Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synergetic effects on the capture and release of CO2 using guanidine and amidine superbases†
Todd
Elliott‡
a,
Luc
Charbonneau‡
a,
Eva
Gazagnaire
a,
Ilkka
Kilpeläinen
 a,
Bianka
Kótai
b,
Gergely
Laczkó
b,
Imre
Pápai
a,
Bianka
Kótai
b,
Gergely
Laczkó
b,
Imre
Pápai
 b and
Timo
Repo
b and
Timo
Repo
 *a
*a
aDepartment of Chemistry, University of Helsinki, P.O. Box 55, FIN-00014, Finland. E-mail: timo.repo@helsinki.fi
bInstitute of Organic Chemistry, Research Centre for Natural Sciences, H-1117 Budapest, Hungary
First published on 29th April 2024
Abstract
The capture of CO2 from air is of utmost importance, not only to reduce its impact on climate change but also for its utilisation as a tremendous, renewable source of C1 building blocks for sustainable chemical synthesis. Novel and known superbase structures are compared in a new selection of solvents for CO2 capture and release. Bicyclic amidine and guanidine superbases with 6–5, 6–6 and 6–7 configurations and many methylated analogues are investigated. As reported here, identified superbase/solvent combinations offer a highly efficient, reversible, and kinetically favourable CO2 capture process from air. The two most beneficial superbase/solvent synergic combinations identified are 1,5,7-triazabicyclo[4.3.0]non-6-ene (TBN) in butyl acetate and 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) in acetonitrile. They reach saturation after 15 min with pure CO2 and after 24 hours under open-air conditions and release CO2 with a CO2/superbase molar ratio of 0.41 and 0.25, respectively. Due to the favourable thermodynamics of the systems, quantitative CO2 release for TBN and DBN occurs under mild conditions at 90 °C and 60 °C within 20 minutes. The required time for a complete absorption–desorption cycle for both TBN-butyl acetate and DBN-acetonitrile was only 48.5 and 38.5 minutes respectively. Superbase–solvent mixtures are recyclable and the system retains its initial CO2 capturing capability after 5 cycles. As this apparently easy emerging system design allows the direct capture of CO2 from air, it has potential for positive utilization on the global scale.
Sustainability spotlightDirect carbon dioxide (CO2) capture from air is not only imperative for addressing the escalating climate crisis, but it also holds significant potential to serve as a sustainable and cost-effective source of C1 building blocks for the chemical industry. This emerging technology represents a crucial step towards mitigating the adverse impacts of anthropogenic carbon emissions and fostering a more sustainable future. On the other hand, current technologies rely on calcium based adsorbents or aqueous alkanolamine which require high energy to recover the captured CO2 for industrial applications. With the aim of developing efficient CO2 capture and release at low energy, the use of amidines and guanidine is becoming an attractive alternative to other processes. Herein, we introduced not only the importance of the structure of the superbases (amidine or guanidine), but also the role of the solvent and its synergetic effects on both capture and release of CO2. The effect of solvent has not been deeply investigated and plays a dual role in the thermodynamics and kinetics of CO2 capture and release. |
Introduction
Carbon dioxide is an overly abundant greenhouse gas, particularly from industrial point sources such as petrochemical, iron and steel manufacturing and the cement industry.1–5 Reducing anthropogenic CO2 emissions is an important step towards carbon neutrality but it is insufficient to reach the anticipated CO2 negativity that will be required.6 Direct CO2 capture from air is not only necessary but it also has great potential to provide a clean source of renewable and low cost C1 building blocks for the chemical industry.7,8 Contributing to added-value products such as urea and inorganic carbonates, innovative processing of methanol and dry reforming with methane to jet fuels have also attracted much recent interest.9–12 CO2 is also essential in industrial scale synthesis of cyclic carbonates, polycarbonates, non-isocyanate polyurethanes (NIPU), and salicylic acid.13–19Different technologies have been developed to capture CO2 from different sources, whether this is direct air capture (DAC) or from point sources such as flue gas.20,21 Inorganic sorbents have been used to capture CO2 using the calcium oxide-calcium carbonate loop. However, the recovery process is energy intensive, posing challenges to future use.22
With the importance of removing CO2 from flue gases as well as from air, different capture technologies were developed with many pros and cons for each of these methods.23
Based on the limitation of solid sorbents, amine in solvent becomes an attractive alternative, since the reaction of an amine with CO2 is a fundamental interaction in chemistry. This interaction leads to the formation of stable ionic species such as carbamate or (bi)carbonate. Currently, the most used absorbents for CO2 capture in industries are amine-based aqueous solutions such as monoethanolamine (MEA), diethanolamine (DEA), diglycolamine (DGA) and N-methyldiethanolamine (MDEA).2,24,25 These amines strongly bind CO2, and high temperatures are needed (130 °C) for its recovery. Due to the high heat capacity of the reaction medium, this step also requires significant amounts of energy.26 Additionally, these absorbents face severe challenges with the loss of organic amines due to high volatility and decomposition during regeneration.2,27,28
Ionic liquids have improved this system by improving thermal stability, lowering loss of solution through lower vapour pressure and by having tuneable polarity in the choice of ion pairs. The choice of ion pairs also allows control over other physical and chemical properties. However, negative ions for ionic liquids tend to be halogenated or toxic, meaning they have little chance of being scalable to the problem at hand.29
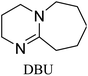
Bicyclic organic amine superbases, particularly amidines and guanidines, have gained attention as promising alternatives to conventional alkanolamine for the capture of CO2. First, Jessop et al. studied 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) with hexanol to form a switchable ionic liquid, reaching a polarity like that of water.30–32 They were also able to recover CO2 by simply heating the solution.33 Not only does this system show reversible capture of CO2, but their results show that the energy consumption of this system is 50% less than that of a water–MEA mixture.31 When amidine or guanidine captures CO2, in the presence of water, a bicarbonate anion is formed over the carbamate zwitterion.34,35 The carbamate zwitterion can allow for stabilising intramolecular hydrogen bonding to occur, whereas the bicarbonate exists as separate ions.36 An example of this can be seen in Fig. 1.
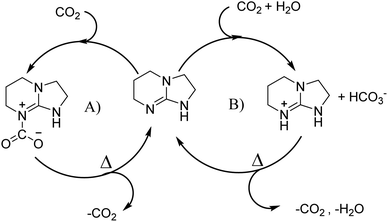 | ||
| Fig. 1 Absorption schematic of CO2 using TBN: (A) without water leading to the formation of zwitterions and (B) with the presence of water forming bicarbonate. | ||
Amidine and guanidine superbases were further studied with different alcohols and hexane revealing further possibilities for reversible capture of CO2.30,32,37–39 A review thoroughly described the effect of alcohols, ionic liquids, and deep eutectic solvent on CO2 capture.39 On the other hand, the use of other organic solvents is not very well studied for CO2 capture. Furthermore, fundamental understanding of the synergistic solvent effect with superbases is lacking and further improvement of the superbase (SB)–CO2 capture system remains highly challenging.40
Herein we report highly reversible and kinetically favourable CO2 capture benefitting from the synergistic SB–solvent combinations. Even more strikingly, this concept also opens a window of opportunity to directly capture CO2 from air (DAC). The lowest temperature of CO2 release and which combinations release the most CO2 in a capture/release cycle would be considered optimal. As much as the structure of the SB is the core of reactivity, the selection of solvent is fundamental to attain maximum absorption and reversibility.
Results and discussion
CO2 capture and release using different solvents
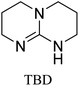
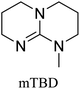
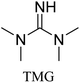
All in all, we evaluated 10 solvents and 8 superbases, totalling up to 80 combinations for the reversible capture of CO2. First, we saturated a 30% weight superbase solution with 15 min of bubbling pure CO2 at room temperature (RT). To identify their reversibility capability in CO2 capture, the release temperature and duration of CO2 released were studied (see the ESI†). Some solvents were excluded from further study as they, regardless of the superbase, demonstrated an irreversible binding of CO2, had limited solubility for the superbases or released CO2 above the boiling point of the solvent. We observed irreversible behaviour for example with TBU and toluene. Based on these observations, SB/solvent combinations demonstrated distinct differences for CO2 release. While the structure of the superbase has a bearing on its ability to bind CO2, the synergetic effects with the solvent play an important role in both its absorption and release. Other physicochemical properties of the solvent, such as viscosity and polarity, also affect the mass transfer of CO2 to the reaction medium. However, the literature regarding interaction of a solvent with a SB–CO2 adduct is sparse, especially regarding its direct effect on the reversibility of the reaction.41For further studies, we focused on five green solvents, ethanol, ethyl acetate, butyl acetate, propylene carbonate and acetonitrile.42,43 DBU was widely investigated and was used as a reference point to compare with 7 bicyclic amidines and guanidines consisting of different ring configurations; 6–5, 6–6 and 6–7. The amidines used in this study were DBU and 1,5-diazabicyclo[4.3.0]non-5-ene (DBN). The guanidines studied were 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), [1,5,7]-triazabicyclo [4.5.0]-undec-5-ene (TBU) and 1,5,7-triazabicyclo[4.3.0]non-6-ene (TBN). Superbases were also compared with their N-methyl substituted analogues for their influence on binding CO2; 7-methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) and the racemic mixture of the isomers 7-methyl-1,5,7-triazabicyclo[4.3.0]non-5-ene (mTBN) and 5-methyl-1,5,7-triazabicyclo[4.3.0]non-6-ene (mTBN) depending on whether the methyl group is on the 5 or 6 membered ring, respectively. 1,1,3,3-Tetramethylguanidine (TMG) was included as a representative of acyclic guanidines. The three major criteria for efficient systems are having a high molar ratio of CO2/SB released, low reversibility temperature and a rapid release.
In the selected aprotic solvents, the superbases reacted with pure CO2 and formed a white precipitate after 5 minutes. When heat is applied, any resulting slurries disappear, and CO2 gas is emitted. However, ethanol forms an ionic liquid, and so no precipitate is observed.38 There is a stark contrast in the SB–CO2 adduct reversibility depending on the solvent used, including two exceptions, TBN/ethyl acetate and TBN/acetonitrile being irreversible (see Tables S2 and S3 in the ESI†).
Compared to acyclic TMG, a bicyclic configuration increases electron density at the sp2-hybridised nitrogen, which increases its nucleophilicity and thus binding strength with CO2. From the series of bicyclic superbases, DBN/acetonitrile and TBN/butyl acetate are the best candidates when combining time, temperature and molar ratio of CO2 bound and released. Based on results in Table 1, TBN and DBN, both having 6–5 heterocyclic ring configurations, are markedly faster in CO2 release than bicyclic amidines and guanidines with 6–6 and 6–7 ring combinations, including classical DBU. The other obvious benefit of TBN and DBN superbases is a higher molar ratio of superbase to CO2 (Table 1).
| Superbase structure | Solvent | Temperature of CO2 release (°C) | Timea (min) | Molar ratio CO2/SB |
|---|---|---|---|---|
| a The CO2 absorption studies used 30 g of a superbase–solvent solution, with a 30% wt superbase. CO2 was sparged through the solution for 30 minutes under vigorous stirring. The flask was then placed in a preheated oil bath at the selected temperature. The volume of CO2 released was measured by using a burette system, and accordingly, the amount of CO2 released and molar ratio of CO2/superbase were calculated. b Solubility issues in all selected solvents as TBU partially precipitates out of the solution after releasing CO2. | ||||
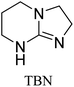
|
Butyl acetate | 90 | 20.5 | 0.41 (±6%) |
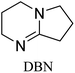
|
Acetonitrile | 60 | 20.5 | 0.25 (±3%) |
|
Acetonitrile | 60 | 39 | 0.25b |
|
Acetonitrile | 60 | 25 | 0.16 (±4%) |
|
Acetonitrile | 65 | 29 | 0.13 (±6%) |
|
Ethanol | 60 | 17 | 0.13 (±6%) |
|
Butyl acetate | 65 | 26 | 0.05 (±4%) |
|
Ethyl acetate | 60 | 32 | 0.11 (±6%) |
The structure of the superbase has a bearing on its ability to reversibly bind CO2. In the same solvent, the change from an amidine structure to its guanidine analogue increases the amount of reversibly released CO2 (see the ESI, Table S3†). Despite similar reversibility temperatures, (when considering differences in solvent) non-methylated guanidines capture more CO2 compared to their methylated analogues (TBD vs. mTBD). Having a H-bond donor and acceptor nitrogen in near proximity in a planar configuration, bicyclic guanidines are considered to bind CO2 stronger than the corresponding amidines.44 Accordingly, we suggest that the N-methylation of TBD and TBN annihilates the intramolecular hydrogen bonding, and the desired zwitterionic SB–CO2 interaction is remarkably decreased. This can be seen by their marked reduction in CO2 adsorption compared to the non-methylated versions (Table 1).
To demonstrate reversibility even under moist conditions including atmospheric humidity, bicarbonate formation was forced by adding 1 mL of water to TBN and DBN in their respective reaction medium. Although the amount of precipitate increased significantly, the CO2/SB ratio remained consistent showing no distinct effect on the reversibility and temperature needed (see Table S4†).
As shown above, besides the structure of the SB as the core of reactivity, the solvent choice becomes critical for efficient CO2 capture and release (Tables 1, S2 and S3 in the ESI†). Based on our observations, polar media are beneficial for CO2 binding to a certain extent as, depending on the SB structure, this can stabilise the SB–CO2 adduct but at the same time, this can inhibit reversibility. This solvent effect is clearly illustrated with TBN; the reversibility is lost in acetonitrile, while in butyl acetate the capacity to absorb and release CO2 is among the best in this study (ESI, Tables S2 and S3†). To gain further insights into this phenomenon and the effect of solvent on the reversibility, we performed DFT calculations to determine the energetics.
DFT calculations
The present computational approach provides reasonably accurate Gibbs free energy data for the interaction of SB molecules with CO2; however, other important processes involved in CO2 capture/release (CO2 transfer from the gas to the solvent phase, diffusion, and precipitation) are not considered in our models. For this reason, the computed energetics can only be used in qualitative terms to interpret the observed trends.To develop our understanding of the factors that determine the reversibility of the examined CO2 capture/release processes, we investigated the interaction of SB molecules with CO2 computationally. We considered three different SB/CO2 systems (SB = DBN, TBN and mTBN) using acetonitrile and butyl acetate as solvent media (see Fig. 2). The applied computational protocol involved solution phase geometry optimizations carried out at the ωB97X-D/6-311G(d,p) level of DFT, where the solvent effects were incorporated via the implicit SMD solvation model.45,46 Additional single-point electronic energy calculations were carried out using the LNO-CCSD(T)/CBS method to provide accurate energetics for the interaction of SB molecules with CO2.47,48 The reported energy data refer to solution phase Gibbs free energies under standard conditions (T = 298.15 K and c = 1 mol L−1). For further details, see the ESI.†
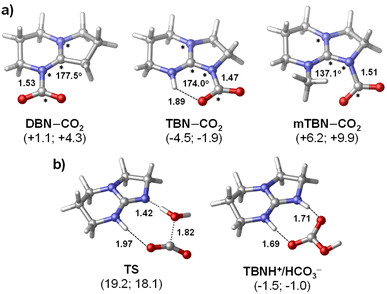
The barriers of SB–CO2 adduct formation are predicted to be fairly low (ΔG‡ = 8–14 kcal mol−1) suggesting that the rate of CO2 capture is likely diffusion controlled. The thermodynamic stability data computed for the three zwitterionic SB–CO2 adducts (Fig. 2a) show a notable variation with the superbase molecule and with the solvent as well. Although the basicities of the three SB molecules as quantified by using the computed solution phase proton affinities are very similar (they are within 1.3 kcal mol−1 for both solvents), the stabilities of the SB–CO2 adducts vary in a much broader energy window (>10 kcal mol−1). In both solvents, TBN–CO2 is predicted to be the most stable adduct followed by DBN–CO2 and mTBN–CO2 in the stability order. As expected, adduct formation is found to be more favoured thermodynamically in acetonitrile (by about 3 kcal mol−1) with all the three bases, since it is significantly more polar than butyl acetate (dielectric constants are 35.7 and 4.62, respectively).
The enhanced stability of TBN–CO2 is associated with the intramolecular H-bonding interaction, which is absent in the other two adducts.36 On the other hand, the N-methyl substituent in mTBN induces steric hindrance for the ideal planar arrangement of the guanidine–CO2 unit in the adduct that would allow extended charge delocalization. Consequently, mTBN–CO2 is predicted to be the least favoured zwitterionic species in the series. The distorted structure of mTBN–CO2 is apparent in Fig. 2a (the highlighted NCNC dihedral angle deviates significantly from 180°). The adduct formation is nearly neutral thermodynamically for DBN/acetonitrile and TBN/butyl acetate combinations (ΔG = +1.1 and −1.9 kcal mol−1), which is in accordance with our observations in the CO2 capture/release experiments that these are the two most efficient systems (Table 1). For the mTBN/CO2 system, computations show slightly higher endergonicity even in acetonitrile (ΔG = +6.2 kcal mol−1), but this is likely compensated by the precipitation of the zwitterionic adduct species. The presented Gibbs free energies of adduct formation by no means can be regarded as a quantitative measure of the reversibility of CO2 capture; however, the computed trend accounts well for the observations.
The reaction of TBN and CO2 in the presence of water was investigated computationally as well (Fig. 2b). The results suggest that the formation of the TBNH+/HCO3− guanidium-bicarbonate ion pair in butyl acetate is also kinetically feasible at room temperature (ΔG‡ = 18.1 kcal mol−1), although the computed barrier points to a slower process as compared to that of TBN–CO2 formation (8.9 kcal mol−1). The overall reaction with water is predicted to be slightly less favoured thermodynamically (ΔG = −1.0 kcal mol−1) implying that this reaction will not affect the reversibility of CO2 capture with TBN.
Design of experiment
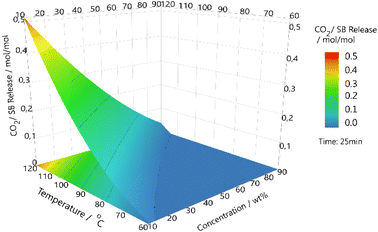
Higher temperatures and higher concentrations are not guaranteed to increase efficiency, and as such it is important to create a simple model for the system.49 A design of experiment model was created and followed to cover TBN in butyl acetate (Fig. 3). The three variables covered were concentration of SB, temperature used to release CO2 and length of exposure time to CO2 for absorption. The results further evidence that higher temperatures do increase the speed and amount of CO2 recovered, as does longer exposure time to CO2. It can also be observed that lower concentrations of SB allow for higher ratios of CO2 to be captured. This further illustrates the mass transfer issue that affects the system.Kinetics
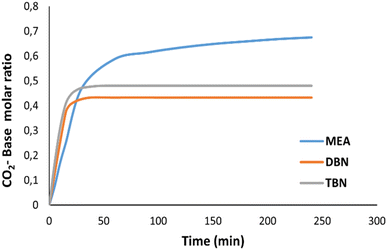
The required time for the absorption for CO2 to reach saturation was measured for TBN-butyl acetate and DBN-acetonitrile and compared with that of MEA in water as a reference (Fig. 4).The required time for TBN-butyl acetate as well as DBN-acetonitrile to reach saturation is only 15 min, compared to MEA in water which required more than 4 h. For DBN, the cycle for the capture and release of CO2 takes only 38.8 minutes compared to that of other systems which require much longer times per cycle.50,51
From the data reported in Table 1 and Fig. 4, it was possible to determine the yield of CO2 recovery per cycle of absorption and release for the best superbase solvent combination (equation in the ESI†). For the absorption, the CO2–SB molar ratio for TBN-butyl acetate reached 0.49 and 0.42 for DBN-acetonitrile. For the release, the yield of CO2 recovery for TBN-butyl acetate and DBN-acetonitrile are 83.6% and 59.5% respectively showing that not all carbon dioxide was fully recovered.
Reusability
An important criterion for CO2 capture is the reusability of the SB–solvent system for multiple cycles. For this reason, the reusability for both TBN and DBN systems was investigated for five consecutive capture/release cycles (Fig. 5).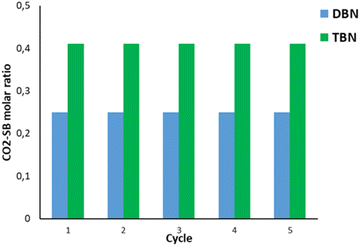 | ||
| Fig. 5 Five consecutive recycling experiments with (a) TBN in butyl acetate (green) and (b) DBN in acetonitrile (blue). | ||
After the sequence of 5 cycles, these two systems do not show any decrease in CO2 capture/release capacity. Also, the five cycles required only tens of minutes compared to hundreds of minutes when other systems were used, showing the better efficiency of our system.52,53
CO2 capture from air
To continue our experimental investigations, we examined the two best candidates also for DAC. Due to low concentration of atmospheric CO2, saturation of the systems required 24 hours under ambient conditions. The quantitative release occurs, as in the case of pure CO2, at 60 °C and in less than 20 min for DBN. The DFT calculations corroborate our experimental results; reversal occurs regardless of if the CO2 adduct is in the carbamate or bicarbonate form. The required transition state energy for the release is nearly the same in the range of 9.3 to 12 kcal mol−1. Although the amount of CO2 captured is 1/8 of the amount captured with pure CO2, this full reversibility, low energy requirement, and fast kinetics makes the system highly intriguing for DAC.General considerations and sensitivity of the parameters
In this study, the focus was to investigate the application of superbases with organic solvents for direct air capture. For this reason, parameters employed in this study were at standard pressure and temperature. However, these parameters can influence the absorption and release of carbon dioxide. First, the concentration of CO2 will play a dramatic role in the kinetics of absorption. By lowering the concentration from pure CO2 to the 400 ppm level, the time required to reach saturation will obviously be much longer going from few minutes to hours. As for the temperature, it is anticipated that a higher temperature will decrease the absorption capacity of the superbase solvent mixture. Another important parameter is the humidity from air which varies in different places around the world. The two systems presented are shown to be moisture tolerant for 5 cycles and can both release the captured CO2 regardless of if it is a carbamate or a bicarbonate. On the other hand, these systems will be tested for multiple cycles and moisture might induce degradation of the superbases.54 If degradation by the presence of water is observed, to maintain constant activity, dry air should be favoured. These parameters will be further investigated in future studies during scale up development.In comparison to MEA, our systems demonstrate lower corrosion and temperature of release, as well as faster absorption and desorption regardless of bicarbonate or carbamate ion formation. For industries this process is expected to have a low energy cost. As this apparently easy emerging system design allows the direct capture of CO2 from air, it has potential for positive utilisation on the global scale.
Conclusion
In summary, we have established that bicyclic 6–5 ring configurations capture CO2 more efficiently than 6–6 and 6–7 configurations. TBN in butyl acetate and DBN in acetonitrile are shown to be the best systems of those in this study. The solvent choice is important as it affects the reversibility and capture of CO2, either aiding capture or impeding CO2 release. DFT calculations show that both the capture and the release of CO2 are thermodynamically favourable for TBN in butyl acetate, which is the best system studied regardless of if it forms a zwitterion or a bicarbonate molecular adduct. The reversibility of TBN and DBN shows that capture-release of CO2 can be performed for 5 cycles without losing the absorption capacity.Future studies will consider prolonged repeatability experiments to account for stability, and degradation from moisture as part of the scale-up considerations. For flue gas applications, NOx and SOx durability would also need to be considered.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was funded by the NordForsk (grant no. 85378) and the members of the Nordic Consortium for CO2 Conversion (NordCO2), the Academy of Finland (project 310767), and Business Finland (8205/31/2022). The authors would like to thank Joseph Install for his help with the design of experiment.References
- S. Chen, J. Liu, Q. Zhang, F. Teng and B. C. McLellan, A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality, Renewable Sustainable Energy Rev., 2022, 167, 112537, DOI:10.1016/j.rser.2022.112537.
- W. Gao, S. Liang, R. Wang, Q. Jiang, Y. Zhang, Q. Zheng, B. Xie, C. Y. Toe, X. Zhu and J. Wang, et al., Industrial carbon dioxide capture and utilization: state of the art and future challenges, Chem. Soc. Rev., 2020, 49(23), 8584–8686, 10.1039/d0cs00025f.
- M. G. Plaza, S. Martínez and F. Rubiera, CO2 Capture, Use, and Storage in the Cement Industry: State of the Art and Expectations, Energies, 2020, 13(21), 28, DOI:10.3390/en13215692.
- S. Yun, M.-G. Jang and J.-K. Kim, Techno-economic assessment and comparison of absorption and membrane CO2 capture processes for iron and steel industry, Energy, 2021, 229, 12, DOI:10.1016/j.energy.2021.120778.
- S. Y. W. Chai, L. H. Ngu, B. S. How, M. Y. Chin, K. Abdouka, M. J. B. A. Adini and A. M. Kassim, Review of CO2 capture in construction-related industry and their utilization, Int. J. Greenhouse Gas Control, 2022, 119, 24, DOI:10.1016/j.ijggc.2022.103727.
- J. Rogelj, D. Shindell, K. Jiang, S. Fifita, P. Forster, V. Ginzburg, C. Handa, H. Kheshgi, S. Kobayashi, E. Kriegler, L. Mundaca,; R. Séférian, and M. V. Vilariño, Mitigation Pathways Compatible with 1.5°C in the Context of Sustainable Development, Global Warming of 1.5°C, An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Trengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, 2018 Search PubMed.
- A. Adamu, F. Russo-Abegão and K. Boodhoo, Process intensification technologies for CO2 capture and conversion – a review, BMC Chem. Eng., 2020, 2(2), 1–18, DOI:10.1186/s42480-019-0026-4.
- H. Ning, Y. Li and C. Zhang, Recent Progress in the Integration of CO(2) Capture and Utilization, Molecules, 2023, 28(11), 4500–4514, DOI:10.3390/molecules28114500.
- J. R. Fernández, S. Garcia and E. S. Sanz-Pérez, CO2 Capture and Utilization Editorial, Ind. Eng. Chem. Res., 2020, 59(15), 6767–6772, DOI:10.1021/acs.iecr.0c01643.
- Y. Yoo, I. Kim, D. Lee, W. Yong Choi, J. Choi, K. Jang, J. Park and D. Kang, Review of contemporary research on inorganic CO2 utilization via CO2 conversion into metal carbonate-based materials, J. Ind. Eng. Chem., 2022, 116, 60–74, DOI:10.1016/j.jiec.2022.09.007.
- R. Shirmohammadi, A. Aslani, R. Ghasempour and L. M. Romeo, CO2 Utilization via Integration of an Industrial Post-Combustion Capture Process with a Urea Plant: Process Modelling and Sensitivity Analysis, Processes, 2020, 8(9), 12, DOI:10.3390/pr8091144.
- M. Marchese, G. Buffo, M. Santarelli and A. Lanzini, CO2 from direct air capture as carbon feedstock for Fischer-Tropsch chemicals and fuels: Energy and economic analysis, J. CO2 Util., 2021, 46, 15, DOI:10.1016/j.jcou.2021.101487.
- X. D. Lang and L. N. He, Green Catalytic Process for Cyclic Carbonate Synthesis from Carbon Dioxide under Mild Conditions, Chem. Rec., 2016, 16(3), 1337–1352, DOI:10.1002/tcr.201500293.
- H. Blattmann, M. Fleischer, M. Bahr and R. Mulhaupt, Isocyanate- and phosgene-free routes to polyfunctional cyclic carbonates and green polyurethanes by fixation of carbon dioxide, Macromol. Rapid Commun., 2014, 35(14), 1238–1254, DOI:10.1002/marc.201400209.
- A. B. Paninho and A. V. M. Nunes, Limonene carbonate synthesis from CO2: Continuous high-pressure flow catalysis with integrated product separation, J. Supercrit. Fluids, 2023, 193, 105827, DOI:10.1016/j.supflu.2022.105827.
- B. Yao, T. Xiao, O. A. Makgae, X. Jie, S. Gonzalez-Cortes, S. Guan, A. I. Kirkland, J. R. Dilworth, H. A. Al-Megren and S. M. Alshihri, et al., Transforming carbon dioxide into jet fuel using an organic combustion-synthesized Fe-Mn-K catalyst, Nat. Commun., 2020, 11(1), 6395, DOI:10.1038/s41467-020-20214-z.
- J. K. Mannisto, L. Pavlovic, T. Tiainen, M. Nieger, A. Sahari, K. H. Hopmann and T. Repo, Mechanistic insights into carbamate formation from CO2 and amines: the role of guanidine–CO2 adducts, Catal. Sci. Technol., 2021, 11(20), 6877–6886, 10.1039/d1cy01433a.
- Á. Mesías-Salazar, J. Martínez, R. S. Rojas, F. Carrillo-Hermosilla, A. Ramos, R. Fernández-Galán and A. Antiñolo, Aromatic guanidines as highly active binary catalytic systems for the fixation of CO2 into cyclic carbonates under mild conditions, Catal. Sci. Technol., 2019, 9(15), 3879–3886, 10.1039/c9cy00667b.
- R. Villa, S. Nieto, A. Donaire and P. Lozano, Direct Biocatalytic Processes for CO(2) Capture as a Green Tool to Produce Value-Added Chemicals, Molecules, 2023, 28(14), 5520–5572, DOI:10.3390/molecules28145520.
- E. S. Sanz-Perez, C. R. Murdock, S. A. Didas and C. W. Jones, Direct Capture of CO(2) from Ambient Air, Chem. Rev., 2016, 116(19), 11840–11876, DOI:10.1021/acs.chemrev.6b00173.
- X. Wu, Y. Yu, Z. Qin and Z. Zhang, The Advances of Post-combustion CO2 Capture with Chemical Solvents: Review and Guidelines, Energy Procedia, 2014, 63, 1339–1346, DOI:10.1016/j.egypro.2014.11.143.
- M. Erans, V. Manovic and E. J. Anthony, Calcium looping sorbents for CO2 capture, Appl. Energy, 2016, 180, 722–742, DOI:10.1016/j.apenergy.2016.07.074.
- A. C. Forse and P. J. Milner, New chemistry for enhanced carbon capture: beyond ammonium carbamates, Chem. Sci., 2020, 12(2), 508–516, 10.1039/d0sc06059c.
- B. Li, Y. Duan, D. Luebke and B. Morreale, Advances in CO2 capture technology: A patent review, Appl. Energy, 2013, 102, 1439–1447, DOI:10.1016/j.apenergy.2012.09.009.
- P. D. Vaidya and E. Y. Kenig, CO2-Alkanolamine Reaction Kinetics: A Review of Recent Studies, Chem. Eng. Technol., 2007, 30(11), 1467–1474, DOI:10.1002/ceat.200700268.
- G. T. R. Stefano Freguia, Modeling of CO2 capture by aqueous monoethanolamine, AIChE J., 2004, 49(7), 1676–1686, DOI:10.1002/aic.690490708.
- P. Luis, Use of monoethanolamine (MEA) for CO 2 capture in a global scenario: Consequences and alternatives, Desalination, 2016, 380, 93–99, DOI:10.1016/j.desal.2015.08.004.
- A. V. Rayer, K. Z. Sumon, T. Sema, A. Henni, R. O. Idem and P. Tontiwachwuthikul, Part 5c: Solvent chemistry: solubility of CO2in reactive solvents for post-combustion CO2, Carbon Manage., 2014, 3(5), 467–484, DOI:10.4155/cmt.12.47.
- M. Aghaie, N. Rezaei and S. Zendehboudi, A systematic review on CO2 capture with ionic liquids: Current status and future prospects, Renewable Sustainable Energy Rev., 2018, 96, 502–525, DOI:10.1016/j.rser.2018.07.004.
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop and L. Phan, Organic liquid CO2 capture agents with high gravimetric CO2 capacity, Energy Environ. Sci., 2008, 1(4), 487–493, 10.1039/b809533g.
- D. J. Heldebrant, C. R. Yonker, P. G. Jessop and L. Phan, CO2 -binding organic liquids (CO2 BOLs) for post-combustion CO2 capture, Energy Procedia, 2009, 1(1), 1187–1195, DOI:10.1016/j.egypro.2009.01.156.
- P. G. H. Jessop, J. D, X. Li, C. A. Eckert and C. L. Liotta, Reversible nonpolar-to-polar solvent, Nature, 2005, 436(7054), 1102, DOI:10.1038/4361102a.
- X. Zhu, M. Song and Y. Xu, DBU-Based Protic Ionic Liquids for CO2 Capture, ACS Sustain. Chem. Eng., 2017, 5(9), 8192–8198, DOI:10.1021/acssuschemeng.7b01839.
- F. S. Pereira, E. R. deAzevedo, E. F. da Silva, T. J. Bonagamba, D. L. da Silva Agostíni, A. Magalhães, A. E. Job and E. R. Pérez González, Study of the carbon dioxide chemical fixation—activation by guanidines, Tetrahedron, 2008, 64(43), 10097–10106, DOI:10.1016/j.tet.2008.08.008.
- L. F. B. Wilm, T. Eder, C. Mück-Lichtenfeld, P. Mehlmann, M. Wünsche, F. Buß and F. Dielmann, Reversible CO2 fixation by N-heterocyclic imines forming water-stable zwitterionic nitrogen-base–CO2 adducts, Green Chem., 2019, 21(3), 640–648, 10.1039/c8gc02952k.
- C. Villiers, J. P. Dognon, R. Pollet, P. Thuery and M. Ephritikhine, An isolated CO2 adduct of a nitrogen base: crystal and electronic structures, Angew Chem. Int. Ed. Engl., 2010, 49(20), 3465–3468, DOI:10.1002/anie.201001035.
- H. Zhou, W. Chen, J.-H. Liu, W.-Z. Zhang and X.-B. Lu, Highly effective capture and subsequent catalytic transformation of low-concentration CO2 by superbasic guanidines, Green Chem., 2020, 22(22), 7832–7838, 10.1039/d0gc03009k.
- D. C. Lam Phan, D. J. Heldebrant, H. Huttenhower, E. John, X. Li, P. Pollet, R. Wang, C. A. Eckert, C. L. Liotta and P. G. Jessop, Switchable Solvents Consisting of Amidine/Alcohol or Guanidine/Alcohol Mixtures, Ind. Eng. Chem. Res., 2008, 47(3), 539–545, DOI:10.1021/ie070552r.
- B. Gabriele, N. Della Ca, R. Mancuso, L. Veltri and I. Ziccarelli, Amidine– and guanidine-based synthetic methods for CO2 capture and utilization, Curr. Opin. Green Sustainable Chem., 2023, 41, 100793, DOI:10.1016/j.cogsc.2023.100793.
- T. N. Borhani and M. Wang, Role of solvents in CO2 capture processes: The review of selection and design methods, Renewable Sustainable Energy Rev., 2019, 114, 109299, DOI:10.1016/j.rser.2019.109299.
- D. Malhotra, D. C. Cantu, P. K. Koech, D. J. Heldebrant, A. Karkamkar, F. Zheng, M. D. Bearden, R. Rousseau and V.-A. Glezakou, Directed Hydrogen Bond Placement: Low Viscosity Amine Solvents for CO2 Capture, ACS Sustain. Chem. Eng., 2019, 7(8), 7535–7542, DOI:10.1021/acssuschemeng.8b05481.
- R. A. Sheldon, The greening of solvents: Towards sustainable organic synthesis, Curr. Opin. Green Sustainable Chem., 2019, 18, 13–19, DOI:10.1016/j.cogsc.2018.11.006.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, CHEM21 selection guide of classical- and less classical-solvents, Green Chem., 2016, 18(1), 288–296, 10.1039/c5gc01008j.
- M. K. Kiesewetter, M. D. Scholten, N. Kirn, R. L. Weber, J. L. Hedrick and R. M. Waymouth, Cyclic Guanidine Organic Catalysts: What Is Magic About Triazabicyclodecene?, J. Org. Chem., 2009, 74(24), 9490–9496, DOI:10.1021/jo902369g.
- J.-D. Chai and M. Head-Gordon, Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections, Phys. Chem. Chem. Phys., 2008, 10(44), 6615–6620, 10.1039/b810189b.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions, J. Phys. Chem. B, 2009, 113(18), 6378–6396, DOI:10.1021/jp810292n.
- P. R. Nagy and M. Kallay, Optimization of the linear-scaling local natural orbital CCSD(T) method: Redundancy-free triples correction using Laplace transform, J. Chem. Phys., 2017, 146(21), 214106, DOI:10.1063/1.4984322.
- P. R. Nagy, G. Samu and M. Kallay, Optimization of the Linear-Scaling Local Natural Orbital CCSD(T) Method: Improved Algorithm and Benchmark Applications, J. Chem. Theory Comput., 2018, 14(8), 4193–4215, DOI:10.1021/acs.jctc.8b00442.
- A. Dashti, M. Raji, A. Razmi, N. Rezaei, S. Zendehboudi and M. Asghari, Efficient hybrid modeling of CO2 absorption in aqueous solution of piperazine: Applications to energy and environment, Chem. Eng. Res. Des., 2019, 144, 405–417, DOI:10.1016/j.cherd.2019.01.019.
- B. Lv, Y. Shi, C. Sun, N. Liu, W. Li and S. Li, CO2 capture by a highly-efficient aqueous blend of monoethanolamine and a hydrophilic amino acid ionic liquid [C2OHmim][Gly], Chem. Eng. J., 2015, 270, 372–377, DOI:10.1016/j.cej.2015.02.010.
- F. Barzagli, F. Mani and M. Peruzzini, A Comparative Study of the CO2 Absorption in Some Solvent-Free Alkanolamines and in Aqueous Monoethanolamine (MEA), Environ. Sci. Technol., 2016, 50(13), 7239–7246, DOI:10.1021/acs.est.6b00150.
- J. Im, S. Y. Hong, Y. Cheon, J. Lee, J. S. Lee, H. S. Kim, M. Cheong and H. Park, Steric hindrance-induced zwitterionic carbonates from alkanolamines and CO2: highly efficient CO2 absorbents, Energy Environ. Sci., 2011, 4(10), 4284–4289, 10.1039/c1ee01801a.
- H. Yan, L. Zhao, Y. Bai, F. Li, H. Dong, H. Wang, X. Zhang and S. Zeng, Superbase Ionic Liquid-Based Deep Eutectic Solvents for Improving CO2 Absorption, ACS Sustain. Chem. Eng., 2020, 8(6), 2523–2530, DOI:10.1021/acssuschemeng.9b07128.
- J. Muzart, DBU: A Reaction Product Component, ChemistrySelect, 2020, 5(37), 11608–11620, DOI:10.1002/slct.202002910.
Footnotes |
| † Electronic supplementary information (ESI) available: General information, the experimental section, CO2 results with other solvents, and DFT calculations. See DOI: https://doi.org/10.1039/d4su00022f |
| ‡ These authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |