Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Improving the stability and activity of a dye-decolourizing peroxidase using NADESs†
Maria
Garbe
 *,
Linnea Ute
Lutz
*,
Linnea Ute
Lutz
 ,
Leander Tom
Lehmann
,
Theresa
Strotmann
,
Ralf G.
Berger
and
Franziska
Ersoy
,
Leander Tom
Lehmann
,
Theresa
Strotmann
,
Ralf G.
Berger
and
Franziska
Ersoy
Institute of Food Chemistry, Leibniz University Hannover, 30167 Hannover, Germany
First published on 8th March 2024
Abstract
Natural Deep Eutectic Solvents (NADESs) are composed of naturally occurring compounds such as organic acids, amines and sugars; they are biodegradable and have low toxicities. NADESs have gained attention not only as environmentally friendly solvents, but also for their enzyme stabilization effects at high temperatures or osmotic stress. Six betaine-based NADESs were tested regarding their impact on enzymatic activity and thermal stability of a dye-decolourizing peroxidase from the basidiomycete Pleurotus sapidus (PsaPOX). Betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sucrose
sucrose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (2
water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) and betaine
10) and betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trehalose
trehalose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (4
water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14) had the best impact on the thermostability. Coloured silk was discoloured more efficiently by PsaPOX after addition of either NADES, which indicates a potential as stain-removal agents. The yield of the enzymatic transformation of trans-anethole to the aroma compound p-anisaldehyde increased by a factor of three with both tested NADESs. Overall, it was shown that betaine-based NADESs serve as sustainable media for reactions using PsaPOX and increase temperature stability as well as enzymatic activity. They might thus improve the industrial usability of PsaPOX.
14) had the best impact on the thermostability. Coloured silk was discoloured more efficiently by PsaPOX after addition of either NADES, which indicates a potential as stain-removal agents. The yield of the enzymatic transformation of trans-anethole to the aroma compound p-anisaldehyde increased by a factor of three with both tested NADESs. Overall, it was shown that betaine-based NADESs serve as sustainable media for reactions using PsaPOX and increase temperature stability as well as enzymatic activity. They might thus improve the industrial usability of PsaPOX.
Sustainability spotlightThe efficient use of resources, their substitution, reuse, or recycling play an increasingly important role in our society. Key are sustainable, biodegradable, low-toxicity, and naturally occurring substances for science and industry. Natural Deep Eutectic Solvents (NADES) possess these characteristics and are crucial for the green solvent technology. The present work demonstrates their utilisation for the temperature-stabilisation of a dye-decolourising peroxidase from the edible mushroom Pleurotus sapidus. Potential industrial applications like dye decolourisation and flavour production profited from the utilisation of NADES. NADES thus enable sustainable consumption and production, and supports measures to combat climate change, all of which are vital cornerstones of the UN’s Sustainable Development Goals. |
1. Introduction
Dye-decolourizing peroxidases (DyPs) (EC 1.11.1.19) represent a novel superfamily of heme peroxidases with the ability to oxidize a wide range of dyes and alkenes and thus numerous applications in various fields.1,2 One of the first described DyPs, from the basidiomycete Thanatephorus cucumeris (formerly Geotrichum candidum), decolourized 18 different reactive, acidic, and dispersing dyes, a majority of which were xenobiotics. The DyP exhibited higher decolourizing activities than classical peroxidases toward anthraquinone dyes.3–5 Some DyPs are also capable of degrading other natural- or azo dyes.6–9 As many dyes are toxic, mutagenic, and carcinogenic, and resistant to conventional wastewater treatments, DyPs are interesting tools for bioremediation.10–12 In addition, some DyPs have a redox potential which is high enough to enable the oxidation of lignin.13Our group recently identified and characterized a DyP from Pleurotus sapidus (PsaPOX). It contained a non-canonical Mn2+-oxidation site on the protein surface and exhibited an alkene cleavage activity.7 The latter enabled its use as a biocatalyst to generate aromatic aldehydes with olfactory properties, such as veratraldehyde or p-anisaldehyde.7,14 These substances are used by the fragrance and flavor industries. It also bleached β-carotene and annatto, which is of interest for the detergent, milk, and baking industries.7,15,16 For industrial utilization, stabilizers are often necessary to improve the biocatalytic efficiency.17,18 One possibility is the use of natural deep eutectic solvents (NADESs).
NADESs are composed of two or more naturally occurring compounds. These can include sugars, amino acids, and organic acids, which, in combination, form a liquid phase at room temperature.19,20 Metabolic analyses have shown that NADESs naturally exist in organisms like plants: a system consisting of sucrose, fructose, and glucose protects cellular systems against freezing and drought.21 NADESs have several benefits over traditional organic solvents: they are biodegradable, have low toxicities, and are not easily flammable.22 In addition, they are simple to prepare and can be derived from renewable resources.23
NADESs have been used for various applications, e.g., biomolecule extraction and separation, as environmentally friendly solvents for chemical reactions, and as protein stabilizers.24–27 They can be customized through an adjustment of their composition and properties to optimize conditions for different types of enzymes.28–33 Studies on horseradish peroxidase (HRP) showed that the NADES betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sorbitol
sorbitol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) had the highest benefit because of its enzymatic activity. Alterations in the composition of the secondary structures illustrated the restructuring of the protein in the presence of NADESs through changes in the hydrogen bond network. Structural studies of HRP were conducted by determining the major secondary structure. The control contained 31% α-helices and 9% β-sheets, while, in the presence of NADESs, the α-helix content was extended by about 13%. This influenced the thermal stability of HRP positively.30,31 DESs and NADESs have shown advantageous effects on the activity and stability of various enzymes.21 Despite the limited industrial application of organophosphorus hydrolase due to its low thermal stability, promising results were observed by incubating the enzyme in ethaline and glyceline based-media.34 Moreover, studies focusing on protein folding and thermostability have reported favourable outcomes. The presence of choline chloride–fructose improved the stability of the therapeutic protein human interferon-a2 (IFN-a2), showcasing the advantageous solvent environment provided by NADESs for storing therapeutic peptides.35
3) had the highest benefit because of its enzymatic activity. Alterations in the composition of the secondary structures illustrated the restructuring of the protein in the presence of NADESs through changes in the hydrogen bond network. Structural studies of HRP were conducted by determining the major secondary structure. The control contained 31% α-helices and 9% β-sheets, while, in the presence of NADESs, the α-helix content was extended by about 13%. This influenced the thermal stability of HRP positively.30,31 DESs and NADESs have shown advantageous effects on the activity and stability of various enzymes.21 Despite the limited industrial application of organophosphorus hydrolase due to its low thermal stability, promising results were observed by incubating the enzyme in ethaline and glyceline based-media.34 Moreover, studies focusing on protein folding and thermostability have reported favourable outcomes. The presence of choline chloride–fructose improved the stability of the therapeutic protein human interferon-a2 (IFN-a2), showcasing the advantageous solvent environment provided by NADESs for storing therapeutic peptides.35
In this work, we utilized betaine-based NADESs to improve the enzymatic activity and stability of PsaPOX. Six different NADESs were characterized regarding their effect on the relative enzymatic activity and the best two were chosen for further work. Their effect on temperature stability and different examples for potential industrial applications of PsaPOX were evaluated. Both the decolourization of silk and the biotransformation of trans-anethole to p-anisaldehyde were investigated. It is presumed that the use of NADESs will lead to an enhancement in the enzymatic activity of PsaPOX and influence its stability at high temperatures. These effects are expected to have an impact on the application possibilities.
2. Experimental
2.1. Materials
Chemicals were obtained from Carl-Roth (Karlsruhe, Germany), Sigma-Aldrich (Seelze, Germany) Merck (Darmstadt, Germany) and Oterra (Nienburg, Germany) in p.a. quality.2.2. Synthesis of NADESs
The used NADESs were derived from Dai et al., Khodaverdian et al., and Tian et al.24,36,37 If NADESs were described as unstable in preliminary experiments, water was added to ensure long-term stability. NADESs were selected with substances known to have enzyme-stabilizing effects, such as sucrose and proline.21,38–40 All NADESs contained betaine as a hydrogen bond acceptor (HBA) and different combinations of hydrogen bond donors (HBD) (Table 1). The components were heated at 70 °C and 300 rpm until the solvents became stable and clear.| Abbreviations | Component A | Component B | Component C | Molar ratio |
|---|---|---|---|---|
| BSucW | Betaine anhydrous | Sucrose | Water | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| BTrehW | Betaine anhydrous | Trehalose dihydrate | Water | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| BProGlu | Betaine anhydrous | D/L-Proline | Glucose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| BSucPro | Betaine anhydrous | D/L-Proline | Sucrose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| BMalPro | Betaine anhydrous | Malic acid | D/L-Proline | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| BMal | Betaine anhydrous | Malic acid | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.3. Protein expression and purification
The PsaPOX gene was expressed in Komagataella phaffii and the recombinant His-tagged enzyme was purified from the culture supernatant using Ni-NTA affinity chromatography as described before.7,41 The purity of the enzyme was determined using SDS (12% resolving, 4% stacking gel) and native PAGE.2.4. Enzyme activity
The enzyme was prepared by mixing 22% NADES and 0.3 g per L peroxidase. The DyP was incubated at room temperature for 5 min. All assays were performed in triplicate. Blanks were carried out with water instead of the enzyme.The peroxidase activity was determined photometrically (EON™ High Performance Microplate Spectrophotometer, BioTek Instruments GmbH, Bad Friedrichshall, Germany) by monitoring the oxidation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt (ABTS) as a substrate in the presence of hydrogen peroxide at 30 °C and 420 nm (ε420 = 3.6 × 104 M−1 cm−1) for 10 min. The samples were mixed with malonic acid buffer (50 mM, pH 4), 50 μM hydrogen peroxide and 0.5 mM ABTS in a total volume of 300 μL.7
2.5. Thermostability
The thermostability of PsaPOX was tested in potassium phosphate buffer (50 mM) with 22% NADES. For the control, 22% 50 mM potassium phosphate buffer with the same pH value as the tested NADESs or 50 mM malonic acid buffer at pH 3.5, the pH-optimum of the enzyme, was added. PsaPOX was incubated at room temperature for 5 min at 200 rpm and 65, 75 or 80 °C for different intervals of time. For the stability tests at 65 °C, samples were taken after 2, 6, 10, 20, 30, 40 and 50 min, at 75 °C, samples were taken after 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 and 60 min and at 80 °C after 2, 4, 6, 8 and 10 min. The samples were immediately placed on ice. All samples were brought to room temperature and their activity was determined using the ABTS assay as described above.2.6. Decolourization assays
The decolourization of annatto, β-carotene, hispidin and laetiporic acid was evaluated. For β-carotene, a 9% (v/v) β-carotene emulsion was prepared with 1 g Tween 80 and 20 mL dichloromethane (DCM), and the solvent was removed by rotary evaporation.429% (v/v) emulsion or annatto (Oterra, Nienburg), 100 μM hydrogen peroxide, 25 mM manganese sulphate, 50 mM malonic acid buffer pH 4, 22% hispidin and laetiporic acid were extracted from fungal biomass using methanol as described elsewhere.43,44 20 mL of these extracts were transferred into an aqueous emulsion as described for β-carotene.
2.7. Silk destaining
Coffee (Extra, Penny), red wine (Chianti Colli Senesi, 2020, Sviando, Italy), and annatto (Oterra, Nienburg) were used to dye 4.5–5.8 g silk. For the dyeing solution, 22.6 g soluble coffee powder was dissolved in 1.2 L boiling water. The coffee drink was cooled to room temperature. For the dyeing with red wine and annatto, 600 mL red wine or 4 mL annatto reagent was mixed with 200 or 800 mL water, respectively. 1 mL white vinegar was added to each dyeing solution. The silk was washed with water and heated with one of the dyeing solutions at 80 °C for 1 h. After cooling down to RT, the silk was washed for 2 min with water and dried for 24 h at RT. For the decolourization experiments, the silk was cut into 4 × 2 cm squares.For decolourizing, a reaction mix containing 4 g NADES, 7.6 mL 50 mM malonate buffer pH 4, 1.58 mL 200 mM manganese sulphate, 4.82 mL purified enzyme (47 U L−1), and 1.8 mL 1 mM hydrogen peroxide was prepared. Controls without the enzyme, as well as without NADESs were included. Three dyed silk pieces were covered with the reaction mix and incubated at RT for 30 min. After incubation, the silk pieces were washed for 2 min with deionized water and dried for 16 h at 30 °C. The colour intensity of the silk was measured after staining and destaining using a spectrophotometer (ColorLite sph900). All changes in colour intensity were calculated in comparison to changes of the controls.
2.8. Biotransformation of trans-anethole
The transformation of trans-anethole to p-anisaldehyde was carried out using 20 U L−1 purified PsaPOX in 4 mL gas tight glass vials at a shaking rate of 200 rpm at 22 °C for 16 h in the absence of light. The reaction was performed in 50 mM Bis–Tris buffer pH 6, 100 μM hydrogen peroxide, 25 mM manganese sulphate and 22% NADES or 50 mM potassium phosphate buffer with the same pH as the NADES sample. In a total volume of 1 mL, 1 μL (6.7 mM) trans-anethole was added. Following the incubation period, trans-anethole and its resultant product, p-anisaldehyde, were extracted using 1 mL of hexane containing 100 mg per L (1 mM) cyclohexanol as an internal standard (IS).7 Blanks were conducted without enzyme (chemical blank) or with heat-inactivated enzyme (2 hours at 95 °C). The organic phase was dried with anhydrous sodium sulphate and subsequently analyzed by gas chromatography (GC). GC measurements were conducted using a GC-2025 Shimadzu Instrument (Kyoto, Japan) equipped with a Cyclosil B column (30 m × 0.32 mm, 0.25 μm, Agilent, Santa Clara, USA), a split/splitless injector port, and a flame ionization detection (FID) system. Hydrogen was employed as the carrier gas at a constant column flow rate of 2.5 mL per minute. A 1 μL sample was injected via an autosampler and analyzed using the following method: 40 °C (3 minutes), a temperature ramp of 5 °C per minute until 220 °C, followed by a final hold time of 10 minutes. Trans-anethole and p-anisaldehyde were semi-quantified based on the area of the internal standard.3. Results and discussion
3.1. Influence of NADESs on the enzymatic activity of PsaPOX
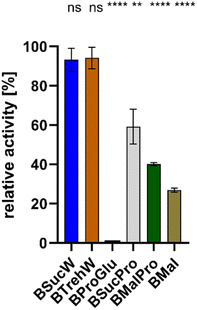
The influence of various NADESs on the activity of PsaPOX was determined. All NADESs were based on betaine as HBA, and sucrose, malic acid, glucose, proline and/or trehalose as HBD. PsaPOX was incubated in NADES-based media at 25 °C for 5 min and its relative activity was compared to that in the reference solution at the pH-optimum of 3.5 (100%, Fig. 1).7PsaPOX exhibited the best activities in BSucW and BTrehW with 93.1 and 94.1%, respectively. Reduced activities were measured in BSucPro (59.2%), BMalPro (40.1%) and BMal (26.9%). No activity could be detected in BProGlu.
PsaPOX did not reach its maximal activity (100%) in any of the NADESs. This was due to the deviation from the pH optimum: Krahe et al. demonstrated the highest activity at pH 3.5, with more than 50% activity conserved between pH 3 and 5. At higher pH ≤ 25% of the activity remained, with only 2.7% activity at pH 8.0, which corresponds to the pH of 7.9 of both BTrehW and BSucW.7 The shift in pH inevitably led to changes in the charge state and will have affected the protein structure. The addition of BSucW and BTrehW prevented this loss of activity. Instead, a high level of activity was maintained, indicating enzyme stabilization under significantly altered pH conditions. The results for BTrehW and BSucW confirm other studies which showed the ability of betaine-based NADESs to improve enzyme stability.24,31 Accordingly, BTrehW and BSucW were chosen for all subsequent analyses.
These two systems were tested with higher addition of water. This led to a change in viscosity and thus affected the enzyme activity. It can influence the formation of hydrogen bonds and may have contributed to an enhanced enzyme stabilization.32
Due to the low pH optimum of PsaPOX, it was supposed that NADESs with a pH of 3–5 should further enhance the yields. Acids as HBD, such as lactic acid, should have reduced the pH value and thus enhanced the activity of PsaPOX, but did not show the desired effect (NADESs BMal and BMalPro) and were thus disregarded for further experiments.
Proline, which is known to protect enzymes in plants, also showed no positive effect on PsaPOX in this study (NADESs BSucPro and BMalPro).45,46
Besides the water content of NADESs, their composition and the individual components also have an important influence on the relative activity. Altering the ratios changes the properties of the NADES and its interaction with the enzyme. For instance, certain DESs like ChCl–glycerol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or ChCl–ethylene glycol (2
1) or ChCl–ethylene glycol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) had a negative impact on the relative activity of HRP but improved its thermal stability in higher concentrations.47 The stability of NADESs themselves also needs to be ensured. NADESs containing sucrose and betaine (1
1) had a negative impact on the relative activity of HRP but improved its thermal stability in higher concentrations.47 The stability of NADESs themselves also needs to be ensured. NADESs containing sucrose and betaine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) without water are not stable at room temperature.36 It is crucial to find the correct concentrations of the respective NADES components. The composition can significantly influence their viscosity and stability, thereby impacting enzyme activity. For the tested NADESs and DyP in this study, the relative enzyme activity could not be increased, but the stability under different temperatures could be positively and significantly influenced.
1) without water are not stable at room temperature.36 It is crucial to find the correct concentrations of the respective NADES components. The composition can significantly influence their viscosity and stability, thereby impacting enzyme activity. For the tested NADESs and DyP in this study, the relative enzyme activity could not be increased, but the stability under different temperatures could be positively and significantly influenced.
For versatile peroxidases (VP) in ChCl–glycerol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and ChCl–urea (1
2) and ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the enzyme activity decreased at different concentrations of DESs. At pH 6 and in the presence of 10% (v/v) ChCl–glycerol NADES, the highest enzyme activity was achieved with about twice that of the control.48,49 Similar effects could also be identified for the DyP.
2), the enzyme activity decreased at different concentrations of DESs. At pH 6 and in the presence of 10% (v/v) ChCl–glycerol NADES, the highest enzyme activity was achieved with about twice that of the control.48,49 Similar effects could also be identified for the DyP.
3.2. Thermal stability of PsaPOX in NADESs
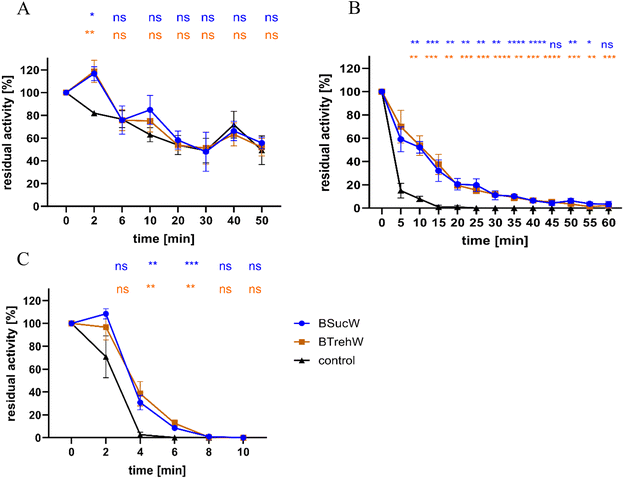
Both NADESs stabilized the enzyme activity of PsaPOX at 75 and 80 °C (Fig. 2). Without NADESs, a complete loss of enzyme activity occurred after 25 min at 75 °C. In contrast, samples containing BSucW and BTrehW showed measurable enzyme activities over the entire period of 60 min. After 15 minutes, activities of 32.1% (BSucW) and 37.5% (BTrehW) were measured in the NADESs, whereas only 1% remained in the control. A similar trend was observed at 80 °C, where a loss of activity of up to 96% was detected within 4 min in the control sample, while the samples containing NADESs showed only 69.2 (BSucW) and 61.5% (BTrehW) loss. No significant changes were observed at 65 °C.Many plants accumulate betaine and proline in stressful situations like heat or high salt conditions.50–53 It was shown that this protected enzymes from heat inactivation.39 This was attributed to the formation of strong molecular interactions that potentially affect molecular mobility, which can also influence the thermal stability of proteins. The enzymatic activity of horseradish peroxidase at different temperatures was studied in betaine-based NADES.30,31 Several of them, including NADESs with trehalose, sucrose and water, resulted in a 60% increase in enzymatic activity. Betaine, trehalose, glycerol and water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) kept HRP activity stable at 60 °C for 24 h.30 One of the highest enzymatic activities was achieved in betaine–sorbitol–water (1
5) kept HRP activity stable at 60 °C for 24 h.30 One of the highest enzymatic activities was achieved in betaine–sorbitol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), which achieved an about two-fold increase in enzyme activity.31 Also ChCl-containing DESs like ChCl–glycerol (2
3), which achieved an about two-fold increase in enzyme activity.31 Also ChCl-containing DESs like ChCl–glycerol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and ChCl–ethylene glycol (2
1) and ChCl–ethylene glycol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were tested to improve thermal stability. Compared to the control, the NADESs slightly increased the relative activity of the HRP, compared to the control. Higher DES concentrations triggered the enzyme to be less active, but much more stable. For example, in the mixture of ChCl–urea (1
1) were tested to improve thermal stability. Compared to the control, the NADESs slightly increased the relative activity of the HRP, compared to the control. Higher DES concentrations triggered the enzyme to be less active, but much more stable. For example, in the mixture of ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), an enhancement in stability of up to 6.0-fold was measured.47 For the tested NADESs in this study, thermal stability appeared to be positively influenced without any significant loss in relative activity. In a study by Wu et al., a choline acetate-based NADES promoted both HRP activity and stability.47
2), an enhancement in stability of up to 6.0-fold was measured.47 For the tested NADESs in this study, thermal stability appeared to be positively influenced without any significant loss in relative activity. In a study by Wu et al., a choline acetate-based NADES promoted both HRP activity and stability.47
Not only did choline-based NADESs show effects on HRP, studies by Chang et al. showed a heat-stabilizing effect of sucrose on HRP.38 In addition, trehalose is important for the induction of thermotolerance in yeast and other microorganisms.54,55 These positive effects of betaine, as well as trehalose and sucrose, were also observed here for PsaPOX.
Essential for the heat stability is the protein's secondary structure that undergoes restructuring in the presence of NADESs by establishing new hydrogen bond networks. CD (circular dichroism) spectroscopy has shown that the NADES which improved enzyme activity the most facilitated higher portions of α-helical structures.30,31 CD spectra showed that trypsin's secondary structure contained less α-helical area and a higher number of β-sheets folded into two domains in the NADES with betaine and trehalose.37 Mamashli et al. measured ultraviolet CD spectra of versatile peroxidase with different DES contents at pH 4.5 and 7. The results showed that the α-helical content increased with the increase of DES concentration.48 To explain the effect of NADESs on PsaPOX, the changes of the protein structure were determined in buffer with different concentrations of guanidine hydrochloride (GdnHCl) (Fig. S3†). The fluorescence of intrinsic aromatic amino acids increased more strongly in the control with higher concentrations of denaturing buffer. It has to be assumed that the addition of NADESs slows down the unfolding of PsaPOX. Gajardo-Parra et al. demonstrated that the addition of NADESs such as BSorbW resulted in an increase in the unfolding temperature of horseradish peroxidase (HRP). The findings suggest that interactions between NADESs and amino acids promote intermediate states during HRP folding, coupled with changes in the protein's secondary structure.31 It is assumed that these intermediate states are associated with a slower unfolding, which is also observed in DyPs.
Some NADESs also reduced enzyme flexibility, thus improving the active site stability. It is also known that NADESs generate an increase in water on the protein surface, which stabilizes the active site.30 Which of these effects led to the increased stability of PsaPOX has to be addressed by future analyses, but the results are promising to run industrial processes over an extended period of time at elevated temperatures.
3.3. Decolourization of dyes in NADESs
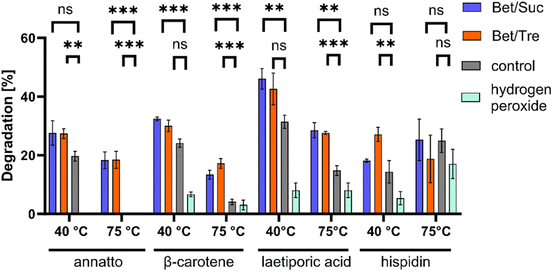
PsaPOX belongs to a unique heme peroxidase family and is known for its ability to decolourize dyes.9 NADESs positively impacted the degradation of natural dyes such as β-carotene and annatto using PsaPOX (Fig. 3). Within 20 min at 40 °C, approximately 32% β-carotene was degraded in NADESs, while only 24% degradation was observed in the buffer solution. The effect became even more pronounced at 75 °C, where NADESs facilitated the degradation of 17% β-carotene, while only 4% degradation was evident in the control. For annatto, no degradation was measured in the control at 75 °C, while 18% degradation occurred in both NADESs. Similar trends were observed with laetiporic acid. No significant differences between NADESs and control were observed for hispidin at 75 °C and at 40 °C, a positive effect on dye degradation was shown only for BTrehW.These findings further support the stabilizing effect of NADESs on enzymatic activities. Different studies have shown that the use of sugars as HBD leads to high viscosities due to the presence of a more pronounced intermolecular hydrogen-bond network. A decreased Km was measured for a lipase in a NADES consisting of choline chloride and sucrose.56 As the NADESs used in the present study contained sugars as HBD this is a possible explanation for the improved decolourisation.
In addition, the active site can be influenced by the change in hydrogen bonding patterns, leading to improved substrate binding.29 This can enhance substrate degradation and alter enzyme stability, as observed for PsaPOX. The literature about solvent–enzyme and NADES–substrate interactions is very limited. Nian et al. showed that the interaction of a lipase with NADESs increased the nucleophilic properties of the substrate, promoting the reaction.57 A possible explanation is the effect of the increased water content in NADESs on solvated enzymes as described for lipases: the mobility of the enzyme increases with the hydration level of NADES.58
The structural differences between hispidin as a polyketide and the other terpenoid dyes could have led to the formation of hydrogen bonds between NADESs and hispidin. A resultant substrate stabilization may prevent effective binding to the active site of PsaPOX or affect the reaction's activation energy and equilibrium. Comparable effects were discussed by Kovács et al.29
In summary, it can be said that the improvement in decolorization may be a result of the enhanced stability of the DyP or an improved enzyme–substrate interaction facilitated by the NADES. It is evident that not all dyes are equally influenced by the interaction with the NADES and the DyP.
3.4. PsaPOX and NADESs as stain removers for textiles
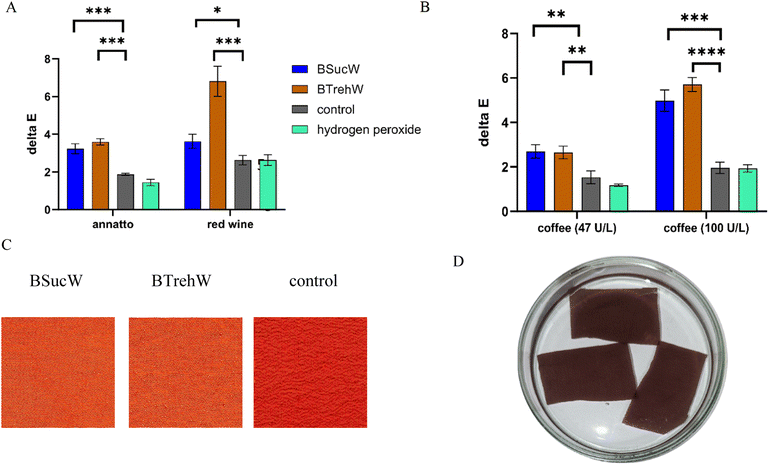
Silk fabric was stained with red wine and coffee as examples for everyday stains and treated with a stain removal solution (NADESs, PsaPOX, buffer, manganese sulphate, and hydrogen peroxide). In addition to the purified enzyme, the culture supernatant was tested as a stain remover, which would save the costs associated with enzyme purification for a potential industrial application. In almost all samples, a two-fold increase in textile decolourization was achieved by the addition of NADESs (Fig. 4A and B). Furthermore, a visually detectable improvement of the destaining of annatto treated silk was observed (Fig. 4C). The addition of hydrogen peroxide had a noticeable destaining effect; however, it was significantly lower than after the addition of PsaPOX. The culture supernatant with higher enzyme activities showed the activity-dependency of the decolourization (Fig. 4B). No inhibitory effect of the culture supernatant was detected, indicating that a combination with other destaining enzymes should be unproblematic. Purification of the enzyme is therefore not necessary, avoiding activity losses and reducing the cost of the approach.3.5. Biotransformation of trans-anethole in NADESs
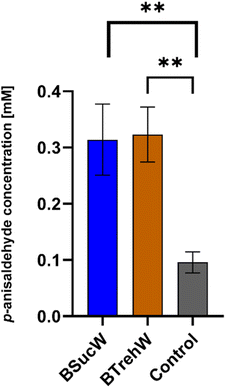
As shown above (Fig. 3), PsaPOX showed alkene cleavage activity towards the natural dyes β-carotene, annatto and laetiporic acid in NADESs. An improved cleavage was also observed for trans-anethole. The product concentration of p-anisaldehyde was increased by a factor of 3 with both NADESs (Fig. 5).As demonstrated in previous experiments, enzyme stabilization may have occurred during the 16 hour incubation period with the NADESs. There was no significant difference between BTrehW and BSucW in this regard. Increased cleavage of trans-anethole increased the production of p-anisaldehyde, which can be used for the synthesis of pharmaceuticals or as a fragrance in the food industry. Another explanation for the improved biotransformation in NADESs is the formation of hydrogen bonds for stabilizing the enzyme–substrate complex.29 Similar results have already been achieved in cannabinoid production. Compared to aqueous systems, the use of NADESs resulted in a two-fold increase of cannabinoid production with a tetrahydrocannabinolic acid synthase and more than a three-fold increase with a cannabichromenic acid synthase.59
A study by Yang et al. explored the feasibility of chemobiocatalytic production of furfuryl alcohol from D-xylose using tandem catalysis, the deep eutectic solvent betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzenesulfonic acid and recombinant Escherichia coli DCF cells. Application of 2.5 wt% DES BE
benzenesulfonic acid and recombinant Escherichia coli DCF cells. Application of 2.5 wt% DES BE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BA catalyzed D-xylose at 170 °C for 0.5 h, yielding 51.1% furfural.60 NADESs hold promise as sustainable media for improving product yields in various industries.
BA catalyzed D-xylose at 170 °C for 0.5 h, yielding 51.1% furfural.60 NADESs hold promise as sustainable media for improving product yields in various industries.
4. Conclusions
This study established that several betaine-based NADESs enhanced PsaPOX stability at temperatures of 75 and 80 °C and a pH of 7.9. Moreover, NADESs improved the degradation of polyene dyes such as β-carotene and annatto and removal of coffee and red wine stains from silk samples. Alkene cleavage of trans-anethole to p-anisaldehyde was also enhanced.The potential of PsaPOX as a biocatalyst for the degradation of β-carotene, as a stain remover and for the generation of aromatic aldehydes with olfactory properties was shown. For all tested applications, NADESs served as sustainable media with a stabilizing effect. Recently, molecular-scale modelling of NADESs and enzymes was established as an alternative to determine the optimal NADES for a specific enzyme based on the predicted intermolecular interactions.29,61 This should simplify and broaden the application of NADESs to improve enzymatic reactions.
Author contributions
M. G. – conceptualization, data curation, investigation, methodology, writing: original draft; L. U. L. – investigation; L. T. L. – investigation, data curation, formal analysis, T. S. – investigation, data curation, formal analysis; R. G. B. and F. E. – methodology, supervision, funding acquisition, writing – review and editing. All authors have read and agreed to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Notes and references
- T. Caldas, N. Demont-Caulet, A. Ghazi and G. Richarme, Microbiology, 1999, 145(pt 9), 2543–2548 CrossRef CAS PubMed.
- Y. Sugano, R. Muramatsu, A. Ichiyanagi, T. Sato and M. Shoda, J. Biol. Chem., 2007, 282, 36652–36658 CrossRef CAS PubMed.
- S.-J. Kim, K. Ishikawa, M. Hirai and M. Shoda, J. Ferment. Bioeng., 1995, 79, 601–607 CrossRef CAS.
- M. Shoda, Commun. Agric. Appl. Biol. Sci., 2003, 68, 269–274 CAS.
- S. Kim and M. Shoda, Appl. Environ. Microbiol., 1999, 65, 1029–1035 CrossRef CAS PubMed.
- G. Rajhans, S. Sen, A. Barik and S. Raut, J. Appl. Microbiol., 2020, 129, 1633–1643 CrossRef CAS.
- N.-K. Krahe, R. G. Berger and F. Ersoy, Molecules, 2020, 25, 69–85 CrossRef PubMed.
- C. Lauber, T. Schwarz, Q. K. Nguyen, P. Lorenz, G. Lochnit and H. Zorn, AMB Express, 2017, 7, 1–15 CrossRef CAS PubMed.
- D. I. Colpa, M. W. Fraaije and E. van Bloois, J. Ind. Microbiol. Biotechnol., 2014, 41, 1–7 CrossRef CAS PubMed.
- A. Bafana, S. S. Devi and T. Chakrabarti, Environ. Rev., 2011, 19, 350–371 CrossRef CAS.
- W.-Y. Li, F.-F. Chen and S.-L. Wang, Protein Pept. Lett., 2010, 17, 621–629 CrossRef CAS PubMed.
- A. K. Singh, M. Bilal, H. M. N. Iqbal, A. S. Meyer and A. Raj, Sci. Total Environ., 2021, 777, 145988 CrossRef CAS PubMed.
- C. Liers, C. Bobeth, M. Pecyna, R. Ullrich and M. Hofrichter, Appl. Microbiol. Biotechnol., 2010, 85, 1869–1879 CrossRef CAS PubMed.
- N.-K. Krahe, R. G. Berger, M. Witt, H. Zorn, A. B. Omarini and F. Ersoy, Int. J. Mol. Sci., 2021, 22(1363), 91–113 Search PubMed.
- R. T. Szweda, K. Schmidt and H. Zorn, Eur. Food Res. Technol., 2013, 237, 377–384 CrossRef CAS.
- C. Harrel, Ind. Eng. Chem., 1952, 44, 95–100 CrossRef CAS.
- A. S. Bommarius and M. F. Paye, Chem. Soc. Rev., 2013, 42, 6534–6565 RSC.
- M. Pätzold, S. Siebenhaller, S. Kara, A. Liese, C. Syldatk and D. Holtmann, Trends Biotechnol., 2019, 37, 943–959 CrossRef PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS PubMed.
- S. M. Taklimi, A. Divsalar, B. Ghalandari, X. Ding, M. L. Di Gioia, K. A. Omar and A. A. Saboury, J. Mol. Liq., 2023, 377, 121562 CrossRef CAS.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustain. Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- S. Khodaverdian, B. Dabirmanesh, A. Heydari, E. Dashtban-Moghadam, K. Khajeh and F. Ghazi, Int. J. Biol. Macromol., 2018, 107, 2574–2579 CrossRef CAS PubMed.
- Y. Ma, Y. Li, S. Ali, P. Li, W. Zhang, M. C. Rauch, S. J. P. Willot, D. Ribitsch, Y. H. Choi and M. Alcalde, ChemCatChem, 2020, 12, 989–994 CrossRef CAS.
- M. Faggian, S. Sut, B. Perissutti, V. Baldan, I. Grabnar and S. Dall'Acqua, Molecules, 2016, 21, 1531 CrossRef PubMed.
- H. Vanda, R. Verpoorte, P. G. Klinkhamer and Y. H. Choi, Deep Eutectic Solvents: Synthesis, Properties, and Applications, 2019, pp. 61–81 Search PubMed.
- A. E. Delorme, J.-M. Andanson and V. Verney, Int. J. Biol. Macromol., 2020, 163, 919–926 CrossRef CAS PubMed.
- A. Kovács, M. Yusupov, I. Cornet, P. Billen and E. C. Neyts, J. Mol. Liq., 2022, 366, 120180 CrossRef.
- L. Meneses, N. F. Gajardo-Parra, E. Cea-Klapp, J. M. Garrido, C. Held, A. R. Duarte and A. Paiva, RSC Sustainability, 2023, 1, 886–897 RSC.
- N. F. Gajardo-Parra, L. Meneses, A. R. C. Duarte, A. Paiva and C. Held, ACS Sustain. Chem. Eng., 2022, 10, 12873–12881 CrossRef CAS PubMed.
- Y. Zhou, Y.-J. Wu, L. Wang, J. Han, J.-C. Wu, C.-M. Li and Y. Wang, Int. J. Biol. Macromol., 2021, 190, 206–213 CrossRef CAS PubMed.
- C. Knudsen, K. Bavishi, K. M. Viborg, D. P. Drew, H. T. Simonsen, M. S. Motawia, B. L. Møller and T. Laursen, Phytochemistry, 2020, 170, 112214 CrossRef CAS PubMed.
- M. Mirzaei, H. Khorshahi, H. Tebyanian, R. S. Moradi, M. Rastegar, S. Panahi, R. Sariri and A. M. Latifi, Biointerface Res. Appl. Chem., 2020, 10, 6488–6497 CAS.
- M. S. Lee, K. Lee, M. W. Nam, K. M. Jeong, J. E. Lee, N. W. Kim, Y. Yin, S. Y. Lim, J. Lee and J. H. Jeong, J. Ind. Eng. Chem., 2018, 65, 343–348 CrossRef CAS.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- Y. Tian, M. Zhu, T. Hu and C. Liu, Int. J. Biol. Macromol., 2023, 125477 CrossRef CAS PubMed.
- B. S. Chang, K. H. Park and D. B. Lund, J. Food Sci., 1988, 53, 920–923 CrossRef CAS.
- D. Nash, L. Paleg and J. Wiskich, Funct. Plant Biol., 1982, 9, 47–57 CrossRef CAS.
- L. Paleg, T. Douglas, A. Van Daal and D. Keech, Funct. Plant Biol., 1981, 8, 107–114 CrossRef CAS.
- A. Nieter, S. Kelle, M. Takenberg, D. Linke, M. Bunzel, L. Popper and R. G. Berger, Food Chem., 2016, 209, 1–9 CrossRef CAS PubMed.
- D. Linke, A. B. Omarini, M. Takenberg, S. Kelle and R. G. Berger, Appl. Biochem. Biotechnol., 2019, 187, 894–912 CrossRef CAS PubMed.
- P. Bergmann, M. Takenberg, C. Frank, M. Zschätzsch, A. Werner, R. G. Berger and F. Ersoy, Fermentation, 2022, 8, 541 CrossRef CAS.
- P. Bergmann, C. Frank, O. Reinhardt, M. Takenberg, A. Werner, R. G. Berger, F. Ersoy and M. Zschätzsch, Fermentation, 2022, 8, 684 CrossRef CAS.
- K. Shah and R. S. Dubey, Biol. Plantarum, 1997, 40, 121–130 CrossRef CAS.
- X. Liang, L. Zhang, S. K. Natarajan and D. F. Becker, Antioxidants Redox Signal., 2013, 19, 998–1011 CrossRef CAS PubMed.
- B.-P. Wu, Q. Wen, H. Xu and Z. Yang, J. Mol. Catal. B: Enzym., 2014, 101, 101–107 CrossRef CAS.
- F. Mamashli, J. Badraghi, B. Delavari, H. Lanjanian, M. Sabbaghian, M. Hosseini and A. A. Saboury, J. Mol. Liq., 2018, 272, 597–608 CrossRef CAS.
- F. Mamashli, J. Badraghi, B. Delavari, M. Sabbaghian, M. Hosseini and A. A. Saboury, J. Solution Chem., 2019, 48, 689–701 CrossRef CAS.
- T. Singh, D. Aspinall and L. Paleg, Nat. New Biol., 1972, 236, 188–190 CrossRef CAS PubMed.
- R. Storey and R. W. Jones, Plant Sci. Lett., 1975, 4, 161–168 CrossRef CAS.
- T. M. Chu, D. Aspinall and L. Paleg, Funct. Plant Biol., 1974, 1, 87–97 CrossRef CAS.
- F. Zulfiqar, M. Ashraf and K. H. Siddique, Agronomy, 2022, 12, 276 CrossRef CAS.
- C. De Virgilio, T. Hottiger, J. Dominguez, T. Boller and A. Wiemken, Eur. J. Biochem., 1994, 219, 179–186 CrossRef CAS PubMed.
- A. Wiemken, Antonie van Leeuwenhoek, 1990, 58, 209–217 CrossRef CAS PubMed.
- A. A. Elgharbawy, A. Hayyan, M. Hayyan, S. N. Rashid, M. R. M. Nor, M. Y. Zulkifli, Y. Alias and M. E. S. Mirghani, Chem. Biochem. Eng. Q., 2018, 32, 359–370 CrossRef CAS.
- B. Nian, G. Liao, Y. Song, Y. Su, C. Cao and Y. Liu, J. Catal., 2020, 384, 159–168 CrossRef CAS.
- H. Monhemi, M. R. Housaindokht, A. A. Moosavi-Movahedi and M. R. Bozorgmehr, Phys. Chem. Chem. Phys., 2014, 16, 14882–14893 RSC.
- F. Thomas and O. Kayser, Biochem. Eng. J., 2022, 180, 108380 CrossRef CAS.
- L. Yang, Y. Liu, Y. Peng, Y.-C. He and C. Ma, Catal. Commun., 2023, 184, 106783 CrossRef CAS.
- A. Kovács, E. C. Neyts, I. Cornet, M. Wijnants and P. Billen, ChemSusChem, 2020, 13, 3789–3804 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00359k |
| This journal is © The Royal Society of Chemistry 2024 |