Open Access Article
Open Access ArticleLife-cycle analysis of lithium chemical production in the United States†
Rakesh Krishnamoorthy
Iyer
 * and
Jarod C.
Kelly
* and
Jarod C.
Kelly

Energy Systems and Infrastructure Analysis Division, Argonne National Laboratory, IL 60439, USA. E-mail: riyer@anl.gov
First published on 17th October 2024
Abstract
To achieve its ambitious national decarbonization goals, the United States has incentivized the domestic production of materials critical to decarbonization technologies, including lithium-ion batteries (LIBs). These materials include battery-grade lithium chemicals (Li-chemicals), for which the U.S. is encouraging domestic production from resources (sedimentary clays and low Li-content brines (LLCBs)) that differ substantially from conventional sources (Salar brines and spodumene ores). Here, we conduct the first-ever comparative life-cycle analysis of Li-chemical production from all alternative resources (in the U.S.) and conventional sources based on data from company literature for U.S.-related production efforts. Two energy sources (electricity and natural gas), four material inputs (HCl, NaOH, Na2CO3, and CaO), and process carbon emissions dominate the life-cycle impacts (≥90% share) of U.S.-based Li-chemical production. Comparatively, the life-cycle impacts of alternative sources-based Li-chemicals lie between those for Li-chemical production from Salar brines and from spodumene ores. At the battery level, the shift in Li-chemical sourcing causes a notable change in LIB's life-cycle impacts (by ∼5–15%), independent of the cathode chemistry employed. Our study highlights the relevance of a decarbonized electric grid and the capture and sequestration of process carbon emissions generated during Li-chemical and upstream material production in decarbonizing Li-chemical production from alternative sources. Further decarbonization would necessitate using decarbonized material inputs and a shift away from natural gas towards renewable energy for alternative resource-based Li-chemical production processes.
Sustainability spotlightTo achieve its goal of net-zero carbon emissions by 2050, the United States has embarked extensively on the domestic production of critical materials for decarbonization technologies, including lithium-ion batteries (LIBs), to ensure their robust, reliable supply chains. These materials include battery-grade lithium chemicals (Li-chemicals) that are used for LIBs across different cathode chemistries. Using life-cycle analysis (LCA), this study highlights that avoidance of process carbon emissions and decarbonized electricity are vital to decarbonizing Li-chemical production from U.S.-based alternative resources. This study aligns with the United Nations Sustainable Development Goals (SDGs), particularly for its emphasis on clean energy (SDG 7), responsible consumption and production (SDG 12), and climate action (SDG 13). |
1. Introduction
1.1. Motivation/context of study
Lithium-ion batteries (LIBs) are central to the United States' objective of achieving net-zero greenhouse gas (GHG) emissions by 2050.1 Based on projections, a multi-fold increase in LIB demand is needed to accomplish this objective over the next few decades.2–5 This increase necessitates a robust supply chain of LIB constituents to meet its demand, given the multifactorial supply chain constraints observed in recent years.4,6 This is especially true for battery-grade lithium chemicals (Li-chemicals) – lithium carbonate (Li2CO3) and lithium hydroxide (LiOH)‡ – that are critical to producing LIB cathodes of different chemistries.4,5Historically, the U.S. was at the forefront of global lithium production.7 However, the present-day Li-chemical industry is dominated by production outside of the U.S., primarily from Salar brines in Chile and Argentina and spodumene ores located in Australia that are processed subsequently in China.7,8 To reduce its dependence on these foreign sources for Li-chemicals and establish a reliable supply chain, the U.S. has introduced incentives under its Inflation Reduction Act (IRA) of 2022 to encourage the domestic production of these chemicals.9,10 Specifically, Section 45X of the IRA provides an advanced manufacturing production credit for the domestic production of various critical components and applicable critical minerals in the U.S., including battery-grade Li-chemicals.9,10 This has resulted in multiple Li-chemical exploration and production projects within the country, including those from low-Li content brines (LLCBs) and sedimentary clays, as shown in Fig. 1.11,12 Most clay-based projects are located in Nevada (Fig. 1) on sedimentary deposits formed post-evaporation of large, ancient, closed lake basins that contained significant amounts of dissolved Li.13–16 These deposits were preserved and/or observed a significant rise in Li-content due to a mix of volcanic, hydrothermal, and tectonic activities.13–16 Conversely, brine-based projects are spread across the U.S., including in Utah, the Salton Sea in California, and Arkansas.11,12
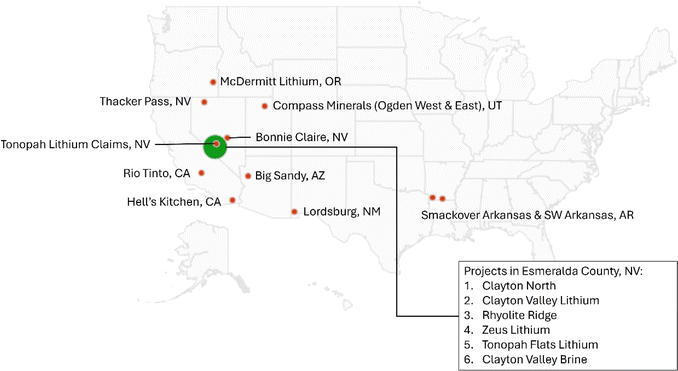 | ||
| Fig. 1 List of Li-chemical production projects from alternative sources (clays and low Li-content brines) in the United States (based on ref. 11 and 12). | ||
While the successful commencement of Li-chemical projects in Fig. 1 is essential to achieving the U.S.'s supply chain objectives, it is also important that Li-chemical production from these sources has low negative effects on the environment. This is pertinent as these projects envisage Li-chemical production from resources (clays and LLCBs) that differ in nature from those used for current production that focus on some of the world's highest concentration (Salar) brines and mineral ore assets (spodumene ores).11,12,17,18 Such differences in resource nature often mandate using alternative technologies over those used for current Li-chemical production. For instance, while solar evaporation is used for Salar brines due to their relatively higher Li-content (∼600–2000 ppm), direct lithium extraction (DLE) is used for LLCBs (≤200 ppm).18 Similarly, Li-chemical production from clays is expected to be less material- and energy-intensive than from spodumene ores because of the weaker chemical bonding of Li with other elements in clays.17
To understand the environmental implications of shifting Li-chemical supply from foreign conventional to U.S.-based alternative sources, it is important to consider all the relevant production steps in the Li-chemical life-cycle (from mining to processing and Li-chemical refining). Life-cycle analysis (LCA) is a tool that enables a detailed estimation of the environmental impacts of products across their complete life-cycle.19 LCA is used to identify the key impact drivers of each life-cycle stage, determine the reasons behind their contributions, evaluate the effectiveness of specific measures to reduce these impacts, and compare the performance of any product produced using different routes/precursors.19 This study uses LCA to evaluate the comparative life-cycle environmental impacts of Li-chemical production in the United States from alternative resources with their conventional counterparts. We also identify prominent contributors to the impacts of Li-chemicals from alternative sources and evaluate the effect of variation in influential parameters on their environmental performance.
1.2. Literature review
Several LCA studies on Li-chemical production have been published to date.8,20–27 However, almost all these studies focus on its production from conventional Salar brines and spodumene ores. Only a handful of studies discuss the impacts of their production from alternative sources like clays28 and LLCBs.29–32 Table S1 (ESI†) compiles these LCA studies, listing their Li-chemical produced (Li2CO3 or LiOH), major objectives, and key results.Per Table S1,† four studies focus on the LCA of Li-chemical production from LLCBs,29–32 while a lone study explores the environmental impacts of their production from clays.28 These studies use one or more of the following methods to determine the material and energy inputs for Li-chemical production from alternative sources:
(a) Up-scaling the inputs used for lab-scale production of Li-chemicals to their industrial-scale counterparts31
(b) Parametric (or other form of) modeling of industrial-scale Li-chemical production;29,30,32 or
(c) Use of data from company and/or academic literature.28–30
Further, these studies also consider the process carbon emissions produced during Li-chemical production from these alternative sources due to associated chemical reactions, e.g., from the reaction of iron sulfate (in LLCBs) with calcium carbonate (CaCO3).32
The studies listed in Table S1† have major gaps that need further investigation. First, the use of up-scaled inputs from lab-scale processing to industrial-scale production, as done in one study,31 may, in some cases, differ from the actual inputs used for commercial production, leading to some major differences in their life-cycle impacts.32,33 A shift from such lab-based estimates to more commercially relevant production data can help arrive at a more realistic estimation of the environmental performance of Li-chemical production from these upcoming sources.32,33 Another study30 reportedly uses input data based on a mix of commercial and academic sources, but the exact nature of sources used to arrive at this data is unclear. Two other studies use input data computed using models that account for several parameters and employ existing literature.29,32 However, these studies do not involve any of the entities that are currently engaged in efforts for commercial production of Li-chemicals from alternative sources.29,32 This can affect the impact results reported in these studies because while such models provide the technical input quantities needed for large-scale production of Li-chemicals, they may not consider scenarios that optimize the cost-effectiveness of such production. For instance, as discussed later in Section 2.1, some companies produce sulfuric acid (H2SO4) within their plant setup from the procured sulfur, as the process generates both acid (for leaching process) and steam (used to generate electricity for plant operations) for Li-chemical production from clays.13,14 Such steam-based electricity will have negligible impacts compared to the baseline grid electricity option for Li-chemical production, thereby affecting the chemicals' GHG profile. Yet, this aspect is not a point of consideration for the models used in the above-mentioned studies,29,32 likely due to the non-involvement of commercial entities engaged in this domain. The last study28 – the lone study based on clays – is the only study to involve commercial entities engaged in this domain and to state the entity explicitly, thus addressing the aforementioned issue(s). However, a comprehensive assessment of Li-chemical production in the U.S. also requires a similar analysis of such projects by other entities or states to provide a more robust comparison with conventional source-based Li-chemicals.
Additionally, the studies in Table S1† suffer from two other drawbacks. First, these studies focus only on one alternative resource type (LLCBs or clays); no study provides/compares the environmental performance of Li-chemical production from both sources with their conventional counterparts. Second, the studies in Table S1† confine themselves to the environmental impacts of Li-chemical production without framing the downstream impact that such production has on LIBs with different cathode chemistries. Only one study reports such analysis for one LIB chemistry (NMC811).32 Thus, these studies offer a limited perspective, given that LIBs are a vital decarbonization technology that needs Li-chemicals for their cathodes.
1.3. Relevance and contribution of this study
We address the aforementioned research gaps here via an LCA of Li-chemical production in the United States. Our study is the first to date to evaluate the environmental impacts of Li-chemical production in the U.S. from both clays and LLCBs. We develop inventory data for six projects from company reports13,14,34–36 – such as preliminary economic analysis (PEA) and pre-feasibility studies – and other literature.11,12 While these projects are yet to commence production, the companies' in-house analyses provide the expected material and energy inputs for commercial-scale Li-chemical production. The literature also highlights materials and energy sources that will be produced within the plant boundary for each project, enabling a more accurate characterization of their embodied impacts on the life-cycle impact calculations for Li-chemicals. We also conduct sensitivity analyses to study the effect of two parameters on these life-cycle impacts: (1) electric grid mix variation and (2) avoiding process carbon emissions associated with the Li-chemical production life-cycle. We then contextualize our LCA results for Li-chemical production from alternative sources by comparing them with the corresponding impacts of Li-chemical production from conventional sources (Salar brines and spodumene ores) and analyze their implications on the life-cycle environmental impacts of LIBs.2. Methods and analysis
2.1. System scope and process description
The primary goal of this paper is to estimate and analyze the life-cycle environmental impacts of Li-chemical production from alternative sources (clays and LLCBs) in the United States. We place these life-cycle results in context by comparing them with current commercial sources and by evaluating the impact of Li-chemical sourcing on the LCA results of LIBs. Our system boundary encompasses all the processes from raw material extraction to the final production of battery-grade Li-chemicals (Fig. 2(a and b)). Based on company reports and other literature, a brief description of the involved processes is given below (in subsections 2.1.1 and 2.1.2).11–14,34–36 Note that some projects produce battery-grade Li2CO3, while others produce battery-grade LiOH (as LiOH·H2O). To enable an appropriate understanding of the environmental impacts of Li-chemical production across different projects, we choose 1 kg of Li2CO3-equivalent (LCE) as the functional unit in this analysis. 1 kg of Li2CO3 equals 1 kg of LCE, while 1 kg of LiOH equals 1.543 kg of LCE (based on calculations considering the same number of moles of Li in LCE as in 1 kg of LiOH). Note that material inputs used for Li-chemical production from clays/LLCBs differ across various projects studied in this work, and Fig. 2(a and b) combines these inputs over these projects and lists them for each process. Also, energy inputs are listed separately as they are used across the different production processes (from mining to battery-grade Li-chemical production). Per company literature, clay-based projects only produce battery-grade Li2CO3, while LLCB-based projects produce both Li2CO3 and LiOH.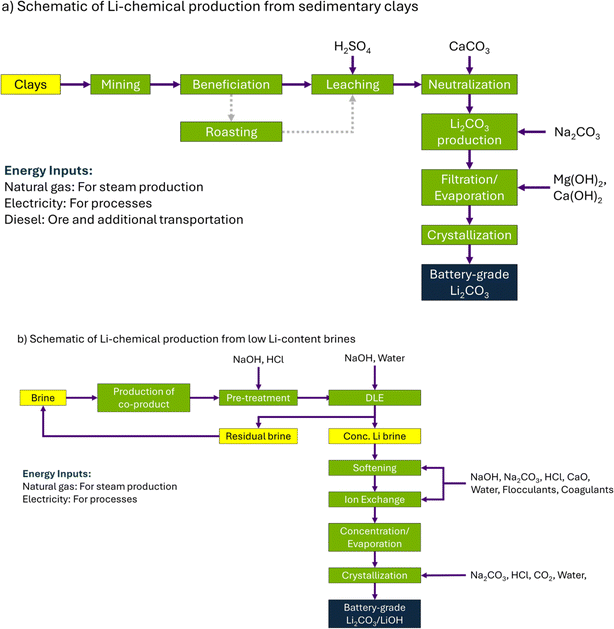 | ||
| Fig. 2 Generalized schematic of Li-chemical production from alternative sources: (a) sedimentary clays and (b) low Li-content brines. | ||
PLS is then neutralized using basic reagents – such as lime (CaO) and limestone (CaCO3) – and then reacted with soda ash (Na2CO3) to produce a lithium carbonate (Li2CO3) solution. This solution is filtered and evaporated to remove water and other impurity ions (mainly as sulfate salts) to increase its Li content.11–14 The residual solution is crystallized to produce battery-grade Li2CO3 or, alternatively, converted to battery-grade LiOH via a reaction with calcium hydroxide (Ca(OH)2). More details on the process schematic are provided in the literature.11–14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm), the solar evaporation process is not an economically viable route to produce Li-chemicals from the U.S. brines (LLCBs) due to their relatively lower Li-content (≤200 ppm).11,37 These LLCBs can be grouped into two categories. The first category comprises Li extraction from brines that are either currently being used to produce other materials or energy sources (such as bromine and magnesium chloride) or were being used for this purpose (e.g., crude oil).35 These brines have sufficient quantities of Li byproduct that can be extracted through additional processing. The second category comprises brines that are currently being examined for geothermal electricity generation but also contain Li (such as the LLCBs in the Salton Sea).37 LLCB-based U.S. projects across both categories expect to produce Li-chemicals using direct lithium extraction (DLE) – a group of technologies that can produce Li-chemicals without affecting the existing/likely production of other materials or energy sources.11,38,39 DLE technologies include precipitation, sorption, ion exchange, solvent extraction, electrochemical separation, and membrane separation, with more details on these technologies provided elsewhere.11,18,38,39 Also, LLCBs can be treated with DLE technologies either before or after the production of these other materials or energy sources to produce Li-chemicals.11,18,35,37–39Fig. 2(b) shows the general schematic of Li-production from LLCBs via the DLE route, with a brief description of this schematic given below based on the literature.11–14,34–36
000 ppm), the solar evaporation process is not an economically viable route to produce Li-chemicals from the U.S. brines (LLCBs) due to their relatively lower Li-content (≤200 ppm).11,37 These LLCBs can be grouped into two categories. The first category comprises Li extraction from brines that are either currently being used to produce other materials or energy sources (such as bromine and magnesium chloride) or were being used for this purpose (e.g., crude oil).35 These brines have sufficient quantities of Li byproduct that can be extracted through additional processing. The second category comprises brines that are currently being examined for geothermal electricity generation but also contain Li (such as the LLCBs in the Salton Sea).37 LLCB-based U.S. projects across both categories expect to produce Li-chemicals using direct lithium extraction (DLE) – a group of technologies that can produce Li-chemicals without affecting the existing/likely production of other materials or energy sources.11,38,39 DLE technologies include precipitation, sorption, ion exchange, solvent extraction, electrochemical separation, and membrane separation, with more details on these technologies provided elsewhere.11,18,38,39 Also, LLCBs can be treated with DLE technologies either before or after the production of these other materials or energy sources to produce Li-chemicals.11,18,35,37–39Fig. 2(b) shows the general schematic of Li-production from LLCBs via the DLE route, with a brief description of this schematic given below based on the literature.11–14,34–36
Briefly, brine is withdrawn from the reservoir and pre-treated with chemicals like caustic soda (NaOH) and hydrochloric acid (HCl). Subsequently, the brine is processed via DLE to produce two solutions: concentrated Li-brine for further processing and the residual brine.§ Concentrated Li-brine is sent for softening and ion exchange processes to remove impurities (such as Ca2+ and Mg2+ ions) and convert the brine into a pregnant stripping solution containing Li as lithium chloride (LiCl). LiCl is reacted with Na2CO3 to produce Li2CO3 or, alternatively, reacted with NaOH to produce LiOH. In either case, the Li-chemical obtained is subjected to evaporative crystallization to be converted to battery-grade purity levels.
2.2. Inventory and impact analysis
Across the U.S., several Li-chemical production projects are under different stages of exploration and production (Fig. 1), although no project has yet begun actual production. Of these, commercial-scale Li-chemical production inventory data from company reports and other literature is available for six projects.11–14,34–36 While these projects have not commenced production, their inventory is based on in-house modeling of commercial-scale production, which should be more robust than the approaches used in other Li LCA studies (listed in Table S1†).28–32 The two clay-based projects produce Li2CO3, while LLCB-based projects are split equally between Li2CO3 and LiOH (two projects each). Table 1 provides the inventory for battery-grade Li-chemical production for all six projects, based on the functional unit (1 kg of LCE). More information on these projects is provided in Section 2 of the ESI† and in the prior literature published by the authors on this subject.11,12| Parameter | Material/energy source | Project identification & output | Unit | |||||
|---|---|---|---|---|---|---|---|---|
| Output: Li2CO3 | Output: LiOH | |||||||
| P1 | P2 | P3 | P4 | P5 | P6 | |||
| a kg = Kilogram; MJ = Megajoules, *Based on in-house calculations (more details are provided in Section 3 of ESI), flocculants and clarifier polymers are used mainly as thickening agents to ensure that colloidal particles generated during processes get collected and can be easily separated from the remaining clay minerals for further processing to produce the final Li-chemical. Projects are as follows: P1: Thacker Pass, NV, P2: Tonopah Lithium, NV, P3: Ogden East, UT, P4: Smackover Arkansas, AR, P5: Ogden West, UT, P6: SW (South-West) Arkansas, AR. | ||||||||
| Asset type | Clay | LLCB | LLCB | |||||
| Output quantity | LCE | 1 | 1 | 1 | 1 | 1 | 1 | kg |
| Input materials | Lime (CaO) | 2.10 | 4.65 | 0.10 | 0.94 | 0.02 | kg | |
| Limestone (CaCO3) | 6.52 | 4.16 | kg | |||||
| Soda ash (Na2CO3) | 3.71 | 1.68 | 1.67 | 2.13 | 1.37 | 0.88 | kg | |
| Hydrochloric acid (HCl) | 0.01 | 0.07 | 0.92 | 0.07 | 0.02 | kg | ||
| Caustic soda (NaOH) | 0.05 | 0.69 | 0.58 | 0.55 | 0.71 | kg | ||
| Flocculant+ | 0.07 | 0.03 | kg | |||||
| Sulfur (S) | 9.81 | 17.60 | kg | |||||
| Clarifier polymer+ | 0.05 | 0.04 | kg | |||||
| Citric acid | 0.03 | kg | ||||||
| Sodium metabisulfite | 0.08 | kg | ||||||
| Nitrogen (N2) | 0.08 | kg | ||||||
| Water | 0.10 | 147.40 | 2.58 | 0.76 | kg | |||
| Input energy | Natural gas | 127.23 | 130.51 | 17.59 | 184.05 | 1.46 | MJ | |
| Diesel | 9.51 | MJ | ||||||
| Gasoline | 0.16 | MJ | ||||||
| Propane | 0.78 | MJ | ||||||
| Electricity(self-generated) | 22.23 | 77.50 | MJ | |||||
| Electricity (grid) | 38.03 | 7.20 | 44.67 | 7.07 | 58.97 | MJ | ||
| Output emissions | CO2* | 2.87 | 1.83 | kg | ||||
To calculate the life-cycle impacts of Li-chemical production in the U.S., we use Argonne National Laboratory's R&D GREET® 2023 (Greenhouse gases, Regulated Emissions, and Energy use in Technologies) model (hereafter referred to as GREET).40 GREET contains an extensive inventory database for materials and energy sources, including those used to produce Li-chemicals. It also contains the inventory and life-cycle impacts of commercial-scale Li-chemical production from conventional sources (Salar brines and spodumene ores) and LIBs with various cathode chemistries produced using these Li-chemicals.40 Salar brine-based Li-chemical production considers data from SQM, while spodumene ore-based production involves the mining of these ores in Australia and their subsequent processing in China per data from a Chinese company.8 Hence, we use GREET to accomplish our objectives, with our impact of focus being GHG emissions.40 GREET also computes other impacts beyond GHG emissions, such as water footprint, particulate emissions (PM10 and PM2.5), and other emissions (such as SOX and NOX). We acknowledge that these impact indicators can be important to consider, but this study focuses only on GHGs.¶
A number of assumptions are made in this study. The first assumption concerns the flocculant and clarifier polymer used in Li-chemical production from alternative sources (Table 1). Since the exact nature of these materials used in various projects is unknown, we use the fuel-cell Nafion dry polymer and average polymer mix in the GREET model40 as their respective substitutes. Further, the six projects studied here are located across the U.S. and use electricity from both the nearby electric grid and via self-generation (Fig. 1 and Table 1). For grid-sourced electricity, we consider the electric grid mix of the North American Electric Reliability Corporation (NERC) region corresponding to the project's location. Section 4 of the ESI† provides more details on the states constituting the different NERC regions within the U.S., the corresponding NERC region for each project studied here (per the project location), and the respective grid mix of each NERC region. The combination of in-house generated electricity and NERC grid mix usage, as discussed in the company literature for each of the projects, represents the baseline scenario for this study.
Clay-based projects (P1 and P2) produce their leaching agent (H2SO4) in-house from externally procured sulfur and use the steam produced in this process to generate electricity for Li-chemical production.13,14 These projects claim that the electricity produced from this steam is carbon-free, i.e., it has no GHG impacts.13,14 We assume this claim to be true, i.e., zero GHG impacts for in-house production of H2SO4 and subsequent electricity generation from the produced steam, and consider only the embodied impacts of the sulfur used or procured. This assumption is based on the nature of H2SO4 production from sulfur – a three-step process – that involves: (a) combustion of sulfur to produce sulfur dioxide (SO2); (b) conversion of SO2 to sulfur trioxide (SO3); and (c) absorption of SO3 in water to produce H2SO4.13,14 Since all these three steps are exothermic reactions, the energy generated as steam can be used to produce electricity.13,14 Further, per the PEA studies of clay-based projects,13,14 the electricity generated from this process is sufficient to provide the initial amount of energy needed for all three steps associated with H2SO4 production once it begins, thus avoiding the need for external electricity procurement for this process in subsequent years/time. Last, we assume that H2SO4 production does not generate any other process emissions, which is in line with the inventory for its production in GREET.40
We compare our life-cycle impact results for Li-chemical production from alternative sources with those from conventional counterparts (Salar brines and spodumene ores), and we extend that analysis to evaluate the life-cycle impacts of LIBs that use these Li-chemicals. Five cathode chemistries are included in this analysis: nickel-manganese-cobalt (NMC – 111, 532, 622, and 811)|| and lithium iron phosphate (LFP or LiFePO4). Section 5 of the ESI† provides details on the material composition (bill of materials) of these LIBs.
We also conduct sensitivity analyses to evaluate the effect of two specific parameters on the life-cycle impacts of Li-chemicals from alternative sources from the perspective of its decarbonization. These parameters are: (a) electric grid mix, and (b) avoiding process carbon emissions during the production of Li-chemicals and upstream materials.
Electric grid mix is reported to have a substantial influence on the environmental impacts of different materials and technologies,41–46 given the substantial carbon emissions associated with electricity generation from fossil fuels. We consider two scenarios for the electric grid mix in this analysis:
(a) Switching from a mix of in-house generated and NERC grid mix for clay-based projects to a 100% NERC grid mix for these projects; and
(b) Substituting the current grid mix to 100% renewables-based electricity for all projects.
Apart from electricity, this analysis involves significant non-combustion process carbon emissions that are generated through chemical reactions from two sources. These sources are:
• The neutralization step associated with clay-based Li-chemical production via reaction between CaCO3 and H2SO4; and.
• Embodied impacts of upstream production of materials used for Li-chemical production, namely, CaO (formed via combustion of limestone, which in turn generates CO2) and Na2CO3 (produced from the decomposition of sodium bicarbonate or NaHCO3), per GREET.40
A potential way to reduce these impacts is by capturing the non-combustion process emissions generated from the aforementioned sources using carbon capture and sequestration (CCS) technologies. We assess this decarbonization potential for Li-chemicals by applying CCS for both sources of process carbon emissions.
Note that our analysis is not meant to compare the environmental impacts of Li-chemical production across different U.S.-based projects, given the difference in the nature of their respective Li-resource base and the preliminary nature of their commercial-scale engineering analyses. Our aim here is rather to understand the environmental performance of Li-chemical production across these projects and to discuss the effect of specific measures toward improving that performance. Also, we do not consider any temporal changes in the electric grid mix over time for simplicity. However, we acknowledge that such changes will affect real-life Li-chemical production plants that will operate for several years over a changing electric grid, which in turn will influence their production-related environmental impacts.
Lastly, while we use 1 kg of LCE (lithium carbonate equivalent) as the functional unit in this study, we do not intend to compare the life-cycle impacts of Li2CO3 and LiOH production. This is because the purpose of Li-chemical production is for use in LIBs, and the two Li-chemicals – Li2CO3 and LiOH – are used for differing battery chemistries. Li2CO3 is used in four of the five LIB chemistries considered in this study (NMC – 111, 532, and 622; and LFP), while LiOH is used in the remaining chemistry (NMC811). Since the end-use of these two Li-chemicals is different, their environmental impacts are not comparable. However, LCA studies in the literature typically use LCE as a common functional unit to understand the environmental impacts of Li-chemicals,28–32 and the same practice has been adopted in this study.
3. Results
3.1. Life-cycle impacts: U.S. projects
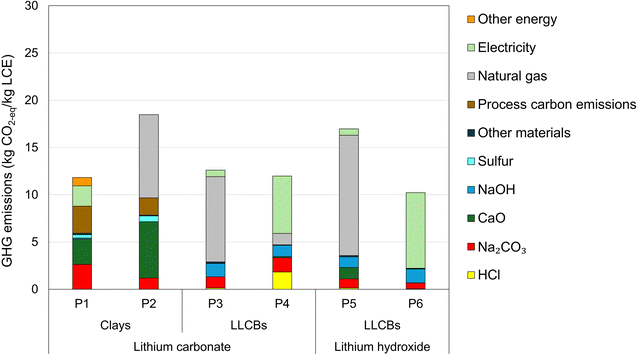
Two energy inputs (electricity and natural gas), four material inputs (Na2CO3, CaO, NaOH, and HCl), and process carbon emissions dominate the life-cycle GHG emissions of Li-chemical production from alternative sources, as shown in Fig. 3. This dominance arises from a mix of significant use of inputs in various Li-chemical production steps, the high carbon intensities of these inputs, and the considerable process carbon emissions generated via chemical reactions during Li-chemical production.For clay-based projects (P1 and P2), Li2CO3 is the final product in the studies investigated here.13,14 For these projects, two input materials (CaO and Na2CO3), energy inputs (electricity and diesel for P1 and natural gas for P2), and process carbon emissions during Li-chemical production are major contributors to life-cycle GHG emissions of Li-chemicals (Fig. 3). Na2CO3 is used to produce Li2CO3 through reaction with Li-salts in clays, while CaO removes impurities, especially Mg2+ ions.13,14 For P1, GHG emissions are dominated by electricity as it accounts for a large majority of the project's energy needs (Table 1).13 P2 consumes a large amount of natural gas (Table 1) to dry Li2CO3 and remove moisture, with residual energy needs met through in-house electricity generation.14 Finally, the neutralization reaction associated with clay-based production – namely, the reaction of H2SO4 with CaCO3 – generates a substantial amount of carbon emissions (discussed in Table 1 here and Section 3 of the ESI†). These process carbon emissions account for ∼10–25% of the life-cycle GHG impacts of clay-based Li-chemical production (Fig. 3).
LLCB-based projects investigated here produce both Li2CO3 (P3 and P4) and LiOH (P5 and P6). Regardless of their final product, energy sources (natural gas and electricity) account for the vast majority (60–80%) of the life-cycle GHG impacts of these projects (Fig. 3). While natural gas is used in all projects (Table 1), it dominates the GHG impacts for P3 and P5 (Fig. 3). The PEA report for P3 and P5 projects suggests the possible use of natural gas to produce electricity within the plant setup without any accompanying details.34,36,47 The life-cycle GHG impacts for P4 and P6 projects are dominated by electricity (Fig. 3).
Apart from energy sources, four materials are influential to the life-cycle impacts of LLCB-based Li-chemicals: NaOH, HCl, Na2CO3, and CaO (Fig. 3). These chemicals are used for one or more of the following processes:
(a) Brine pretreatment: HCl and NaOH are used to reduce and increase the brine's pH value, respectively.
(b) Chemical softening and ion exchange: One or more of these materials are used to remove impurities from brines (e.g., Ca2+ and Mg2+ ions) and/or to regenerate ion exchange resins.
(c) Li-chemical production: Na2CO3 reacts with Li-salts to produce Li2CO3 (for P3 and P4).
The aforementioned materials contribute substantially to the life-cycle GHG impacts of Li-chemicals because of two reasons per GREET.40 The first reason is the highly energy-intensive nature of the respective production of these materials. The second reason is the high process carbon emissions generated via chemical reactions during the production of specific materials (CaO and Na2CO3).
3.2. Life-cycle impacts: comparison with conventional sources
To place our impact results for Li-chemicals from alternative sources in context, we compare them with the life-cycle GHG impacts of Li-chemical production from conventional sources (Salar brines and spodumene ores) in GREET.40 Per GREET, Li-chemicals are produced from Salar brines entirely in Chile, while spodumene ores are mined in Australia and then processed in China, as mentioned earlier.8,40Fig. 4(a and b) shows the life-cycle GHG emissions for Li-chemical production from all sources.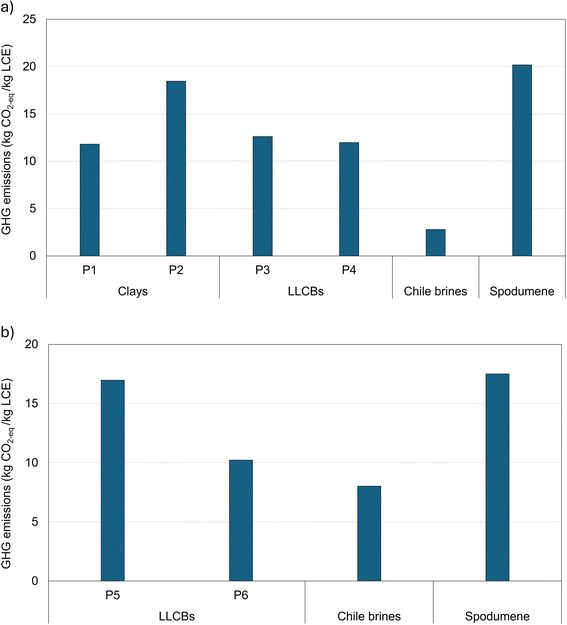 | ||
| Fig. 4 Comparative life-cycle GHG impacts of Li-chemical production from alternative sources with those from conventional counterparts for (a) Li2CO3 production, (b) LiOH production. | ||
Life-cycle GHG emissions for the investigated U.S.-based Li-chemical production lie between the impacts of its production from Salar brines and that from spodumene ores for both Li2CO3 and LiOH (Fig. 4). Except for P6, the life-cycle impacts of Li-chemicals from alternative sources are substantially higher than those from their Salar brine counterpart (∼2–6.5 times that of Salar brine-based Li-chemicals; Fig. 4). For P6, the life-cycle GHG emissions are only ∼30% higher than that for Salar brine-based Li-chemicals (Fig. 4). In contrast, the life-cycle impacts of alternative source-based Li-chemicals are comparable to impacts for their production from spodumene ores (3–60% lower; Fig. 4).
One primary reason for the low GHG impacts of Li-chemical production from Chilean brines is its very low fossil energy usage during the Li concentration phase, with a major share of this phase's energy needs met using solar energy.8,17,40 Conversely, spodumene-based Li-chemicals are extracted by employing energy-intensive processes with significant chemical inputs to release it from its tightly bonded ore.8,17,40 Simultaneously, the gap in impacts of Li-chemical production from alternative sources and spodumene ores is much lower than the corresponding gap in impacts from Salar brines and alternative sources (Fig. 4). This indicates that the bond strength of Li with other elements in clays is closer to that in spodumene ores and significantly higher than that in Salar brines.
3.3. Life-cycle impacts: effects on battery impacts
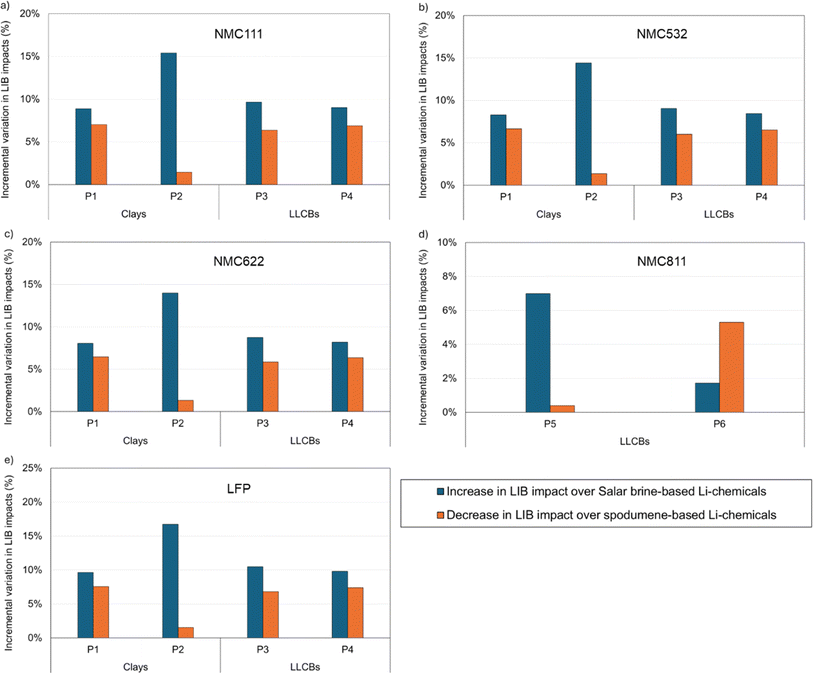
Since LIBs use Li-chemicals in their cathodes, a change in Li-chemical production source merits an investigation into the effect of this shift on the life-cycle impacts of LIBs. We investigate this for five LIB cathode chemistries: NMC (111, 532, 622, and 811) and LFP (produced via solid-state processing), using GREET in tandem with the GREET Battery Module.48 While GREET has an extensive inventory database for LIBs and their constituents to compute the battery's environmental impacts, the GREET Battery Module offers a detailed breakdown of these impacts by contributions from different materials and energy sources.Fig. 5(a–e) provides the incremental variation in life-cycle impacts of various LIB chemistries upon shifting Li-chemical sourcing from conventional sources (Salar brines and spodumene ores) to their alternative counterparts located in the U.S. Separately, Fig. S3 (ESI†) provides the life-cycle impacts of these LIB chemistries using Li-chemicals from different sources, with a breakdown of impact contributions from Li-chemicals and other sources (material and energy contributors). Table S6† also provides the life-cycle GHG impacts of LIBs when sourcing Li-chemicals for their cathodes from conventional sources. Note that four chemistries in Fig. 5 (NMC – 111, 532, and 622; and LFP) use Li2CO3 for cathode production, while one (NMC811) uses LiOH. For all cathode chemistries, the life-cycle GHG emissions of LIBs using Li-chemicals from alternative sources show an intermediate rank in between the corresponding emissions for LIBs based on Li-chemicals from Salar brines and from spodumene ores (Fig. 5). This is in line with the relative order of life-cycle GHG impacts for Li-chemical production from these sources (Fig. 4).
Barring a few exceptions, the shift in Li-chemical production from conventional sources (Salar brines and spodumene ores) to their alternative counterparts (clays and LLCBs) alters the life-cycle impacts of LIBs by ≥5% (Fig. 5). The exceptions include P2 over spodumene for Li2CO3, P5 over spodumene for LiOH, and P6 over Salar brines for LiOH sourcing (Fig. 5). Further, LIB chemistries that show larger increases in life-cycle GHG impacts upon sourcing Li-chemicals from alternative sources instead of Salar brines also exhibit smaller decreases in LIB impacts when compared to using Li-chemicals from spodumene ores, independent of the final Li-chemical used (Fig. 5).
3.4. Sensitivity analyses
Two energy sources (electricity and natural gas), process carbon emissions, and specific materials dominate the life-cycle GHG emissions for U.S.-based Li-chemical production (Fig. 3). To understand how changes in parameters associated with these major impact contributors influence the life-cycle impacts of Li-chemical production from alternative sources, we conduct sensitivity analyses on two parameters. These are: (a) electricity source; and (b) avoiding process CO2 emissions while producing Li-chemicals and/or materials used for its production.(a) Switching from in-house generated electricity to a fully NERC-specific grid for clay-based projects (Scenario 1); and
(b) Switching from the baseline grid to 100% renewables-based electricity for all projects (Scenario 2).
Note that in the baseline scenario, in-house electricity is generated in clay-based projects using steam produced from the sulfuric acid plant within the overall plant setup.14,49 If this electricity is replaced by the external (NERC) grid mix, it is unlikely that sulfur will be procured to produce the sulfuric acid in-house, as the associated steam generated in this process may not get used for any purpose, and, thus, may be lost as waste. Hence, the aforementioned Scenario 1 will likely result in the direct use of externally produced H2SO4 for Li-chemical production. However, here our focus is only on evaluating the effect of the change in electricity source on the life-cycle GHG impacts of Li-chemical production from alternative sources. Hence, we do not consider any change in H2SO4 production from the baseline scenario to Scenario 1. Lastly, we consider Scenario 2, given the influential role of electricity in the life-cycle GHG impacts of Li-chemical production across different U.S. projects in the baseline scenario (Fig. 3). This scenario helps to evaluate the environmental benefits of using clean electricity/energy for Li-chemical production in the U.S.
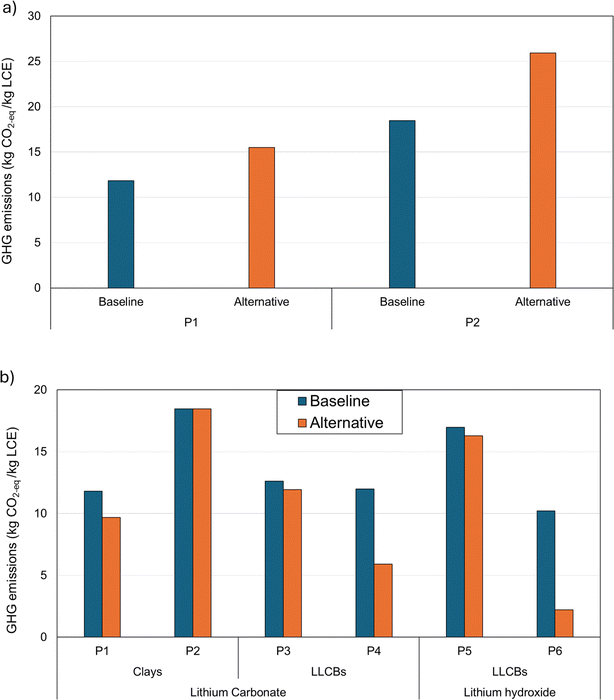
Fig. 6(a) shows the change in the life-cycle GHG impacts of Li-chemical production under Scenario 1 (for clay-based projects P1 and P2). Here, the change in grid mix from self-generated to NERC grid electricity significantly increases the life-cycle impacts of clay-based Li-chemicals (by ∼30–40%; Fig. 6(a)). In the baseline scenario, P1 and P2 meet a part or all of their electricity demand from in-house electricity generation using steam from the H2SO4 production plant. This electricity is assumed to be 100% carbon-free in this study as it is produced from steam, which is a byproduct of the exothermic H2SO4 production process. Hence, shifting to the NERC grid (which also uses fossil fuels) from such in-house electricity will lead to an increase in the life-cycle GHG impacts in this scenario (Fig. 6(a)).
Fig. 6(b) shows the result of switching to 100% renewables-based electricity on the life-cycle GHG impacts of Li-chemical projects in the U.S. Three projects (P1, P4, and P6) show significant GHG impact reductions (∼15–75%) upon switching to renewables-based electricity from the baseline grid. Since electricity accounts for a considerable share of process energy use and life-cycle GHG impacts of Li-chemical production from these projects (Table 1 and Fig. 3), the use of renewable energy for such production facilitates sizeable impact reductions (Fig. 6(b)). Conversely, other projects show a negligible or marginal change in impacts (∼0–6%) due to the shift in Scenario 2 (Fig. 6(b)). For P3 and P5, this result stems from the relatively small role of electricity in the baseline life-cycle impacts of Li-chemical production (Fig. 3). For P2, as explained above, the in-house generated electricity is assumed to be 100% carbon-free. Hence, any shift from this form of electricity to renewable energy will have an insignificant effect on the life-cycle impacts of Li-chemical production (Fig. 6(b)) due to negligible differences in their respective carbon emission profiles.
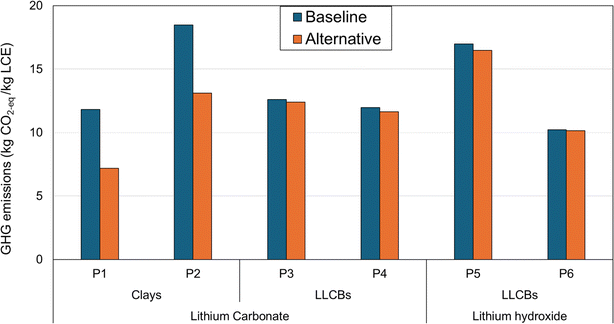
Substantial reductions (∼30–40%) are observed in the life-cycle impacts of Li-chemical production from clay-based projects (P1 and P2) upon using CCS for the production of Li-chemicals, CaO, and Na2CO3 (Fig. 7). This is a consequence of a prominent chunk of combined contributions from these materials/process emissions to the life-cycle impacts of Li-chemicals in the baseline scenario (∼50–70%; Fig. 3). Conversely, LLCB-based projects do not involve any process carbon emission generation during Li-chemical production, and CaO and Na2CO3 together comprise a much smaller share of the life-cycle impacts of Li-chemicals (∼5–15%; Fig. 3). This leads to a marginal decrease in these impacts (< 5%) on switching to CCS-based CaO and Na2CO3 for Li-chemical production (Fig. 7).
4. Discussion
4.1. Comparative performance: our study v/s literature
Table 2 shows our life-cycle GHG results for Li-chemical production from alternative sources with those from studies listed in Table S1,† highlighting that at a broader level, the two sets of impacts are comparable. For clay-based Li-chemical production, our impacts are in line with that in the literature, with the difference in the higher range of impacts (16.6 in our study v/s 25.4 in the literature) due to the use of energy-intensive roasting for Li-chemical production in the literature versus its non-use here.28 For DLE-based projects, a wide variation is observed in the life-cycle GHG impacts of Li-chemical production in the literature, especially vis-à-vis our results in this work. This can be ascribed to the variation in the electricity sources used for Li-chemical production (geothermal, solar, natural gas, and grid-based electricity in different countries)30,32 and/or the use of scaled-up inventory from lab-scale production to commercial-scale.29,31 Additionally, the divergence in our results with the literature can be ascribed to differences in the inventory used for analysis. Some studies use a slightly older inventory for the same projects as ours, and the differences in associated inventories cause variation in the final impacts. For instance, our inventory for P1 considers a higher consumption of Na2CO3 and sulfur (by 30–100%) based on a more recent PEA study13 than that used in the literature28 based on an earlier company report.| Source | Li-chemical produced | Study | GHG impacts (kg CO2-eq/kg LCE) | Notes |
|---|---|---|---|---|
| Clays | Li2CO3 | Our results | 8.9–16.6 | |
| 28 | 8.4–25.4 | The lower value is for the acid leaching route (used here), while the higher value is for roasting route | ||
| Low Li-content brines (DLE) | Li2CO3 | Our results | 12–12.6 | |
| 30 | 7.6–22.0 | Values depend on the nature of the energy source used (solar + grid mix, 100% grid mix, and 100% diesel) | ||
| 31 | 1.2 | |||
| 29 | 15.1 | |||
| 32 | 5.3–46 | For geothermal brines in Germany | ||
| 18–59 | For geothermal brines in the U.S. | |||
| LiOH | Our results | 15.8–26.2 | ||
| 31 | 3.4 |
4.2. Comparative performance of U.S.-based production with existing production
Typically, the life-cycle GHG impacts of Li-chemical production from different sources are expected to be related to the strength of the chemical bond of Li with other elements/minerals in these sources.17 Such bonding is the weakest for brines (regardless of their Li-content) and the strongest for spodumene ores, with clays showing an intermediate bond strength.17 A weak bond strength reduces the energy needed to separate Li from source minerals and produce Li-chemicals, lowering the life-cycle impacts of its production and vice versa.17 Hence, the expected order of life-cycle GHG impacts of Li-chemical production from the sources considered in this analysis is: brines < clays < spodumene ores, i.e., brines and spodumene ores show the lowest and the highest life-cycle impacts, respectively. Our results for clay-based projects conform to this expected trend (Fig. 3), with the life-cycle GHG impacts of Li-chemical production from these projects being in between the corresponding impacts of Li-chemicals from Salar brines and spodumene ores.Our analysis also considers two brine types: high Li-content Salar brines and LLCBs in the U.S. High-Li content Salar brines employ solar evaporation to increase the brine's Li-content, which leads to a lower life-cycle GHG footprint for the Li-chemical produced.50 In contrast, the DLE route employed for LLCBs requires energy – mainly natural gas and electricity – that increases the GHG footprint of Li-chemicals that employ typical U.S. electrical grids (Table 1 and Fig. 3).50 This explains the higher life-cycle GHG impacts of Li-chemical production from DLE-based LLCBs than from Salar brines, despite the weak Li-bonding with other elements in both brine types (Fig. 4). Simultaneously, the lower life-cycle GHG footprint of Li-chemical production from LLCBs over that from spodumene ores (Fig. 4) can be ascribed to the relatively weaker bond strength of Li with other elements in these brines.
An outright comparison of our Li-chemical production impacts from clays and LLCBs is beyond the scope of this study, given the preliminary nature of such clay assets in the commercial production of Li-chemicals. Nevertheless, based on the above-mentioned logic about the strength of Li-bond with other elements in these sources, we can expect clay-based production to have higher life-cycle impacts. Interestingly, in the baseline scenario, of all the projects that produce Li2CO3, one clay project (P2) adheres to this trend by exhibiting higher GHG impacts than LLCB-based projects (P3 and P4), while the other clay project (P1) shows lower impacts (Fig. 3). Further, a switch from in-house generated electricity to the NERC grid mix for clay-based Li2CO3 production (P1 and P2) increases its life-cycle impacts beyond that of DLE-based Li2CO3 (for both P1 and P2) and spodumene-based Li2CO3 (for P2 only) (see Fig. 3, 4, and 6(a)).
Overall, our impact results are broadly in line with the expected trends for Li-chemical production from different sources based on the strength of Li-bonding with other elements in these sources. At the same time, our results indicate that it is possible for Li-chemical production from certain projects to violate this expected trend for a number of reasons. These include:
(a) Project-specific factors, such as the avoidance of natural gas for P1 and the use of in-house generated electricity, that cause the impacts of Li2CO3 production to be lower than that of DLE-based production (Fig. 3); and
(b) Nascent nature of Li-chemical production from clays and LLCBs, which can result in a non-accounting of specific impacts that may be revealed in the future.
Such conditions must be considered while understanding the reasons for variations in results from the expected trend. These variations also represent opportunities to reduce the GHG impacts of Li-chemical production from sources that are otherwise expected to be more GHG-intensive per the expected trend.
4.3. Measures for impact reductions
Two factors emerge as critical to reducing the life-cycle GHG impacts of Li-chemical production from alternative sources:(a) Use of renewables-based electricity and/or in-house generated electricity from carbon-free sources (such as from steam produced in H2SO4 plants for clay-based projects13,14);
(b) Utilization of specific processing materials that are produced via low-carbon methods, including CCS.
The importance of these factors is compounded by a third factor: the relevance of these different contributors towards the baseline life-cycle impacts of Li-chemical production in the U.S.
The significance of avoiding the use of fossil fuels and/or substituting them with renewable energy constitutes a key pillar of global decarbonization strategies aimed at a wide range of technologies,41 sectors,51 and materials.44,52 For projects in this study on which electricity constitutes a prominent chunk of the life-cycle impacts of Li-chemical production (P1, P4, and P6; Fig. 3), our Scenario 2 results in Section 3.4.1 confirm the benefits of using renewables-based electricity to decarbonize this production (Fig. 6(b)). The increasing incorporation of renewable energy across all the NERC grid regions within the U.S. – a trend expected to continue in the future53 – will be pivotal to realizing the potential GHG reductions observed in the sensitivity analysis in Scenario 2 in Section 3.4.1.
Apart from energy sources, specific materials and processes are known to contribute substantially to the life-cycle impacts of various energy-related materials, products, and technologies. In this study, such substantive contributions to the life-cycle impacts of Li-chemicals come in part from process carbon emissions generated during the production of Li2CO3 from clays (during the neutralization step) and from specific inputs (CaO and Na2CO3) used for Li-chemical production (Fig. 3). Carbon capture and sequestration (CCS) technologies are often highlighted as an important tool to capture these process carbon emissions towards accomplishing the global aim of net-zero GHG emissions.51 Our work shows a sizeable reduction in life-cycle GHG emissions of clay-based Li-chemical production via the use of CCS for both Li2CO3 production and upstream CaO and Na2CO3 production (Fig. 7). This result highlights the potential of CCS in decarbonizing Li-chemical production from alternative sources on a life-cycle basis, bolstering its position in the global efforts for a decarbonized world. However, while CCS is critical to global deep decarbonization and also provides the option for the use of carbon to produce other chemicals, the high costs of this technology impede its widespread application, including for concentrated CO2 streams.54 A combination of specific policies and regulations, dedicated private investment, and technological advancements is critical for a significant scale-up and adoption of CCS technologies across different sectors, including for the processes related to clay-based Li-chemical production in this study.54 Such policies and advancements are also needed to keep in check the cost of Li-chemical production from alternative resources and make them economically competitive vis-à-vis Li-chemical production from their conventional resource counterparts.
In addition to renewable energy usage and avoidance of process carbon emissions, another major contributor to the life-cycle impacts of Li-chemical production is natural gas (primarily for projects P2, P3, and P5; Fig. 3). For P2, natural gas is used to dry Li2CO3,14 while for P3 and P5, the exact use of natural gas remains unspecified, although the PEA study for these projects mentions that natural gas is converted to steam.36 Thus, natural gas is likely used in P3 and P5 projects either for heating or for drying processes associated with Li-chemical production. In recent years, attempts to decarbonize the industrial sector have focused on the electrification of processes that have conventionally used fossil fuels, with the electricity used for such processes based on renewable energy.44–46 Such processes also include heating, suggesting that it may be possible to substitute natural gas for Li-chemical production with electricity. If achieved, this would offer a third prominent measure towards a decarbonized production of Li-chemicals from alternative sources in the U.S., provided the electricity used is based on renewable energy only.
Overall, our study highlights the benefits of a decarbonized electric grid mix and the use of carbon capture technologies in improving the environmental outcomes of alternative resource-based Li-chemical production. The combined application of these two measures can significantly reduce the life-cycle GHG impacts of Li-chemicals (by ∼60–80%) across all the projects studied here (results are shown in Fig. S4,† ESI†). If achieved, these impact reductions will make Li-chemical production from alternative resources environmentally competitive or superior to its production from conventional sources (Salar brines and spodumene ores; Fig. S4†). However, it should be noted that such initiatives could also benefit the traditional production routes toward their own decarbonization.
Lastly, our study suggests an important benefit of domestically sourced U.S.-based Li-chemicals. Switching from spodumene-based to U.S.-based Li-chemicals can establish a reliable supply chain of these LIB materials that also reduce the battery's GHG intensity. This is subject to the condition that Li-chemical production parameters for U.S.-based sources are in line with those provided in the company literature and used for this study.
4.4. Limitations of this study
Given the nascent state of commercial Li-chemical production from clays and LLCBs – both in the U.S. and globally – it is important to consider the limitations that influence the impact results in this study.First, we use production inventory per company literature based on their in-house modeling.13,14,34–36 However, the projects considered here are yet to begin commercial production, and it is possible that the material and energy inputs used for actual production vary from those provided in the company reports (and used here for analysis). These variations, especially for major GHG impact contributors that are highlighted in Section 3.1 (Fig. 3), can affect both the life-cycle impacts of Li-chemicals from alternative sources and their comparative performance with conventional source-based counterparts.
A second important limitation is the lack of data on non-combustion process emissions during the production of Li-chemicals from U.S.-based sources for any of the projects studied here, either in company literature13,14,34–36 or in the prior LCA studies published on this subject (listed in Table S1†). Although we have considered the process carbon emissions from the neutralization reaction step of Li-chemical production from clays, this may not be the sole source of carbon emissions for this production route. For LLCBs, no process emissions are considered here due to the lack of data, even though GREET highlights multiple process emissions during Li-chemical production from Salar brines that contribute towards GHG impacts (such as VOC and CO). The possible omission of such emissions means that our life-cycle GHG impact results for such Li-chemicals may be somewhat underestimated. Nevertheless, our results are still the most comprehensive impact values on this subject to date in light of the available inventory.
Finally, a key requirement of process-based LCA studies is the availability of a step-by-step LCI that provides a comprehensive understanding of material flows across different process steps to produce the concerned material. Given the lack of such data for individual projects in the company literature,13,14,34–36 as well as the use of specific materials in multiple steps (e.g., NaOH for brine pre-treatment, chemical softening, and ion exchange for DLE-based brines), we are unable to provide such a flowchart for Li-chemical production in this study. Future studies based on commercial Li-chemical production will hopefully address this research gap and offer a detailed analysis of the distribution of its life-cycle impacts across different steps, and the additional measures needed to lower these impacts beyond those offered in this study.
5. Conclusions
We used LCA to assess the environmental impacts of commercial-scale production of Li-chemicals from clays and low Li-content brines (LLCBs) in the United States – alternative resource types that are vital to ensuring their future robust supply chain towards national decarbonization. We used the material and energy inputs from company reports and other literature to develop a process-based inventory for six U.S. projects under development (two from clays and four from LLCBs). The Li-chemicals here refer to battery-grade lithium carbonate (Li2CO3) and lithium hydroxide (LiOH). We also compared our impact results with those for Li-chemical production from conventional sources (Salar brines and spodumene ores) to study the environmental implications of shifting Li-chemical supply from present-day external sources to U.S.-based sources in the future. Further, we evaluated the resultant effect of this shift on the life-cycle impacts of lithium-ion batteries (LIBs) – as the Li-chemical is finally used to produce LIB cathodes – as well as of variation in influential parameters on impacts of alternative source-based Li-chemicals.Two energy sources (natural gas and electricity) and two materials (Na2CO3 and CaO) dominate the life-cycle greenhouse gas (GHG) impacts of Li-chemical production across different alternative resource types and Li-chemicals. Additionally, HCl and NaOH are influential on the GHG impacts of Li-chemical production from LLCBs, while process carbon emissions play a similar role for clay-based Li-chemical production. These impacts stem from a combination of factors, including the: (a) use of fossil fuels (directly as natural gas; indirectly as electricity); (b) sizeable use of material and energy inputs; and (c) considerable non-combustion process carbon emissions generated via chemical reactions during the production of Li2CO3 (from clays) and of upstream material impact contributors to Li-chemicals (CaO and Na2CO3). On a comparative basis, Li-chemical production from alternative sources has lower GHG impacts than spodumene-based Li-chemicals but higher than the corresponding impacts of Salar brine-based Li-chemicals. This trend is in line with the respective order of bonding strength of Li with other elements in these different sources. The life-cycle impacts of LIBs employing Li-chemicals from different production sources show a similar trend. Our results are also in line with the limited literature on this subject.
Overall, our study highlights the benefits of switching to renewables-based electricity over energy from fossil fuels and of using carbon capture and sequestration (CCS) technologies to avoid process emissions while producing Li-chemicals and upstream process materials. Also, our work indicates that a switch from spodumene-based Li-chemicals to their alternative source-based counterparts can help decarbonize LIBs and associated sectors of application. Further GHG reductions in Li-chemical production from alternative sources, particularly with respect to Salar brine-based counterparts, will necessitate the complete decarbonization of all other energy and process materials used during the production of Li-chemicals. At the same time, the cost implications of such decarbonization measures, especially of using CCS technologies, will have to be considered to ensure the commercial viability of Li-chemical production from U.S.-based alternative resources.
Abbreviations
| AR | Arkansas |
| Ca | Calcium |
| CaCO3 | Calcium carbonate (limestone) |
| Ca(OH)2 | Calcium hydroxide |
| CaO | Calcium oxide (lime) |
| CCS | Carbon capture and sequestration |
| CO2 | Carbon dioxide |
| Cs | Cesium |
| DLE | Direct lithium extraction |
| GHG | Greenhouse gas |
| GREET | Greenhouse gases, regulated emissions, and energy use in technologies |
| HCl | Hydrochloric acid |
| IRA | Inflation Reduction Act |
| LCA | Life-cycle analysis |
| LCI | Life-cycle inventory |
| Li | Lithium |
| Li2CO3 | Lithium carbonate |
| Li-chemical | Lithium chemical |
| LiCl | Lithium chloride |
| LiOH | Lithium hydroxide |
| LFP (LiFePO4) | Lithium iron phosphate |
| LIB | Lithium-ion battery |
| LLCB | Low Li-content brines |
| Mg | Magnesium |
| Mg(OH)2 | Magnesium hydroxide |
| NV | Nevada |
| NMC | Nickel manganese cobalt |
| N2 | Nitrogen |
| NOX | Nitrogen oxides |
| NERC | North American Electric Reliability Corporation |
| PM10 | Particulate matter (size < 10 microns) |
| PM2.5 | Particulate matter (size < 2.5 microns) |
| ppm | Parts per million |
| K | Potassium |
| PEA | Preliminary economic analysis |
| R&D | Research and development |
| Rb | Rubidium |
| Na | Sodium |
| NaHCO3 | Sodium bicarbonate |
| Na2CO3 | Sodium carbonate (soda ash) |
| NaOH | Sodium hydroxide (caustic soda) |
| SW | South-west |
| S | Sulfur |
| SO2 | Sulfur dioxide |
| SOX | Sulfur oxides |
| SO3 | Sulfur trioxide |
| H2SO4 | Sulfuric acid |
| SDG | Sustainable Development Goal |
| U.S. | United States |
| UT | Utah |
Data availability
The data supporting this article is either provided within this manuscript, the associated ESI document,† or in the R&D GREET 2023 model (available at: https://greet.anl.gov/) and associated literature (referred to in the manuscript). Impact calculations are based on the R&D GREET 2023 model.Author contributions
Rakesh Krishnamoorthy Iyer: conceptualization, methodology, investigation, data curation, visualization, formal analysis, writing – original draft, writing – review & editing. Jarod C. Kelly: conceptualization, methodology, data curation, visualization, funding acquisition, supervision, writing – review & editing.Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
This research effort was supported by the Vehicle Technologies Office (VTO), Office of Energy Efficiency and Renewable Energy (EERE), United States Department of Energy (U.S. DOE) under Contract Number DE-AC02-06CH11357. The authors would like to thank Steven Boyd and Brian Cunningham of that Office for their guidance and support. The authors would also like to thank Ramsharan Pandey and Siddharth Shukla of Argonne National Laboratory for providing their comments on this manuscript and support on the GREET Battery Module, respectively. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the U.S. Government or any agency thereof. Neither the U.S. Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights.References
- FCAB, National Blueprint for Lithium Batteries 2021-2030, Washington D.C., 2021 Search PubMed.
- C. Zhang, X. Zhao, R. Sacchi and F. You, Nat. Commun., 2023, 14, 1616 CrossRef CAS PubMed.
- L. Usai, J. J. Lamb, E. Hertwich, O. S. Burheim and A. H. Strømman, Environ. Res.: Infrastruct. Sustainability, 2022, 2, 011002 CAS.
- D. J. Bauer, R. T. Nguyen and B. J. Smith, Critical Materials Assessment 2023, Washington D.C., 2023 Search PubMed.
- J. Fleischmann, M. Hanicke, E. Horetsky, D. Ibrahim, S. Jautelat, M. Linder, P. Schaufuss, L. Torscht and A. van de Rijt, Battery 2030: Resilient, sustainable, and circular, 2023 Search PubMed.
- T. Igogo, America's Strategy to Secure the Supply Chain for a Robust Clean Energy Transition, 2022 Search PubMed.
- A. Miatto, B. K. Reck, J. West and T. E. Graedel, Resour., Conserv. Recycl., 2020, 162, 105034 CrossRef.
- J. C. Kelly, M. Wang, Q. Dai and O. Winjobi, Resour., Conserv. Recycl., 2021, 174, 105762 CrossRef CAS.
- USC, Inflation Reduction Act of 2022, 2022 Search PubMed.
- J. A. [D-K.-3] Yarmuth, Text – H. R.5376 – 117th Congress (2021–2022): Inflation Reduction Act of 2022, U. S. Congress, United States of America, 2022 Search PubMed.
- R. K. Iyer and J. C. Kelly, Lithium Production from North American Brines, Argonne, IL (United States), 2022 Search PubMed.
- R. K. Iyer and J. C. Kelly, Lithium Production in North America: A Review, Argonne, IL (United States), 2023 Search PubMed.
- D. Roth, L. Tahija, E. Iasillo, K. Martina, B. Chow, W. Mutler, K. Bahe, P. Kaplan, T. Cluff and B. Shannon, Lithium Americas: Feasibility Study - National Instrument 43-101 Technical Report for the Thacker Pass Project, 2022 Search PubMed.
- DRA Pacific, Tonopah Lithium Claims Project NI 43-101 Technical Report – Preliminary Economic Assessment, Perth, 2023 Search PubMed.
- S. B. Castor and C. D. Henry, Minerals, 2020, 10, 68 CrossRef CAS.
- Y. Cui, H. Wen, Z. Zhou, K. Ling, L. Xu, S. Liu and F. Xu, Acta Geochim., 2024, 43, 478–488 CrossRef CAS.
- A. Grant, The Sedimentary Lithium Opportunity, https://www.jadecove.com/research/, accessed 8 July 2022.
- J. Farahbakhsh, F. Arshadi, Z. Mofidi, M. Mohseni-Dargah, C. Kök, M. Assefi, A. Soozanipour, M. Zargar, M. Asadnia, Y. Boroumand, V. Presser and A. Razmjou, Desalination, 2024, 575, 117249 CrossRef CAS.
- S. Hellweg, L. M. i Canals, L. Milà i Canals and L. M. i Canals, Science, 2014, 344, 1109–1113 CrossRef CAS PubMed.
- A. Stamp, D. J. Lang and P. A. Wäger, J. Cleaner Prod., 2012, 23, 104–112 CrossRef CAS.
- M. Chordia, S. Wickerts, A. Nordelöf and R. Arvidsson, Resour., Conserv. Recycl., 2022, 187, 106634 CrossRef CAS.
- R. T. Halkes, A. Hughes, F. Wall, E. Petavratzi, R. Pell and J. J. Lindsay, Resour., Conserv. Recycl., 2024, 207, 107554 CrossRef CAS.
- V. Schenker, C. Oberschelp and S. Pfister, Carbon footprint of current and future lithium carbonate production in South America, https://www.bbc.com/future/article/20201124-how-geothermal-lithium-could-revolutionise-green-energy, accessed 23 May 2024.
- A. Di Maria, Z. Elghoul and K. Van Acker, Procedia CIRP, 2022, 105, 672–677 CrossRef.
- S. Khakmardan, M. Rolinck, F. Cerdas, C. Herrmann, D. Giurco, R. Crawford and W. Li, Procedia CIRP, 2023, 116, 606–611 CrossRef.
- A. Mas-Fons, R. Horta Arduin, P. Loubet, T. Pereira, A. M. Parvez and G. Sonnemann, J. Cleaner Prod., 2024, 452, 142108 CrossRef CAS.
- S. Jiang, L. Zhang, F. Li, H. Hua, X. Liu, Z. Yuan and H. Wu, J. Environ. Manage., 2020, 262, 110253 CrossRef CAS PubMed.
- V. Roy, M. P. Paranthaman and F. Zhao, Lithium from Clay: Assessing the Environmental Impacts of Extraction, SSRN, preprint, 2024, DOI:10.2139/ssrn.4782093.
- J. Bonk, Ex-Ante Comparative Life Cycle Assessment of Membrane Distillation-Crystallization for Lithium, Master thesis, Utrecht University, 2022, https://studenttheses.uu.nl/bitstream/handle/20.500.12932/43165/Final%20Draft%20Master%20Thesis_John%20Patrick%20Bonk_%202930136.pdf?sequence=1 Search PubMed.
- S. Mousavinezhad, S. Nili, A. Fahimi and E. Vahidi, Resour., Conserv. Recycl., 2024, 205, 107583 CrossRef CAS.
- T. Y. Huang, J. R. Pérez-Cardona, F. Zhao, J. W. Sutherland and M. P. Paranthaman, ACS Sustain. Chem. Eng., 2021, 9, 6551–6560 CrossRef CAS.
- V. Schenker, P. Bayer, C. Oberschelp and S. Pfister, Renewable Sustainable Energy Rev., 2024, 199, 114456 CrossRef CAS.
- V. Goldberg, T. Kluge and F. Nitschke, Grundwasser, 2022, 27, 239–259 CrossRef CAS.
- F. Gay, M. Dworzanowski, R. M. Brush, R. E. Williams, C. Mutschler, D. Johnson and C. Campbell, NI 43-101 Technical Report South West Arkansas Project Pre-feasibility Study, Lewisville, 2023 Search PubMed.
- R. M. Brush, C. Daniel, F. Gay, S. B. Patton, M. Rockandel and R. E. Williams, NI 43-101 Technical Report for the Definitive Feasibility Study for Commercial Lithium Extraction Plant at LANXESS South Plant, Vancouver, 2023 Search PubMed.
- Compass Minerals, Technical Report Summary Updated Initial Assessment Lithium and LCE Mineral Resource Estimate, Ogden, 2022 Search PubMed.
- W. T. Stringfellow and P. F. Dobson, Energies, 2021, 14, 6805 CrossRef CAS.
- M. Lee, W. Jung, H. J. Kwon and G. Lim, J. Mater. Chem. A, 2022, 10, 4621–4633 RSC.
- M. R. Mojid, K. J. Lee and J. You, Sustainable Mater. Technol., 2024, 40, e00923 CrossRef CAS.
- M. Wang, A. Elgowainy, U. Lee, K. H. Baek, S. Balchandani, P. T. Benavides, A. Burnham, H. Cai, P. Chen, Y. Gan, U. R. Gracida-Alvarez, T. R. Hawkins, T.-Y. Huang, R. K. Iyer, S. Kar, J. C. Kelly, T. Kim, C. P. Kolodziej, K. Lee, X. Liu, Z. Lu, F. H. Masum, M. Morales, C. Ng, L. Ou, T. K. Poddar, K. Reddi, S. Shukla, U. Singh, L. Sun, P. Sun, P. Vyawahare and J. Zhang, Summary of Expansions and Updates in R&D GREET® 2023, Argonne, IL (United States), 2023 Search PubMed.
- R. K. Iyer and S. Pilla, Sci. Total Environ., 2021, 752, 141674 CrossRef CAS PubMed.
- R. K. Iyer, Thermoelectrics: Ecological Profile and Fundamental Principles for Sustainability, Dissertation thesis, Clemson University, 2020, https://www.proquest.com/openview/987f42a362fddaacba1f2382e747dad5/1?cbl=44156&pq-origsite=gscholar&parentSessionId=0msv488Q1CQ8IIUjWyL2%2BT3DWTpG8RUgj921tsvtQ1c%3D Search PubMed.
- A. B. Kousaalya, R. K. Iyer and S. Pilla, SAE Int. J. Sustain. Transp. Energy, Environ. Policy, 2020, 1, 39–57 Search PubMed.
- C. Y. Loh, R. Huang, R. Bell and M. Xie, RSC Sustainability, 2023, 1, 2287–2295 RSC.
- U. Sultan, K. Städtke, A. Göpfert, D. Lemmen, E. Metwali, S. Maiti, C. Schlumberger, T. Yokosawa, B. Apeleo Zubiri, E. Spiecker, N. Vogel, T. Unruh, M. Thommes and A. Inayat, RSC Sustainability, 2023, 1, 1449–1461 RSC.
- B. Davies, J. A. Llamas-Orozco, F. Meng, I. D. Posen, H. L. MacLean, A. F. N. Abdul-Manan and J. McKechnie, RSC Sustainability, 2024, 2(8), 2275–2288 RSC.
- Respec Company LLC, Technical Report Summary for the Tonopah Flats Lithium Project, Esmeralda and Nye Counties, Nevada, USA, Reno, 2023 Search PubMed.
- S. Shukla, T. Sykora, J. C. Kelly and H. Cai, GREET Battery Module: Beta Version, Argonne, IL, 2023 Search PubMed.
- Lithium Americas, Thacker Pass, https://www.lithiumamericas.com/usa/thacker-pass/, accessed 25 April 2023.
- M. L. Vera, W. R. Torres, C. I. Galli, A. Chagnes and V. Flexer, Nat. Rev. Earth Environ., 2023, 43(4), 149–165 CrossRef.
- IEA, Net Zero by 2050 – A Roadmap for the Global Energy Sector, 2021 Search PubMed.
- S. M. Kernaghan, T. Coady, M. Kinsella and C. M. Lennon, RSC Sustainability, 2024, 2, 578–607 RSC.
- US EIA, Annual Energy Outlook 2023, Washington DC, 2023 Search PubMed.
- IEA, Energy Technology Perspectives 2020: Special Report on Carbon Capture Utilisation and Storage, 2020 Search PubMed.
- IPCC, Sixth Assessment Report — IPCC, 2023 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00446a |
| ‡ Battery-grade LiOH refers to lithium hydroxide monohydrate (LiOH·H2O), but for this manuscript, we simplify all impact analysis in terms of anhydrous LiOH production. |
| § The residual brine is reinjected back to the reservoirs either after processing it further to produce other materials or energy sources or, alternatively, without doing so if these materials/energy sources have already been produced from it. |
| ¶ GHG impacts in this analysis are computed using the life-cycle emissions of different greenhouse gases and characterization factors of these gases from the GREET model.40 While the emissions are calculated within the GREET model, characterization factors are based on the Sixth Assessment Report (AR6) of the Intergovernmental Panel on Climate Change (IPCC).55 |
| || The chemical formula of different NMC chemistries is as follows: (a) NMC111 – LiNi1/3Mn1/3Co1/3O2; (b) NMC532 – LiNi0.5Mn0.3Co0.2O2; (c) NMC622 – LiNi0.6Mn0.2Co0.2O2; and (d) NMC811 – LiNi0.8Mn0.1Co0.1O2. |
| This journal is © The Royal Society of Chemistry 2024 |