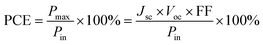Open Access Article
Open Access ArticleValidating the “greenness” of chemicals via life cycle assessment: the case of anisole as an anti-solvent in perovskite solar cells†
A. Kamal
Kamali
*a,
Nilanka M.
Keppetipola‡
b,
Yuka
Yoshihara
c,
Ajay Kumar
Jena
 d,
Satoshi
Uchida
d,
Satoshi
Uchida
 e,
Hiroshi
Segawa
f,
Guido
Sonnemann
e,
Hiroshi
Segawa
f,
Guido
Sonnemann
 a,
Thierry
Toupance
a,
Thierry
Toupance
 b and
Ludmila
Cojocaru
b and
Ludmila
Cojocaru
 *bd
*bd
aUniv. Bordeaux, CNRS, Bordeaux INP, ISM, UMR 5255, Groupe Analyse du Cycle de Vie et Chimie Durable (CyVi), 351 Cours de la Libération, F-33405 Talence, France. E-mail: ahmad-kamal.kamali@u-bordeaux.fr; guido.sonnemann@u-bordeaux.fr
bUniv. Bordeaux, CNRS, Bordeaux INP, ISM, UMR 5255, Groupe Chimie Moléculaire et Matériaux (C2M), 351 Cours de la Libération, F-33405 Talence, France. E-mail: nilanka.keppetipola@college-de-france.fr; cojocaru@g.ecc.u-tokyo.ac.jp; thierry.toupance@u-bordeaux.fr
cThe University of Tokyo, Department of Chemical System Engineering, Graduate School of Engineering, 7-3-1, Hongo, Bunkyo-ku, Tokyo 113-8656, Japan. E-mail: yoshihara_y@chemsys.t.u-tokyo.ac.jp
dThe University of Tokyo, Komaba Institute for Science, Graduate School of Arts and Sciences, 4-6-1, Komaba, Meguro, Tokyo 153-8904, Japan. E-mail: ajayjena@g.ecc.u-tokyo.ac.jp; cojocaru@g.ecc.u-tokyo.ac.jp
eThe University of Tokyo, Research Center for Advanced Science and Technology, 4-6-1, Komaba, Meguro, Tokyo 153-8904, Japan. E-mail: uchida@rcast.u-tokyo.ac.jp
fThe University of Tokyo, Department of General Systems Studies, Graduate School of Arts and Sciences, 3-8-1, Komaba, Meguro-ku, Tokyo 153-8902, Japan. E-mail: csegawa@mail.ecc.u-tokyo.ac.jp
First published on 16th September 2024
Abstract
Technological development is increasingly driven by environmental sustainability, with labels like ‘green’ gaining traction. However, the complex interactions in a product's life cycle make the environmental impact of materials and chemicals highly context-dependent, highlighting the need for context-specific environmental assessments. Anisole has been popularly used as a “green” alternative to chlorobenzene for perovskite solar cell (PSC) fabrication. This work validates the technical and environmental performance of anisole in the fabrication of PSCs. PSCs exhibiting conversion efficiencies exceeding 22% can be attained by using anisole as an antisolvent. Moreover, around 50% reduction in the potential toxicity is obtained when substituting chlorobenzene for anisole embodied in the carcinogenic human and ecosystem toxicity impact categories. Nonetheless, anisole embodies higher impact in all 14 remaining impact categories. This is due to anisole's multistep synthesis procedure that contributes to more than double the climate change impact of chlorobenzene, synthesized by a single-step method. To reduce the emissions several recommendations and strategies are proposed. Ultimately, it has been proved that context-specific and transparent environmental assessments are needed to make informed decisions in research and development leading to environmentally sustainable solutions.
Sustainability spotlightPerovskite solar cells have rapidly emerged as promising alternatives to other established solar cell technologies and as they are currently at the trial stage of commercial development it is essential to verify and propose sustainable practices that ensure their widespread adoption without compromising the environment. In the antisolvent treatment of the perovskite layer, anisole has been proposed as a green alternative to chlorobenzene. Efficiencies over 22% and halved carcinogenic toxicity have been achieved by substituting chlorobenzene for anisole according to life cycle assessment. However, other environmental impacts have doubled, such as climate change impact. This highlights the need for transparent, context-specific environmental assessments. This work contributes to access to clean energy (SDG 7), industry innovation (SDG 9), and climate action (SDG 13) by contributing to the development of environmentally sustainable commercializable solar cell technology. Ultimately, anisole use in antisolvent treatment is one of many optimizations needed in PSC development. |
Introduction
Terminologies such as “Green,” “Eco-efficient,” and “Environmentally friendly” are commonly employed to convey that certain materials, products, or processes are environmentally sustainable. However, the results of environmental assessments are typically comparative and dependent on the conditions and assumptions specified,1 rendering their outcomes predominantly relative rather than absolute. Hence, the utilization of a “green” material or adherence to a “green” process does not automatically confer “green” status upon a product; rather, it may simply reduce its environmental impact compared to an alternative. Researchers across various fields prioritize the integration of “green” materials into their technologies to achieve environmental sustainability. This is evident in the clean energy generation sector, particularly in developing perovskite solar cells (PSCs).2–4 Recommendations made by solvent selection guides, namely CHEM21 and GlaxoSmithKline (GSK),5,6 are usually applied. These guides score solvents based on environmental, health, and safety criteria, including physical properties. Solvents are assigned scores, and those with the highest scores are color-coded green. Unfortunately, this color coding can be misinterpreted. Chemicals marked as green are sometimes mistakenly assumed to be environmentally sustainable. This misconception arises because the scoring system considers multiple criteria, with the environmental aspect being just one of them. A chemical could have excellent health and safety performance but poor environmental performance and still receive a high score. Moreover, reducing the environmental aspect to a single score hampers a thorough understanding of what the score actually represents. In other words, the green color-coding of these solvents does not inherently ensure a reduced environmental impact of the resulting product. The actual environmental impact depends on various factors, including the solvent's life cycle, and the performance of the produced PSCs, among other aspects.Researchers worldwide are working on developing photovoltaic cells exhibiting high power conversion efficiency (PCE) and low environmental footprint. Generally, PCE is defined as the ratio of energy output from solar cells (Pmax) to the energy input (Pin) of sunlight and is defined as:
PSCs have emerged as promising alternatives to other established solar cell technologies.7 The efficiency of PSCs has now reached 26.1% in single junction and 33.9% in tandem configuration with Si-solar cells.8 A major contribution to such a fast rise in PCE was the development of methods producing high-quality perovskite films exhibiting a pinholes-free and flat surface.9–12 As the morphology of the perovskite film influences the performance of devices significantly a lot of efforts during the initial days have been devoted to controlling the crystallization of perovskite.13 Several techniques such as sequential deposition,14 antisolvent methods,15 solvent-assisted crystallization,16 additives included in the precursors, and co-evaporation,17 have been developed to enhance the quality of the perovskite film. The community's dedication to advancing the environmental sustainability of PSCs is apparent through environmental assessment studies published.18,19 These studies evaluate the environmental impact of PSCs across diverse fabrication methods and configurations, while also considering variations in PCEs. Furthermore, decent efficiencies have been achieved by integrating a variety of “green” solvents into the fabrication process of solar cells.20–30
The solvent-engineering method employing antisolvent treatment is one of the most popular and commonly used perovskite deposition methods,9,31 leads to high efficiencies but faces environmental challenges. A one-step solvent-assisted deposition approach for producing uniform CH3NH3PbI3 thin films through rapid crystallization was developed.15 In this process, chlorobenzene, the antisolvent employed, is promptly introduced after applying a perovskite precursor solution in dimethylformamide (DMF) during spin coating. The role of chlorobenzene is to rapidly reduce the solubility of perovskite in mixed solvent and promote fast nucleation of the crystals in the film. During this antisolvent treatment, the density of nuclei increases resulting in a uniform and pinhole-free film with large perovskite grains up to a size of microns.32 The antisolvent step significantly improves solar cell efficiency, reduces hysteresis, and enhances the stability of the devices. However, chlorobenzene, widely used as an antisolvent, is known to be a toxic substance with suppressive and anesthetic impacts on the human central nervous system.33 Owing to its high toxicity, the use of chlorobenzene constitutes a major concern28 for industrial-scale manufacturing. Thus, alternative solvents have been explored to not only reduce toxicity but also achieve high solar cell efficiency. For example, claimed to be “green” antisolvents such as ethanol,25 tetraethyl orthocarbonate,34 anisole,24,35etc., have been studied and applied as antisolvents to obtain high-efficiency PSCs. Nonetheless, the actual “greenness” of these solvents has yet to be verified using standardized environmental assessment methods.
In this work, we employ the solvent-engineering technique to fabricate PSCs, comparing the use of chlorobenzene and anisole as antisolvents. Our investigation delves into the technical and performance aspects of the fabrication of PSCs, focusing on the morphology, crystallography, and surface composition of the perovskite film. Additionally, we apply the life cycle assessment (LCA) methodology to uncover the environmental implications of substituting chlorobenzene for anisole during the antisolvent treatment phase of PSC production. In this context, various characterization techniques are employed to ensure that PSCs fabricated using antisolvents have the same characteristics and offer identical performance of over 22% with excellent reproducibility. The conducted experiments and detailed characterization are paramount for a valid LCA comparison, as they demonstrate that the PSCs fabricated using the two antisolvents are identical in structure and performance. Our objective is to ascertain whether adopting anisole compared to alternative antisolvents can reduce the environmental footprint attributed to the antisolvent treatment step. In accordance with the study objective, the LCA boundary focuses solely on the chemicals used in the antisolvent treatments, i.e., anisole and chlorobenzene. It does not assess any components of the PSC, as these components have been proven to be identical in both performance and composition, regardless of whether anisole or chlorobenzene is used in the antisolvent treatment step.
This case study shows the importance of conducting lab-scale LCAs to validate the potential positive impact associated with the adoption of “green” materials and processes. Our motivation stems from recognizing that the environmental footprint of materials can vary depending on their utilization and end-of-life management. Hence, while the integration of “green” materials holds promise for reducing environmental impact in emerging technologies, it does not inherently guarantee such an outcome. Moreover, several ideas are explored and discussed such as material efficiency, bio-based synthesis routes, and solvent recovery demonstrating the relative “greenness” of solvents.
Experimental
In this section, the employed materials and followed processes for the fabrication of PSCs are detailed. Next, the LCA, a leading method in environmental assessment, is introduced. LCA modelling parameters are transparently communicated along with the built life cycle inventories to facilitate the results' replication.Materials
Fluorine-doped tin oxide (SnO2:F, FTO) glass substrates were purchased from Nippon Sheet Glass Co. Ltd (NSG), and VisionTek Systems Ltd. Acetone, ethanol, and propan-2-ol were provided by Fisher. SnO2 (15 wt%) in H2O colloidal dispersion was bought from Alfa Aesar while PbI2 (99.99%) was obtained from Kojundo Chemical Laboratory Co. LTD. PbI2, FAI, and KI were obtained from Tokyo Chemical Industry (TCI), CsI from Sigma-Aldrich or TCI, N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) from TCI. Titanium di-isopropoxide bis (acetylacetonate) (75 wt% in isopropanol), lithium bis (trifluoro methane sulfonyl) imide (LiTFSI), and 4-tert-butylpyridine (tBP) were purchased from Sigma-Aldrich. TiO2 (24 nm) was purchased from JGC C&C and spiro-OMeTAD ((2,2′,7,7′-tetrakis-(N,N-di-4-methoxyphenylamino)-9,9′-spiro-bifluorene)) from Merck. Anisole and chlorobenzene were obtained from Wako Pure Chemical Industries, LTD. All chemicals were used without any further purification.Fabrication of PSCs
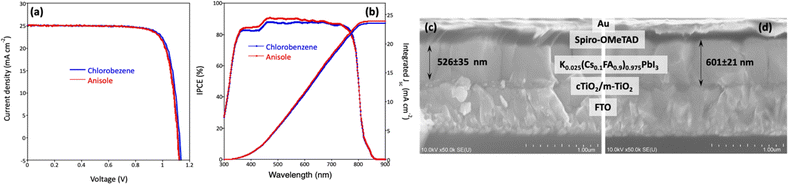
Compact TiO2, mesoporous-TiO2, spiro-OMeTAD, and Au layers were prepared following a method earlier reported by Mariotti et al.36 A precursor solution of perovskite of composition K0.025(Cs0.1FA0.9)0.975PbI3 was made by dissolving 54.6 mg of CsI, 325 mg of FAI, and 1016.6 mg of PbI2 in 1.2 ml of DMF and 300 μl of DMSO. Then, 35 μl of KI (49.8 mg KI in 200 μl DMSO) was added to it. After that, 100 μl of this solution was spin-coated at 1000 r.p.m. for 10 seconds and at 3000 r.p.m. for 30 seconds. During the spin coating, 500 μl of chlorobenzene or anisole was dropped during the second spin coating step, 8 seconds before completing the spinning, which was followed by annealing the substrates at 155 °C for 15 min. According to the industrial solvent selection guide, anisole is categorized as a minimal health hazardous material than chlorobenzene.37Material characterization and solar cells evaluation
The scanning electron microscopy (SEM) images of the perovskite layer surface and perovskite solar cells were recorded using a field emission scanning microscope (FE-SEM JSM6700F, Jeol 6700F). The I–V characteristics and photovoltaic parameters were measured under AM1.5G conditions at 100 mW cm−2 using a 300 W Xenon solar simulator (YSS-80A; Yamashita Denso Co., Ltd, Japan). The light source was calibrated using a Si photodiode of BS-520 (Bunkokeiki Co., Ltd, Japan). The active area of solar cells was 0.18 cm2. The incident photon to electron conversion efficiency (IPCE) was measured using Bunkokeiki, CEP-2000MLQ Instruments system.Life cycle assessment (LCA)
LCA is a standardized method frequently used for evaluating the environmental performances of products, processes, or services over their full life cycle. This LCA follows the framework of ISO standards (14040 and 14044).38,39 LCA consists of four stages: goal and scope definition, life cycle inventory (LCI) analysis, life cycle impact assessment (LCIA), and interpretation. In this section, the first three stages are described, while the LCIA results are presented and interpreted in the Results and discussion section.The goal of this LCA was to evaluate the environmental performance of anisole and compare it to chlorobenzene, thereby verifying whether the substitution of chlorobenzene for anisole can reduce the environmental impact of the antisolvent treatment step. This analysis focuses on the use of these two chemicals as antisolvents in the laboratory production process of PSCs. The functional unit selected is carrying an antisolvent treatment on the perovskite layer, resulting in a PSC with a PCE of 22%. For this goal, chlorobenzene and anisole were compared on a 1-to-1 basis, given that equal volumes of these two solvents are used to obtain identical results. The absolute environmental results correspond to 1 kg of chemicals. This mass-based functional unit is justified because both chemicals are used in equal quantities and produce PSCs of similar characteristics and efficiencies.
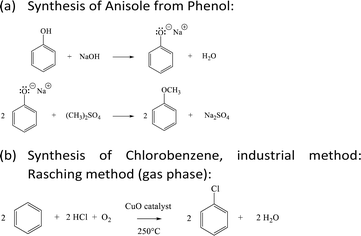
The scope of this LCA encompasses the entire life cycle of the antisolvents (anisole and chlorobenzene) noted as a cradle-to-grave study. It considers impacts arising from material extraction, chemical synthesis, transportation, use, and end-of-life stages. Notably, there are no impacts associated with the use phase, as the antisolvents evaporate during the thermal treatment of the perovskite layer. It is assumed that the entire amount of anisole or chlorobenzene is evaporated into the atmosphere. The LCA boundary focuses solely on the chemicals used in the antisolvent treatments. It does not assess any components of the PSC, as these components have been proven to be identical in both performance and composition, regardless of whether anisole or chlorobenzene is used in the antisolvent treatment step. The geographical scope of the study has been set to solvent production outside of Europe, and their use in southern France. Additionally, this is a contemporary study, where background and foreground data have been sourced from the latest available version of the ecoinvent database at the time of conducting the research. Life cycle inventories have been developed for anisole's most common production route, which relies on petrochemicals.40 The foreground subsystem representing anisole40,41 and chlorobenzene production has been constructed based on the following chemical reactions.
The synthetic reactions of phenol, used as a precursor for anisole, is available in ESI† along with the LCI in Table S1.† The synthesis route has been optimized and scaled up to emulate industrial production conditions, by assuming that 80% of benzene is recycled and calculating the heating and distillation energy necessary during anisole production based on engineering equations.42 The energy calculation has been carried out according to eqn (S1) and (S2)† and according to information disclosed in Tables S2–S4.† The background subsystem including raw material acquisition and chlorobenzene production has been modelled based on generic datasets. An LCI has been also built to account for the impacts arising during the chlorobenzene life cycle (Table S5†).
The life cycle impact assessment method used to conduct this analysis is the environmental footprint method version 3.1 (adapted).43 This LCIA method has been selected due to its wide coverage of chemical toxicity embodied in three impact categories namely, human carcinogenic and non-carcinogenic toxicity as well as ecotoxicity.44 The ecoinvent version 3.9.1 APOS was used to model background inventory processes.45 Modeling was carried out using SimaPro 9.5 software.
Results and discussion
Processing, morphology, and PSCs performances, perovskite film prepared using chlorobenzene and anisole as antisolvents
To prepare PSCs, the K0.025(Cs0.1FA0.9)0.975PbI3 composition was selected and spin-coated on the SnO2 layer (electron transport layer) followed by a quick application of the antisolvents, chlorobenzene or anisole at a fixed time of 8 seconds before the end of the spin-coating procedure (total time of 30 seconds). In both cases, a volume of 500 μL was dripped quickly through a micropipette tip. The substrates coated with perovskite were annealed at 155 °C for 15 min followed by the coating of spiro-OMeTAD and Au layers36 to complete the devices. The fabrication process of perovskite film is depicted in Fig. 1.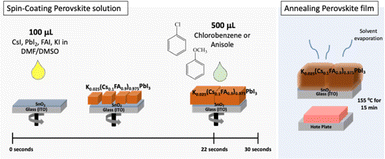 | ||
| Fig. 1 Schematic representation of the processing method of K0.025(Cs0.1FA0.9)0.975PbI3 layer using chlorobenzene and anisole as antisolvents. | ||
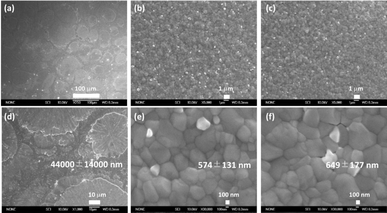
As shown in Fig. 2 the surface morphology of the untreated films (without antisolvent, Fig. 2a and d) shows uneven surfaces with aggregated particles and poor coverage while antisolvent-treated chlorobenzene (Fig. 2b and e) and anisole (Fig. 2c and f) films show homogeneous smooth surfaces with well-defined perovskite grain. It has been established that the antisolvent nature governs the perovskite crystallization, and thus the grain size and surface roughness of the perovskite films.46 A similar trend was observed in our experiments.
According to SEM images, the average grain size was estimated to be 574 ± 131 nm for chlorobenzene-treated film while that for the anisole-treated film was found to be 649 ± 177 nm. Larger grains in the case of anisole can be ascribed to its higher boiling point, i.e. 153.8 °C, and higher viscosity, leading to slower crystallization resulting in larger grains. It has been also reported that anisole forms intermolecular interaction with DMF and DMSO via hydrogen bonding that can play a role in the formation of perovskite films.47 According to the X-ray diffraction analysis (Fig. S1†), both prepared films show the expected diffraction lines for quasi-cubic structure K0.025(Cs0.1FA0.9)0.975PbI3 perovskite films. The surface composition of perovskite films after each antisolvent step was investigated by X-ray photoelectron spectroscopy. As depicted in Fig. S2(a) and (b),† the expected core level emission lines for Cs, C, N, Pb, and I elements were clearly detected. Moreover, a surface composition of K0(Cs0.12FA0.88)1PbI2.88 and K0.04(Cs0.18FA0.81)0.95PbI2.94 was determined for anisole and chlorobenzene-treated films, respectively (Table S6†). These compositions are not far behind the K0.025(Cs0.1FA0.9)0.975PbI3 perovskite nominal composition introduced by Mariotti et al.36
Before assessing the environmental and technical impacts of the antisolvents used for fabrication of perovskite solar cells the power conversion efficiency (PCE) of solar cells which is crucial parameter for demonstrating comparable or superior efficiencies using anisole, a proposed green solvent, compared to the toxic chlorobenzene. In this context, the devices with perovskite layer treated with chlorobenzene and anisole, planar n-i-p devices with ITO/SnO2/K0.025(Cs0.1FA0.9)0.975PbI3/spiro-OMeTAD/Au structure were fabricated. Fig. 3 and Table 1. display the statistical distribution of photovoltaic parameters for both cases. The J–V curves, incident photon-to-current efficiency (IPCE), and cross-section SEM images of the best-efficient devices for chlorobenzene and anisole-treated perovskite films are shown in Fig. 4a–d. Devices made using chlorobenzene as antisolvent exhibited a PCE of 22.6% with an open circuit voltage (Voc) of 1.12 V, a short circuit current (Jsc) of 24.9 mA cm−2, and a fill factor (FF) of 0.81 under reverse scan and a PCE of 22.4% with a Voc of 1.12 V, Jsc of 25.0 mA cm−2 and FF of 0.80 under forward scan (Table 1). The PSCs with K0.025(Cs0.1FA0.9)0.975PbI3 layer prepared using anisole as antisolvent yielded similar photovoltaic responses, PCE of 22.5% with a Voc of 1.12 V, Jsc of 25.1 mA cm−2, and FF of 0.80 under reverse scan and PCE of 22.1% with Voc of 1.11 V, Jsc of 25.2 mA cm−2, and FF of 0.79 under forward scan, but in average with smaller hysteresis index and narrow distribution of data. The integrated photocurrent values (from IPCE), were 23.6 and 24.0 mA cm−2 for the devices using chlorobenzene and anisole, respectively, showing quite a close match with the Jsc values extracted from J–V curves shown. We found that the efficiency of over 22%, obtained in the present work, is higher than others reported for slightly different perovskite absorbers and device architectures. Besides, IV hysteresis is minimal compared to others which can be related to the device structure.24,35 The efficiencies of 20.01% (18.95%) and 19.0% (18.1%) reported by Wei et al.,24 and 20.4% (19.04%) and 20.53% (19.11) reported by Saliba et al.35 for chlorobenzene and anisole, respectively.
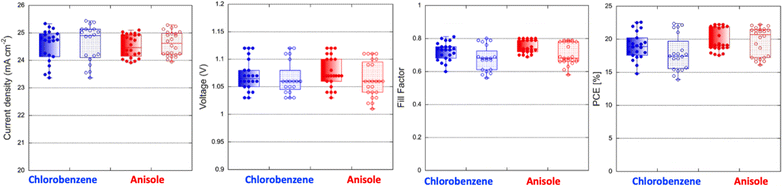 | ||
| Fig. 3 Statistics of photovoltaic parameters (reverse scan full dots, forward scan open dots) of the perovskite solar cells, K0.025(Cs0.1FA0.9)0.975PbI3 layer treated with chlorobenzene and anisole. | ||
| Antisolvent | Scan direction | J (mA cm−2) | V (V) | FF | PCE (average) (%) |
|---|---|---|---|---|---|
| Chlorobenzene | Reverse | 24.9 (24.5 ± 0.6) | 1.12 (1.06 ± 0.03) | 0.81 (0.72 ± 0.06) | 22.6 (19.0 ± 2.1) |
| Forward | 25.0 (24.6 ± 0.7) | 1.12 (1.06 ± 0.03) | 0.80 (0.67 ± 0.08) | 22.4 (17.9 ± 2.7) | |
| Anisole | Reverse | 25.1 (24.5 ± 0.4) | 1.12 (1.07 ± 0.03) | 0.80 (0.75 ± 0.04) | 22.5 (19.8 ± 1.7) |
| Forward | 25.2 (24.6 ± 0.4) | 1.11 (1.06 ± 0.03) | 0.79 (0.70 ± 0.07) | 22.1 (19.7 ± 2.2) |
Life cycle impact assessment results
As shown above, anisole appeared to be a suitable alternative to chlorobenzene as an antisolvent to fabricate efficient PSCs. The question, therefore, arises about the environmental impact of the use of these solvents. In this context, LCIA results of anisole and chlorobenzene, normalized to 1 kg of the chemicals consumed during the fabrication of perovskite solar cells, have been produced and are shown in Table 2 and displayed in Fig. 5.| Impact category | Anisole | Chlorobenzene | Unit |
|---|---|---|---|
| Human toxicity, cancer | 1.85 × 10−8 | 4.17 × 10−8 | CTUh |
| Human toxicity, non-cancer | 1.15 × 10−7 | 1.00 × 10−7 | CTUh |
| Ecotoxicity, freshwater | 2.13 × 102 | 3.74 × 102 | CTUe |
| Climate change | 6.20 × 100 | 2.83 × 100 | kg CO2 eq. |
| Ionizing radiation | 3.16 × 10−1 | 1.23 × 10−1 | kBq U-235 eq. |
| Ozone depletion | 6.93 × 10−7 | 5.91 × 10−7 | kg CFC11 eq. |
| Particulate matter | 3.56 × 10−7 | 1.83 × 10−7 | Disease inc. |
| Photochemical ozone formation | 2.78 × 10−2 | 2.22 × 10−2 | kg NMVOC eq. |
| Water use | 4.27 × 100 | 3.04 × 100 | m3 depriv. |
| Acidification | 4.13 × 10−2 | 1.81 × 10−2 | mol H+ eq. |
| Eutrophication, marine | 6.72 × 10−3 | 3.52 × 10−3 | kg N eq. |
| Eutrophication, freshwater | 1.71 × 10−3 | 7.00 × 10−4 | kg P eq. |
| Eutrophication, terrestrial | 7.09 × 10−2 | 3.72 × 10−2 | mol N eq. |
| Land use | 1.47 × 101 | 4.80 × 100 | Pt |
| Resource use, fossils | 1.37 × 102 | 6.84 × 101 | MJ |
| Resource use, minerals and metals | 1.38 × 10−4 | 1.25 × 10−5 | kg Sb eq. |
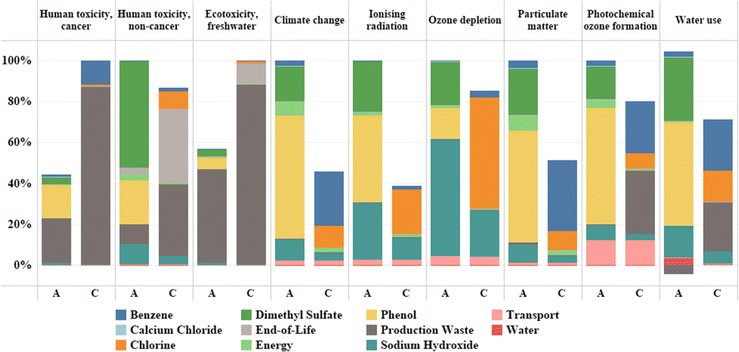 | ||
| Fig. 5 Comparative and contribution analysis of the potential toxicity and other environmental impacts of anisole (A) and chlorobenzene (C). | ||
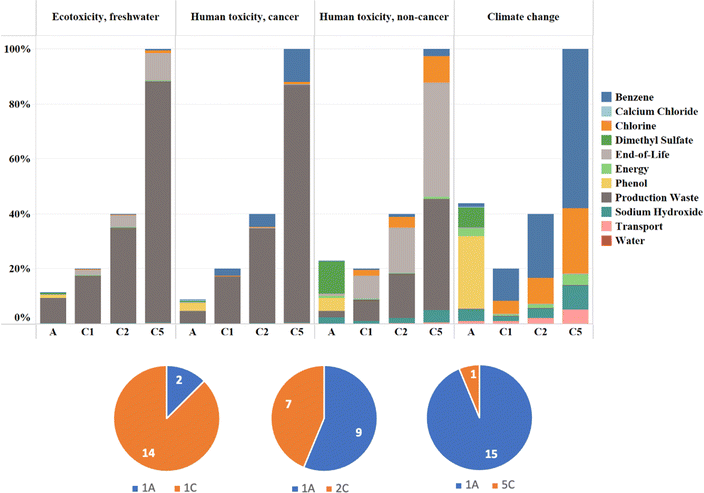
It is obvious that compared to chlorobenzene, anisole has reduced potential toxicity on human health and the environment. Expressed in comparative toxic units, anisole is responsible for carcinogenic human toxicity of 1.85 × 10−8 CTUh kg−1 compared to 4.17 × 10−8 CTUh kg−1 for chlorobenzene. Similarly, ecotoxicity of anisole was estimated to be 2.13 × 102 CTUe kg−1 whereas the same for chlorobenzene was 3.74 × 102 CTUe kg−1. Moreover, both chemicals have similar non-carcinogenic toxicity with chlorobenzene having a slight edge over anisole embodied in a 13.19% reduced non-carcinogenic toxicity. However, the impact of anisole on climate change was found to be substantially higher than that of chlorobenzene, emitting 6.20 kg CO2 equivalent per kg of solvent, which was about twice the amount released for chlorobenzene (2.83 kg CO2 equivalent per kg of solvent). All the remaining categories such as ionizing radiation, ozone depletion, particulate matter, photochemical ozone formation, and water use, that have an indirect effect on human health follow the trend of climate change.48 A comparative chart with an embedded contribution analysis (Fig. 5) demonstrates the potential toxicity effects, both direct and indirect, of the two antisolvents. This pinpoints toxicity and environmental hotspots, including potential toxicity hotspots in the life cycle of anisole and chlorobenzene used for laboratory-scale PSC fabrication.
In the following sections, several ideas are explored to reduce the environmental impact of anisole: material efficiency, alternative synthetic routes (bio-sourced precursors), and antisolvent recovery.
Material efficiency, lowering the amount of antisolvents
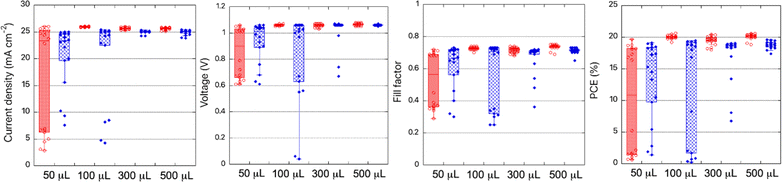
The possibility of reducing the quantity of antisolvent was investigated by experimenting with various amounts: 50, 100, 300, and 500 μL. The corresponding performances of PSCs were then evaluated (Fig. 6) and the resulting average photovoltaic parameters are listed in Table S7.† Initially, 500 μL of anisole was used to treat the perovskite absorber on a 25 mm × 25 mm substrate.As shown in Fig. 6, decreasing the amount of anisole from 500 μL to 100 μL led to comparable performances, an average PCE of 20.1 ± 0.5% with Jsc of 25.6 ± 0.2 mA cm−2, Voc of 1.06 ± 0.01 V, and FF of 0.74 ± 0.02 and average PCE of 20.0 ± 0.3% with Jsc of 25.9 ± 0.1 mA cm−2, Voc of 1.06 ± 0.01 V, and FF of 0.73 ± 0.01, respectively (Table S7†). However, at 50 μL, the efficiency of the solar cells collapsed, and reproducibility became poor, resulting in a wide distribution of efficiencies (9.8 ± 8.5%). This volume was insufficient to cover the entire perovskite surface, leading to poor morphology on the 25 mm × 25 mm substrate. For chlorobenzene, lower-performing solar cells were obtained when the quantity was reduced to 300 μL, an average PCE of 17.1 ± 3.8% with Jsc of 24.9 ± 0.1 mA cm−2, Voc of 1.02 ± 0.12 V, and FF of 0.66 ± 0.1 were registered. A more significant distribution of data was observed with 100 μL (average PCE of 12.2 ± 8.6%) and 50 μL of chlorobenzene (average PCE of 13.3 ± 6.4%), likely due to its faster evaporation compared to anisole (Fig. 6, Table S7†). These results demonstrate that lower amounts of anisole (to 100 μL) can be used to obtain higher-quality perovskite compared to chlorobenzene.
A sensitivity analysis was conducted to evaluate the environmental impact of variations in the use of chlorobenzene and anisole, as shown in Fig. 6. Initially, a 1-to-1 comparison was made using 500 μL of each solvent. However, data displayed in Fig. 6 suggest that the amount of anisole can be reduced significantly, either by half or even to one-fifth of the volume of chlorobenzene. Consequently, comparisons were also made on 1-to-2 and 1-to-5 bases. The results, presented in Fig. 7 and Table S8,† demonstrate that reducing the amount of anisole by half makes it more advantageous than chlorobenzene in 9 out of 16 environmental indicators. However, anisole's impact on climate change remains higher compared to chlorobenzene. When the anisole volume is reduced to one-fifth, anisole becomes less environmentally impactful than chlorobenzene across all assessed indicators except for the resource use in the minerals and metals category (1.38 × 10−4 kg Sb eq. for anisole versus 6.24 × 10−5 kg Sb eq. for chlorobenzene), as shown in Table S7.†
Bio-based synthesis routes
The lower human carcinogenic and ecosystem toxicities of anisole are attributed to two main factors: the reduced potential toxicity of its production waste and its lower direct toxicity when released into the air, especially in comparison to chlorine-containing compounds like chlorobenzene. However, the multistep synthesis procedure of anisole contributes to a higher environmental impact due to waste (i.e., benzene used for anisole extraction) while the one-step synthesis of chlorobenzene from chlorine and benzene has a lower environmental impact. Therefore, the adoption of anisole in large-scale manufacturing of PSCs might be beneficial only when produced with higher efficiencies and/or from renewable sources.49–51Although the bio-based synthesis of anisole holds promise for reducing environmental impact, it remains an unexplored domain. As of the time of writing, no research articles have been published on the bio-based synthesis of anisole. However, some publications have investigated the bio-based synthesis of phenol,52–54 which is a key precursor for anisole production. Presently, bio-oil can be utilized to produce renewable phenol,52 but the process is highly inefficient. According to our calculations, 263.15 grams of bio-oil extracted from approximately 1.253 kilograms of corn cobs are required to produce just 1 gram of phenol. Aside from economic concerns, scaling this process to an industrial level could have severe environmental implications, especially in terms of land use. Additionally, the energy-intensive pyrolysis process reaches temperatures of up to 800 °C,52 and catalysis further contributes to the environmental impact of this bio-based synthesis route. Another approach involves synthesizing anisole from lignin, but this method yields only 5% and relies on a ZrO2-FeOx catalyst.53 It's evident that the synthesis of anisole from biomass still requires significant optimization to become a viable substitute for the petroleum route.
Antisolvent recovery
Antisolvent treatment, like any other production process, can be made more environmentally friendly by incorporating recycling processes. In this case, the recycling of antisolvent could be facilitated through the compartmentalization of the PSC production step. Each material would be deposited on the substrate in a dedicated, closed compartment, allowing for the capture of chemical vapors and their subsequent recycling. For the antisolvents used in this study, this could be achieved via condensation, a well-known solvent recovery method.55 Research on the vapor recovery of anisole and chlorobenzene in the context of PSC production is currently unexplored. Since these solvents interact with perovskite materials during antisolvent treatment, chemical mixing could occur in the vapors, resulting in impure solvent vapor. Techniques for the separation and recycling of such vapors exist and could be optimized for scaling up this treatment step.56,57 This would benefit the environmental performance of both anisole and chlorobenzene treatments, with greater benefits for the solvent with a higher recovery rate. Therefore, future research needs to focus not only on achieving high-performance PSCs but also on the recovery of anisole, paving the way for industrial scale-up.The relative “greenness” of chemicals
Labeling chemicals as “green” requires a thorough, context-specific assessment rather than relying on general guidelines. Achieving absolute “greenness” is difficult because chemicals can behave differently under various conditions, leading to diverse outcomes. In other words, a chemical that is environmentally friendly in one synthetic route might be harmful in another. This discrepancy often stems from factors such as its production method, the yield it produces when used as a reactant, the quality of its resulting products, and other factors such as the need for catalysis. For example, the production method can substantially impact the environmental footprint of a chemical. Petrol-based ethanol has around three times the carbon emissions of bio-sourced ethanol.45 On the other hand, hydrogen peroxide illustrates how yield can vary across different processes. Thus, the oxidation of propylene with hydrogen peroxide, also known as the HPPO process, yielded high-yield and high-quality propylene oxide at the industrial scale,58,59 while wastewater treatment using hydrogen peroxide via the Fenton reaction still requires improvements in terms of efficiency to be economically viable.60 Consequently, a context-specific and transparent environmental assessment is essential for making informed decisions when developing new technologies and considering potential substitutes for chemicals. Transparent assessments enable researchers to tailor evaluations to their specific process conditions, enhancing the accuracy and relevance of their results.Conclusions
The environmental viability of chlorobenzene substitution with anisole has been investigated, shedding light on the need for context-specific and transparent environmental assessment. Solvent selection guides showed anisole as a better alternative to chlorobenzene. Based on this recommendation, researchers have employed anisole as an antisolvent in the fabrication of PSC. A claim that anisole is a “green” antisolvent is made, lacking a scientific foundation. This work evaluates the technical and environmental performance of anisole in the fabrication of PSC.The use of anisole as a less toxic alternative to chlorobenzene in the processing of PSCs based on K0.025(Cs0.1FA0.9)0.975PbI3 was assessed by comparison of solar cell performance. PSCs (ITO/SnO2/K0.025(Cs0.1FA0.9)0.975PbI3/spiro-OMeTAD/Au) made using anisole led to slightly better performances, showing an efficiency over 22% with improved reproducibility and minimum hysteresis, than those fabricated with chlorobenzene. Performance improvement in the case of anisole was related to a quasi-cubic crystallite, larger grain sizes, i.e. 649 ± 177 nm, smooth even surface, resulting in higher Voc and FF. Furthermore, experiments were conducted to determine if smaller volumes of anisole and chlorobenzene could achieve high-performance PSC. The results showed that the volume of anisole could be reduced by up to five times compared to chlorobenzene, while still producing high-performance PSC with excellent reproducibility.
A cradle-to-grave LCA study of anisole and chlorobenzene shows that anisole is less ecotoxic (2.13 × 102 CTUe kg−1) than chlorobenzene (3.74 × 102 CTUe kg−1). Anisole demonstrates significantly low carcinogenic human toxicity, (1.85 × 10−8 CTUh kg−1) in comparison to chlorobenzene (4.17 × 10−8 CTUh kg−1). However, the LCA results revealed other environmental impacts of anisole to be higher than chlorobenzene's, arising from anisole's multi-step production process which is more complex than chlorobenzene. Nonetheless, the ability to use lower quantities of anisole than chlorobenzene in the fabrication of PSC can lower its environmental impact. A sensitivity analysis shows that anisole amount should be lowered by fivefold to provide a higher environmental performance than chlorobenzene, with reservation to the resource use impact category.
This study illustrates that context-specific and transparent environmental assessments are needed to make informed decisions in research and development leading to environmentally sustainable solutions. The environmental friendliness or so-called “greenness” of anisole is investigated. As PSCs are currently at a trial stage of commercial development, we believe that this work of addressing the solvent potential toxicity during perovskite film fabrication contributes to the development of environmentally conscious industrial production.
Data availability
Data discussing the results of this article are available as a part of the main text and the data supporting this article have been included as a part of ESI.†Author contributions
N. M. K. and Y. Y. performed device fabrication, evaluation, and data analysis of the devices. A. K. K. and G. S. assess the potential toxicity among other environmental impacts using LCA. N. M. K. wrote the manuscript and revised it by Y. Y., A. K. K., A. K. J., T. T., G. S., H. S., S. U., L. C. All authors approved the final version of the manuscript. L. C. T. T. contributed to the conceptualization and project supervision. All authors contributed to manuscript development.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work benefited from State assistance managed by the National Research Agency under the “Programme d’Investissements d’Avenir” (ANR-19-MPGA-0006), supported by ELORPrintTec ANR-10-EQPX-28-01, and the New Energy and Industrial Technology Development Organization (NEDO) PV R&D programs. The authors would like to acknowledge IRP-NextPV-II a CNRS and RCAST/University Tokyo collaboration. Dr Christine Labrugère (PLACAMAT) is thanked for her precious assistance in recording XPS data and Philippe Legros (PLACAMAT) for SEM measurements.References
- Bjørn, M. Diamond, M. Owsianiak, B. Verzat and M. Z. Hauschild, Environ. Sci. Technol., 2015, 49, 6370–6371 CrossRef.
- C. Wu, K. Wang, J. Li, Z. Liang, J. Li, W. Li, L. Zhao, B. Chi and S. Wang, Matter, 2021, 4(3), 775–793 CrossRef CAS.
- J. Doolin, R. G. Charles, C. S. P. De Castro, R. G. Rodriguez, E. V. Péan, R. Patidar, T. Dunlop, C. Charbonneau, T. Watson and M. L. Davies, Green Chem., 2021, 23(6), 2471–2486 RSC.
- M. Zhang, D. Xin, X. Zheng, Q. Chen and W. H. Zhang, ACS Sustain. Chem. Eng., 2020, 8(35), 13126–13138 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada, P. J. Dunn and P. CHEM21, Green Chem., 2016, 18(1), 288–296 RSC.
- M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18(13), 3879–3890 RSC.
- J. Y. Kim, J. W. Lee, H. S. Jung, H. Shin and N. G. Park, Chem. Rev., 2020, 120, 7867–7918 CrossRef CAS PubMed.
- https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf .
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS.
- F. Ma, Y. Zhao, Z. Qu and J. You, Acc. Mater. Res., 2023, 4, 716–725 CrossRef CAS.
- N. Ahn, D. Y. Son, I. H. Jang, S. M. Kang, M. Choi and N. G. Park, J. Am. Chem. Soc., 2015, 137, 8696–8699 CrossRef CAS.
- W. S. Yang, J. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. Seok, Science, 2015, 348, 1234–1237 CrossRef CAS.
- A. Srivastava, J. A. K. Satrughna, M. K. Tiwari, A. Kanwade, S. C. Yadav, K. Bala and P. M. Shirage, Mater. Today Commun., 2023, 35, 105686 CrossRef CAS.
- J. Burschka, N. Pellet, S. J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- M. Xiao, F. Huang, W. Huang, Y. Dkhissi, Y. Zhu, J. Etheridge, A. Gray-Weale, U. Bach, Y.-B. Cheng and L. Spiccia, Angew. Chem., Int. Ed., 2014, 53, 9898–9903 CrossRef CAS PubMed.
- S. Dinghan, S. Yu, X. Cai, M. Peng, Y. Ma, X. Su, L. Xiao and D. Zou, J. Mater. Chem. A, 2014, 2, 20454–20461 RSC.
- L. Cojocaru, K. Wienands, T. W. Kim, S. Uchida, A. J. Bett, S. Rafizadeh, J. C. Goldschmidt and S. Glunz, ACS Appl. Mater. Interfaces, 2018, 10, 26293–26302 CrossRef CAS.
- R. Vidal, J. Alberola-Borràs, N. Sánchez-Pantoja and I. Mora-Seró, Adv. Energy Sustainability Res., 2021, 2(5), 2000088 CrossRef CAS.
- S. Weyand, C. Wittich and L. Schebek, Energies, 2019, 12(22), 4228 CrossRef CAS.
- T. Bu, L. Wu, X. Liu, X. Yang, P. Zhou, X. Yu, T. Qin, J. Shi, S. Wang and S. Li, Adv. Energy Mater., 2017, 7(20), 1700576 CrossRef.
- N.-G. Park, Nat. Sustain., 2021, 4(3), 192–193 CrossRef.
- J. Lee, M. Malekshahi Byranvand, G. Kang, S. Y. Son, S. Song, G. W. Kim and T. Park, J. Am. Chem. Soc., 2017, 139(35), 12175–12181 CrossRef CAS.
- M. Wang, Q. Fu, L. Yan, J. Huang, Q. Ma, M. Humayun, W. Pi, X. Chen, Z. Zheng and W. Luo, Chem. Eng. J., 2020, 387, 123966 CrossRef CAS.
- X. Cao, L. Hao, G. Su, X. Li, T. Dong, P. Chao, D. Mo, Q. Zeng, X. He and J. Wei, RSC Sustainability, 2023, 1(5), 1290–1297 RSC.
- W. Xu, Y. Gao, W. Ming, F. He, J. Li, X. Zhu, F. Kang, J. Li and G. Wei, Adv. Mater., 2020, 32(38), 2003965 CrossRef CAS.
- G. Jang, H. Kwon, S. Ma, S. Yun, H. Yang and J. Moon, Adv. Energy Mater., 2019, 9(36), 1901719 CrossRef.
- Y. Cui, S. Wang, C. Li, A. Wang, J. Ren, C. Yang, B. Chen, Z. Wang and F. Hao, Green Chem., 2021, 23(10), 3633–3641 RSC.
- M. Zhang, Z. Wang, B. Zhou, X. Jia, Q. Ma, N. Yuan, X. Zheng, J. Ding and W. Zhang, Sol. RRL, 2018, 2(2), 1700213 CrossRef.
- M. T. Hoang, F. Ünlü, W. Martens, J. Bell, S. Mathur and H. Wang, Green Chem., 2021, 23(15), 5302–5336 RSC.
- Z. Tang, S. Wang, W. Zhu, L. Ding and F. Hao, Green Chem., 2023, 25(3), 1150–1156 RSC.
- D. Taylor, Q. Sun, K. P. Goetz, Q. An, T. Schramm, Y. Hofstetter, M. Litterst, F. Paulus and Y. Vaynzof, Nat. Commun., 2021, 12, 1–11 CrossRef.
- S. Ghosh, S. Mishra and T. Singh, Adv. Mater. Interfaces, 2020, 7, 2000950 CrossRef CAS.
- Y. Yun, F. Wang, H. Huang, Y. Fang, S. Liu, W. Huang, Z. Cheng, Y. Liu, Y. Cao, M. Gao, L. Zhu, L. Wang, T. Qin and W. Huang, Adv. Mater., 2020, 32, 1907123 CrossRef CAS.
- M. Wang, Q. Fu, L. Yan, J. Huang, Q. Ma, M. Humayun, W. Pi, X. Chen, Z. Zheng and W. Luo, Chem. Eng. J., 2020, 387, 123966 CrossRef CAS.
- M. Yavari, M. Mazloum-Ardakani, S. Gholipour, M. Mahdi Tavakoli, S.-H. Turren-Cruz, N. Taghavinia, M. Grätzel, A. Hagfeldt and M. Saliba, Adv. Energy Mater., 2018, 8, 1800177 CrossRef.
- S. Mariotti, D. Mantione, S. Almosni, M. Ivanović, T. Bessho, M. Furue, H. Segawa, G. Hadziioannou, E. Cloutet and T. Toupance, J. Mater. Chem. C, 2022, 10, 16583–16591 RSC.
- R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. S. R. Alston, G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC.
- ISO – 14044 – Environmental Management – Life Cycle Assessment – Requirements and Guidelines, Geneva, 2006 Search PubMed.
- ISO – 14040 — Environmental Management — Life Cycle Assessment — Principles and Framework, Geneva, 2006 Search PubMed.
- Anisole, Organic Syntheses, Coll, 1941, 1, 58 Search PubMed.
- https://www.essentialchemicalindustry.org/chemicals/phenol.html .
- F. Piccinno, R. Hischier, S. Seeger and C. Som, J. Cleaner Prod., 2016, 135, 1085–1097 CrossRef CAS.
- B. A. Andreasi, F. Biganzoli, N. Ferrara, A. Amadei, A. Valente, S. Sala and F. Ardente, European Commission, Joint Research Centre, Publications Office of the European Union, 2023 Search PubMed.
- M. Damiani, N. Ferrara and F. Ardente, European Commission, Joint Research Centre, Publications office of the European Union, Luxembourg, LUX, 2022 Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- Y. Yu, S. Yang, L. Lei, Q. Cao, J. Shao, S. Zhang and Y. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 3667–3676 CrossRef CAS.
- P. Zhao, B. J. Kim, X. D. Ren, D. G. Lee, G. J. Bang, J. B. Jeon, W. B. Kim and H. S. Jung, Adv. Mater., 2018, 30, 1802763 CrossRef.
- Joint Research Centre, Institute for Environment and Sustainability, International Reference Life Cycle Data System (ILCD) Handbook – Framework and Requirements for Life Cycle Impact Assessment Models and Indicators, Publications Office, 2010 Search PubMed.
- H. Taghvaei and M. R. Rahimpour, RSC Adv., 2016, 6, 98369–98380 RSC.
- R. C. Runnebaum, T. Nimmanwudipong, D. E. Block and B. C. Gates, Catal. Sci. Technol., 2011, 2, 113–118 RSC.
- A. Ould Hamou and J. B. Giorgi, J. Phys. Chem. C, 2019, 123, 8122–8132 CrossRef.
- L. Dai, Z. Zeng, Q. Yang, S. Yang, Y. Wang, Y. liu, R. Ruan, C. He, Z. Yu and L. Jiang, Fuel, 2020, 279, 118532 CrossRef CAS.
- H. Ishimaru, T. Yoshikawa, Y. Nakasaka, E. Fumoto, S. Sato and T. Masuda, Catal. Today, 2023, 407, 194–200 CrossRef CAS.
- M. Wojcieszyk, L. Knuutila, Y. Kroyan, M. balsemao, R. Tripthi, J. Kskivali, A. Karvo, A. Santasalo-Aarnio, O. Blomstedt and M. Larmi, Sustainability, 2021, 13(16), 8729 CrossRef CAS.
- M. Smallwood, Solvent Recovery Handbook, CRC, 2002 Search PubMed.
- V. Nabok, A. K. Hassan and A. K. Ray, J. Mater. Chem., 2000, 10, 189–194 RSC.
- M. Leemann, G. Eigenberger and H. Strathmann, J. Membr. Sci., 1996, 113, 313–322 CrossRef CAS.
- F. Schmidt, M. Bernhard, H. Morell and M. Pascaly, Chim. Oggi – Chem. Today, 2014, 32, 31–35 CAS.
- V. Russo, R. Tesser, E. Santacesaria and M. Di Serio, Ind. Eng. Chem. Res., 2013, 52, 1168–1178 CrossRef CAS.
- P. Vinosh Muthukumar, B. Gopalakrishnan and B. Bharathiraja, J. Ind. Chem. Soc., 2022, 99, 100622 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00361f |
| ‡ Current position: Chimie du solide et de l'énergie (CSE), UMR 8260, Collège de France, 05, Paris 75231 CEDEX, France. |
| This journal is © The Royal Society of Chemistry 2024 |