Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green synthesis of thioamide derivatives in an environmentally benign deep eutectic solvent (DES)†
Susmita
Mandal
 a,
Archana
Jain
a,
Archana
Jain
 *b and
Tarun K.
Panda
*b and
Tarun K.
Panda
 *a
*a
aDepartment of Chemistry, Indian Institute of Technology Hyderabad, Kandi, Sangareddy, Telangana 502284, India. E-mail: tpanda@chy.iith.ac.in; Tel: +91-40-2301-6254
bDepartment of Physics and Chemistry, Mahatma Gandhi Institute of Technology, Gandipet, Hyderabad, Telangana 500 075, India. E-mail: archanajain_chem@mgit.ac.in
First published on 27th May 2024
Abstract
A mild, direct, simple, and highly efficient green protocol is described for synthesizing thioamides. A wide variety of thioamides are obtained in good-to-excellent yields by the reaction of readily available starting materials, substituted aldehydes/ketones, secondary amines, and elemental sulfur in a choline chloride–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)-based deep eutectic solvent (DES). This protocol, which can be applied without an additional catalyst support, contributes significantly to enhancing sustainability by reducing energy consumption and waste reduction, involving biodegradability, improved reactivity, and versatility. The DES can be recycled and used several times without any significant loss in terms of its activity.
2)-based deep eutectic solvent (DES). This protocol, which can be applied without an additional catalyst support, contributes significantly to enhancing sustainability by reducing energy consumption and waste reduction, involving biodegradability, improved reactivity, and versatility. The DES can be recycled and used several times without any significant loss in terms of its activity.
Sustainability spotlightWith the growing need for sustainable chemistry, deep eutectic solvents (DESs) drive the field of synthetic chemistry towards more green practices. In most conventional methods, organic solvents are used which have adverse effects on the environment. In this paper, we report the synthesis of a wide variety of thioamides in good to excellent yield under mild conditions using a mixture of choline chloride and urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (DES) as an environmentally benign solvent as well as a catalyst without using any toxic organic solvent and additional catalyst. Our work aligns with the 12 principles of green chemistry and the following UN sustainable development goals: SDG 9 (industry), SDG 12 (responsible consumption and production), and SDG 13 (climate action). 2) (DES) as an environmentally benign solvent as well as a catalyst without using any toxic organic solvent and additional catalyst. Our work aligns with the 12 principles of green chemistry and the following UN sustainable development goals: SDG 9 (industry), SDG 12 (responsible consumption and production), and SDG 13 (climate action).
|
Introduction
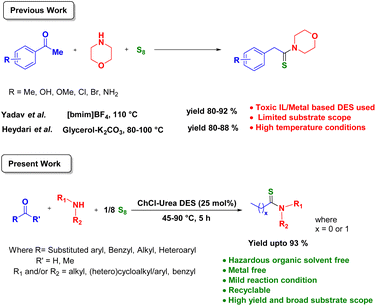
The development of environmentally benign and sustainable synthetic methodologies is undeniably necessary in the modern era of organic synthesis, owing to ever-increasing concerns over environmental contamination and its impact. In addition to our scientific obligations, it is now a moral and ethical responsibility to protect our environment for future generations. In this context, the 12 principles of green chemistry, first proposed by Anastas and Warner in 1998,1 have transformed the way we approach chemical synthesis. The principles of green chemistry promote the design and development of chemical processes that minimize environmental impact, reduce waste, and maximize efficiency.2,3 Recently, deep eutectic solvents (DESs) have emerged as a potential new class of solvents that not only follow the principles of green chemistry, but also have a wide range of applications in various fields.4,5 DESs, first reported by Abbott and co-workers,6 exhibit numerous unique properties such as low volatility, high thermal stability, and variable polarity.7,8 Additionally, they are easily accessible, relatively nontoxic, low-cost, and biodegradable.9 DESs have been employed as media/catalysts in a variety of chemical reactions, such as biomass processing,10,11 polymerizations,12 material synthesis,13,14 electrochemistry,15,16 nanotechnology,17 and multicomponent reactions (MCRs).18,19 Literature reports show that DESs have the potential to play an important part in enhancing the sustainability of MCRs.20 Their green solvent properties and versatility render them valuable for researchers striving to develop more sustainable chemical synthetic methods.21Thioamides, characterized by the presence of the –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)NH– functional group, have been employed as indispensable building blocks in the synthesis of bioactive compounds,22,23 pharmaceutical agents,24–26 agrochemicals,27 and functional materials with tailored properties.28 Among the myriad of various traditional synthetic methods reported in the literature,29–39 one of the most efficient methods for thioamide synthesis is the Willgerodt–Kindler (WK) reaction, comprising the three-component condensation of a carbonyl compound, a sulfur reagent, and an amine (primary or secondary) in one pot.40,41 The reported protocols produce good results in many cases. However, most of them involve the use of expensive chemicals, such as sulfur reagents, metal catalysts, and toxic organic solvents, as well as long reaction times, excess reactants, harsh reaction conditions, and strenuous work-up methods. In some instances, the yield of products is also low. To avoid such limitations, there is a significant trend toward substituting most traditional organic solvents or harsh reaction conditions with ones that have a reduced impact on the environment while still achieving the same outcomes. Ionic liquids and DESs have recently emerged as potential substitutes for toxic organic solvents.42 Hitherto, only a few examples of thioamide synthesis using ionic liquids and DESs have been reported (Scheme 1).43–45 The protocols reported involved the use of relatively toxic ionic liquid/metal-based DESs, high temperature conditions, presence of toxic organic solvents, an excess of reactants, and limited substrate scope.
S)NH– functional group, have been employed as indispensable building blocks in the synthesis of bioactive compounds,22,23 pharmaceutical agents,24–26 agrochemicals,27 and functional materials with tailored properties.28 Among the myriad of various traditional synthetic methods reported in the literature,29–39 one of the most efficient methods for thioamide synthesis is the Willgerodt–Kindler (WK) reaction, comprising the three-component condensation of a carbonyl compound, a sulfur reagent, and an amine (primary or secondary) in one pot.40,41 The reported protocols produce good results in many cases. However, most of them involve the use of expensive chemicals, such as sulfur reagents, metal catalysts, and toxic organic solvents, as well as long reaction times, excess reactants, harsh reaction conditions, and strenuous work-up methods. In some instances, the yield of products is also low. To avoid such limitations, there is a significant trend toward substituting most traditional organic solvents or harsh reaction conditions with ones that have a reduced impact on the environment while still achieving the same outcomes. Ionic liquids and DESs have recently emerged as potential substitutes for toxic organic solvents.42 Hitherto, only a few examples of thioamide synthesis using ionic liquids and DESs have been reported (Scheme 1).43–45 The protocols reported involved the use of relatively toxic ionic liquid/metal-based DESs, high temperature conditions, presence of toxic organic solvents, an excess of reactants, and limited substrate scope.
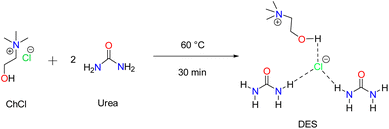
Given this context, we were interested in showcasing the pivotal role of metal-free DESs in the synthesis of a series of biologically and pharmaceutically important thioamide derivatives via a multicomponent approach (i.e., Willgerodt–Kindler reaction), while aligning with the principles of green chemistry. Recently, we reported the application of a choline chloride (ChCl)–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)-based DES in the synthesis of α-aminophosphorus derivatives.46 This DES is composed of the naturally occurring hydrogen-bond acceptor (HBA) choline chloride and hydrogen-bond donor (HBD) urea in a 1
2)-based DES in the synthesis of α-aminophosphorus derivatives.46 This DES is composed of the naturally occurring hydrogen-bond acceptor (HBA) choline chloride and hydrogen-bond donor (HBD) urea in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio.
2 molar ratio.
Here, we report a green methodology for the synthesis of thioamide derivatives via a Willgerodt–Kindler (W–K) reaction between aldehydes/ketones, different secondary amines, and sulfur powder in a ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)-based DES under mild conditions. This methodology positively contributes to enhancing sustainability by: (i) reducing the need for additional toxic solvents/catalysts, thereby improving the overall efficiency of the reaction in terms of high yield and selectivity, (ii) cutting energy consumption through low-temperature reactions due to its relatively low melting point, (iii) lowering waste generation by using reagents in stoichiometric ratio and since the DES is easily recyclable, it allows the recovery of reactants and products, and (iv) reducing the environmental burden due to its biodegradable nature. We also describe mechanistic insights and versatility of the DES with various substrates (up to 40 examples). Furthermore, we evaluate the reusability of the DES.
2)-based DES under mild conditions. This methodology positively contributes to enhancing sustainability by: (i) reducing the need for additional toxic solvents/catalysts, thereby improving the overall efficiency of the reaction in terms of high yield and selectivity, (ii) cutting energy consumption through low-temperature reactions due to its relatively low melting point, (iii) lowering waste generation by using reagents in stoichiometric ratio and since the DES is easily recyclable, it allows the recovery of reactants and products, and (iv) reducing the environmental burden due to its biodegradable nature. We also describe mechanistic insights and versatility of the DES with various substrates (up to 40 examples). Furthermore, we evaluate the reusability of the DES.
Results and discussion
In this investigation, we present a simple and green methodology for synthesizing a series of thioamide derivatives via a multicomponent approach (i.e., Willgerodt–Kindler reaction) using a DES [ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)] as the reaction medium as well as a catalyst, without adding any other organic solvent or catalyst, under mild reaction conditions.
2)] as the reaction medium as well as a catalyst, without adding any other organic solvent or catalyst, under mild reaction conditions.
The DES was synthesized by following the previously reported method of heating a mixture of choline chloride (1 mol) and urea (2 mol) at 60 °C for 30 minutes (Scheme 2).47 The colorless liquid formed was used for thioamidation reaction as such without any purification.
To begin our investigation, we selected the thioamidation reaction between p-tolualdehyde (1a), diethylamine (2a), and sulfur powder as the model reaction. The three reactants, 1a, 2a, and sulfur powder, in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.125 molar ratio, were reacted in 25 mol% of DES [ChCl–urea (1
0.125 molar ratio, were reacted in 25 mol% of DES [ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)] in one pot with constant stirring at 45 °C for five hours. Progress of the reaction was monitored by thin-layer chromatography (TLC). After completion, the mixture was cooled to room temperature and then diluted with water and ethyl acetate. To our delight, the corresponding thioamide derivative was isolated in a yield of 93% (Table 1, entry 3). The DES could easily be recycled by evaporating the water under vacuum.
2)] in one pot with constant stirring at 45 °C for five hours. Progress of the reaction was monitored by thin-layer chromatography (TLC). After completion, the mixture was cooled to room temperature and then diluted with water and ethyl acetate. To our delight, the corresponding thioamide derivative was isolated in a yield of 93% (Table 1, entry 3). The DES could easily be recycled by evaporating the water under vacuum.
| Entry | 1a (equiv.) | 2a (equiv.) | S (equiv.) | DES | Loading of DES (mol%) | Time (h) | Temp. (°C) | Yielda (%) |
|---|---|---|---|---|---|---|---|---|
| a Isolated yield. OA-oxalic acid, PTSA-p-toluenesulfonic acid. | ||||||||
| 1 | 1 | 1 | 1 | — | — | 24 | 45 | — |
| 2 | 1 | 1 | 1 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
25 | 24 | rt | — |
| 3 | 1 | 1 | 1 |
ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 2) 2)
|
25 | 5 | 45 | 93 |
| 4 | 1 | 1 | 2 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
25 | 5 | 45 | 93 |
| 5 | 1 | 2 | 1 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
25 | 5 | 45 | 93 |
| 6 | 1 | 1 | 1 | ChCl–OA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
25 | 5 | 45 | — |
| 7 | 1 | 1 | 1 | ChCl–PTSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
25 | 5 | 45 | — |
| 8 | 1 | 1 | 1 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
25 | 5 | 45 | 74 |
| 9 | 1 | 1 | 1 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
30 | 5 | 45 | 93 |
| 10 | 1 | 1 | 1 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
20 | 5 | 45 | 83 |
| 11 | 1 | 1 | 1 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
25 | 5 | 60 | 93 |
| 12 | 1 | 1 | 1 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
25 | 4 | 45 | 86 |
| 13 | 1 | 1 | 1 | ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
25 | 8 | 45 | 93 |
The thioamidation reaction can be optimized by altering several parameters, viz., reaction temperature, reaction time, DES dosage, and subtraction of DES (Table 1). Initially, when the reaction between 1a, 2a, and sulfur powder in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.125 molar ratio was performed in the absence of DES at 45 °C for 24 hours under neat conditions, no product was formed (Table 1, entry 1). This explains the role of the DES as an effective catalyst as well as medium. The high polarity and unique hydrogen-bonding properties of the DES influence the reactivity of reactants. It also accelerates the reaction speed and improves selectivity by solvating and stabilizing reactive intermediates or transition states in the catalytic reactions.48 Even in the DES, this reaction at room temperature did not afford any product (Table 1, entry 2). This may be because at 45 °C, mass transfer of reactants in the DES increases, facilitating more effective reactant contact. Other DESs, such as ChCl–oxalic acid (1
0.125 molar ratio was performed in the absence of DES at 45 °C for 24 hours under neat conditions, no product was formed (Table 1, entry 1). This explains the role of the DES as an effective catalyst as well as medium. The high polarity and unique hydrogen-bonding properties of the DES influence the reactivity of reactants. It also accelerates the reaction speed and improves selectivity by solvating and stabilizing reactive intermediates or transition states in the catalytic reactions.48 Even in the DES, this reaction at room temperature did not afford any product (Table 1, entry 2). This may be because at 45 °C, mass transfer of reactants in the DES increases, facilitating more effective reactant contact. Other DESs, such as ChCl–oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and ChCl–PTSA (1
1) and ChCl–PTSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were also tested, but we were unsuccessful in obtaining the products (Table 1, entries 6, 7). However, using the DES in lower proportion ChCl
1) were also tested, but we were unsuccessful in obtaining the products (Table 1, entries 6, 7). However, using the DES in lower proportion ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) led to the formation of the corresponding product in lower yield (74%) under similar conditions (Table 1, entry 8). For further screening, we changed the quantities of the starting materials, and the best result was obtained when 1a, 2a, and sulfur were used in a 1
1) led to the formation of the corresponding product in lower yield (74%) under similar conditions (Table 1, entry 8). For further screening, we changed the quantities of the starting materials, and the best result was obtained when 1a, 2a, and sulfur were used in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.125 molar ratio (Table 1, entries 3–5). Next, we optimized the amount of DES, and the highest yield was obtained with 25 mol% of DES (Table 1, entries 3, 9, and 10). Other parameters, such as reaction temperature (Table 1, entries 3, 10, 11) and time (Table 1, entries 11–13) were also analyzed. The ideal reaction temperature and time were 45 °C and five hours, respectively.
0.125 molar ratio (Table 1, entries 3–5). Next, we optimized the amount of DES, and the highest yield was obtained with 25 mol% of DES (Table 1, entries 3, 9, and 10). Other parameters, such as reaction temperature (Table 1, entries 3, 10, 11) and time (Table 1, entries 11–13) were also analyzed. The ideal reaction temperature and time were 45 °C and five hours, respectively.
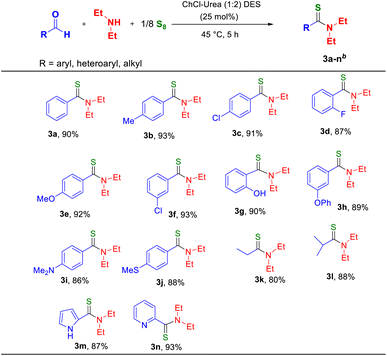
With the established optimized conditions, an extensive study was undertaken to examine the scope of this protocol, using a variety of aldehydes/ketones and amines in the DES [ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)]. At first, the reactions of various aldehydes with diethylamine and sulfur powder were performed in the DES ChCl–urea (1
2)]. At first, the reactions of various aldehydes with diethylamine and sulfur powder were performed in the DES ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) in 1
2) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.125 molar ratio at 45 °C for five hours. After the completion of each reaction, the corresponding thioamide (3a–3n) was isolated and characterized by 1H and 13C NMR spectroscopy (see the ESI†). The results are shown in Table 2. We observed that aromatic aldehydes containing electron-releasing and electron-withdrawing groups reacted smoothly under mild conditions, and the corresponding thioamides were obtained in good-to-excellent yields (Table 2, 3a–3j). Aliphatic aldehydes also reacted well and gave good yields (Table 2, 3k and 3l). Interestingly, heteroaromatic aldehydes such as pyrrole 2-carboxaldehyde and pyridine 2-carboxaldehyde also performed well when our method was used and afforded good-to-excellent yields (Table 2, 3m and 3n).
0.125 molar ratio at 45 °C for five hours. After the completion of each reaction, the corresponding thioamide (3a–3n) was isolated and characterized by 1H and 13C NMR spectroscopy (see the ESI†). The results are shown in Table 2. We observed that aromatic aldehydes containing electron-releasing and electron-withdrawing groups reacted smoothly under mild conditions, and the corresponding thioamides were obtained in good-to-excellent yields (Table 2, 3a–3j). Aliphatic aldehydes also reacted well and gave good yields (Table 2, 3k and 3l). Interestingly, heteroaromatic aldehydes such as pyrrole 2-carboxaldehyde and pyridine 2-carboxaldehyde also performed well when our method was used and afforded good-to-excellent yields (Table 2, 3m and 3n).
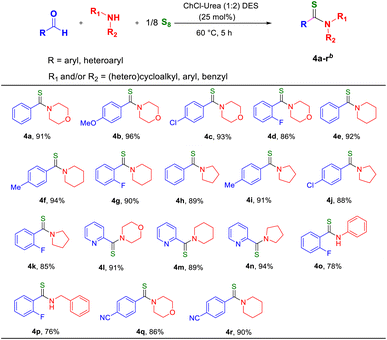
Next, we expanded our protocol to demonstrate the scope of the DES with the different types of amines. Under the given optimized conditions, the cyclic secondary amines (viz., morpholine, piperidine, and pyrrolidine) reacted well with aromatic aldehydes containing electron-releasing as well as electron-withdrawing groups.
In all cases, the corresponding thioamides were obtained in good-to-excellent yields (Table 3, 4a–4k). We also tested the reactivity of cyclic secondary amines with a heterocyclic aldehyde, pyridine 2-carboxyaldehyde, under similar conditions and it afforded the pyridine-based thioamide motifs in excellent yields (Table 3, 4l–4n). Primary amines, such as aniline and benzyl amine, also gave thioamides in good yields (Table 3, 4o and 4p). The thioamidation reaction was also performed with benzaldehyde bearing a reducible functional group such as nitro or cyano to show the limitations of this methodology. It was observed that 4-nitro benzaldehyde did not give any product when reacted with diethyl amine as well as heterocyclic amines such as morpholine and piperidine under the optimized conditions. However, 4-cyano benzaldehyde gave the corresponding products in good yield with morpholine and piperidine (Table 3, 4q and 4r) but did not afford any product with diethylamine. The corresponding thioamide compounds (4a–4r) were isolated and characterized using 1H and 13C NMR spectroscopy (see the ESI†). This study demonstrated that our methodology is compatible with a broad range of aldehydes and amines bearing a variety of functional groups including methyl, methoxy, chloro, and fluoro, under mild conditions (Tables 2 and 3).
Upon achieving these results, we were keen to extend our methodology to various ketones. The W–K reaction with alkyl aryl ketones is quite interesting and challenging as it involves the reversal of polarity where the carbonyl –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)– group is reduced and the terminal methyl group is oxidized, and new C
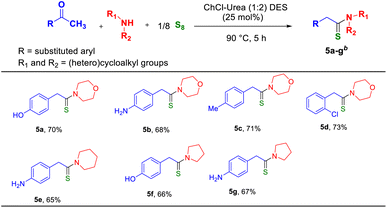
O)– group is reduced and the terminal methyl group is oxidized, and new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C–N bonds are formed. For the thioamidation reaction, different cyclic secondary amines, such as morpholine, piperidine, and pyrrolidine, were employed with the ketones bearing electron-donating and withdrawing groups, which afforded the corresponding thioamides (5a–5g) in moderate-to-good yields. For the ketones, the reaction conditions were similar to those used for aldehydes, except that the reaction was performed at a slightly higher temperature of 90 °C (Table 4). All the compounds (5a–5g) were isolated and characterized using 1H and 13C NMR spectroscopy (see the ESI†).
S and C–N bonds are formed. For the thioamidation reaction, different cyclic secondary amines, such as morpholine, piperidine, and pyrrolidine, were employed with the ketones bearing electron-donating and withdrawing groups, which afforded the corresponding thioamides (5a–5g) in moderate-to-good yields. For the ketones, the reaction conditions were similar to those used for aldehydes, except that the reaction was performed at a slightly higher temperature of 90 °C (Table 4). All the compounds (5a–5g) were isolated and characterized using 1H and 13C NMR spectroscopy (see the ESI†).
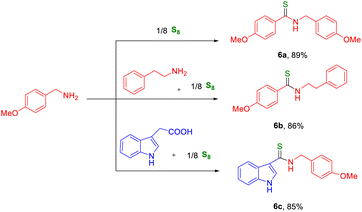
We continued our exploration of the thioamidation reaction using different types of primary amines and acids. In one case, we used two same and different types of amines and sulfur powder in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.125 molar ratio and obtained the corresponding thioamide products in good yields (Table 5, 6a and 6b). In another case we used acid, primary amine, and sulfur powder in a 1
0.125 molar ratio and obtained the corresponding thioamide products in good yields (Table 5, 6a and 6b). In another case we used acid, primary amine, and sulfur powder in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.125 molar ratio and obtained the corresponding thioamide in good yield (Table 5, 6c). Here, we tried the reaction using aliphatic and aromatic secondary amines as well as with aliphatic acid but failed to obtain any product.
0.125 molar ratio and obtained the corresponding thioamide in good yield (Table 5, 6c). Here, we tried the reaction using aliphatic and aromatic secondary amines as well as with aliphatic acid but failed to obtain any product.
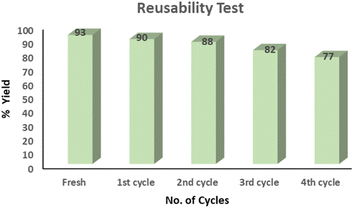
Recycling the reaction media and catalysts is crucial for waste reduction – it also aligns with green chemistry principles and contributes to sustainability.49 To assess reusability, we conducted the MCR using p-tolualdehyde, diethylamine, and sulfur powder in the DES under optimized conditions (at 45 °C for five hours). After the reaction was completed, the DES was recovered from the water phase by evaporation at 85 °C under reduced pressure. The recovered DES was further dried at 70 °C for three hours under reduced pressure to remove any traces of water and then subjected to the next run of the same reaction without adding more DES. We were able to reuse the DES medium four times for the same reaction with negligible loss of product yields (Fig. 1).
We also tested this methodology for industrial applications by performing a gram-scale reaction between 3.7 g of p-tolualdehyde, 2.3 g of aniline, and 1 g of sulfur powder in DES ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (25 mol%) under solvent-free conditions with constant stirring at 45 °C for five hours. The corresponding thioamides generated had a yield of 93%. These results highlight the efficacy of DES ChCl–urea (1
2) (25 mol%) under solvent-free conditions with constant stirring at 45 °C for five hours. The corresponding thioamides generated had a yield of 93%. These results highlight the efficacy of DES ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) for the large-scale, ecofriendly synthesis of a series of biologically and pharmaceutically important thioamide derivatives. Additionally, we evaluated green chemistry metrics to demonstrate the environment-friendly nature of our methodology.50 Ideally, high atom economy and a low E-factor value, together with high process mass intensity is crucial for green reactions. In this study, we found that the reaction has a small E-factor value (0.17), a high reaction mass efficiency value (RME = 85.5%), high atom economy (AE = 92%), and high process mass intensity (PMI = 1.17) (see the ESI† for green metrics calculation). This reaction avoids toxic solvents, expensive reagents, and harsh conditions.
2) for the large-scale, ecofriendly synthesis of a series of biologically and pharmaceutically important thioamide derivatives. Additionally, we evaluated green chemistry metrics to demonstrate the environment-friendly nature of our methodology.50 Ideally, high atom economy and a low E-factor value, together with high process mass intensity is crucial for green reactions. In this study, we found that the reaction has a small E-factor value (0.17), a high reaction mass efficiency value (RME = 85.5%), high atom economy (AE = 92%), and high process mass intensity (PMI = 1.17) (see the ESI† for green metrics calculation). This reaction avoids toxic solvents, expensive reagents, and harsh conditions.
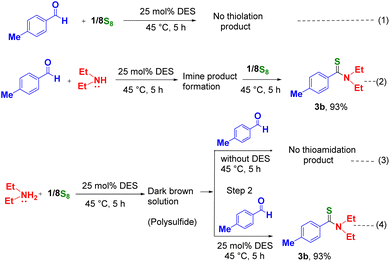
To clarify the mechanism of this reaction, we performed some control reactions under the optimized conditions. At first, the p-tolualdehyde and sulfur powder were heated at 45 °C for five hours in the DES, we observed that no product was generated by the thiolation reaction (Scheme 3, eqn (1)). Later when aldehydes and diethylamine were reacted together at 45 °C for five hours in the DES, we observed the formation of the imine product which yielded thioamide on reaction with sulfur under the similar reaction conditions (Scheme 3, eqn (2)). Then, diethylamine was reacted with elemental sulfur powder at both room temperature and 45 °C for one hour. In both cases, a dark, brown-colored solution was obtained due to the formation of polysulfides. Subsequently, when we added aldehyde to the brown-colored solution obtained in each case (in the absence of the DES) (Scheme 3, eqn (3)), we observed that the expected thioamide product was not formed. Only when the aldehyde was added to the reaction mixture of diethylamine and sulfur and heated at 45 °C in the presence of the DES (Scheme 3, eqn (4)), the expected thioamide product was formed. Results from our control experiments suggest that the formation of polysulfide (I) may take place even at room temperature under neat conditions and thioamide formation may be feasible in the DES medium at a slightly elevated temperature (45 °C) only. In this catalytic reaction, the DES may play a crucial role in inducing the reactivity of reactants by solvating and stabilizing reactive intermediates or transition states due to its high polarity and unique hydrogen-bonding properties. Therefore, the presence of the DES may be essential for the thioamidation reaction.
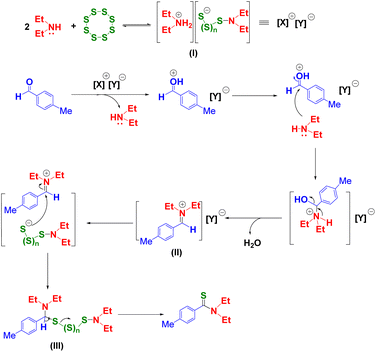
Based on results from our control experiments and the previous literature, we propose a most plausible mechanism of thioamidation reaction in the DES (Scheme 4).41,51–53 The first step in this reaction involves a cleavage of the S–S bond of elemental sulfur by the nucleophilic attack of a lone nitrogen on the corresponding secondary amine to form the polysulfide anion (I) in a reversible way. Next, upon addition of the aldehyde, the protonation of aldehyde molecule enhances the electrophilicity of carbonyl carbon of the aldehyde and it thus reacts easily with secondary amine to form the imine intermediate (II). We anticipate that the driving force in the formation of the imine compound in situ might be the hydrogen bond interaction in the OH group of the intermediate formed with the DES.47 Thereafter, the nucleophilic attack of polysulfide anion on the imine intermediate (II) led to intermediate III in which the hydrogen on the methylene group oxidizes and gives the final product.
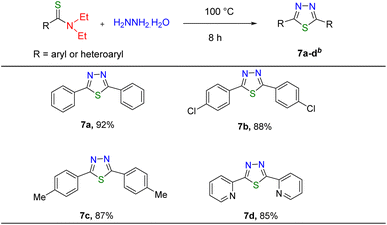
We also present here the application of thioamide derivatives in the synthesis of thiadiazoles. Thiadiazoles are five-membered heterocyclic compounds which are extensively applied in medicinal, agricultural, industrial, and polymer chemistry. In particular, 1,3,4-thiadiazole compounds are important owing to their characteristics – antiviral,54 antitubercular,55 anti-depressant,56 anti-microbial,57 and anticancer.58 1,3,4-Thiadiazole derivatives have been used in the synthesis of different drugs like acetazolamide,59 furidiazine, megazol,60etc. There are various reports on thiadiazole derivative synthesis.61–64 Here, we have demonstrated the synthesis of 2,5-disubstituted 1,3,4-thiadiazole compounds from the reaction between thioamides we prepared with hydrazine hydrate at 100 °C for eight hours under neat conditions (Table 6). The corresponding thiadiazole compounds (7a–7d) were isolated and characterized using 1H and 13C NMR spectroscopy (see the ESI†).
Conclusion
In summary, we have reported here a simple, novel, efficient, and scalable methodology for thioamide synthesis in a ChCl–urea DES without any other additive or organic solvent. Thioamides were obtained at the 45–60 °C temperature range within five hours with yields of 76–93% under optimized conditions using aldehyde, secondary amine, and sulfur powder in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.125 molar ratio. We also used this protocol for ketones and obtained the corresponding thioamides in ∼65–90% yield. Furthermore, we demonstrated the synthesis of thiadiazole derivatives using the thioamides prepared. The main benefits of this protocol are the use of the DES as a green solvent as well as a non-toxic catalyst, lower reaction temperature, and most importantly, easy recovery and reusability of the DES. We also performed a gram-scale reaction, which yielded good results. To the best of our knowledge, this is the first report on thioamidation of aldehydes using a metal-free basic DES.
0.125 molar ratio. We also used this protocol for ketones and obtained the corresponding thioamides in ∼65–90% yield. Furthermore, we demonstrated the synthesis of thiadiazole derivatives using the thioamides prepared. The main benefits of this protocol are the use of the DES as a green solvent as well as a non-toxic catalyst, lower reaction temperature, and most importantly, easy recovery and reusability of the DES. We also performed a gram-scale reaction, which yielded good results. To the best of our knowledge, this is the first report on thioamidation of aldehydes using a metal-free basic DES.
Author contributions
Susmita Mandal: methodology, validation, formal analysis, data curation. Archana Jain: writing (original draft), data curation, project administration. Tarun K. Panda: funding acquisition, supervision, project administration, reviewing, and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Science and Engineering Research Board (SERB), Government of India, under the Teaching Associateship for Research Excellence (TARE) scheme (project no. TAR/2020/000003). Instrumental facilities were provided by the Indian Institute of Technology, Hyderabad (IITH).References
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, p. 30, By permission of Oxford University Search PubMed.
- A. DeVierno Kreuder, T. House-Knight, J. Whitford, E. Ponnusamy, P. Miller, N. Jesse, R. Rodenborn, S. Sayag, M. Gebel, I. Aped, I. Sharfstein, E. Manaster, I. Ergaz, A. Harris and L. Nelowet Grice, ACS Sustain. Chem. Eng., 2017, 5, 2927–2935 CrossRef CAS.
- O. V. Kharissova, B. I. Kharisov, C. M. O. González, Y. P. Méndez and I. López, R. Soc. Open Sci., 2019, 6, 191378 CrossRef PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- L. Lomba, C. B. García, M. P. Ribate, B. Giner and E. Zuriaga, Appl. Sci., 2021, 11, 10156 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 1, 2010–2011 RSC.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- C. Ruß and B. König, Green Chem., 2012, 14, 2969–2982 RSC.
- T. El Achkar, H. Greige-Gerges and S. Fourmentin, Environ. Chem. Lett., 2021, 19, 3397–3408 CrossRef CAS.
- M. Francisco, A. Van Den Bruinhorst and M. C. Kroon, Green Chem., 2012, 14, 2153–2157 RSC.
- S. Xia, G. A. Baker, H. Li, S. Ravula and H. Zhao, RSC Adv., 2014, 4, 10586–10596 RSC.
- F. Del Monte, D. Carriazo, M. C. Serrano, M. C. Gutiérrez and M. L. Ferrer, ChemSusChem, 2014, 7, 999–1009 CrossRef CAS PubMed.
- D. V. Wagle, H. Zhao and G. A. Baker, Acc. Chem. Res., 2014, 47, 2299–2308 CrossRef CAS PubMed.
- D. Carriazo, M. C. Serrano, M. C. Gutiérrez, M. L. Ferrer and F. del Monte, Chem. Soc. Rev., 2012, 41, 4996–5014 RSC.
- A. R. Hillman, K. S. Ryder, C. J. Zaleski, V. Ferreira, C. A. Beasley and E. Vieil, Electrochim. Acta, 2014, 135, 42–51 CrossRef CAS.
- P. Sebastián, E. Vallés and E. Gómez, Electrochim. Acta, 2014, 123, 285–295 CrossRef.
- A. Abo-Hamad, M. Hayyan, M. A. H. AlSaadi and M. A. Hashim, Chem. Eng. J., 2015, 273, 551–567 CrossRef CAS.
- F. G. Calvo-Flores and C. Mingorance-Sánchez, ChemistryOpen, 2021, 10, 815–829 CrossRef CAS PubMed.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC.
- L. S. Longo and M. V. Craveiro, J. Braz. Chem. Soc., 2018, 29, 1999–2025 CAS.
- P. Liu, J. W. Hao, L. P. Mo and Z. H. Zhang, RSC Adv., 2015, 5, 48675–48704 RSC.
- Z. Moussa, Z. M. A. Judeh, M. A. M. S. El-Sharief and A. M. S. El-Sharief, ChemistrySelect, 2020, 5, 764–798 CrossRef CAS.
- H. Verma, B. Khatri, S. Chakraborti and J. Chatterjee, Chem. Sci., 2018, 9, 2443–2451 RSC.
- P. Zoumpoulakis, C. Camoutsis, G. Pairas, M. Soković, J. Glamočlija, C. Potamitis and A. Pitsas, Bioorg. Med. Chem., 2012, 20, 1569–1583 CrossRef CAS PubMed.
- F. Wang, R. Langley, G. Gulten, L. G. Dover, G. S. Besra, W. R. Jacobs and J. C. Sacchettini, J. Exp. Med., 2007, 204, 73–78 CrossRef CAS PubMed.
- J. R. Kirshner, S. He, V. Balasubramanyam, J. Kepros, C. Y. Yang, M. Zhang, Z. Du, J. Barsoum and J. Bertin, Mol. Cancer Ther., 2008, 7, 2319–2327 CrossRef CAS PubMed.
- J. Yu and X. Jiang, Adv. Agrochem, 2023, 2, 3–14 CrossRef.
- Y. Huang, R. Hu and B. Z. Tang, Sulfur-Containing Polymers: from Synthesis to Functional Materials, WILEY-VCH GmbH, 2021, pp. 1–37 Search PubMed.
- W. Liu, C. Chen and H. Liu, Beilstein J. Org. Chem., 2015, 11, 1721–1726 CrossRef CAS PubMed.
- J. Li, X. Ren, G. Li, H. Liang, Y. Zhao, Z. Wang, H. Li and B. Yuan, J. Sulfur Chem., 2020, 41, 229–237 CrossRef CAS.
- T. B. Nguyen, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2012, 14, 4274–4277 CrossRef CAS PubMed.
- T. B. Nguyen, M. Q. Tran, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2014, 16, 310–313 CrossRef CAS PubMed.
- R. Liboska, D. Zyka and M. Bobek, Synthesis, 2002, 6–09 Search PubMed.
- B. Kaboudin, V. Yarahmadi, J. Y. Kato and T. Yokomatsu, RSC Adv., 2013, 3, 6435–6441 RSC.
- A. D. Kale and D. S. Dalal, ChemistrySelect, 2022, 7, e202203497 CrossRef CAS.
- J. Wei, Y. Li and X. Jiang, Org. Lett., 2016, 18, 340–343 CrossRef CAS PubMed.
- Y. Bian, X. Qu, Y. Chen, J. Li and L. Liu, Molecules, 2018, 23, 2225 CrossRef PubMed.
- N. Borthakur and A. Goswami, Tetrahedron Lett., 1995, 36, 6745–6746 CrossRef CAS.
- D. Brillon, Synth. Commun., 1992, 22, 1397–1401 CrossRef CAS.
- O. I. Zbruyev, N. Stiasni and C. O. Kappe, J. Comb. Chem., 2003, 5, 145–148 CrossRef CAS PubMed.
- K. Okamoto, T. Yamamoto and T. Kanbara, Synlett, 2007, 2687–2690 CAS.
- D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramón, Eur. J. Org Chem., 2016, 2016, 612–632 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, G. Kondaji, J. S. S. Reddy and K. Nagaiah, J. Mol. Catal. A: Chem., 2007, 266, 249–253 CrossRef CAS.
- I. Radfar, S. Abbasi, M. K. Miraki, E. Yazdani, M. Karimi and A. Heydari, ChemistrySelect, 2018, 3, 3265–3267 CrossRef CAS.
- A. Gupta, J. K. Vankar, J. P. Jadav and G. N. Gururaja, J. Org. Chem., 2022, 87, 2410–2420 CrossRef CAS PubMed.
- S. Mandal, R. Narvariya, S. L. Sunar, I. Paul, A. Jain and T. K. Panda, Green Chem., 2023, 25, 8266–8272 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- S. Khandelwal, Y. K. Tailor and M. Kumar, J. Mol. Liq., 2016, 215, 345–386 CrossRef CAS.
- V. Hessel, N. N. Tran, M. R. Asrami, Q. D. Tran, N. Van Duc Long, M. Escribà-Gelonch, J. O. Tejada, S. Linke and K. Sundmacher, Green Chem., 2022, 24, 410–437 RSC.
- J. Martínez, J. F. Cortés and R. Miranda, Processes, 2022, 10, 1274 CrossRef.
- A. Gutiérrez-Hernández, A. Richaud, L. Chacón-García, C. J. Cortés-García, F. Méndez and C. A. Contreras-Celedón, J. Org. Chem., 2021, 86, 223–234 CrossRef PubMed.
- X. Li, Q. Pan, R. Hu, X. Wang, Z. Yang and S. Han, Asian J. Org. Chem., 2016, 5, 1353–1358 CrossRef CAS.
- M. Carmack, J. Heterocycl. Chem., 1989, 26, 1319–1323 CrossRef CAS.
- D. Kumar, H. Kumar, V. Kumar, A. Deep, A. Sharma, M. G. Marwaha and R. K. Marwaha, Med. Drug Discov., 2023, 17, 100150 CrossRef CAS.
- S. G. Alegaon, K. R. Alagawadi, P. V. Sonkusare, S. M. Chaudhary, D. H. Dadwe and A. S. Shah, Bioorg. Med. Chem. Lett., 2012, 22, 1917–1921 CrossRef CAS PubMed.
- F. Clerici, D. Pocar, M. Guido, A. Loche, V. Perlini and M. Brufani, J. Med. Chem., 2001, 44, 931–936 CrossRef CAS PubMed.
- G. L. Almajan, S. F. Barbuceanu, G. Bancescu, I. Saramet, G. Saramet and C. Draghici, Eur. J. Med. Chem., 2010, 45, 6139–6146 CrossRef CAS PubMed.
- N. A. Al-Masoudi and Y. A. Al-Soud, Nucleosides, Nucleotides Nucleic Acids, 2008, 27, 1034–1044 CrossRef CAS PubMed.
- J. R. A. Diaz, G. E. Camí, M. Liu-González, D. R. Vega, D. Vullo, A. Juárez, J. C. Pedregosa and C. T. Supuran, J. Enzyme Inhib. Med. Chem., 2016, 31, 1102–1110 CrossRef CAS PubMed.
- A. Abdel-Aziem, M. S. El-Gendy and A. O. Abdelhamid, Eur. J. Chem., 2012, 3, 455–460 CrossRef CAS.
- B. Stanovnik, Compr. Org. Funct. Group Transform., 1995, 5, 805–864 CAS.
- A. R. Sayed, Tetrahedron Lett., 2010, 51, 4490–4493 CrossRef CAS.
- M. Zarei, Tetrahedron, 2017, 73, 1867–1872 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, Tetrahedron Lett., 2008, 49, 879–883 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00206g |
| This journal is © The Royal Society of Chemistry 2024 |