Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tailored sulfonated carbons: unraveling enhanced catalytic dynamics for fructose dehydration under conventional and microwave heating†
Gabrielle Mathias
Reis
 ,
Letícia Ferreira Lima
Machado
,
Renan Silva
Nunes
,
Dalmo
Mandelli
,
Letícia Ferreira Lima
Machado
,
Renan Silva
Nunes
,
Dalmo
Mandelli
 and
Wagner Alves
Carvalho
and
Wagner Alves
Carvalho
 *
*
Centre for Natural Sciences and Humanities, Federal University of ABC (UFABC), 09280-560 Santo André, Brazil. E-mail: wagner.carvalho@ufabc.edu.br
First published on 15th March 2024
Abstract
This investigation is centered on the synthesis and evaluation of sulfonated carbons (SCs) employed as catalysts in the transformation of fructose into 5-hydroxymethylfurfural (5-HMF). The sulfonated carbons were synthesized through carbonization and in situ sulfonation in the presence of H2SO4, using glycerol and glucose as precursors. Experimental conditions were systematically optimized to achieve an appropriate quantity and density of surface acidic sites, primarily sulfonic and carboxylic groups, pivotal for the fructose dehydration reaction. The catalytic performance of these sulfonated carbons was assessed under both conventional and microwave heating. Notably, the most favorable outcomes were observed in a microwave-assisted system, achieving an 80% conversion at 120 °C within a concise 5 min reaction time. This feat was realized using a 5 wt% fructose solution in dimethylsulfoxide (DMSO) and a 5 wt% catalyst, with selectivity to 5-HMF reaching values as high as 98%. The findings elucidate the impact of the heating mode, alongside the textural properties and surface density of acid groups, providing valuable insights for the optimization of catalysts in fructose conversion processes. This investigation provides pertinent insights into the domain, underscored by the significance of fine-tuning synthesis conditions and reaction parameters for the development of effective catalysts in the context of fructose-to-5-HMF conversion.
| Gabrielle Mathias Reis Gabrielle Mathias Reis is a PhD student in science and technology/chemistry (UFABC-in progress) and scholarship holder of PRH/ANP N°49 from UFABC (FINEP/FUNDEP Management). He has a master’s degree in science and technology in the field of chemistry from the Federal University of ABC (2021) and a bachelor's degree in chemistry from Universidade Federal Fluminense (2018). He has experience in chemistry in catalysis and adsorption aimed at green chemistry, mainly on the following topics: determination and quantification of heavy metals through zeolites, treatment of industrial waste and biomass, development of new catalysts for the conversion of biomass, acid carbon, and characterization of solids. |
| Letícia Ferreira Lima Machado Letícia Ferreira Lima Machado is a master's student in chemistry at the Federal University of ABC-UFABC. She graduated with a bachelor's degree in chemistry with an emphasis on technological chemistry from the Fluminense Federal University-UFF (2020). In the period 2017-2018 she was a CNPq Scientific Initiation Fellow at the Molecular Modeling Laboratory of the Institute of Exact Sciences-UFF, having worked with molecular modeling of active compounds against HSV-1. |
| Renan Silva Nunes Renan Silva Nunes is a PhD student in science and technology in the area of chemistry at UFABC through the Industrial Academic Doctorate Program with a sandwich period in Portugal at the Instituto Superior Técnico of the University of Lisbon, having also worked at the Instituto Superior de Engenharia de Lisboa (ISEL). He has experience in the areas of physical chemistry, materials engineering, and chemometrics. He has worked on the following topics: (1) the development of catalysts, adsorbents, and nanomaterials with applications in catalysis, adsorption, and disinfection; (2) advanced treatment of sanitary and industrial effluents; (3) valorization of biomass and industrial waste; (4) nanotechnology; (5) chemometrics and design of experiments (DoE); (6) optimization and multivariate modeling of chemical processes; (7) technological innovation, patents and applied research. |
| Dalmo Mandelli Dalmo Mandelli has a bachelor's degree, master’s degree, and doctorate in chemistry from UNICAMP (1992, 1994, and 1999, respectively). He developed part of his PhD at the Delft University of Technology (Netherlands). He interned at the University of Bergen (Norway), Rheinisch-Westfaelische Technische Hochschule (Aachen, Germany), and Katholieke Universiteit Leuven (Belgium). He is currently an international relations advisor, professor, and researcher at the Federal University of ABC, working in catalysis (alkene metathesis reactions, oxidation, hydrogenation, hydrogenolysis, esterification, and etherification of organic compounds, including natural products) using environmentally friendly processes to produce inputs for the petroleum industry, plastics, flavors, and pharmaceutical products. He has experience in chemometrics and the identification and quantification of organic compounds by GC, GC-MS, HPLC, NMR, and IR. In 2021 he won the Kurt Politzer Prize for the Technology and Innovation-Researcher Category. |
| Wagner Alves Carvalho Wagner Alves Carvalho has a bachelor’s degree in chemistry from the State University of Campinas (1990), a master’s degree in chemistry from the State University of Campinas (1992) and Doctor in Sciences from the State University of Campinas (1997). He did a post-doctoral internship at the Faculty of Chemical Engineering at the State University of Campinas and an internship as a visiting professor at the Center for Surface Chemistry and Catalysis, University of Leuven (K.U.Leuven), Belgium. He is currently an associate professor at the Federal University of ABC and master's/doctorate advisor in the postgraduate program in science and technology/chemistry at UFABC. He was Vice-Rector from 2018 to 2022 and is currently Pro-Rector of Research at UFABC. He has experience in chemistry, with an emphasis on catalysis and adsorption processes, with research on the following topics: hydrogenation and catalytic oxidation, polyol and lignin conversion reactions, environmental control, effluent treatment, molecular sieves, natural zeolites, clays, niobia, and activated carbons. |
Sustainability spotlightFructose dehydration stands out as an appealing method for 5-HMF production, but sustainable practices require addressing challenges in selecting environmentally friendly solvents and catalysts. Dimethylsulfoxide (DMSO) emerges as a widely utilized solvent, prized for its low toxicity, affordability, and effective fructose solubilization. However, DMSO's drawback lies in its potential to encourage cross-polymerization, leading to undesired humin formation and reducing 5-HMF selectivity. To counter this, we refine the preparation of acid catalysts, employing microwave heating for swift, high-activity outcomes. This optimization is pivotal in mitigating the negative impact of DMSO, advancing sustainable 5-HMF production via fructose dehydration. |
Introduction
The dehydration of fructose emerges as an attractive approach for synthesizing 5-hydroxymethylfurfural (5-HMF), recognized as a carbon-neutral feedstock for the production of fuels and other chemicals.1 However, ensuring sustainable production necessitates overcoming several challenges, notably the utilization of environmentally friendly solvents and catalysts.2 Dimethylsulfoxide (DMSO) has been widely used as a solvent due to its low toxicity, low cost, and good fructose solubilization capacity. Typically the fructose dehydration in DMSO systems results in high yields of 5-HMF and selectivities greater than 90%.3The role of this solvent in the dehydration of fructose has been extensively investigated, including in our previous work,4 resulting in the emergence of numerous fresh insights within the literature.5,6 DMSO can either enhance or hinder the efficiency of the reaction, depending on the catalyst and applied conditions. This solvent plays a crucial role in stabilizing the produced 5-HMF, preventing its rehydration and the formation of levulinic and formic acids,7–9 Furthermore, it modifies the distribution of fructose isomers, predominantly favoring the presence of the β-D-fructofuranose isomer. This isomer is more susceptible to the selective dehydration reaction.10
Nevertheless, our discoveries4,11,12 and findings in the literature8,13 reveal that DMSO can promote cross-polymerization among the solvent, substrate, and products, resulting in the undesirable formation of humins and reduced selectivity towards 5-HMF. This phenomenon proves detrimental to reaction efficiency, as humins become the predominant product. In addition to diminishing the yield of 5-HMF, humins strongly interact with and obstruct the Brønsted acidic sites of various catalyst types.11,14–16 The higher the reaction temperature and time, the greater the adverse contribution of DMSO. One approach to minimize the adverse impact of DMSO is by diminishing the reaction time, and an effective method for achieving this is using microwave heating (MH) as opposed to conventional heating (CH). The application of microwave-assisted reactions not only addresses the challenges associated with DMSO but also tends to result in shorter reaction times and higher selectivities. This reduction in reaction time can be instrumental in mitigating issues related to product separation from DMSO, ultimately minimizing the exposure of the main product to conditions that could potentially lead to its degradation.17 One strategy to inhibit the catalytic activity of DMSO is to perform the reaction under an inert atmosphere and mild conditions (<100 °C).4 Another strategy reported in our previous work12 is to perform the reaction under microwave heating using optimized conditions to minimize the contact time of the reaction products with DMSO. Therefore, optimizing the reaction conditions (i.e. temperature, time, concentration of the substrate, and amount of the catalyst) is an important step towards obtaining a high 5-HMF yield and selectivity.
Among the catalysts employed in fructose dehydration, SCs stand out as particularly appealing, given that carbon possesses Brønsted acid groups. These acid functionalities on the carbon catalyst surface play a pivotal role in facilitating the conversion of fructose to 5-HMF, making SCs effective and environmentally friendly catalysts in this chemical transformation.18 These materials are commonly derived from agricultural waste, making a significant contribution to the circular economy,19–21 especially in emerging countries. Moreover, there is compelling evidence suggesting that SCs do not contribute to undesirable cross-polymerization reactions. This is attributed to the low affinity of 5-HMF for the hydrophilic surface of these catalysts.22,23
Progress in the synthesis methodology of SCs has enabled the creation of a novel catalyst characterized by high acidity, utilizing fewer reactants, shortening the required time, and reducing energy consumption.11,24 Moreover, the physicochemical characteristics of SCs, including acidity, chemical composition, and textural properties, can be precisely adjusted through careful selection of the raw material and optimization of the in situ carbonization conditions. This was emphasized in our recent report,12 where 5-HMF was obtained from a concentrated fructose solution in DMSO in just 10 seconds at 120 °C using MH. The resulting 5-HMF yield reached 93.3%, showcasing a remarkable selectivity of up to 98%.
However, recent reports in the literature show that the surface acidic sites of SCs, particularly carboxylic and sulfonic groups, can synergistically collaborate to significantly enhance the efficiency of fructose dehydration.12,25 Wang et al.26 investigated the selectivity of the Glu-TsOH catalyst, which contains both carboxylic and sulfonic groups, in converting fructose to 5-HMF. Deliberate experiments were subsequently carried out using a mixture of Amberlyst-15 (containing only sulfonic groups) and acetic acid to partially emulate the Glu-TsOH catalyst. The authors demonstrated a higher 5-HMF yield in the mixture compared to the sum of that of the individual catalysts, indicating a synergistic effect between sulfonic and carboxylic groups, facilitating dehydration and improving selectivity for 5-HMF.
Moreover, when designing an efficient SC for fructose dehydration, various characteristics such as surface area, porosity, acidic site density, and diffusional limitations must be considered. Catalysts featuring a well-developed porous structure can facilitate the removal of the product from the catalyst surface.27 It is essential to highlight that a well-established pore structure, especially with a significant presence of micropores, poses challenges in efficiently removing the target product from the vicinity of active sites. As a result, the probability of undesirable reactions, leading to the formation of humins, tends to increase. SCs characterized by numerous acidic sites and a high surface area, though aligning with the attributes of a theoretically ideal catalyst, may not necessarily embody an efficient catalyst for fructose dehydration.
This study aimed to enhance the valorisation of biomass by producing SCs using various raw materials, such as glucose and glycerol, via in situ carbonization. We employed modified methodologies to optimize catalyst preparation under mild conditions. The use of readily available and well-defined precursors, such as glycerol and glucose, offers advantages in terms of reproducibility while expanding the possibilities of raw materials that can be used. Glycerol, a byproduct of the biodiesel industry, and glucose, a widely found monosaccharide, provide consistent starting materials for catalyst synthesis. This approach aims to minimize potential variations arising from the inherent heterogeneity of naturally derived precursors, ensuring greater control over catalyst properties and performance. We employed modified methodologies to optimize catalyst preparation under mild conditions. The fructose dehydration reaction was finely tuned using both CH and MH in a DMSO system. A comprehensive characterization of the carbon-based catalysts was conducted, and a comparative analysis was performed with our recent studies and existing literature reports, yielding novel insights for the intelligent design of SCs in the context of fructose dehydration.
Experimental section
Catalyst preparation
In a standard procedure, 10 g of glycerol or glucose was blended with the requisite volume of concentrated H2SO4 in an 80 mL Teflon-coated stainless-steel reactor. Subsequently, the reactor was hermetically sealed and subjected to heating in an oil bath, accompanied by magnetic stirring for a specified time interval, thereby enabling simultaneous carbonization and sulfonation. The resulting solids underwent successive washes with distilled water and acetone (utilizing a Soxhlet system) before undergoing drying at 60 °C. SCs were denoted as “OL(x)y − z” or “OSE(x)y − z”, where “OL” and “OSE” signified the precursor used (OL for glycerol and OSE for glucose), “x” represented the precursor to H2SO4 mass ratio, “y” indicated the carbonization time, and “z” stood for the carbonization temperature. For instance, a sulfonated carbon produced with glucose, with a glucose to H2SO4 mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, a carbonization time of 1 hour, and a carbonization temperature of 180 °C, was denoted as “OSE(3)1h-180”.
3, a carbonization time of 1 hour, and a carbonization temperature of 180 °C, was denoted as “OSE(3)1h-180”.
Catalyst characterization
Surface acidity was measured according to the Boehm method,28 with a modification to differentiate sulfonic and carboxylic groups as proposed by Tamborini et al.29 Elemental analysis was performed using a ThermoScientific Flash EA1112 elemental analyzer. The X-ray photoelectron spectroscopy (XPS) spectra were obtained using a ThermoFisher Scientific spectrometer model K-alpha+. Thermogravimetric analysis (TGA/DTA) was carried out using Netzsch TG209F1 equipment. Micrographs and energy dispersive X-ray (EDX) spectra were obtained using a JSM-6010LA JEOL scanning electron microscope. Fourier transform infrared spectroscopy (FTIR) analyses were performed using a Varian-Agilent 640-IR FT-IR spectrometer (ATR mode). Textural properties were investigated using a Quantachrome Autosorb 1-MP surface area analyzer.Fructose dehydration
Catalytic tests were conducted using two distinct heating methods: (a) MH and (b) CH (in an oil bath). For method (a), the sealed reactor (35 mL) was heated using a CEM discover microwave synthesizer in a power range of 20 to 40 W until the desired temperature was reached, remaining within this power range during the temperature study while for method (b) the reaction was carried out in a round-bottom flask equipped with a reflux system. In a typical run, 5 mL of a 5 wt% fructose solution (in DMSO) and 5 wt% (mcatalyst/mfructose) of the catalyst were introduced into the reactor. The systems were maintained under magnetic stirring (500 rpm) at the desired temperature and time. For method (a), the reactor attained the predetermined temperature in approximately 120 s, while in method (b), the oil batch had already stabilized at the set value before introduction of the reactor. Upon completion of the tests, the reactor was rapidly cooled using mechanical ventilation.To evaluate the adsorption behavior of fructose and 5-HMF on the catalysts' surface, experiments were carried out under the same conditions as a typical reaction at room temperature. Upon completion of the tests, fructose and 5-HMF that remained in the solution were quantified accordingly.
The identification and quantification of reaction products were performed through high-performance liquid chromatography (HPLC) using an Agilent 1220 Infinity LC liquid chromatograph equipped with a Rezex ROA organic acid column H+ (250 × 4.6 mm) and detectors for the refractive index and diode array. Throughout the analysis, the column was maintained at 65 °C, and the flow rate of the mobile phase (H2SO4 5 mmol L−1) was kept at 0.2 mL min−1.
Fructose conversion (%), 5-HMF yield (%), and selectivity (%) were calculated using the following equations:
 | (1) |
 | (2) |
 | (3) |
Recycling tests
Recycling tests were performed under optimized conditions determined in this work. The spent catalyst was washed with water (2 × 30 mL) and acetone (2 × 10 mL) and then dried at 100 °C for 24 h before the next run.Results and discussion
Surface acidity
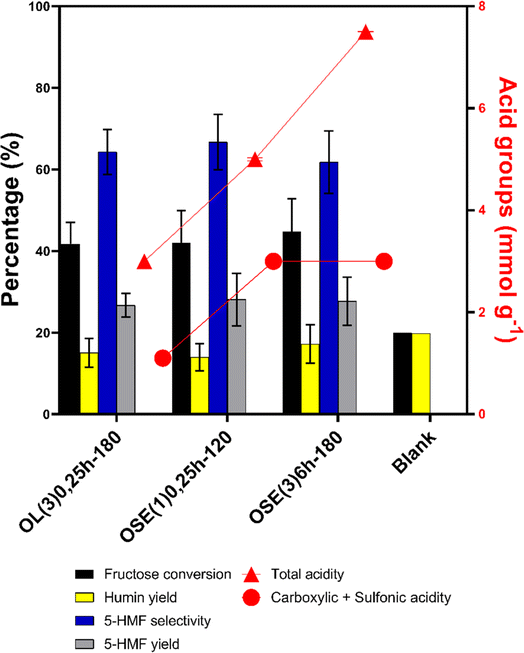
Various synthesis parameters were investigated to optimize the acidity of the SC under the mildest conditions possible (Fig. 1). Preliminarily, the SC derived from glycerol were compared with those obtained in our earlier study.24 Specifically, a comparison was made with glycerol-derived SC identified as OL(3)0.25h-180-M in our earlier work, wherein an oven was employed as the heating system instead of the oil batch utilized in this study for a comparison of the CH methods. The synthesis parameters remained unchanged, except for the heating method. As illustrated in Fig. 1a, both SC samples exhibited a similar amount of carboxylic + sulfonic groups (strong acid groups). The carboxylic and sulfonic groups are recognized as strong acid sites in the context of carbohydrate dehydration, which is related to their Brønsted acidity.24,30–35However, the SC produced in our previous work (using an oven) exhibited a significantly higher total acid group content compared to that produced using an oil batch. This discrepancy suggests that the OL(3)0.25h-180-M carbon possesses a higher concentration of phenolic + lactonic surface groups. In our previous study, the time required to reach the operating temperature was extended, ranging from 30 to 60 min.24 In contrast, in the present study, the oil batch had already stabilized at the intended carbonization temperature before introduction of the reactor. As a result, the prolonged preheating time in the oven, as observed in our previous work, contributed to heightened oxidation of the carbon surface, resulting in an increased concentration of phenolic and lactonic groups. Despite this distinction, both SCs exhibited a comparable amount of strong acid groups, which constitute the primary active sites for fructose dehydration. This suggests the suitability of the oil batch heating method, significantly reducing the synthesis time. Under identical synthesis conditions, OSE(3)0.25h-180 exhibited significantly higher acidity compared to OL(3)0.25h-180. As illustrated in Fig. 1b, there was a consistent increase in the acidity of the SC with extended carbonization times. This is in agreement with the recent findings from Morais et al.,36 suggesting that extended activation times in glucose-derived carbons contribute to heightened porosity and larger surface areas. The prolonged contact time between the carbon material and the activating agent led to improved microporosity and widened pore sizes. These findings emphasize the influence of activation duration on the structural characteristics of carbon materials.
Carbons derived from glucose, prepared with the lowest glucose to H2SO4 mass ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) across all investigated time intervals, displayed reduced amounts of strong acid groups. Interestingly, extending carbonization times did not promote the incorporation of acid groups into the solids, suggesting that oxidation processes in glucose-derived carbons occur more rapidly compared to those observed with glycerol as a precursor.
1) across all investigated time intervals, displayed reduced amounts of strong acid groups. Interestingly, extending carbonization times did not promote the incorporation of acid groups into the solids, suggesting that oxidation processes in glucose-derived carbons occur more rapidly compared to those observed with glycerol as a precursor.
The optimal conditions in this study were identified as a carbonization time of 0.25 h and a glucose-to-acid mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This selection enabled the production of highly acidic SCs with a reduced carbonization time and a lower quantity of H2SO4. The carbonization temperature was investigated within the range of 120–180 °C, as depicted in Fig. 1c. The total acid groups exhibited no significant variation within the tested temperature range. However, the quantity of carboxylic + sulfonic groups was notably influenced by this parameter. As the temperature increased from 120 to 180 °C, there was a decrease in the amount of strong acid groups, reaching a maximum value (3.0 mmol g−1) at 120 °C. Flores et al.37 suggested that at higher temperatures, the reduction in sulfonic groups occurs due to biomass graphitization and carbon structure cross-linking. Many C–H bonds are eliminated before the fixation of these groups, resulting in a more rigid carbon structure. As a result, introducing oxygenated acid groups becomes more challenging when the carbon structure is already rigid and stable. However, we observed an increase in the amount of phenolic and lactonic groups with temperature, contrasting the behaviour observed with sulfonic groups. The introduction of oxygenated functional groups on the carbon surface is favored by the presence of structural defects, such as around empty spaces and on the edges of planes.38 Upon initiation of the treatment with sulfuric acid, its oxidizing nature facilitates the incorporation of various types of previously observed oxygenated groups, which migrate or hop on the surface of the basal plane. However, it should be noted that the carbon surface undergoes gradual deterioration during direct treatment with sulfuric acid. In this process, there is a propensity to augment the amount of oxygenated functional groups, particularly lactonic and phenolic groups. Conversely, sulfonic groups are leached, leading to a reduction in the sulfur (S) content in the solid. It is noteworthy that this effect is more pronounced at a carbonization temperature close to 200 °C. In this range, carboxylic and sulfonic groups are susceptible to thermal decomposition, as observed through TGA/DTA and Boehm titration.28 On the other hand, phenolic and lactonic groups tend to decompose only at temperatures above 350–400 °C. Consequently, the incorporation of phenolic and lactonic groups continues to occur at temperatures close to 200 °C, while the quantity of carboxylic and sulfonic groups tends to decrease with prolonged contact time.
1. This selection enabled the production of highly acidic SCs with a reduced carbonization time and a lower quantity of H2SO4. The carbonization temperature was investigated within the range of 120–180 °C, as depicted in Fig. 1c. The total acid groups exhibited no significant variation within the tested temperature range. However, the quantity of carboxylic + sulfonic groups was notably influenced by this parameter. As the temperature increased from 120 to 180 °C, there was a decrease in the amount of strong acid groups, reaching a maximum value (3.0 mmol g−1) at 120 °C. Flores et al.37 suggested that at higher temperatures, the reduction in sulfonic groups occurs due to biomass graphitization and carbon structure cross-linking. Many C–H bonds are eliminated before the fixation of these groups, resulting in a more rigid carbon structure. As a result, introducing oxygenated acid groups becomes more challenging when the carbon structure is already rigid and stable. However, we observed an increase in the amount of phenolic and lactonic groups with temperature, contrasting the behaviour observed with sulfonic groups. The introduction of oxygenated functional groups on the carbon surface is favored by the presence of structural defects, such as around empty spaces and on the edges of planes.38 Upon initiation of the treatment with sulfuric acid, its oxidizing nature facilitates the incorporation of various types of previously observed oxygenated groups, which migrate or hop on the surface of the basal plane. However, it should be noted that the carbon surface undergoes gradual deterioration during direct treatment with sulfuric acid. In this process, there is a propensity to augment the amount of oxygenated functional groups, particularly lactonic and phenolic groups. Conversely, sulfonic groups are leached, leading to a reduction in the sulfur (S) content in the solid. It is noteworthy that this effect is more pronounced at a carbonization temperature close to 200 °C. In this range, carboxylic and sulfonic groups are susceptible to thermal decomposition, as observed through TGA/DTA and Boehm titration.28 On the other hand, phenolic and lactonic groups tend to decompose only at temperatures above 350–400 °C. Consequently, the incorporation of phenolic and lactonic groups continues to occur at temperatures close to 200 °C, while the quantity of carboxylic and sulfonic groups tends to decrease with prolonged contact time.
The optimal conditions for producing glucose-derived SCs were identified as 15 min of carbonization at 180 °C with a glucose: H2SO4 mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Interestingly, the OSE(1)0.25h-120 carbon exhibited a number of strong acid groups comparable to that of OSE(3)6h-180, which was prepared under the most extreme condition studied in this work. However, the total acid group content in OSE(3)6h-180 was notably higher, with the difference reaching 2.42 mmol g−1.
1. Interestingly, the OSE(1)0.25h-120 carbon exhibited a number of strong acid groups comparable to that of OSE(3)6h-180, which was prepared under the most extreme condition studied in this work. However, the total acid group content in OSE(3)6h-180 was notably higher, with the difference reaching 2.42 mmol g−1.
In Fig. 1d, it is evident that OL(3)0.25h-180 and OSE(1)0.25h-120 exhibit comparable levels of sulfonic groups, notwithstanding a higher overall presence of strong acid groups in the glucose's carbon. As anticipated, intensifying the severity of the acid treatment conditions led to a more substantial incorporation of sulfonic groups. Notably, OSE(3)6h-180 demonstrates a sulfonic group concentration 22.4% higher than that observed in OSE(1)0.25h-120. These observations align with the expected result of increased sulfonic group insertion under more rigorous acid treatment conditions. Recent literature on carbons prepared through various carbonization/sulfonation methods consistently reports total acidity values usually below 5 mmol g−1, with strong acid groups reaching up to 2 mmol g−1.37–42
Higher total acidity values have been achieved only under extreme conditions, such as a precursor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acid mass ratio as high as 1
acid mass ratio as high as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.43 The carbon OSE(1)0.15h-120 exhibits a surface acid group content typically higher than values reported in the literature, achieved through a simpler one-step method under mild conditions. Notably, the most acidic carbon derived from glucose, OSE(3)6h-180, demonstrates a remarkable total acid group content of 7.5 mmol g−1 and 3.0 mmol g−1 for strong acid groups. This exceeds the acidity observed in our previous work, where kraft lignin was employed as the precursor. The relatively simple structure of glucose, in contrast to kraft lignin, facilitates the incorporation of acid groups into the carbon structure, yielding a highly acidic sulfonated carbon even under milder conditions.
36.43 The carbon OSE(1)0.15h-120 exhibits a surface acid group content typically higher than values reported in the literature, achieved through a simpler one-step method under mild conditions. Notably, the most acidic carbon derived from glucose, OSE(3)6h-180, demonstrates a remarkable total acid group content of 7.5 mmol g−1 and 3.0 mmol g−1 for strong acid groups. This exceeds the acidity observed in our previous work, where kraft lignin was employed as the precursor. The relatively simple structure of glucose, in contrast to kraft lignin, facilitates the incorporation of acid groups into the carbon structure, yielding a highly acidic sulfonated carbon even under milder conditions.
Characterization of the selected SC
For characterization and catalytic tests in the fructose dehydration reaction, we utilized OL(3)0.25h-180 to make a comparison with our previous study,24 OSE(3)0.25h-180 (the most acidic SC, produced under the more drastic conditions in this work), and OSE(1)0.25h-120 (produced under the mildest and optimized conditions in this work) were selected. Table 1 shows the main properties of the selected SC.| Carbon | OL(3)0.25h-180 M** | OL(3)0.25h-180 | OSE(1)0.25h-120 | OSE(3)6h-180 | |
|---|---|---|---|---|---|
| Ccc | (%) | 64.8 | 69.3 ± 0.94 | 62.7 ± 0.37 | 61.5 ± 0.01 |
| Hcc | (%) | 3.8 | 4.8 ± 0.38 | 4.1 ± 0.15 | 2.6 ± 0.15 |
| Scc | (%) | 6.1 | 1.50 ± 0.19 | 0.7 ± 0.02 | 0.4 ± 0.04 |
| Occ | (%)* | 25.3 | 23.3 ± 1.13 | 31.4 ± 0,5 | 34.5 ± 0.2 |
| Ash content | (%) | — | 0.9 ± 0.01 | 0.9 ± 0.01 | 0.9 ± 0.02 |
| Carbon-based yield | (%) | — | 81.0 | 85.9 | 74.5 |
| Mass yield | (%) | 35.0 | 45.7 | 54.8 | 48.5 |
| CXPS | (%) | — | — | 74.0 ± 0.2 | 72.3 ± 0.3 |
| OXPS | (%) | — | — | 25.3 ± 0.1 | 25.2 ± 0.4 |
| SXPS | (%) | — | — | 0.9 ± 0.3 | 2.6 ± 0.1 |
| SEDX | (%) | — | 1.98 ± 0.08 | 0.4 ± 0.08 | 1.89 ± 0.02 |
| Average pore diameter | (Å) | — | 30.6 | 27.3 | 22.0 |
| Total pore volume | (cm3 g−1) | — | 0.0667 | 0.198 | 0.262 |
| BET surface area | (m2 g−1) | — | 90 | 290 | 480 |
| Total acid groups | (mmol g−1) | — | 3.0 ± 0.1153 | 5.0 ± 0.0368 | 7.5 ± 0.0056 |
| (μmol m−2) | — | 33 | 17 | 17 | |
| Carboxylic + sulfonic groups | (mmol g−1) | — | 1.14 ± 0.1661 | 2.99 ± 0.0514 | 3.00 ± 0.1069 |
| (μmol m−2) | — | 13 | 10 | 6 | |
| Sulfonic groups | (mmol g−1) | — | 0.56 ± 0.07 | 0.59 ± 0.08 | 0.76 ± 0.005 |
| (μmol m−2) | — | 6 | 2 | 2 | |
| Carboxylic groups | (mmol g−1) | — | 0.58 ± 0.05 | 2.40 ± 0.08 | 2.24 ± 0.006 |
| (μmol m−2) | — | 6 | 8 | 5 | |
The mass yield achieved for OL(3)0.25h-180 in this study reached 45.7%, surpassing the previously reported value of 35.0%.24 This difference is likely attributed to variations in the heating method employed during the sulfonated carbon (SC) preparation process, as elucidated previously.
After a 15 min washing period with acetone in a Soxhlet system (Fig. S1†), a brownish coloration, attributed to the presence of unpolymerized organic matter in the solid, was observed for OL(3)0.25h-180 and OSE(1)0.25h-120. In contrast, no coloration was noted for OSE(3)6h-180. When comparing the yields of glucose-derived carbons, acid content, and carbonization time and temperature, there was an approximate 10% variation in the obtained mass yield (Table 1). This implies that an increase in carbonization temperature led to a more efficient glucose carbonization process, resulting in a reduction in the amount of less polymerized organic matter, particularly evident as the temperature increased from 120 to 180 °C.
The quantity of sulfur incorporated into the sulfonated carbon (SC) structure correlates with the amount of acid employed. In a previous investigation, Mantovani et al.24 achieved the incorporation of a higher amount of sulfur (6.1%) into the structure. However, a significant portion was not catalytically active, manifesting as exposed sulfonic groups on the surface. Notably, Table 1 reveals substantial disparities in mass percentages obtained by energy dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS) for glucose-derived carbons, particularly concerning sulfur. It is important to consider that EDX provides an analysis depth of 1–2 μm, depending on the material, while XPS reaches up to 10 nm.44 Therefore, glucose-derived carbons exhibited a higher sulfur content in a more superficial layer, indicating that a significant portion of sulfur atoms were successfully inserted on the surface and are readily accessible for fructose interactions.
Fig. S2† shows the deconvolution of the C 1s peak in the XPS spectra which unveiled peaks centered at 284.6, 286.3, and 288.7 eV. These can be attributed to carbon atoms in graphitized regions (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and those bonded to hydroxyl groups (C–OH), and carboxylic groups (COOH), respectively. Moreover, in the S region, two peaks centered at 163 and 168 eV were identified, corresponding to reduced sulfur forms (–SH) and sulfonic groups, respectively.11,24,41,45,46 Fraga et al.45 observed the presence of non-oxygenated sulfur compounds, such as –SH, in carbons prepared at higher temperatures. Consistent with this behavior, the preparation of OSE(3)6h-180 under more drastic conditions in this work allowed the observation of the formation of reduced forms of sulfur in this solid.
C) and those bonded to hydroxyl groups (C–OH), and carboxylic groups (COOH), respectively. Moreover, in the S region, two peaks centered at 163 and 168 eV were identified, corresponding to reduced sulfur forms (–SH) and sulfonic groups, respectively.11,24,41,45,46 Fraga et al.45 observed the presence of non-oxygenated sulfur compounds, such as –SH, in carbons prepared at higher temperatures. Consistent with this behavior, the preparation of OSE(3)6h-180 under more drastic conditions in this work allowed the observation of the formation of reduced forms of sulfur in this solid.
Confirming the presence of oxygenated groups, the FTIR spectra (Fig. S3†) revealed bands centered at 1043, 1173, and 1396 cm−1, corresponding to symmetrical and asymmetrical O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching from –SO3H groups. Additionally, bands at 1700 and 3400 cm−1 were observed, indicating the presence of C
O stretching from –SO3H groups. Additionally, bands at 1700 and 3400 cm−1 were observed, indicating the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups from carboxylic and lactonic groups, and O–H groups from phenolic and carboxylic groups.47–49
O groups from carboxylic and lactonic groups, and O–H groups from phenolic and carboxylic groups.47–49
SEM images (Fig. S4†) unveiled irregular particle sizes and morphologies of the SC, characteristics typically associated with the in situ processes of carbonization and sulfonation. This concurrent carbonization and sulfonation promoted the precursor's transformation, resulting in the observed irregularities in particle size and morphology.
Thermal analysis (Fig. S5†) revealed three primary regions of mass loss, occurring within the temperature ranges of 50–100 °C, 200–300 °C, and 400–700 °C, respectively. The initial mass loss (50–100 °C) is attributed to the release of volatile gases and moisture adsorbed on the surface of the sulfonated carbon (SC). The second region (200–300 °C) corresponds to the degradation of oxygenated groups, while the third region (>400 °C) signifies the graphitization of the material.14,50,51 The overall mass loss for all SCs ranged from 42% to 49%. In the temperature range of 50–150 °C, the mass losses for OL(3)0.25h-180, OSE(3)6h-180, and OSE(1)0.25h-120 were approximately 3.68%, 4.45%, and 2.70%, respectively. This phenomenon is associated with the hydrophilicity of the solids, influenced by the varying degrees of incorporation of oxygenated groups on their surfaces.
Textural property analysis revealed that the surface area increased with both carbonization time and temperature, consistent with data reported in the literature for other precursors.39,41,52 As indicated in Table 1, OL(3)0.25h-180 exhibits less developed textural characteristics compared to glucose-derived carbons. This suggests that the acidic sites of OL(3)0.25h-180 are primarily located in the external surface area of the catalyst, enhancing substrate contact. In contrast, glucose-derived carbons displayed higher values for both surface area and pore volume. As highlighted by Wang et al.,27 in catalysts with pronounced textural characteristics, a fraction of the active sites is located within the pores, making them less accessible to fructose molecules. It is anticipated that glucose-derived carbons impede the internal molecular diffusion of reagents, thereby increasing resistance to mass transfer. This makes it difficult for fructose to reach the active sites situated within the pores.
Evaluation of catalytic activity
The chosen SCs were employed as catalysts in the fructose dehydration reaction to produce 5-HMF. The reactions were conducted using two heating methods: conventional and microwave irradiation.Conventional heating
The initial evaluation of catalytic activity took place at 100 °C with a 1 hour reaction time, employing DMSO as the solvent, conducted in the presence of O2. Fig. 2 illustrates the outcomes of fructose conversion, yield, and selectivity of 5-HMF in both the absence (blank reactions) and presence of the catalysts.In the blank reaction, a fructose conversion of 20% was noted, likely attributed to the catalytic activity of the DMSO solvent used.4 However, there was no substantial production of 5-HMF, and humins were obtained with a selectivity exceeding 98%.
The catalysts demonstrated an enhancement in fructose conversion, as well as in the yield and selectivity to 5-HMF. OSE(3)6h-180 exhibited the most favorable outcomes, achieving a conversion of 44.8% and a 5-HMF yield and selectivity of 27.7% and 61.8%, respectively. These findings align with the elevated content of both strong and total acid groups observed in this solid (Fig. 1). According to the literature,34,53 phenolic and lactonic groups interact with fructose and its reaction intermediate, favoring the contact with the strong acid groups.
As observed, although the amount of strong acid groups for glucose-derived carbons is comparable (3.0 mmol g−1), there is a disparity in the total acid groups between these catalysts (5.0 and 7.5 mmol g−1, respectively, for OSE(1)0.25h-120 and OSE(3)6h-180). This variation of approximately 2.5 mmol g−1 likely facilitated the interaction of the fructose intermediate (β-D-fructofuranose) with the strong acid groups in OSE(3)6h-180, promoting the dehydration reaction to 5-HMF. Consequently, the conversion of fructose in the presence of OSE(1)0.25h-120 reached 42.1%, while OSE(3)6h-180 achieved a conversion of 44.8%. Concerning the OL(3)0.25h-180 carbon, it was noted that despite exhibiting lower surface acidity than the other catalysts, this carbon allowed a fructose conversion similar to that of glucose-derived carbons. As previously reported, OL(3)0.25h-180 has less developed textural properties than the other catalysts. The smaller average pore diameter in the glucose-derived carbons can make the active sites less accessible. Consequently, even though OL(3)0.25h-180 has less acidity, its active sites may be more accessible than those in glucose-derived carbons. The smaller surface area and more accessible active sites favor the interaction between acid groups, even if present in smaller quantities. It should also be noted that the incorporation of oxygenated sites makes the carbon surface more hydrophilic. In this scenario, fructose adsorption becomes much more effective, reaching 11% of the initial concentration present in the reaction medium, while the adsorption of 5-HMF remains below 1%. Consequently, it is expected that during the reaction, fructose will be more favorably adsorbed in the vicinity of the active sites on the catalyst's surface with a more hydrophilic character. Conversely, the 5-HMF produced during the reaction would be more favorably moved away from the catalyst surface. Since the resulting 5-HMF could potentially undergo both rehydration and cross-linking reactions leading to the formation of humins, its faster removal from the solid surface may significantly enhance product selectivity.
Concerning the selectivity of 5-HMF, there was a slight decrease from OSE(1)0.25h-120 to OSE(3)6h-180. Despite the arrangement of the DMSO solvent around the fructose, the production of water molecules during the dehydration stage of fructose can lead to the generation of by-products and humins through the rehydration of 5-HMF, contributing to a reduction in selectivity.13,54 Given that OSE(3)6h-180 facilitated a higher conversion of fructose, there is a greater amount of water molecules resulting from the dehydration reaction. This increase in water content can explain the decline in selectivity compared to that of OSE(1)0.25h-120.
Microwave heating
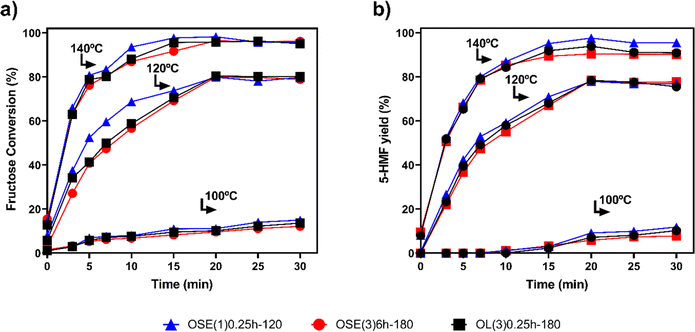
The effect of temperature and reaction time on fructose dehydration was investigated in the range of 100 to 140 °C for 30 min. The results are shown in Fig. 3.The use of MH promoted fructose dehydration quickly and more selectively than the CH. In addition, the formation of humins was strongly suppressed in the presence of the SC. An increase in fructose conversion (Fig. 3a) and 5-HMF yield (Fig. 3b) was observed as temperature increased for all catalysts. The selectivity of 5-HMF remained very close to 100% throughout the reaction, indicating that 5-HMF did not suffer from side reactions.
The results indicate, once again, the influence of total acid groups on the catalytic process, improving the results of fructose dehydration, besides increasing the reaction rate. In addition, the results demonstrate that the textural characteristics (Table 1) also contributed to the catalytic study. Among the prepared SCs, OSE(3)6h-180 exhibited a higher total pore volume (0.262 cm3 g−1) and a greater surface area (480 m2 g−1) compared to the others. Thus, OSE(3)6h-180 stands out for presenting higher quantities of acidic groups and a considerably elevated surface area compared to the other catalysts. However, it was observed that the greater distance between these acidic groups may result in a weakening of the synergistic effect between catalytic sites. So, the density of acidic sites in OSE(3)6h-180, when compared to the others, shows that its acidic sites are more spaced out, contributing to the reduction of synergy among the acidic groups.
When examining the other catalysts, OL(3)0.25h-180 (90 m2 g−1) and OSE(1)0.25h-120 (290 m2 g−1), even though OL(3)0.25h-180 has a smaller area, indicating that its acidic groups are closer, the density of carboxylic acids in OSE(1)0.25h-120 reveals a greater synergy among the groups. Therefore, despite the distance being crucial for the proximity of sites, as observed in OSE(3)6h-180, which has many groups and a large area, its distant groups did not act cooperatively. On the other hand, in OL(3)0.25h-180, with a smaller area and closer sites, the insufficient number of sites prevented effective cooperative action. Thus, there is a balance in the presence of types of acidic sites and the size of the area, which directly influences the catalytic outcome. According to Gan et al.,38 the textural properties can favor the structural orientation of the fructose molecules in the pores and quickly release the 5-HMF formed to the reaction medium causing an increase in catalytic activity and a decrease in side reactions. In this way, a synergistic effect between the amount of acid groups and the textural properties can be expected, favoring the yield and selectivity to 5-HMF. In addition, the increase in hydrophilicity on the surface of the solid is also beneficial, as already discussed.
Unlike CH, selectivity for 5-HMF reached values higher than 98% under MH. Among the advantages provided by the use of MH, it can be mentioned that due to the uniform and fast heating provided by this method, some substrates can be converted more quickly when exposed to radiation heating,55 avoiding the production of humins. MH favors the occurrence of effective shocks between the substrate and the catalyst, resulting in faster conversion and greater selectivity, as the molecules are under homogeneous chaotic movement.56–58 Several authors have reported the beneficial effects of using microwave radiation to replace CH.59–62
In all studies, the authors attribute the best results to the fast and homogeneous heating mode, promoted by microwave radiation, which allows the reaction to occur in less time and with greater selectivity to the products of interest. A comparison between the catalyst's behaviour under CH and MH must be highlighted. Acidic sites are located mainly on the external surface of the glycerol's carbon, while in the glucose's carbons, the acidic sites are also located inside the pores, being less accessible. Under CH, diffusion of the substrate throughout the pores is difficult due to the lower kinetic energy of the molecules. On the other hand, MH can favor the diffusion of the substrate inside the pores, and, consequently, its collision with the acidic sites located inside them. Considerations about the relationship between surface area/porosity and acidity have been made for several catalysts, and agree with the observations made in this work. The performance of carbon-based catalysts is determined not only by their structural parameters but also by their chemical compositions and surface functional groups. It has been linked to synergistic effects between a greater amount of acid groups and a larger surface area of the solid.63 However, the alternative of combining a high surface area (typically activated carbon) with a high number of acidic sites is not feasible, since there is great difficulty in functionalizing the surface of activated carbon. Foo and Sievers67 investigated structural and chemical modifications in the activated carbon after oxidation by H2O2 and H2SO4. The authors obtained carbons with a surface area as high as 695 m2 g−1 and a total acidity of 0.89 mmol g−1 (1.28 μmol m−2). This reduced density of acid groups allowed a glucose yield lower than 10 wt%. Even under more drastic preparation conditions, in which the number of sulfonic groups was substantially increased, there was no improvement in the glucose yield. Wang et al.68 prepared a series of carbons by using templates, carbonization, HF attack, and sulfonation. The most active solid, with a density of acid groups as high as 7.2 μmol m−2, converted 96.1% of fructose with 93.4% selectivity to 5-HMF. Weerasai et al.69 prepared SCs to investigate the hydrolysis of eucalyptus wood chips. The density of acid groups ranged from 9.1 to 19.2 μmol m−2. The catalyst with the smallest density of acid groups presented a restriction on the interaction of active sites with the substrate, thus affecting their conversion. All authors reaffirmed the need for a compromise between textural properties and the number of acid groups (represented here as the density of surface groups) to optimize catalyst activity.
Comparison of fructose dehydration under microwave radiation
In summary, this study compared its results with existing literature (Table 2), particularly focusing on the reaction conditions (140 °C for 10 min, using 5 wt% catalyst OSE(1)0.25h-120 and 5% m/v fructose in DMSO). As shown in entry 1, Qi et al.64 evaluated the catalytic performance of titanium oxide in the dehydration of fructose using a homemade microwave as the heating source for the reaction medium. As shown in Table 2, the authors used water as a solvent and increased the temperature to 200 °C. Despite achieving a considerable fructose conversion, the authors obtained a low yield of 5-HMF. Although this value was achieved in just 3 min, it is important to consider that the temperature used was extremely high, contributing to an increased kinetic rate of molecules and promoting the dehydration of fructose molecules. As shown i entry 2, Doan et al.65 investigated a carbon catalyst for the dehydration of fructose, using microwave radiation as a heating source. The preparation method adopted in this study is similar to that used by them, although with a significant difference in the synthesis temperature of the material, which was set at 200 °C. However, it is observed, as evidenced in this work, that temperatures close to 200 °C initiate the decomposition of sulfonic carboxylic groups, which are active in the dehydration of fructose. Despite achieving satisfactory results in terms of catalytic performance, optimal selectivity was not achieved. Furthermore, details about the specific temperature used in this research were neither addressed nor clarified. The authors limited themselves to assessing the microwave power, fixed at 80 W, without providing information about the temperature studied. In the study by Antonetti et al.66 (Entry 3), the use of phosphated niobium in the dehydration of fructose via microwave radiation was evaluated. Similar to the work presented in Entry 1, Antonetti et al. (Entry 3) also employed a high temperature, but used a larger initial amount of fructose, as well as a more significant quantity of catalyst. Although they achieved an optimal conversion value, a satisfactory yield of 5-HMF was not observed. In 8 min of the reaction, the authors obtained a 5-HMF yield of 32.2%, despite achieving almost complete fructose conversion. However, the selectivity result was 33%, indicating that a selective reaction was not achieved using this catalyst. In the study presented in Entry 4, Jia et al.62 evaluated the dehydration of fructose using a zeolite under microwave radiation.| Entry | Fructose dosage (%) | Catalyst | Catalyst dosage (% m m−1) | Solvent | Time (min) | Temperature or power | C (%) | Y (%) | S (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | TiO2 | 2 | Water | 3 | 200 °C | 73.1 | 27.4 | 37,5 | 64 |
| 2 | 6 | CC-oxa | 2.8 | DMSO | 10 | 80 W | 76.1 | 60.7 | 79.7 | 65 |
| 3 | 10 | NbPO | 6 | Water | 8 | 190 °C | 97.7 | 32.2 | 33 | 66 |
| 4 | 5 | Ypho zeolite | 40 | DMSO/water | 20 | 160 °C | 72.4 | 49.2 | 68 | 62 |
| 5 | 5 | OL(3)0.25h-180 | 5 | DMSO | 10 | 140 °C | 88.1 | 84.3 | 95.6 | This work |
| 6 | 5 | OSE(1)0.25h-120 | 5 | DMSO | 10 | 140 °C | 93.5 | 86.9 | 92 | This work |
| 7 | 5 | OSE(3)6h-180 | 5 | DMSO | 10 | 140 °C | 86.9 | 85.2 | 98 | This work |
Despite achieving a satisfactory outcome with a fructose conversion of 72.4%, a 5-HMF yield of 49.2%, and a 5-HMF selectivity of 68%, it is worth noting the use of a high temperature and a substantial amount of catalyst in relation to the initial fructose dosage.
The analysis highlights the unique effectiveness of the conditions and catalyst employed in this study, resulting in a significant improvement in efficiency, measured by using the yield of 5-HMF and fructose conversion. This research distinctly contributes to the advancement in material synthesis, showcasing both innovation and relevance. The potential applications arising from these findings promise to pioneer innovative and sustainable methodologies, revealing that the catalytic system proposed in this work is extremely efficient for fructose dehydration.
Recycling test
The stability and recycling capacity of OSE(1)0.25h-120 were investigated through successive catalytic runs. This was achieved by subjecting the material to consecutive cycles, as illustrated in Fig. 4. | ||
| Fig. 4 Recycling test using OSE(1)0.25h-120. (Reaction conditions: 140 °C, 10 min, 5% wt% catalyst and 5% m/v fructose in DMSO). | ||
OSE(1)0.25h-120 exhibited a gradual decline in catalytic performance following the initial run. Specifically, from the first to the second run, there was a notable decrease in fructose conversion, dropping from around 90% to 60%, accompanied by a 30% reduction in the percentage points of 5-HMF yield. Interestingly, the selectivity remained constant. However, starting from the second cycle onward, there were no significant alterations observed in the conversion of fructose, 5-HMF yield, and 5-HMF selectivity. In our prior investigation, we found that catalyst deactivation involves the loss of –COOH/–SO3H groups and the deactivation of acidic sites due to humins accumulating or anchoring on the carbon surface.12
Humin polymers are formed during the acid-catalyzed conversion of fructose to 5-hydroxymethyl furfural (5-HMF).70–72 These humins can be impregnated in sulfonated carbon porous materials.73 The formation of humins involves the condensation of key intermediate molecules such as fructose, 5-HMF, and other derived species.74 The mechanism of humin formation includes reactions such as aldol addition and condensation between 5-HMF and other species. The presence of the furan ring and the hydroxy methyl group of 5-HMF in humins suggests that aldol addition and condensation reactions are the main reactions initiating humin formation.
The catalyst deactivation appears to stem from a robust interaction between the acidic sites on the catalyst and the firmly anchored humins on its surface.70 Although Perez and Dumont13 showed that washing the catalyst can eliminate humins, this work which attempts to remove humins through the washing process has proven ineffective, underscoring their strong attachment to the catalyst.13 The acidity of the catalyst emerges as a pivotal factor influencing the strength of the interaction with humins and active sites.11 This observation suggests that humins possess the capacity to impregnate sulfonated carbon porous materials. In summary, the presence of humins on a catalyst surface can significantly contribute to deactivation, prompting the need for further research to comprehensively understand and address this phenomenon.
The comprehensive analysis of the stability and recycling capacity of OSE(1)0.25h-120 highlights the gradual decline in catalytic performance observed over successive runs. This decline, especially notable in fructose conversion and 5-HMF yield during the initial runs, was attributed to the accumulation or anchoring of humins on the carbon surface. The persistence of catalyst deactivation, even with attempted washing, underscores the strong attachment of humins to the catalyst.
Further exploring the intricate aspects of catalyst textural characteristics, it is crucial to consider the potential entrapment of H2SO4 and humins within the porous matrices due to limited accessibility. This entrapment is closely linked to the inadequacy of the porous structure of the OSE(1)0.25h-120 catalyst. The confined space within the pores poses challenges for efficient removal during washing processes, potentially leading to the retention of H2SO4 and humins.
In addition to the challenges posed by the catalyst's porous structure, the utilization of MH to enhance accessibility to acidic sites within the pores introduces further considerations. While MH facilitates substrate interaction with acidic sites, it also presents diffusional challenges for 5-HMF navigating an extensive path to exit the pores. The confined space within the pores acts as an additional barrier, contributing to the complexity of 5-HMF release and exacerbating humin generation within the pores. This phenomenon further obstructs active sites, impacting the overall catalytic process.
The findings presented in this study underscore the significance of addressing these challenges for catalyst reutilization and recycling. Specifically, the potential entrapment of H2SO4 and humins within the porous matrices, combined with the inadequacies of the porous structure of OSE(1)0.25h-120, emphasizes the need for an understanding of textural characteristics and their implications for catalytic performance. Integrating this knowledge will contribute to advancing the efficiency and sustainability of the proposed catalytic system, aligning with the primary objective of minimizing environmental impact in both catalyst preparation and catalytic tests.
Moreover, as previously mentioned in this work, when designing an efficient SC for fructose dehydration, various characteristics such as surface area, porosity, acidic site density, and diffusional limitations must be considered. In catalysts with pronounced textural characteristics, a fraction of the active sites is often located within the pores, making them less accessible to fructose molecules. It is noteworthy that carbons derived from glucose may impede the internal molecular diffusion of reactants, leading to increased resistance to mass transfer. This poses challenges for fructose to effectively reach the active sites situated within these pores. Addressing this concern can be facilitated by the utilization of MH. MH possesses the capacity to enhance accessibility to acidic sites within the pores, thereby optimizing the substrate's interaction with these sites. Nevertheless, it's crucial to acknowledge that in microwave reactions, diffusional challenges may arise as 5-HMF navigates an extensive path to exit the pores. The confined space within the pores serves as an additional barrier for 5-HMF, contributing to the complexity of its release. This prolonged diffusion pathway not only obstructs the efficient exit of 5-HMF but also exacerbates the generation of humins within the pores. This phenomenon potentially obstructs active sites, further impeding the overall catalytic process. Our findings support this explanation, indicating that the consistent results across various runs may be attributed to the fact that only the accessible surface sites may have remained unaffected by humins. It is noteworthy to emphasize that the primary objective of this study was to implement synthesis conditions that minimize environmental impact in both catalyst preparation and catalytic tests. Concerning the catalyst, it is derived from cost-effective and abundant raw materials, specifically sourced from agro-industrial by-products. Furthermore, the procedural steps involved in preparing the catalyst exhibit notable simplicity, cost-effectiveness, and a reduced number of operational steps. This meticulous selection inherently aligns with our commitment to refining the synthetic pathway, thereby advancing the overall efficiency and sustainability of the proposed catalytic system.
Conclusions
The preparation of carbons was carried out under milder conditions, which made it possible to obtain high yields, both for systems based on glycerol and glucose. Carbon OSE(3)6h-180 presented 17 μmol m−2 of total acid groups and 6 μmol m−2 of strong acid groups.The evaluation of the catalytic activity indicated that the SCs are promising catalysts in the selective fructose dehydration to 5-HMF. Reactions carried out under CH favored the formation of humins. The use of MH made the reaction faster and more selective, as the short reaction time prevented undesirable cross-linking reactions. Under these conditions, a fructose conversion of 50% by OSE(1)0.25h-120 with a selectivity for 5-HMF higher than 98% was obtained after only 300 s at 120 °C.
This study explores the complexity of optimizing catalysts for the selective dehydration of fructose to 5-HMF, emphasizing the interplay between acidic site density, textural properties, and heating methods. It underscores the significance of striking a balance between acid group quantity and catalyst textural characteristics, promoting an inclusive design approach to enhance catalytic efficiency. The synergy between accessible acid sites and textural properties emerges as pivotal for facilitating fructose diffusion and increasing 5-HMF production, offering valuable insights for future catalyst design and application in this specific context. Moreover, the high density of acid groups on the carbon surface, expressed as μmol m−2, proves influential in expediting fructose conversion, serving as an additional parameter to enhance acid catalyst activity.
The recyclable catalyst, OSE(1)0.25h-120, emphasizes the study's commitment to environmentally friendly synthesis conditions. Utilizing a cost-effective catalyst derived from agro-industrial by-products, along with a streamlined preparation process, reflects a dedicated effort to improve overall efficiency and sustainability.
The investigation on the OSE(1)0.25h-120 catalyst reveals a gradual decline in catalytic performance attributed to humins' persistent attachment, even after washing attempts. Textural challenges, such as limited accessibility leading to H2SO4 and humin entrapment, further impact efficiency. While microwaves are proposed to enhance accessibility, they introduce diffusional challenges hindering 5-HMF exit and exacerbating humin generation within pores. Addressing these challenges is critical for effective catalyst reutilization and recycling, necessitating a comprehensive understanding of textural characteristics. The study aims to minimize environmental impact through a sustainable synthesis pathway.
Abbreviations
| 5-HMF | 5-hydroxymethylfurfural |
| BET | Brunauer, Emmett, and Teller method |
| SC | Sulfonated carbon |
| DAD | Diode array detector |
| DMSO | Dimethylsulfoxide |
| XRD | X-ray diffraction |
| DTA | Differential thermogravimetric analysis |
| EDX | Energy dispersive X-ray analysis |
| FTIR | Fourier-transform infrared spectroscopy |
| HPLC | High-performance liquid chromatography |
| SEM-EDX | Scanning electron microscopy (SEM) with energy dispersive X-ray analysis (EDX) |
| RID | Refractive index detector |
| TGA | Thermogravimetric analysis |
| XPS | X-Ray photoelectron spectroscopy |
| MH | Microwave heating |
| CH | Conventional heating |
Author contributions
Gabrielle M. Reis: conceptualization, investigation, visualization, writing – original draft preparation. Letícia F. L. Machado: investigation, methodology. Renan S. Nunes: investigation, methodology. Dalmo Mandelli: conceptualization, methodology, resources. Wagner A. Carvalho: supervision, conceptualization, methodology, resources, writing – review & editing. The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the FAPESP (Project No. 2017/24931-4, 2016/05006-5 and 2021/1234-0), CAPES (Finance Code 001), and CNPq (Project No. 404843/2018-2). The authors also acknowledge the Multi-User Central Facilities (CEM/UFABC) for experimental support.Notes and references
- X. Liu, X. Min, H. Liu, Y. Cao, Y. Liu, M. Han, Z. M. Sun and S. Ji, Efficient Conversion of Cellulose to 5-Hydroxymethylfurfural Catalyzed by a Cobalt-Phosphonate Catalyst, Sustainable Energy Fuels, 2020, 4(11), 5795–5801, 10.1039/d0se01006e.
- X. Kong, Y. Zhu, Z. Fang, J. A. Kozinski, I. S. Butler, L. Xu, H. Song and X. Wei, Catalytic Conversion of 5-Hydroxymethylfurfural to Some Value-Added Derivatives, Green Chem., 2018, 20(16), 3657–3682, 10.1039/c8gc00234g.
- M. Sajid, Y. Bai, D. Liu and X. Zhao, Organic Acid Catalyzed Production of Platform Chemical 5- Hydroxymethylfurfural from Fructose: Process Comparison and Evaluation Based on Kinetic Modeling, Arabian J. Chem., 2020, 13, 7430–7444, DOI:10.1016/j.arabjc.2020.08.019.
- T. C. Tudino, R. S. Nunes, D. Mandelli and W. A. Carvalho, Influence of Dimethylsulfoxide and Dioxygen in the Fructose Conversion to 5-Hydroxymethylfurfural Mediated by Glycerol's Acidic Carbon, Front. Chem., 2020, 8, 1–11, DOI:10.3389/fchem.2020.00263.
- D. Licursi, A. M. Raspolli Galletti, B. Bertini, L. Ardemani, N. Scotti, N. Di Fidio, S. Fulignati and C. Antonetti, Design Approach for the Sustainable Synthesis of Sulfonated Biomass-Derived Hydrochars and Pyrochars for the Production of 5-(Hydroxymethyl)Furfural, Sustainable Chem. Pharm., 2023, 35, 101216, DOI:10.1016/j.scp.2023.101216.
- F. Saghandali, M. Kazemeini and S. Sadjadi, Halloysite-Supported Silicotungstic Acid as an Efficient Catalyst for Dehydration of Fructose to 5-Hydroxymethylfurfural, J. Phys. Chem. Solids, 2024, 184, 111697, DOI:10.1016/j.jpcs.2023.111697.
- S. Wang, T. L. Eberhardt and H. Pan, Efficient Dehydration of Fructose into 5-HMF Using a Weakly-Acidic Catalyst Prepared from a Lignin-Derived Mesoporous Carbon, Fuel, 2022, 316, 123255, DOI:10.1016/j.fuel.2022.123255.
- M. Sayed, N. Warlin, C. Hulteberg, I. Munslow, S. Lundmark, O. Pajalic, P. Tunå, B. Zhang, S. H. Pyo and R. Hatti-Kaul, 5-Hydroxymethylfurfural from Fructose: An Efficient Continuous Process in a Water-Dimethyl Carbonate Biphasic System with High Yield Product Recovery, Green Chem., 2020, 22(16), 5402–5413, 10.1039/d0gc01422b.
- A. H. Motagamwala, K. Huang, C. T. Maravelias and J. A. Dumesic, Solvent System for Effective Near-Term Production of Hydroxymethylfurfural (HMF) with Potential for Long-Term Process Improvement, Energy Environ. Sci., 2019, 12(7), 2212–2222, 10.1039/c9ee00447e.
- L. Li, Y. Hu, H. Li, P. Hu, Z. Xue, D. Wu, C. Hu and L. Zhu, Probing the Formation of Soluble Humins in Catalytic Dehydration of Fructose to 5-Hydroxymethylfurfural over HZSM-5 Catalyst, Fuel, 2023, 344, 1–10, DOI:10.1016/j.fuel.2023.128133.
- R. S. Nunes, T. C. Tudino, L. M. Vieira, D. Mandelli and W. A. Carvalho, Rational Production of Highly Acidic Sulfonated Carbons from Kraft Lignins Employing a Fractionation Process Combined with Acid-Assisted Hydrothermal Carbonization, Bioresour. Technol., 2020, 122882, DOI:10.1016/j.biortech.2020.122882.
- R. S. Nunes, G. M. Reis, L. M. Vieira and D. Mandelli, Ultra-Fast and Selective Fructose Dehydration Promoted by a Kraft Lignin Sulfonated Carbon under Microwave Heating in DMSO System, Catal. Lett., 2020, 0123456789, DOI:10.1007/s10562-020-03305-w.
- G. Portillo Perez, A. Mukherjee and M. J. Dumont, Insights into HMF Catalysis, J. Ind. Eng. Chem., 2019, 70, 1–34, DOI:10.1016/j.jiec.2018.10.002.
- G. Portillo Perez and M. J. Dumont, Production of HMF in High Yield Using a Low Cost and Recyclable Carbonaceous Catalyst, Chem. Eng. J., 2020, 382, 122766, DOI:10.1016/j.cej.2019.122766.
- Y. Hu, H. Li, P. Hu, L. Li, D. Wu, Z. Xue, L. Zhu and C. Hu, Probing the Effects of Fructose Concentration on the Evolution of Humins during Fructose Dehydration, React. Chem. Eng., 2022, 8(1), 175–183, 10.1039/d2re00324d.
- Q. Hou, X. Qi, M. Zhen, H. Qian, Y. Nie, C. Bai, S. Zhang, X. Bai and M. Ju, Biorefinery Roadmap Based on Catalytic Production and Upgrading 5-Hydroxymethylfurfural, Green Chem., 2021, 23(1), 119–231, 10.1039/d0gc02770g.
- R. Tomer and P. Biswas, Dehydration of glucose over sulfate impregnated ZnO (hexagonal-monoclinic) catalyst in dimethyl sulfoxide (DMSO) medium: Production, separation, and purification of 5-hydroxymethylfurfural (5-HMF) with high purity, Catal. Today, 2022, 404, 219–228, DOI:10.1016/j.cattod.2022.02.009.
- I. S. Omodolor, N. O. Ofole, S. A. Walz, M. R. Coleman, R. Gogar, S. Viamajala, F. C. F. Marcos and A. C. Alba-Rubio, Polystyrene-Based Catalysts with Simultaneous Brønsted and Lewis Acidity for Hydroxymethylfurfural Production from Starch : Molecular Weight and Solvent Effects, Sustainable Energy Fuels, 2024, 8, 272–285, 10.1039/d3se01164j.
- R. K. Srivastava, N. P. Shetti, K. R. Reddy, M. N. Nadagouda, M. Badawi, A. Bonilla-Petriciolet and T. M. Aminabhavi, Valorization of Biowastes for Clean Energy Production, Environmental Depollution and Soil Fertility, J. Environ. Manage., 2023, 117410 CrossRef CAS PubMed.
- A. S. Yusuff, K. A. Thompson-Yusuff and J. Porwal, Sulfonated Biochar Catalyst Derived from Eucalyptus Tree Shed Bark: Synthesis, Characterization and Its Evaluation in Oleic Acid Esterification, RSC Adv., 2022, 12(17), 10237–10248, 10.1039/d1ra09179d.
- Z. Liu, Y. Qi, M. Gui, C. Feng, X. Wang and Y. Lei, Sulfonated Carbon Derived from the Residue Obtained after Recovery of Essential Oil from the Leaves of: Cinnamomum Longepaniculatum Using Brønsted Acid Ionic Liquid, and Its Use in the Preparation of Ellagic Acid and Gallic Acid, RSC Adv., 2019, 9(9), 5142–5150, 10.1039/c8ra08685k.
- W. R. Silva, E. Y. Matsubara, J. M. Rosolen, P. M. Donate and R. Gunnella, Pd Catalysts Supported on Different Hydrophilic or Hydrophobic Carbonaceous Substrate for Furfural and 5-(Hydroxymethyl)-Furfural Hydrogenation in Water, Mol. Catal., 2021, 504, 111496, DOI:10.1016/j.mcat.2021.111496.
- H. N. Anchan and S. Dutta, Recent Advances in the Production and Value Addition of Selected Hydrophobic Analogs of Biomass-Derived 5-(Hydroxymethyl)Furfural, Biomass Convers. Biorefin., 2023, 13(4), 2571–2593, DOI:10.1007/s13399-021-01315-1.
- M. Mantovani, D. Mandelli, M. Gonçalves and W. A. Carvalho, Fructose Dehydration Promoted by Acidic Catalysts Obtained from Biodiesel Waste, Chem. Eng. J., 2018, 348, 860–869, DOI:10.1016/j.cej.2018.05.059.
- X. Yu, Y. Chu, L. Zhang, H. Shi, M. Xie, L. Peng, X. Guo, W. Li, N. Xue and W. Ding, Adjacent Acid Sites Cooperatively Catalyze Fructose to 5-Hydroxymethylfurfural in a New, Facile Pathway, J. Energy Chem., 2020, 47, 112–117, DOI:10.1016/j.jechem.2019.11.020.
- J. Wang, W. Xu, J. Ren, X. Liu, G. Lu and Y. Wang, Efficient Catalytic Conversion of Fructose into Hydroxymethylfurfural by a Novel Carbon-Based Solid Acid, Green Chem., 2011, 15, 2678–2681, 10.1039/c1gc15306d.
- J. Wang, L. Zhu, Y. Wang, H. Cui, Y. Zhang and Y. Zhang, Fructose Dehydration to 5-HMF over Three Sulfonated Carbons: Effect of Different Pore Structures, J. Chem. Technol. Biotechnol., 2017, 92(6), 1454–1463, DOI:10.1002/jctb.5144.
- H. P. Boehm, Surface Oxides on Carbon and Their Analysis: A Critical Assessment, Carbon, 2002, 40(2), 145–149, DOI:10.1016/S0008-6223(01)00165-8.
- L. H. Tamborini, M. P. Militello, J. Balach, J. M. Moyano, C. A. Barbero and D. F. Acevedo, Application of Sulfonated Nanoporous Carbons as Acid Catalysts for Fischer Esterification Reactions, Arabian J. Chem., 2019, 12(8), 3172–3182, DOI:10.1016/j.arabjc.2015.08.018.
- L. J. Konwar, P. Mäki-Arvela and J. P. Mikkola, SO3H-Containing Functional Carbon Materials: Synthesis, Structure, and Acid Catalysis, Chem. Rev., 2019, 119(22), 11576–11630, DOI:10.1021/acs.chemrev.9b00199.
- G. Delgado, C. Eddine, F. Ammari, S. Ivanova, M. Angel, M. Isabel and A. Monz, Fructose Dehydration Reaction over Functionalized Nanographitic Catalysts in MIBK/H2O Biphasic System, Catal. Today, 2021, 366, 68–76 CrossRef.
- S. Wang, T. L. Eberhardt and H. Pan, Efficient Dehydration of Fructose into 5-HMF Using a Weakly-Acidic Catalyst Prepared from a Lignin-Derived Mesoporous Carbon, Fuel, 2022, 316, 123255, DOI:10.1016/j.fuel.2022.123255.
- P. A. Russo, M. M. Antunes, P. Neves, P. V. Wiper, E. Fazio, F. Neri, F. Barreca, L. Mafra, M. Pillinger, N. Pinna and A. A. Valente, Solid Acids with SO3H Groups and Tunable Surface Properties: Versatile Catalysts for Biomass Conversion, J. Mater. Chem. A, 2014, 2(30), 11813–11824, 10.1039/c4ta02320j.
- X. Xiong, I. K. M. Yu, S. S. Chen, D. C. W. Tsang, L. Cao, H. Song, E. E. Kwon, Y. S. Ok, S. Zhang and C. S. Poon, Sulfonated Biochar as Acid Catalyst for Sugar Hydrolysis and Dehydration, Catal. Today, 2018, 314, 52–61, DOI:10.1016/j.cattod.2018.02.034.
- M. Nahavandi, K. Tirumala, V. Rao, Z. Yuan, C. C. Xu and S. Rohani, Efficient Conversion of Glucose into 5-Hydroxymethylfurfural Using a Sulfonated Carbon-Based Solid Acid Catalyst: An Experimental and Numerical Study, ACS Sustainable Chem. Eng., 2019, 7(14), 11970–11984, DOI:10.1021/acssuschemeng.9b00250.
- R. G. Morais, N. Rey-Raap, J. L. Figueiredo and M. F. R. Pereira, Glucose-Derived Carbon Materials with Tailored Properties as Electrocatalysts for the Oxygen Reduction Reaction, Beilstein J. Nanotechnol., 2019, 10, 1089–1102, DOI:10.3762/BJNANO.10.109.
- K. P. Flores, J. L. O. Omega, L. K. Cabatingan, A. W. Go, R. C. Agapay and Y. H. Ju, Simultaneously Carbonized and Sulfonated Sugarcane Bagasse as Solid Acid Catalyst for the Esterification of Oleic Acid with Methanol, Renewable Energy, 2019, 130, 510–523, DOI:10.1016/j.renene.2018.06.093.
- L. Gan, L. Lyu, T. Shen and S. Wang, Sulfonated Lignin-Derived Ordered Mesoporous Carbon with Highly Selective and Recyclable Catalysis for the Conversion of Fructose into 5-Hydroxymethylfurfural, Appl. Catal., A, 2019, 574, 132–143, DOI:10.1016/j.apcata.2019.02.008.
- B. K. Ozsel, D. Ozturk and B. Nis, One-Pot Hydrothermal Conversion of Different Residues to Value-Added Chemicals Usıng New Acidic Carbonaceous Catalyst, Bioresour. Technol., 2019, 289, 121627, DOI:10.1016/j.biortech.2019.121627.
- R. O. Araujo, J. d S. Chaar, L. S. Queiroz, G. N. da Rocha Filho, C. E. F. da Costa, G. C. T. da Silva, R. Landers, M. J. F. Costa, A. A. S. Gonçalves and L. K. C. de Souza, Low Temperature Sulfonation of Acai Stone Biomass Derived Carbons as Acid Catalysts for Esterification Reactions, Energy Convers. Manage., 2019, 196, 821–830, DOI:10.1016/j.enconman.2019.06.059.
- S. K. Sangar, O. N. Syazwani, M. S. A. Farabi, S. M. Razali, G. Shobhana, S. H. Teo and Y. H. Taufiq-Yap, Effective Biodiesel Synthesis from Palm Fatty Acid Distillate (PFAD)Using Carbon-Based Solid Acid Catalyst Derived Glycerol, Renewable Energy, 2019, 142, 658–667, DOI:10.1016/j.renene.2019.04.118.
- I. M. Lokman, U. Rashid, Y. H. Taufiq-Yap and R. Yunus, Methyl Ester Production from Palm Fatty Acid Distillate Using Sulfonated Glucose-Derived Acid Catalyst, Renewable Energy, 2015, 81, 347–354, DOI:10.1016/j.renene.2015.03.045.
- Z. Wen, Z. Ma, F. Mai, F. Yan, L. Yu, M. Jin, Y. Sang, Y. Bai, K. Cui, K. Wu, M. Chen, H. Chen and Y. Li, Catalytic Ethanolysis of Microcrystalline Cellulose over a Sulfonated Hydrothermal Carbon Catalyst, Catal. Today, 2020, 355, 272–279, DOI:10.1016/j.cattod.2019.05.070.
- D. N. G. Krishna and J. Philip, Review on Surface-Characterization Applications of X-Ray Photoelectron Spectroscopy (XPS): Recent Developments and Challenges, Appl. Surf. Sci. Adv., 2022, 12, 100332, DOI:10.1016/j.apsadv.2022.100332.
- A. d C. Fraga, C. P. B. Quitete, V. L. Ximenes, E. F. Sousa-Aguiar, I. M. Fonseca and A. M. B. Rego, Biomass Derived Solid Acids as Effective Hydrolysis Catalysts, J. Mol. Catal. A: Chem., 2016, 422, 248–257, DOI:10.1016/j.molcata.2015.12.005.
- N. Li, Q. Wang, S. Ullah, X. C. Zheng, Z. K. Peng and G. P. Zheng, Esterification of Levulinic Acid in the Production of Fuel Additives Catalyzed by Porous Sulfonated Carbon Derived from Pine Needle, Catal. Commun., 2019, 129, 105755, DOI:10.1016/j.catcom.2019.105755.
- M. G. Mazzotta, D. Gupta, B. Saha, A. K. Patra, A. Bhaumik and M. M. Abu-Omar, Efficient Solid Acid Catalyst Containing Lewis and Brønsted Acid Sites for the Production of Furfurals, ChemSusChem, 2014, 7(8), 2342–2350, DOI:10.1002/cssc.201402007.
- J. Yang, H. Zhang, Z. Ao and S. Zhang, Hydrothermal Carbon Enriched with Sulfonic and Carboxyl Groups as an Efficient Solid Acid Catalyst for Butanolysis of Furfuryl Alcohol, Catal. Commun., 2019, 123, 109–113, DOI:10.1016/j.catcom.2019.02.016.
- J. Zhang, S. Yang, Z. Zhang, L. Cui, J. Jia, D. Zhou and B. Zhu, An Excellent Solid Acid Catalyst Derived from Microalgae Residue for Fructose Dehydration into 5-Hydroxymethylfurural, ChemistrySelect, 2019, 4(4), 1259–1265, DOI:10.1002/slct.201803528.
- J. M. Fonseca, L. Spessato, A. L. Cazetta, C. da Silva and V. d C. Almeida, Sulfonated Carbon: Synthesis, Properties and Production of Biodiesel, Chem. Eng. Process., 2022, 170, 108668, DOI:10.1016/j.cep.2021.108668.
- N. Li, X. L. Zhang, X. C. Zheng, G. H. Wang, X. Y. Wang and G. P. Zheng, Efficient Synthesis of Ethyl Levulinate Fuel Additives from Levulinic Acid Catalyzed by Sulfonated Pine Needle-Derived Carbon, Catal. Surv. Asia, 2019, 23(3), 171–180, DOI:10.1007/s10563-019-09270-8.
- H. M. Kefas, R. Yunus, U. Rashid and Y. H. Taufiq-Yap, Enhanced Biodiesel Synthesis from Palm Fatty Acid Distillate and Modified Sulfonated Glucose Catalyst via an Oscillation Flow Reactor System, J. Environ. Chem. Eng., 2019, 7(2), 102993, DOI:10.1016/j.jece.2019.102993.
- D. P. Yang, Z. Li, M. Liu, X. Zhang, Y. Chen, H. Xue, E. Ye and R. Luque, Biomass-Derived Carbonaceous Materials: Recent Progress in Synthetic Approaches, Advantages, and Applications, ACS Sustainable Chem. Eng., 2019, 7(5), 4564–4585, DOI:10.1021/acssuschemeng.8b06030.
- M. R. Whitaker, A. Parulkar, P. Ranadive, R. Joshi and N. A. Brunelli, Examining Acid Formation During the Selective Dehydration of Fructose to 5-Hydroxymethylfurfural in Dimethyl Sulfoxide and Water, ChemSusChem, 2019, 12(10), 2211–2219, DOI:10.1002/cssc.201803013.
- M. Pineiro, L. D. Dias, L. Damas, G. L. B. Aquino, M. J. F. Calvete and M. M. Pereira, Microwave Irradiation as a Sustainable Tool for Catalytic Carbonylation Reactions, Inorg. Chim. Acta, 2017, 455, 364–377, DOI:10.1016/j.ica.2016.06.043.
- N. E. Leadbeater, Organic Synthesis Using Microwave Heating, Elsevier Ltd, 2014, vol. 9, DOI:10.1016/B978-0-08-097742-3.00920-4.
- B. Das and K. Mohanty, Microwave Induced One-Pot Conversion of d-Glucose to 5-Hydroxymethylfurfural by a Novel Sulfate-Functionalized Sn-Red Mud Catalyst, Sustainable Energy Fuels, 2020, 4(12), 6030–6044, 10.1039/d0se01476a.
- G. R. Gomes and J. C. Pastre, Microwave-Assisted HMF Production from Water-Soluble Sugars Using Betaine-Based Natural Deep Eutectic Solvents (NADES), Sustainable Energy Fuels, 2020, 4(4), 1891–1898, 10.1039/c9se01278h.
- L. Jiang, Y. Wang, L. Dai, Z. Yu, Q. Wu, Y. Zhao, Y. Liu, R. Ruan, L. Ke, Y. Peng, D. Xia and L. Jiang, Integrating Pyrolysis and Ex-Situ Catalytic Reforming by Microwave Heating to Produce Hydrocarbon-Rich Bio-Oil from Soybean Soapstock, Bioresour. Technol., 2020, 302, 122843, DOI:10.1016/j.biortech.2020.122843.
- B. Eryildirim, H. Arbag, N. Oktar and G. Dogu, Comparison of Microwave and Conventionally Heated Reactor Performances in Catalytic Dehydrogenation of Ethane, Int. J. Hydrogen Energy, 2021, 46(7), 5296–5310, DOI:10.1016/j.ijhydene.2020.11.067.
- S. Uruş, H. Eskalen, M. Çaylar and M. Akbulut, Highly Effective Aldose Reductase Mimetics: Microwave-Assisted Catalytic Transfer Hydrogenation of d-Glucose to D-Sorbitol with Magnetically Recoverable Aminomethylphosphine-Ru(II) and Ni(II) Complexes, J. Mol. Struct., 2021, 1237, 130313, DOI:10.1016/j.molstruc.2021.130313.
- X. Jia, I. K. M. Yu, D. C. W. Tsang and A. C. K. Yip, Functionalized Zeolite-Solvent Catalytic Systems for Microwave-Assisted Dehydration of Fructose to 5-Hydroxymethylfurfural, Microporous Mesoporous Mater., 2019, 284, 43–52, DOI:10.1016/j.micromeso.2019.04.022.
- G. Li, W. Liu, C. Ye, X. Li and C. L. Si, Chemocatalytic Conversion of Cellulose into Key Platform Chemicals, Int. J. Polym. Sci., 2018, 2018, 4723573, DOI:10.1155/2018/4723573.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Catalytical Conversion of Fructose and Glucose into 5-Hydroxymethylfurfural in Hot Compressed Water by Microwave Heating, Catal. Commun., 2008, 9(13), 2244–2249, DOI:10.1016/j.catcom.2008.04.025.
- V. T. C. Doan, T. H. Nguyen, H. B. Phan and P. H. Tran, Microwave-Assisted Conversion of Fructose to 5-HMF Using Carbonaceous Acidic Catalysts, Mol. Catal., 2023, 549, 113518, DOI:10.1016/j.mcat.2023.113518.
- C. Antonetti, M. Melloni, D. Licursi, S. Fulignati, E. Ribechini, S. Rivas, J. C. Parajó, F. Cavani and A. M. Raspolli Galletti, Microwave-Assisted Dehydration of Fructose and Inulin to HMF Catalyzed by Niobium and Zirconium Phosphate Catalysts, Appl. Catal., B, 2017, 206, 364–377, DOI:10.1016/j.apcatb.2017.01.056.
- G. S. Foo and C. Sievers, Synergistic Effect between Defect Sites and Functional Groups on the Hydrolysis of Cellulose over Activated Carbon, ChemSusChem, 2015, 8(3), 534–543, DOI:10.1002/cssc.201402928.
- J. G. Wang, Y. Y. Zhang, Y. Wang, L. W. Zhu, H. Y. Cui and W. M. Yi, Catalytic Fructose Dehydration to 5-Hydroxymethylfurfural over Sulfonated Carbons with Hierarchically Ordered Pores, J. Fuel Chem. Technol., 2016, 44(11), 1341–1348, DOI:10.1016/s1872-5813(16)30058-5.
- K. Weerasai, V. Champreda, C. Sakdaronnarong, A. Shotipruk and N. Laosiripojana, Hydrolysis of Eucalyptus Wood Chips under Hot Compressed Water in the Presence of Sulfonated Carbon-Based Catalysts, Food Bioprod. Process., 2018, 110, 136–144, DOI:10.1016/j.fbp.2018.05.005.
- S. Liu, Y. Zhu, Y. Liao, H. Wang, Q. Liu, L. Ma and C. Wang, Advances in Understanding the Humins: Formation, Prevention and Application, Appl. Energy Combust. Sci., 2022, 10, 100062, DOI:10.1016/j.jaecs.2022.100062.
- A. Modak, A. R. Mankar, K. K. Pant and A. Bhaumik, Mesoporous Porphyrin-Silica Nanocomposite as Solid Acid Catalyst for High Yield Synthesis of HMF in Water, Molecules, 2021, 26(9), 1–13, DOI:10.3390/molecules26092519.
- P. S. Divya, S. Nair and S. Kunnikuruvan, Identification of Crucial Intermediates in the Formation of Humins from Cellulose-Derived Platform Chemicals Under Brønsted Acid Catalyzed Reaction Conditions, ChemPhysChem, 2022, 23(11), e202200057, DOI:10.1002/cphc.202200057.
- M. Du, A. M. Agrawal, S. Chakraborty, S. J. Garibay, R. Limvorapitux, B. Choi, S. T. Madrahimov and S. T. Nguyen, Matching the Activity of Homogeneous Sulfonic Acids: The Fructose-to-HMF Conversion Catalyzed by Hierarchically Porous Sulfonic-Acid-Functionalized Porous Organic Polymer (POP) Catalysts, ACS Sustainable Chem. Eng., 2019, 7(9), 8126–8135, DOI:10.1021/acssuschemeng.8b05720.
- J. C. Velasco Calderón, J. S. Arora and S. H. Mushrif, Mechanistic Investigation into the Formation of Humins in Acid-Catalyzed Biomass Reactions, ACS Omega, 2022, 7(49), 44786–44795, DOI:10.1021/acsomega.2c04783.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00007b |
| This journal is © The Royal Society of Chemistry 2024 |