
| 7c | Sc(OTf)3 instead of Ni(OTf)2 | 25 | |
| 8c | Fe(OTf)2 instead of Ni(OTf)2 | 30 | |
| 9c | Zn(OTf)2 instead of Ni(OTf)2 | 20 | |
| 10c | Co(OTf)2 instead of Ni(OTf)2 | 33 | |
| 11c | BF3·Et2O instead of Ni(OTf)2 | 0 | |
| 12d | Without Ni(OTf)2 | 0 | |
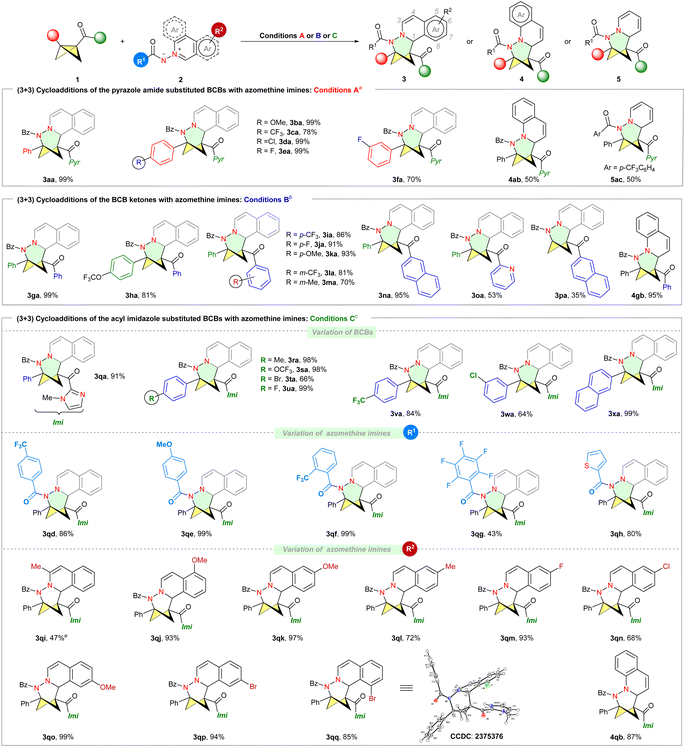
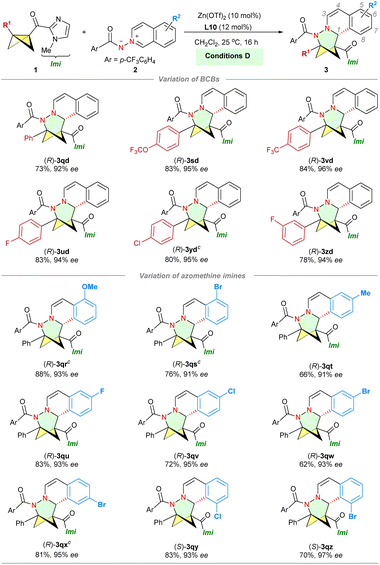
With the optimized conditions in hand, we investigated the BCB scope (Scheme 2, top). Different substituents at the phenyl moiety, such as the methoxy group, halides and the CF3 group, were well tolerated (3aa–fa, 70–99% yield). As a trend, electron-rich 1b afforded relatively better results than electron deficient 1c and 1f. In addition to isoquinoline azomethine imine 2a, both quinoline azomethine imine 2b and N-aminopyridinium ylide 2c are compatible with conditions A, yielding the corresponding products smoothly (4ab and 5ac). During our investigation of BCB ketones under conditions A, we discovered that a (3+3) cycloadduct 3ga was obtained with a 9% NMR yield. To our delight, excellent yield (99% yield of 3ga) was achieved in CH3CN at 50 °C using Sc(OTf)3 as the catalyst (conditions B, see Table S2 in the ESI†).
The substrate scope of BCBs containing benzoyl groups was subsequently explored under conditions B (Scheme 2, middle). The steric and electronic properties of para-substituents on the phenyl group of BCBs had minor impact on the yields (3ga–ka, 81–99% yield). BCBs with substituents at the meta-position of the phenyl group were well tolerated (3la–ma). Besides phenyl ketones, naphthyl (3na) and heteroaryl ketones (3oa) were also suitable for this (3+3) reaction, yielding the corresponding N,N-BCHeps in moderate to excellent yield. The (3+3) cycloaddition of monosubstituted BCB 1p with 2a produced the desired product 3pa, although the yield was low. Quinoline azomethine imine 2b was successfully employed in the reaction, resulting in the formation of 4gb with a yield of 95%. Additionally, we further investigated the scope of substrates containing BCBs with an acyl imidazole group by employing the Fe(OTf)2 catalyst (conditions C, Scheme 2, bottom). BCBs 1q–1w featuring various substituted phenyl rings (p-Me, p-OCF3, p-Br, p-F, p-CF3, m-Cl) furnished the corresponding cycloadducts (3qa–wa) in very high yields (64–99%). Naphthyl-substituted BCB 1x also reacted smoothly. Of note, in contrast to Glorius's observation that a BCB with a strong electron-withdrawing trifluoromethyl group on the phenyl ring resulted in a poor yield of the product,25 our study indicates that the electronic properties of substituents on the phenyl group had minimal influence on the yields, suggesting that a benzylic carbocation intermediate may not be involved in our catalytic system.
We then evaluated the compatibility of aromatic azomethine imines 2 bearing various benzoyl protecting groups. The benzoyl protecting group of N-iminoisoquinolinium ylides exhibited good tolerance towards both electron-withdrawing and electron-donating substituents (R1), with the exception of 2g. Azomethine imine 2h with a thiophene-2-carbonyl group was well tolerated. Unfortunately, the (3+3) reactions are not compatible with N-tosyliminoisoquinolinium ylides. Furthermore, the current (3+3) protocol is amenable to a series of azomethine imines 2 bearing different R2 substituents, including alkyl (3qi and 3ql), OMe (3qj–qk, 3qo) and halogen (3qm–qn and 3qp–qq)27 groups at the C3 and C5–C8 positions of isoquinoline moieties, and led to the corresponding polysubstituted N,N-BCHeps in good yield (47–99%). The azomethine imine 2b was successfully employed in the reaction, resulting in the formation of 4qb with a yield of 87%.
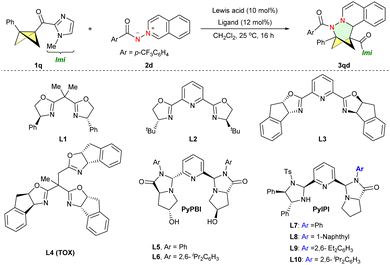
After establishing the non-asymmetric (3+3) cycloadditions of BCBs with aromatic azomethine imines, we proceeded to develop the asymmetric (3+3) version. BCB 1q containing bidentate chelating groups and 2d with the 4-(trifluoromethyl)benzoyl-protecting group were selected as the model substrates. In the presence of Co(OTf)2 and L1, a 6% ee was detected (Table 2, entry 1), prompting us to explore different oxazoline-based chiral ligands for the cycloaddition reaction (entries 2–4). The use of L2 led to an enhancement in enantiomeric excess (22% ee, entry 2). Nevertheless, when transitioning to alternative ligands such as L3–L4, the outcomes were unsatisfactory. The TOX ligand L4 provided excellent enantioselective control in Tang's (3+3) cycloadditions of cyclopropanes,4b but it resulted in racemic 3qd in the current cycloadditions of BCBs. Recently, Xie and Guo developed two novel tridentate nitrogen ligands, PyBPI and PyIPI, which exhibited effective stereoselective control in Lewis acid catalysis.28 Thus these ligands were synthesized and evaluated (L5–L10). While PyBPI L6 showed poor results in the enantioselective dearomative (3+3) reaction, the PyIPI ligand L10 produced 3qd with a promising ee value (entry 6 versus entry 10). After systematically screening various reaction parameters (entries 11–17), we successfully conducted the enantioselective (3+3) reaction using L10, resulting in the synthesis of the target compound (R)-3qd with a 78% NMR yield and 92% ee when Zn(OTf)2 was utilized in place of Co(OTf)2.
| Entry | Lewis acid | Ligand | Yieldb (%) | eec | |
|---|---|---|---|---|---|
| a 1q (0.1 mmol), 2d (0.12 mmol), Lewis acid (10 mol%) and ligand (12 mol%) in CH2Cl2 (2 mL) at room temperature for 16 h. b NMR yield with CH2Br2 as an internal standard. c Based on chiral HPLC analysis. d Performed in 1,4-dioxane. e Run at 0 °C. f Run at 40 °C. | |||||
| 1 | Co(OTf)2 | L1 | 79 | 6 | |
| 2 | Co(OTf)2 | L2 | 68 | 22 | |
| 3 | Co(OTf)2 | L3 | 18 | 17 | |
| 4 | Co(OTf)2 | L4 | 87 | 1 | |
| 5 | Co(OTf)2 | L5 | 73 | 0 | |
| 6 | Co(OTf)2 | L6 | 78 | 16 | |
| 7 | Co(OTf)2 | L7 | 81 | 13 | |
| 8 | Co(OTf)2 | L8 | 77 | 34 | |
| 9 | Co(OTf)2 | L9 | 85 | 64 | |
| 10 | Co(OTf)2 | L10 | 85 | 70 | |
| 11 | Fe(OTf)2 | L10 | 65 | 88 | |
| 12 | Ni(OTf)2 | L10 | 83 | 28 | |
| 13 | Sc(OTf)3 | L10 | 63 | 0 | |
| 14 | Zn(OTf) 2 | L10 | 78 | 92 | |
| 15d | Zn(OTf)2 | L10 | 99 | 88 | |
| 16e | Zn(OTf)2 | L10 | 29 | 86 | |
| 17f | Zn(OTf)2 | L10 | 90 | 51 | |
With the identified reaction conditions D in hand, we examined the scope of this enantioselective (3+3) reaction (Scheme 3). Initially, the variation in BCB was evaluated. BCBs bearing trifluoromethyloxy (1s) and trifluoromethyl (1v) substituents on the aryl ring, which are popular in pharmaceuticals and agrochemicals, were found to be compatible in the reaction, resulting in the desired product with 95–96% ee ((R)-3sd–vd). The presence of halogen substituents at the para- or meta-position of the benzene ring did not negatively impact the reaction outcome ((R)-3ud, 3yd and 3zd). The N-iminoisoquinolinium ylides 2 bearing methoxy, halogen (F, Cl, Br), or methyl groups at the C5–C8 positions of isoquinoline moieties yielded the desired products 3qr–3qz with high efficiency and outstanding enantioselectivity.27 Notably, in some instances, poor yields (<30% NMR yield) of the cycloadducts (3qs and 3qx) under conditions D may be attributed to the low solubility of azomethine imines in CH2Cl2. However, high yields (76–81% yield) and stereoselectivity (91–95% ee) were successfully attained in CH2Cl2/1,4-dioxane (10/1).
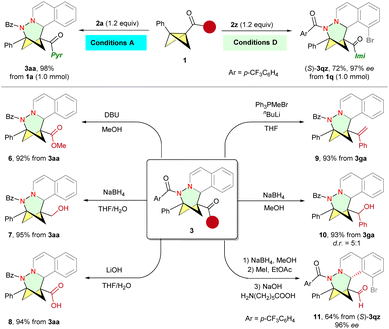
To showcase the synthetic utility of these cycloadducts, we initially performed scale-up experiments. Both non-asymmetric and asymmetric systems were successfully scaled up to a 1.0 mmol scale using standard conditions, while preserving efficiency and selectivity. The pyrazole amide group of 3aa can be easily converted into various valuable functional groups, such as ester 6 and carboxylic acid 8, smoothly. Primary alcohol 7 and secondary alcohol 10 can be obtained with excellent yield through the NaBH4 reduction of 3aa and 3ga, respectively. The reaction of 3ga with the Wittig reagent afforded 9 in 93% yield. Finally, cleavage of the imidazole moiety gave rise to the desired aldehyde 11 in 64% yield and 96% ee (Scheme 4).
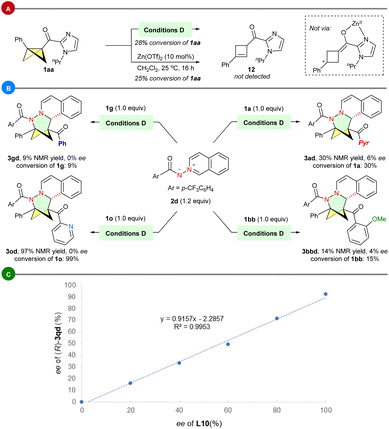
Control experiments were conducted to gain insight into this asymmetric transformation. When subjecting BCB 1aa to conditions D with or without ligand L10, we did not observe the rapid decomposition of 1aa, nor did we observe the corresponding side product cyclobutene 12 (Scheme 5A). This result suggests that the benzylic carbocation enolate intermediate may not be involved in the reaction, aligning with the electronic effect observed in BCB substrates. BCBs containing electron-withdrawing groups (e.g., p- and m-CF3) on the phenyl ring also yield good results. While BCB 1g and 1o generated the corresponding products 3gd and 3od with 0% ee, the (3+3) reactions of 1a or 1bb, which contain a bidentate chelating group, with 2d produced 3ad with 6% ee and 3bbd with 4% ee, respectively. The results indicate that forming a stable chiral environment through the chelation of a bidentate acyl imidazole group on BCB with a Zn-Lewis acid catalyst is essential for achieving enantioselective control (Scheme 5B). Furthermore, the correlation between the enantiomeric purities of ligands L10 and (R)-3qd was assessed. The study uncovered a linear correlation, indicating that the active catalyst/ligand is monomeric in nature (Scheme 5C).28e
Conclusions
In conclusion, we have established a dearomative (3+3) cycloaddition for synthesizing novel diaza-BCHep derivatives from BCBs and aromatic azomethine imines. The good to high overall yields and excellent enantioselectivities are governed by the catalysts and reaction conditions. This straightforward approach operates under mild conditions and exhibits good functional group tolerance. The synthetic utility and practicality were also highlighted by the scale-up experiment and diverse synthetic transformations of the cycloadducts. Notably, this study presents a rare example of Lewis acid-catalyzed asymmetric (3+3) cycloadditions of BCBs, paving the way for the enantioselective synthesis of other valuable bicyclo[n.1.1]alkanes through chiral Lewis acid catalysis.Data availability
The data supporting this article have been included as part of the ESI.† All detailed procedures, characterization data and NMR spectra are available in the ESI.†Author contributions
X.-C. Y. and F. W. performed all of the experiments and analysed their results. W.-B. W., X. Z. and J.-J. F. wrote the manuscript. J.-J. F. conceived the catalytic system and directed the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Fundamental Research Funds for the Central Universities, National Natural Science Foundation of China (22471068), Natural Science Foundation of Hunan Province (2024JJ6126) and China Postdoctoral Science Foundation (2024M750865) for financial support. The 1H, 13C NMR spectroscopy, HRMS (ESI) and single crystal X-ray diffraction were performed at the Analytical Instrumentation Center of Hunan University.Notes and references
-
(a) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed
; (b) C. M. Marshall, J. G. Federice, C. N. Bell, P. B. Cox and J. T. Njardarson, J. Med. Chem., 2024, 67, 11622–11655 CrossRef CAS PubMed
.
- For selected reviews, see:
(a) T.-B. Hua, Q.-Q. Yang and W.-J. Xiao, Chin. J. Org. Chem., 2020, 40, 3559–3595 CrossRef CAS
; (b) D. Li, X.-C. Chen and W. Gao, Synthesis, 2020, 52, 3337–3355 CrossRef CAS
; (c) C. Nájera, J. M. Sansano and M. Yus, Org. Biomol. Chem., 2015, 13, 8596–8636 RSC
; (d) G. Qiu, Y. Kuang and J. Wu, Adv. Synth. Catal., 2014, 356, 3483–3504 CrossRef CAS
; (e) X. Xu and M. P. Doyle, Acc. Chem. Res., 2014, 47, 1396–1405 CrossRef CAS PubMed
; (f) J. J. Mousseau and A. B. Charette, Acc. Chem. Res., 2013, 46, 412–424 CrossRef CAS PubMed
.
-
(a) W. Liu, S. Liu, R. Jin, H. Guo and J. Zhao, Org. Chem. Front., 2015, 2, 288–299 RSC
; (b) J. D. Scott and R. M. William, Chem. Rev., 2002, 102, 1669–1730 CrossRef CAS PubMed
.
-
(a) C. Perreault, S. R. Goudreau, L. E. Zimmer and A. B. Charette, Org. Lett., 2008, 10, 689–692 CrossRef CAS PubMed
; (b) Y.-Y. Zhou, J. Li, L. Ling, S.-H. Liao, X.-L. Sun, Y.-X. Li, L.-J. Wang and Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 1452–1456 CrossRef CAS PubMed
. For selected reviews on D–A cyclopropanes, see:; (c) D. B. Werz and A. T. Biju, Angew. Chem., Int. Ed., 2020, 59, 3385–3398 CrossRef CAS PubMed
; (d) A. U. Augustin and D. B. Werz, Acc. Chem. Res., 2021, 54, 1528–1541 CrossRef CAS PubMed
; (e) Y. Xia, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2021, 60, 9192–9204 CrossRef CAS PubMed
; (f) L. Wang and Y. Tang, Isr. J. Chem., 2016, 56, 463–475 CrossRef CAS
; (g) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS PubMed
; (h) F. de Nanteuil, F. De Simone, R. Frei, F. Benfatti, E. Serrano and J. Waser, Chem. Commun., 2014, 50, 10912–10928 RSC
; (i) C. A. Carson and M. A. Kerr, Chem. Soc. Rev., 2009, 38, 3051–3060 RSC
; (j) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151–1196 CrossRef CAS PubMed
.
- S. Liao, X.-L. Sun and Y. Tang, Acc. Chem. Res., 2014, 47, 2260–2272 CrossRef CAS PubMed
.
-
(a) X. Xu, P. Y. Zavalij and M. P. Doyle, Angew. Chem., Int. Ed., 2013, 52, 12664–12668 CrossRef CAS PubMed
; (b) K. O. Marichev, F. G. Adly, A. M. Carranco, E. C. Garcia, H. Arman and M. P. Doyle, ACS Catal., 2018, 8, 10392–10400 CrossRef CAS
; (c) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316–4319 CrossRef CAS PubMed
; (d) C. Guo, M. Fleige, D. Janssen-Müller, C. G. Daniliuc and F. Glorius, Nat. Chem., 2015, 7, 842–847 CrossRef CAS PubMed
.
-
(a) N. Frank, J. Nugent, B. R. Shire, H. D. Pickford, P. Rabe, A. J. Sterling, T. Zarganes-Tzitzikas, T. Grimes, A. L. Thompson, R. C. Smith, C. J. Schofield, P. E. Brennan, F. Duarte and E. A. Anderson, Nature, 2022, 611, 721–726 CrossRef CAS PubMed
; (b) R. I. Revie, B. J. Whitaker, B. Paul, R. C. Smith and E. A. Anderson, Org. Lett., 2024, 26, 2843–2846 CrossRef CAS PubMed
; (c) T. Iida, J. Kanazawa, T. Matsunaga, K. Miyamoto, K. Hirano and M. Uchiyama, J. Am. Chem. Soc., 2022, 144, 21848–21852 CrossRef CAS PubMed
; (d) D. Dibchak, M. Snisarenko, A. Mishuk, O. Shablykin, L. Bortnichuk, O. Klymenko-Ulianov, Y. Kheylik, I. V. Sadkova, H. S. Rzepa and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2023, e202304246 CAS
; (e) P. K. Mykhailiuk, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC
.
- Representative examples for the synthesis of BCHs, see:
(a) A. Cairncross and E. P. Blanchard Jr, J. Am. Chem. Soc., 1966, 88, 496–504 CrossRef CAS
; (b) P. Wipf and M. A. A. Walczak, Angew. Chem., Int. Ed., 2006, 45, 4172–4175 CrossRef CAS PubMed
; (c) M. Wang, Y. Huang, C. Li and P. Lu, Org. Chem. Front., 2022, 9, 2149–2153 RSC
; (d) B. D. Schwartz, A. P. Smyth, P. E. Nashar, M. G. Gardiner and L. R. Malins, Org. Lett., 2022, 24, 1268–1273 CrossRef CAS PubMed
; (e) R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc and F. Glorius, Nature, 2022, 605, 477–482 CrossRef CAS PubMed
; (f) R. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard and M. K. Brown, J. Am. Chem. Soc., 2022, 144, 7988–7994 CrossRef CAS PubMed
; (g) Y. Liu, Z. Wu, J.-R. Shan, H. Yan, E.-J. Hao and L. Shi, Nat. Commun., 2024, 15, 4374 CrossRef CAS PubMed
; (h) S. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. E. M. Crisenza and D. J. Procter, Nat. Chem., 2023, 15, 535–541 CrossRef CAS PubMed
; (i) H. Ren, T. Li, J. Xing, Z. Li, Y. Zhang, X. Yu and J. Zheng, Org. Lett., 2024, 26, 1745–1750 CrossRef CAS PubMed
; (j) M. Xu, Z. Wang, Z. Sun, Y. Ouyang, Z. Ding, T. Yu, L. Xu and P. Li, Angew. Chem., Int. Ed., 2022, 61, e202214507 CrossRef CAS PubMed
; (k) Y. Liu, S. Lin, Y. Li, J.-H. Xue, Q. Li and H. Wang, ACS Catal., 2023, 13, 5096–5103 CrossRef CAS
; (l) R. Kleinmans, S. Dutta, K. Ozols, H. Shao, F. Schäfer, R. E. Thielemann, H. T. Chan, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2023, 145, 12324–12332 CrossRef CAS PubMed
; (m) J. L. Tyler, F. Schäfer, H. Shao, C. Stein, A. Wong, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2024, 146, 16237–16247 CrossRef CAS PubMed
; (n) Q.-Q. Hu, L.-Y. Wang, X.-H. Chen, Z.-X. Geng, J. Chen and L. Zhou, Angew. Chem., Int. Ed., 2024, 63, e202405781 CrossRef CAS PubMed
; (o) J.-Y. Su, J. Zhang, Z.-Y. Xin, H. Li, H. Zheng and W.-P. Deng, Org. Chem. Front., 2024, 11, 4539–4545 RSC
; (p) J.-J. Wang, L. Tang, Y. Xiao, W.-B. Wu, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202405222 CrossRef CAS PubMed
; (q) N. Radhoff, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2023, 62, e202304771 CrossRef CAS PubMed
; (r) D. Ni, S. Hu, X. Tan, Y. Yu, Z. Li and L. Deng, Angew. Chem., Int. Ed., 2023, 62, e202308606 CrossRef CAS PubMed
; (s) M. de Robichon, T. Kratz, F. Beyer, J. Zuber, C. Merten and T. Bach, J. Am. Chem. Soc., 2023, 145, 24466–24470 CAS
; (t) Q. Fu, S. Cao, J. Wang, X. Lv, H. Wang, X. Zhao and Z. Jiang, J. Am. Chem. Soc., 2024, 146, 8372–8380 CrossRef CAS PubMed
; (u) M. Reinhold, J. Steinebach, C. Golz and J. C. L. Walker, Chem. Sci., 2023, 14, 9885–9891 RSC
; (v) L. Yang, H. Wang, M. Lang, J. Wang and S. Peng, Org. Lett., 2024, 26, 4104–4110 CrossRef CAS PubMed
; (w) K. J. Woelk, K. Dhake, N. D. Schley and D. C. Leitch, Chem. Commun., 2023, 59, 13847–13850 RSC
.
-
(a) A. Denisenko, P. Garbuz, N. M. Voloshchuk, Y. Holota, G. Al-Maali, P. Borysko and P. K. Mykhailiuk, Nat. Chem., 2023, 15, 1155–1163 CrossRef CAS PubMed
; (b) V. V. Levterov, Y. Panasyuk, V. O. Pivnytska and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2020, 59, 7161–7167 CrossRef CAS PubMed
; (c) V. V. Levterov, O. Michurin, P. O. Borysko, S. Zozulya, I. V. Sadkova, A. A. Tolmachev and P. K. Mykhailiuk, J. Org. Chem., 2018, 83, 14350–14361 CrossRef CAS PubMed
; (d) V. V. Levterov, Y. Panasiuk, O. Shablykin, O. Stashkevych, K. Sahun, A. Rassokhin, I. Sadkova, D. Lesyk, A. Anisiforova, Y. Holota, P. Borysko, I. Bodenchuk, N. M. Voloshchuk and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2024, 63, e202319831 CrossRef CAS PubMed
.
- For reviews on BCBs, see:
(a) M. Golfmann and J. C. L. Walker, Commun. Chem., 2023, 6, 9 CrossRef CAS PubMed
; (b) C. B. Kelly, J. A. Milligan, L. J. Tilley and T. M. Sodano, Chem. Sci., 2022, 13, 11721–11737 RSC
; (c) A. Fawcett, Pure Appl. Chem., 2020, 92, 751–765 CrossRef CAS
; (d) J. Turkowska, J. Durka and D. Gryko, Chem. Commun., 2020, 56, 5718–5734 RSC
; (e) M. A. A. Walczak, T. Krainz and P. Wipf, Acc. Chem. Res., 2015, 48, 1149–1158 CrossRef CAS PubMed
; (f) S. J. Sujansky and X. Ma, Asian J. Org. Chem., 2024, 13, e202400045 CrossRef CAS
; (g) Q.-Q. Hu, J. Chen, Y. Yang, H. Yang and L. Zhou, Tetrahedron Chem, 2024, 9, 100070 CrossRef CAS
; (h) J.-J. Feng, Synlett, 2024 DOI:10.1055/a-2406-3243
; (i) X. Zhan, H.-X. He, Q. Peng and J.-J. Feng, Synthesis, 2024 DOI:10.1055/a-2402-6920
. For selected examples on cycloadditions of BCBs, see:; (j) D. Sarkar, S. Deswal, R. C. Das and A. T. Biju, Chem. Sci., 2024, 15, 16243–16249 RSC
; (k) S. Deswal, A. Guin and A. T. Biju, Angew. Chem., Int. Ed., 2024, 63, e202408610 Search PubMed
. For selected examples on ring-openings of BCBs, see:; (l) H.-C. Shen, M. V. Popescu, Z.-S. Wang, L. de Lescure, A. Noble, R. S. Paton and V. K. Aggarwal, J. Am. Chem. Soc., 2023, 145, 16508–16516 CrossRef CAS PubMed
; (m) S.-L. Lin, Y.-H. Chen, H.-H. Liu, S.-H. Xiang and B. Tan, J. Am. Chem. Soc., 2023, 145, 21152–21158 CrossRef CAS PubMed
; (n) A. Guin, S. Bhattacharjee, M. S. Harariya and A. T. Biju, Chem. Sci., 2023, 14, 6585–6591 RSC
; (o) A. Guin, S. Deswal, M. S. Harariya and A. T. Biju, Chem. Sci., 2024, 15, 12473–12479 RSC
.
- Y. Zhang, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 23685–23690 CrossRef PubMed
.
- T. V. T. Nguyen, A. Bossonnet, M. D. Wodrich and J. Waser, J. Am. Chem. Soc., 2023, 145, 25411–25421 CrossRef CAS PubMed
.
- T. Yu, J. Yang, Z. Wang, Z. Ding, M. Xu, J. Wen, L. Xu and P. Li, J. Am. Chem. Soc., 2023, 145, 4304–4310 CrossRef CAS PubMed
.
- Y. Liu, S. Lin, Z. Ding, Y. Li, Y.-J. Tang, J.-H. Xue, Q. Li, P. Li and H. Wang, Chem, 2024, 10, 1–10 Search PubMed
.
- Z. Lin, H. Ren, X. Lin, X. Yu and J. Zheng, J. Am. Chem. Soc., 2024, 146, 18565–18575 CrossRef CAS PubMed
.
- J. Zhang, J.-Y. Su, H. Zheng, H. Li and W.-P. Deng, Angew. Chem., Int. Ed., 2024, 63, e202318476 CrossRef CAS PubMed
.
- Y. Liang, R. Nematswerani, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2024, 63, e202402730 CrossRef CAS PubMed
.
- Y. Xiao, F. Wu, L. Tang, X. Zhang, M. Wei, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202408578 CAS
.
- J.-L. Zhou, Y. Xiao, L. He, X.-Y. Gao, X.-C. Yang, W.-B. Wu, G. Wang, J. Zhang and J.-J. Feng, J. Am. Chem. Soc., 2024, 146, 19621–19628 CrossRef CAS PubMed
.
- X. Wang, R. Gao and X. Li, J. Am. Chem. Soc., 2024, 146, 21069–21077 CrossRef CAS PubMed
.
-
(a) L. Tang, Y. Xiao, F. Wu, J.-L. Zhou, T.-T. Xu and J.-J. Feng, Angew. Chem., Int. Ed., 2023, 62, e202310066 CrossRef CAS PubMed
; (b) L. Tang, Q.-N. Huang, F. Wu, Y. Xiao, J.-L. Zhou, T.-T. Xu, W.-B. Wu, S. Qu and J.-J. Feng, Chem. Sci., 2023, 14, 9696–9703 RSC
; (c) Y. Xiao, T.-T. Xu, J.-L. Zhou, F. Wu, L. Tang, R.-Y. Liu, W.-B. Wu and J.-J. Feng, Chem. Sci., 2023, 14, 13060–13066 RSC
; (d) X.-Y. Gao, L. Tang, X. Zhang and J.-J. Feng, Chem. Sci., 2024, 15, 13942–13948 RSC
.
- For groundbreaking research on the Lewis acid-catalyzed cycloadditions of BCBs, see:
(a) K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny and D. C. Leitch, Angew. Chem., Int. Ed., 2022, 61, e202204719 CrossRef CAS PubMed
. For base promoted non-asymmetric dearomative (3+3) cycloaddition of BCBs, see:; (b) K. Dhake, K. J. Woelk, L. D. N. Krueckl, F. Alberts, J. Mutter, M. O. Pohl, G. T. Thomas, M. Sharma, J. Bjornerud-Brown, N. P. Fernández, N. D. Schley and D. C. Leitch, Chem. Commun., 2024, 60, 13008–13011 RSC
. The preprint of this manuscript, see: ; (c) X.-C. Yang, F. Wu, W.-B. Wu, X. Zhang and J.-J. Feng, ChemRxiv, 2024 DOI:10.26434/chemrxiv-2024-zgffb
. After the preprint of this manuscript was posted on August 6, 2024, our group published two studies on Lewis acid-catalyzed enantioselective cycloadditions of BCBs:; (d) F. Wu, W.-B. Wu, Y. Xiao, Z. Li, L. Tang, H.-X. He, X.-C. Yang, J.-J. Wang, Y. Cai, T.-T. Xu, J.-H. Tao, G. Wang and J.-J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202406548 Search PubMed
; (e) W.-B. Wu, B. Xu, X.-C. Yang, F. Wu, H.-X. He, X. Zhang and J.-J. Feng, Nat. Commun., 2024, 15, 8005 CrossRef CAS PubMed
. During the peer review of our manuscript, Studer reported elegant (3+3) cycloadditions of BCBs with aziridines:; (f) S. Dutta, C. G. Daniliuc, C. Mück-Lichtenfeld and A. Studer, J. Am. Chem. Soc., 2024, 146, 27204–27212 CrossRef CAS PubMed
.
- For reviews on dearomatization reactions, see:
(a) Y.-Z. Cheng, Z. Feng, X. Zhang and S.-L. You, Chem. Soc. Rev., 2022, 51, 2145–2170 RSC
; (b) C. Zheng and S.-L. You, ACS Cent. Sci., 2021, 7, 432–444 CrossRef CAS PubMed
; (c) C. J. Huck and D. Sarlah, Chem, 2020, 6, 1589–1603 CrossRef CAS PubMed
; (d) F.-T. Sheng, J.-Y. Wang, W. Tan, Y.-C. Zhang and F. Shi, Org. Chem. Front., 2020, 7, 3967–3998 RSC
; (e) W. C. Wertjes, E. H. Southgate and D. Sarlah, Chem. Soc. Rev., 2018, 47, 7996–8017 RSC
; (f) C. Zheng and S.-L. You, Chem, 2016, 1, 830–857 CrossRef CAS
; (g) R. Remy and C. G. Bochet, Chem. Rev., 2016, 116, 9816–9849 CrossRef CAS PubMed
; (h) C.-X. Zhuo, C. Zheng and S.-L. You, Acc. Chem. Res., 2014, 47, 2558–2573 CrossRef CAS PubMed
; (i) Q. Ding, X. Zhou and R. Fan, Org. Biomol. Chem., 2014, 12, 4807–4815 RSC
; (j) S. P. Roche and J. A. Porco Jr, Angew. Chem., Int. Ed., 2011, 50, 4068–4093 CrossRef CAS PubMed
.
-
(a) K. W. Quasdorf and L. E. Overman, Nature, 2014, 516, 181–191 CrossRef CAS PubMed
; (b) D. Pierrot and I. Marek, Angew. Chem., Int. Ed., 2020, 59, 36–49 CrossRef CAS PubMed
; (c) S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626–2704 CrossRef CAS PubMed
; (d) F. Zhou, L. Zhu, B.-W. Pan, Y. Shi, Y.-L. Liu and J. Zhou, Chem. Sci., 2020, 11, 9341–9365 RSC
.
- Y. Liang, F. Paulus, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2023, 62, e202305043 CrossRef CAS PubMed
.
- L. Pitzer, F. Schäfers and F. Glorius, Angew. Chem., Int. Ed., 2019, 58, 8572–8576 CrossRef CAS PubMed
.
- CCDC 2375376 (for 3qq) and 2375377 (for (R)-3qw).
-
(a) X.-B. Wang, Y. Tian, L. Zhou, M.-S. Xie, G.-R. Qu and H.-M. Guo, Org. Lett., 2022, 24, 3861–3866 CrossRef CAS PubMed
; (b) B.-Y. Xue, C.-Y. Hou, X.-B. Wang, M.-S. Xie and H.-M. Guo, Org. Chem. Front., 2023, 10, 1910–1914 RSC
; (c) H.-X. Wang, C. Yang, B.-Y. Xue, M.-S. Xie, Y. Tian, C. Peng and H.-M. Guo, Nat. Commun., 2023, 14, 2270 CrossRef CAS PubMed
; (d) X.-Y. Wang, X.-B. Wang, Y. Tian, C. Peng, M.-S. Xie and H.-M. Guo, ACS Catal., 2023, 13, 11528–11540 CrossRef CAS
; (e) H.-L. Wang, T. Wei, R.-Y. Bi, M.-S. Xie and H.-M. Guo, Adv. Synth. Catal., 2024, 366, 3188–3193 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2375376 and 2375377. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc06334a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |