Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Liquid–liquid phase transition as a basis for novel materials for skin repair and regeneration
Shunfeng
Wang†
 a,
Meik
Neufurth†
a,
Meik
Neufurth†
 a,
Hadrian
Schepler
b,
Rafael
Muñoz-Espí
a,
Hadrian
Schepler
b,
Rafael
Muñoz-Espí
 c,
Hiroshi
Ushijima
d,
Heinz C.
Schröder
a,
Xiaohong
Wang
c,
Hiroshi
Ushijima
d,
Heinz C.
Schröder
a,
Xiaohong
Wang
 *a and
Werner E. G.
Müller
*a and
Werner E. G.
Müller
 *a
*a
aERC Advanced Investigator Grant Research Group at the Institute for Physiological Chemistry, University Medical Center of the Johannes Gutenberg University, Duesbergweg 6, 55128 Mainz, Germany. E-mail: wang013@uni-mainz.de; wmueller@uni-mainz.de
bDepartment of Dermatology, University Medical Center of the Johannes Gutenberg University, Langenbeckstraße 1, 55131 Mainz, Germany
cInstitute of Materials Science (ICMUV), Universitat de València, C/Catedràtic José Beltrán 2, 46980 Paterna-València, Spain
dNihon University, Division of Microbiology, Department of Pathology and Microbiology, Nihon University-School of Medicine, Tokyo, Japan
First published on 29th August 2024
Abstract
Inorganic materials are of increasing interest not only for bone repair but also for other applications in regenerative medicine. In this study, the combined effects of energy-providing, regeneratively active inorganic polyphosphate (polyP) and also morphogenetically active pearl powder on wound healing were investigated. Aragonite, the mineralic constituent of pearl nacre and thermodynamically unstable form of crystalline calcium carbonate, was found to be converted into a soluble state in the presence of a Ca2+-containing wound exudate, particularly upon addition of sodium polyP (Na-polyP), driven by the transfer of Ca2+ ions from aragonite to polyP, leading to liquid–liquid phase separation to form an aqueous Ca-polyP coacervate. This process is further enhanced in the presence of Ca-polyP nanoparticles (Ca-polyP-NP). Kinetic studies revealed that the coacervation of polyP and nacre aragonite in wound exudate is a very rapid process that results in the formation of a stronger gel with a porous structure compared to polyP alone. Coacervate formation, enabled by phase transition of crystalline aragonite in the presence of Na-polyP/Ca-polyP-NP and wound exudate, could also be demonstrated in a hydroxyethyl cellulose-based hydrogel used for wound treatment. Furthermore, it is shown that Na-polyP/Ca-polyP-NP together with nacre aragonite strongly enhances the proliferation of mesenchymal stem cells and promotes microtube formation in the in vitro angiogenesis assay with HUVEC endothelial cells. The latter effect was confirmed by gene expression studies, applying real-time polymerase chain reaction, using the biomarker genes VEGF (vascular endothelial growth factor) and hypoxia-inducible factor-1 α (HIF-1α). Division of Escherichia coli is suppressed when suspended in a matrix containing Na-polyP/Ca-polyP-NP and aragonite. The potential medical relevance of these findings is supported by an animal study on genetically engineered diabetic mice (db/db), which demonstrated a marked increase in granulation tissue and microvessel formation in regenerating experimental wounds treated with Ca-polyP-NP compared to controls. Co-administration of aragonite significantly accelerated the wound healing-promoting effect of polyP in db/db mice. Based on these results, we propose that the ability of polyP to form a mixed coacervate with aragonite, in addition to its energy (ATP)-generating function, can decisively contribute to the regenerative activity of this polymer in wound repair.
1. Introduction
Advanced products for skin repair and regeneration make use of the whole range of skin protective materials available for the treatment of acute, critical and chronic wounds, from materials used for treatment of venous leg ulcers, diabetic foot ulcers, pressure ulcers, burns and post-operative wounds.1 They also include regeneratively active skin creams that reinforce the protective potency of the skin, the largest organ of the body, against external influences, microorganisms, fungi and viruses. Their aim is in particular to prevent and act as a protective barrier against external pathogens from entering the body via the bloodstream. The physiological, innate immune system of the skin eliminates most of these pathogens and prevents the human body from cutaneous and systemic infections.2 The regenerative potential of advanced skin repair products prevents superficial wounds from developing into chronic wounds or even chronic skin ulcers. The healing process in these progressed clinical pictures is disrupted in patients suffering from venous stasis, diabetes mellitus or other chronic insufficiencies.Advanced products for skin repair meet these requirements at least partially due to their active constituents, such as anti-inflammatory, moisturizing and cleansing ingredients. However, the formulations applied usually lack compounds that provide the cells with a metabolic fuel that can substitute the deficiency of energy in the extracellular space and body fluids. Certainly, the energy status of the skin is a prominent factor in skin defense,3 and more specifically, it is the mitochondria in particular that play an essential role in regulating the ATP homeostasis in skin physiology.4 This nucleotide is considered the most important intracellular energy-providing metabolite due to its two high-energy anhydride bonds and is the driving force for exergonic reactions. After enzymatic cleavage, 30.5 kJ mol−1 of energy is released per energy-rich bond in the ATP molecule.5 It is important to stress that the intracellular ATP pool is high at 3–10 mM, while the extracellular ATP levels are only ≈10 nM.6,7 However, in every living organism and certainly also in humans,8 there is another high-energy molecule in addition to ATP, namely inorganic polyphosphate (polyP).9,10 Importantly, while ATP only has two high-energy bonds, polyP with a physiological chain length of ≈100 Pi (phosphate) units11 contains a much larger number of energy-rich phosphoanhydride linkages. On a molar base, the energy density in this polymer is 50 times higher than that in ATP.12 The highest concentration of polyP in humans and other mammalian organisms is found in the blood platelets, encapsulated in the dense granules of these cell fragments in addition to large amounts of ADP and ATP as well as pyrophosphate.13,14
The polyP in platelets serves as ATP generator. The free energy stepwise released during the successive enzymatic degradation of the polymer with alkaline phosphatase (ALP) is first stored in ADP, followed by the up-phosphorylation of ADP to ATP by adenylate kinase (ADK).12 These two enzymes are exposed on the outer cell surface.15 It is also noteworthy that polyP not only functions as an ATP generator extracellularly, but also positively affects intracellular ATP generation. After entry into the cells, probably coupled to ion transport,16 polyP causes a significant accumulation of mitochondria,15 resulting in an increased ATP formation through the mitochondrial FoF1-ATP synthase system.17
The property of polyP to act as a generator for the metabolic fuel ATP is enhanced by its property to transform into a coacervate phase (Fig. 1) in the presence of divalent cations such as Ca2+ or Mg2+, whereby polyP and the cations undergo a liquid–liquid phase separation with a denser polyP-rich phase and a phase rich in Ca2+ or Mg2+.18,19 Liquid–liquid phase separation in a polymer-rich and polymer-poor phase has been considered as the second step during complex coacervation.20,21 In the coacervate stage, the polymers can be exploited for their inherent biological potency.22 Furthermore, during the process of coacervation, microbial cells can be enveloped and ultimately killed.23 A final remarkable property of polyP is that the polymer can stabilize the amorphous phase of Ca-carbonate24 and can thereby preserve the biological/osteogenic activity of Ca-carbonate even in its nanoparticulate form.25
The morphogenetic, regeneration-activating activity of polyP is well established26–28 and has been proven in a proof-of-concept study even for human application.23,29 For the Ca-polyP used, it is evident that the Ca2+ ions are essential for regeneration.30 As outlined above, the polyP backbone with its high-energy phosphoanhydride bonds provides the metabolic energy source for any cell-based regeneration process.12
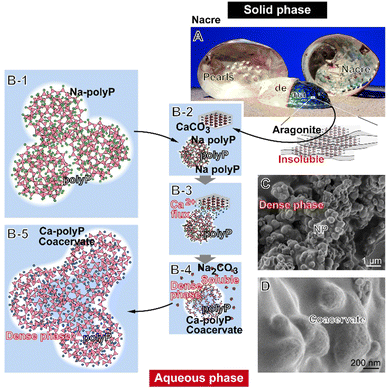
In addition to its property to stabilize amorphous Ca-carbonate and decelerate the crystallization process from amorphous to crystalline Ca-carbonate, polyP also enhances the beneficial effect of calcium on wound healing.31 In the amorphous state, the Ca2+ ions promote the healing of skin wounds, especially chronic wounds, by activating the proliferation, differentiation and migration of keratinocytes as well as the proliferation and collagen deposition of fibroblasts.32,33 Furthermore, preliminary data suggest that carbonate also promotes wound healing.34 Carbonate can be released from the less stable form of crystalline Ca-carbonate, from aragonite, which subsequently buffers the systems.35 Previously, it was reported that soluble Na-carbonate is able to form spherical coacervate droplets with a size of ≈200 nm in the presence of Ca2+.36 In turn, we postulated that aragonite, as the thermodynamically unstable phase of crystalline Ca-carbonate, could also form coacervate deposits like Ca-polyP. A biomaterial that consists of >95% aragonite is nacre/pearls, which is formed by freshwater bivalve molluscs such as from the hybrid species (Hyriopsis schlegeli/Hyriopsis cumingi) as an inner shell layer.37–39 For biomedical application, the formulation as pearl powder is frequently used.40 It is interesting and suggestive that the nacre (Fig. 1(A)), the smooth outer shell surfaces, can serve as a platform for mineralization of nanoscale amorphous and crystalline Ca-carbonate mineral precursors.41 Pearl medicine has a history of over 2000 years in both Europe and Asia, and pearls are used in Europe in therapy of skin sufferings, as mentioned by Vallés,42 as medical cleansing agents [de compositione medimecatorum expurgantium] for treatment of skin diseases [podest nunc exmodo morbi]. An even wider application is reported from China, in which the powder is used, for example, to calm the mind, clear the eyes or detoxify organs.43
The aim of the present study was to combine the energy-generating and regeneratively active potential of polyP with the inherent morphogenetic activities of pearl powder.44 This mineralic material, in the nanoparticle (NP) form, stimulates human mesenchymal stem cells (MSC), promotes osteogenic differentiation in vitro by inducing the ALP activity and causes an upregulation of genes. Moreover, pearl powder implanted into the dermis of rats causes an activation of skin fibroblasts, in parallel with an enhanced extracellular matrix synthesis and the organization of the filamentous structures and, associated with this, the formation a dense network rich in collagen I and collagen III.25,45 Slices of pearl powder, seeded with MSC start to secrete ALP in an extent that exceeds the level induced by bone morphogenetic protein (BMP-2) treatment.46
Originally, it was reported that activated platelets release polyP with a chain length of 60–100 Pi residues, which initiate the blood clotting contact pathway.47 However, later it was argued to the contrary that this effect is not relevant.48 The technical difficulty is that polyP in the circulating blood is enzymatically hydrolyzed by ALP, which shortens the chain lengths of the polymer.49 It has been reported that very long polyP molecules (>500 Pi units), such as those found in microorganisms, interfere with the blood clotting cascade, while shorter polymers do not exhibit this property.50 It is most likely that polyP is released from the platelets either as Na-polyP or as Ca-polyP-NP51 due to the high Ca2+ level in the platelets.52,53 The Ca-polyP-NP with the divalent metal ions remain on the surface of the platelets as insoluble spherical nanoparticles. For chemical synthesis of Ca-polyP, a sintering procedure is usually used, which usually ends up in the crystalline form of the material.54 Likewise, the second component mixed with polyP, the nacreous aragonite, exists in the crystalline aragonite phase.37
However, in order to exploit the biomedical potential of the basically solid aragonite with its excellent strength and toughness for bio-medical skin repair, such as in acute and chronic wounds, the powder must be converted into a soluble phase. In this state, pearl powder exhibits distinct wound healing activity by promoting cell migration and inducing the expression of type III collagen.55
The concept, which has been experimentally proven, is outlined here and is achieved by immersing the crystalline aragonite in a nonbuffered Na-polyP solution as sketched (Fig. 1). Solid aragonite (Fig. 1(A)) is converted into the soluble form upon immersion together with Na-polyP (Fig. 1(B-1)), a soluble and weakly basic salt that dissociates into Na+ cations and polyP anions.56 In medium/serum, the Ca2+ ions from aragonite (Ca-carbonate) are then chelated by Na-polyP, resulting in the conversion of the solid phase into an aqueous Ca-polyP coacervate (Fig. 1(B-2)–(B-5)). If the incubation system contains Ca-polyP-NP, which are characterized by a high zeta potential,18 the coacervate formation is amplified due to the conversion of the fairly indissoluble NP into a soluble state. It can be assumed that such a process also occurs in the presence of body fluids such as sweat and wound secretions, which are rich in peptides/proteins and Ca2+,57 components that affect and lower the high zeta potential18 of the indissoluble polyP particles [dense phase] (Fig. 1(C)) and transform them into the more fluid, aqueous coacervate (Fig. 1(D)).
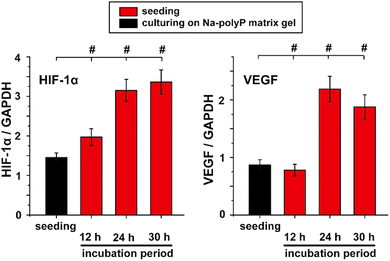
In the present contribution, this concept of producing a co-coacervate from calcareous nacre of pearls and Na-polyP is experimentally supported and it is shown that the mixed coacervate phases (Ca-carbonate/Na-polyP) accelerate the proliferation of MSC and trigger the subsequent organization of these cells for microtube formation, the onset of blood vessel formation. These results are further supported by reverse transcription-quantitative real-time polymerase chain reaction (RT-qPCR) using the biomarker genes VEGF (vascular endothelial growth factor) and hypoxia-inducible factor-1α (HIF-1α). The VEGF is a pivotal proangiogenic regulator of angiogenesis,58 while HIF-1α is the oxygen-regulated subunit that determines HIF-1 activity.59 Both genes are strongly expressed during angiogenesis.59
2. Experimental section
2.1. Components
Crushed powdered pearls (aragonite) were purchased from Dura Green (Nanjing; China); the particles had a size from 3 to 10 μm. The freshwater mussel hybrid species (Hyriopsis schlegeli/Hyriopsis cumingi) has been used to obtain the pearls; they were cultured in the pearl farm in Zhuji (Zhejinag, China). There, a rectangular piece of mantle tissue was routinely prepared from the interior of their shells and reinserted into the mantle epithelium, where a pearl sac is formed and initiates the deposition of the aragonite structure of the shell.60 The shape of the pearls is caused by the shape of the inserted implant.Na-polyphosphate (Na-polyP) with an average chain length of 40 Pi units was purchased from Chemische Fabrik Budenheim (Budenheim; Germany).
2.2. Preparation of Ca-polyP-NP
Amorphous Ca-polyP nanoparticles (Ca-polyP-NP) were prepared from Na-polyP and CaCl2 (#A537.1; Roth, Karlsruhe; Germany) as described.61 A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight ratio of both components was used as start concentration.
3 weight ratio of both components was used as start concentration.
2.3. Application forms
PolyP (either Na-polyP or Ca-polyP-NP) with or without pearl powder was applied to the system either directly or supplemented with a hydrogel or wound exudate removed from human chronic wounds.29 The samples were collected in accordance with the rules of the declaration of Helsinki. In addition to these recommendations, the consent of the patients has been granted. The respective weight ratio is given with the corresponding experiments. If not mentioned otherwise, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio between polyP and aragonite (pearl powder) was used.
1 weight ratio between polyP and aragonite (pearl powder) was used.
2.4. Hydrogel-based gel formulation
The formulation of the polyP-containing hydrogel was prepared as outlined before.23 The hydrogel consisted of a 2% (wt/wt) hydroxyethyl cellulose (Caelo, Hilden; Germany) solution prepared with 10% (wt/wt) 1,2-propandiol (#2554; Caelo). The system was buffered with phosphate buffer to pH 6.5. If indicated, a polyP suspension (200 mg of Na-polyP and 20 mg of Ca-polyP-NP per 10 mL) was prepared and added to the hydrogel. The final formulation contained 600 μg mL−1 of Na-polyP and 60 μg mL−1 of Ca-polyP-NP.2.5. Analytical methods
2.6. Cell biological studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt/wt). After termination, the cell proliferation was assessed microscopically by staining with Calcein AM (#223506; Sigma) and visualized microscopically.65
1 (wt/wt). After termination, the cell proliferation was assessed microscopically by staining with Calcein AM (#223506; Sigma) and visualized microscopically.65
A quantitative determination was achieved by staining with the cell viability reagent PrestoBlue (Life Technologies, Dreieich; Germany) at the end of the incubation; the relative fluorescence units (RFU) were determined at 570 nm and for reference at the wavelength of 595 nm.66 Ten parallel experiments were performed; data are means ± SD (P < 0.01).
In another series of experiments to determine the effect of the hydrogel without or with supplements on cell morphology, 300 μL MSC cultures, in the middle of exponential growth phase (150 × 103 cells mL−1), were added to 100 μL of wound exudate and 100 μL of hydrogel either containing no polyP or aragonite or supplemented with the polyP suspension (final concentration in the assay 120 μg mL−1 of Na-polyP) together with aragonite (final concentration: 120 μg mL−1). The system was buffered with a phosphate buffer to pH 6.5. The cultures were incubated for 12 h; then the cells were freeze-died and inspected by SEM.
The bacterial suspensions were centrifuged at 1700 × g for 10 min (Centrifuge 5810R, Eppendorf, Germany) to separate bacterial pellets. After removing the supernatant, the pellets were rinsed in 3 mL of distilled water (Bio-Rad Laboratories, United States), followed by a second centrifugation under the same conditions. Five to 10 μL of condensed bacterial suspensions were collected.
The condensed bacterial suspension was applied to the surface of a 15 mm × 35 mm Silicon (Si) wafer (Siltronix, France) and dried under a biosafety hood. Dried spots of bacterial deposits with a typical thickness of 5–15 μm were obtained depending on the bacterial concentration after rinsing.
Optical density (OD600) measurements were performed to determine the effect of the polyP/aragonite coacervate on the growth of E. coli. Bactericidal kinetics were measured at an optical density (OD) of 600 nm. E. coli [∼106 cfu mL−1] suspended in 5 mL bacterial culture were shaken overnight at 30 °C. Aliquots of 100 μL were removed at different time intervals and the respective densities were measured.69
For the amplification of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene, the forward primer GTCTCCTCTGACTTCAACAGCG and the reverse primer ACCACCCTGTTGCTGTAGCCAA were selected.73 The annealing temperature was 62 °C. The amplifications were performed in an iCycler (Bio-Rad, Hercules, CA, USA) with the respective iCycler software. Finally the Ct values of the expressions of the respective transcripts were calculated.74
2.8. Animal experiments
Details comprising the animal studies have been given earlier.75
2.9. Microscopic analysis
The high resolution SEM images were taken with a Zeiss Gemini 1530 (Zeiss Oberkochem; Germany). For low resolution SEM studies an ESEM XL-30 machine (Philips, Eindhoven; Netherlands) was used. The samples were freeze-dried prior to inspection. In the SEM-ESEM experiments the samples were coated with gold.2.10. Statistical analysis
For statistical analyses, the Student's t test or the Mann–Whitney U-test were applied. Accordingly, the GraphPad Prism 7.0 software (GraphPad, La Jolla, CA, USA) was used.3. Results and discussion
3.1. Coacervation of crushed powdered pearls by polyP
The complex coacervate technology, based on interactions of oppositely charged polyelectrolytes in aqueous solution, has been successfully applied for drug delivery.77–79 Formulations prepared by complex coacervation have the advantage that the active molecules are completely surrounded by the enveloping coacervate film. PolyP coacervates can be formed from polyP even in the presence of divalent cations such as Ca2+.18,19 The advantage of the polyP-based coacervate is that it is stable in a wide pH range from pH 5 (healthy individuals) to even the alkaline range with pH 8.19 Covering the alkaline pH range is particularly important for the treatment of chronic wounds.80,81 A further distinguished property of polyP is its high binding affinity to histidine-rich peptides, which control the organization of supramolecular biomaterials, especially those that bind to inorganic matrices,82,83 implying that polyP-based coacervates are suitable for binding of aragonite calcareous nacre.The finding that polyP and polyP coacervate cause a transformation of solid Ca-carbonate (crystalline aragonite) into a phase that is likewise soluble (Fig. 1) is used in nature to self-assemble mineralic carbonate crystallites. This principle allows the formation of strong composite materials with filigree morphology, such as those used for construction of mollusk shells.37 Conversely, in the present contribution the polyP polymer is used to re-convert the crystalline aragonite into a soluble phase. During this process, polyP acts as an anionic polymer, like the aspartic acid rich proteins in shells,37 and as a platform for the phase transition of the crystalline aragonite plates.84
As thoroughly documented,37,38,85 mussel shells are formed from crystalline aragonite, a metastable phase formed during the crystallization steps to stable calcite.86 Exposure to polyP abolishes the thermodynamic “pump” mechanism and alters or even blocks phase transformation.87,88 In addition, divalent cations such as Mg2+ and certainly also Ca2+ cause a change in the surface free energy and in turn the nucleation kinetics.89
As outlined in Fig. 1, co-incubation of pearl aragonite with polyP ends up in the formation of a coacervate, a process driven by Na-polyP and the subsequent Ca2+ flux in the aqueous medium. The Ca2+ ions shuttle between Ca-carbonate and the phosphate polymer in a non-enzymatic way.90 As a result, polyP, together with Ca2+ and carbonate, is converted into the biologically active coacervate phase, resulting in stimulation of the migratory ability of myofibroblasts, a key process in wound regeneration, especially of chronic wounds.55,91
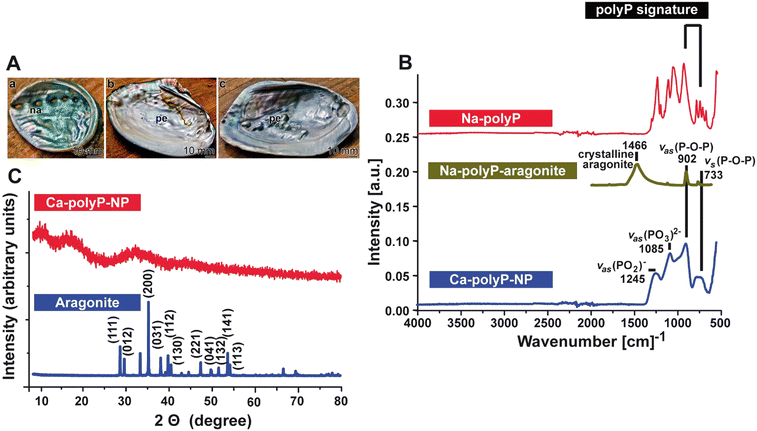
The image of the inner shell abalone (Haliotis spec.) mollusc covered with an organic–inorganic nacreous, iridescent composite layer, is shown in Fig. 2(A)-a. In response to injury, pearls are formed below the Mantel and above the nacre layer (H. schlegeli/H. cumingii);39Fig. 2(A)-b and c.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to aragonite (pearl powder) to wound exudate (Fig. 2(B)).
1 to aragonite (pearl powder) to wound exudate (Fig. 2(B)).
The Ca-polyP-NP are completely amorphous, as proven by the XRD diffractogram, which shows three smooth broad humps without any sharp diffraction peaks (Fig. 2(C)).61 In contrast, the XRD aragonite (nacre) pattern shows the characteristic peaks and reflects the crystalline nature as described.93
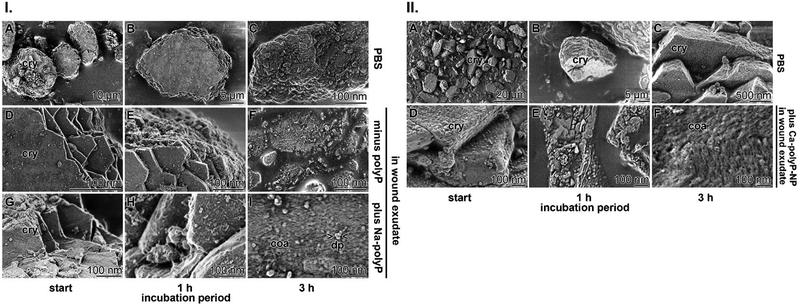
Crushed powdered pearls with or without Na-polyP were suspended in either phosphate-buffered saline (PBS) or wound exudate at a final concentration 120 μg mL−1 each. The suspension in PBS did not significantly change the irregular hexagonal crystal tablets that form the nacre pearls even after immersion for 3 h (Fig. 3(I)-A–C). The surfaces of the lamellae also retained their crystal morphology. In contrast, when the crystalline tablets were added to the wound exudate, the crystals began to dissolve after 3 h even in the absence of Na-polyP (Fig. 3(I)-D–F). Very pronounced is this process when the wound exudate is supplemented with Na-polyP (Fig. 3(I)-G–I). Dissolution started after an incubation of 1 h and was complete after 3 h. After this period, coacervate droplets with a size of ≈20 nm appeared (Fig. 3(I)-I).
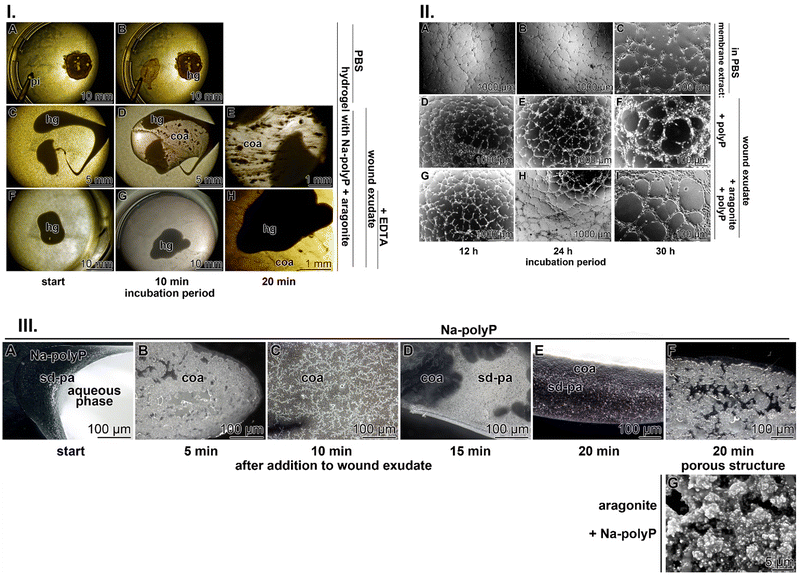
3.1.4.1 Viscosity. In the formulation tested on patients,23 the hydroxyethyl cellulose-based hydrogel was supplemented with polyP (Na-polyP and Ca-polyP-NP), a composition that forms a stable coacervate (Fig. 3) in the presence body fluids such as wound exudate with its Ca2+.95 Importantly, the viscosity of the hydroxyethyl cellulose hydrogel increases after addition of polyP (100 μg mL−1 of Na-polyP and 20 μg mL−1 of Ca-polyP-NP) from 20 Pa s to 25 Pa s.23 Further addition nacreous aragonite (120 μg mL−1) to the mixture stabilized the hydrogel even further to a viscosity of 50 Pa s.
3.1.4.2 Coacervate formation. Injection of the hydrogel wound dressing supplemented with polyP and aragonite in PBS (containing only the monovalent cations Na+ and K+) does not result in bulging of the initial morphology of the roundish hydrogel drop (Fig. 4(I)-A and B). In contrast, if the hydrogel is injected into wound exudate, strong and distinct and time-dependent current flow patterns appear, which are typical for coacervate formation23 (Fig. 4(I)-C–E). To demonstrate that Ca2+, present in the wound exudate at ≈1.5 mM, is the causative component in the wound exudate that triggers coacervation, the wound fluid was supplemented with 1.5 mM Na2–EDTA (ethylene–diamine–tetraacetic acid) in order to chelate divalent cations.95,100 Under these conditions, coacervate formation is drastically suppressed (Fig. 4(I)-F–H)).
These data proved the concept sketched in Fig. 1 that polyP, particularly in the Na-polyP form, triggers a phase transition from crystalline nacreous aragonite to the amorphous phase. This transformation is based on the strong binding affinity of the divalent Ca2+ cations to the anionic polymer. This interaction is accelerated by the property of Ca-polyP to be insoluble, which shifts the equilibrium towards destabilization of the crystalline state of aragonite. In the presence of an organic environment, the NP form of Ca-polyP undergoes coacervation and, concomitantly, also the nacreous aragonite, causing both deposits to turn into an aqueous phase. This reaction is the basis for the morphogenetic properties, with their inherent biological functions, of the two inorganic compounds.
Importantly, after adding nacreous aragonite (150 μg mL−1) to the Na-polyP solution at the beginning of the coacervation process, the more gelatinous structure of the gel is solidified, with pores around 5 μm, most likely stabilized by the remaining 1 μm large, cobble-like particles (Fig. 4(III)-G).
3.2. Effect of soluble aragonitic Ca-carbonate on biological function
The coacervate, formed together with Na-polyP in the presence of Ca2+ shows distinct biological activities.A stimulatory effect on tube formation can be modeled in vitro by HUVEC endothelial cells embedded in extracellular matrix components.109 In the studies summarized here, the matrix extract either remained nonsupplemented or was supplemented with either the polyP cocktail alone (Na-polyP and Ca-polyP-NP) or the mixture of polyP with the nacreous aragonite (Fig. 4(II)). In the controls without polyP and aragonite, the degree of tube formation was incomplete even after an incubation period of 30 h (Fig. 4(II)-A–C). In contrast, when the system was supplemented with the polyP cocktail alone, already after 12 h, the cells assumed a lobulated morphology and began to spread, forming distinct, although often incomplete, tube structures after 12 h, which became complete after 24 h and 30 h (Fig. 4(II)-D–F). Addition of polyP together with aragonite accelerated the tube-like pattern formation and most of the tube-like arrangements of the HUVEC cells were complete after 12 h and 24 h. Further extending the incubation to 30 h allowed the formation of cobblestones framing the tubes and smoothly arranged in monolayered rings (Fig. 4(II)-G–I).
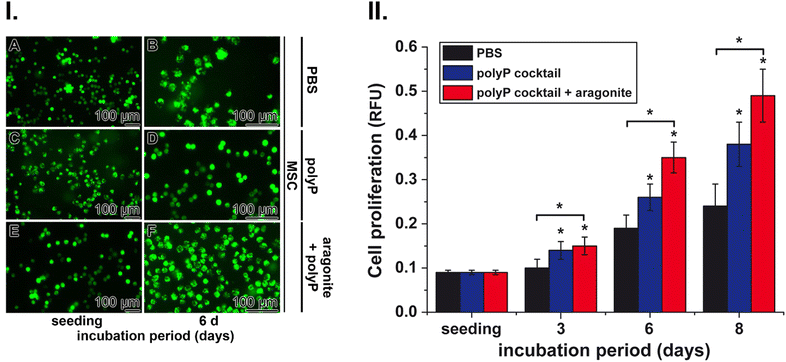
These cells were used to determine the effects of polyP and nacreous aragonite on the growth rate of cells. Incubation of MSC in the controls (with PBS) showed only a marginal increase in cell density under the conditions used compared to the positive potency of the polyP cocktail (Na-polyP and Ca-polyP-NP) and especially the polyP suspension together with nacreous aragonite (Fig. 6(I)). In all three series, with PBS (Fig. 6(I)-A and B), polyP cocktail (Fig. 6(I)-C and D) and with polyP together with nacreous aragonite (Fig. 6(I)-E and F) the densities of the MSC increased during the inspection period between the time of seeding and after 6 d of incubation.
Quantitative determination was possible by staining the cells with the cell viability reagent PrestoBlue (Fig. 6(II)). In the PBS controls, the proliferation increased two-fold after an incubation period of 6 d and continued to ≈3-fold after 8 d of cultivation (Fig. 6(II)). The proliferation-inducing potency of polyP together with aragonite was significantly stronger compared to polyP alone and reached a 2-fold greater efficiency after an incubation period of 6 d and 8 d.
Here, the effect of the wound hydrogel without or with polyP or polyP in combination with nacreous aragonite was tested using MSC as eukaryotic cell model and Escherichia coli M15 as bacterial system.
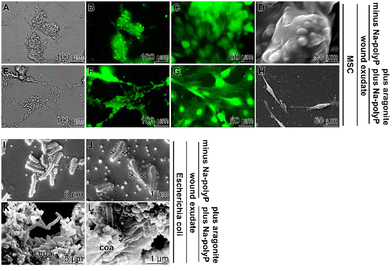
The MSC were added in the middle of the exponential growth phase to the wound exudate supplemented with the hydrogel either containing no polyP or supplemented with the polyP cocktail (Na-polyP and Ca-polyP-NP together with nacreous aragonite as outlined in the “Experimental section”. After overnight incubation, the samples were fixed by freeze-drying and inspected by SEM and fluorescence microscopy (Fig. 7(A)–(H). The difference in cell morphology between the incubation series without and with polyP and aragonite is strong. In the absence of these additives the cells have a globular morphology (Fig. 7(A)–(D)), reflecting reduced cell–cell contact followed by reduced proliferation potency.116 In contrast, MSC in cultures with polyP and aragonite spread vigorously and often showed strong cell–cell contacts (Fig. 7(E)–(H)). This morphology is an indication for a viable cell metabolism.117
In a parallel series, E. coli M15 either remained unembedded (Fig. 7(I) and (J)) or were embedded in the polyP cocktail together with aragonite (Fig. 7(K) and (L)). In the latter polyP/aragonite assays, the bacterial cells were bricked in a coacervate. In this series, no signs of bacterial divisions were observed, whereas constrictions are present in the control series that does not develop a coacervate.
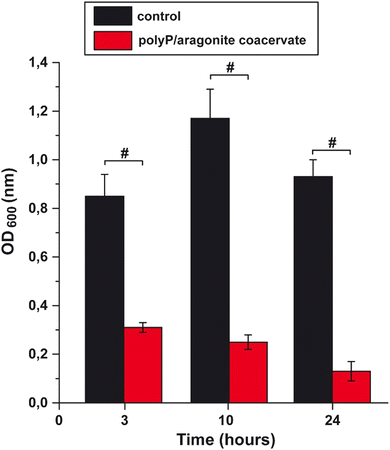
The antibacterial effect of the polyP cocktail together with aragonite was further demonstrated by determining the growth rate of E. coli in medium in the absence or presence of polyP and aragonite (Fig. 8). Already after a 3-h incubation period, the bacteria readily grow in the control (medium in the absence of the polyP/aragonite coacervate) to a density (at 600 nm) of 0.85 ± 0.09, while in the presence of the coacervate a density of 0.31 ± 0.02 was measured. The difference is even more distinct and significant after an incubation period of 10 h. At this stage, the density of the polyP/aragonite-treated cultures is only 20% of the rate reached in the polyP/aragonite-free control (Fig. 8).
The rational explanation for the documented success of polyP in chronic wound healing is the transformation of polyP into its coacervate phase, as already concluded from in vitro studies.18 Important, and documented here, is the result that this phase transition is driven by a liquid–liquid phase separation, initiated by Ca2+, under formation of a thermodynamically reversible association between the oppositely charged ions, the polyanion polyP and the Ca2+ cations.20 During coacervation, the aqueous phase is squeezed out, resulting in a more porous, concentrated macromolecular structure.119 The matrix of the gel-like, porous structure of the polyP coacervate becomes more stable through the inclusion of nacreous aragonite into the material. This property allows for a more efficient blood supply, particularly when this formulation is used to heal chronic wounds.
Based on the fact that wound healing is an energy-demanding process that requires both an extracellular ATP supply19 and an intracellular ATP supply controlled by adenine nucleotide translocase-2 (ANT-2),120,121 it was evident that polyP, especially Na-polyP, is an efficient component of the wound-healing hydrogel due to its coacervate-forming potency. While in healthy individuals skin injuries are restored within 2–3 weeks, this is different in diabetic patients. There, wounds fail to heal within about six months and can become chronic.2 It is well documented that in chronic wounds the four different phases of wound healing, hemostasis, inflammation, proliferation and remodeling, are interrupted at one step or another. Frequently, a discontinuation of the healing process leads to persistent infections and peripheral vascular problems caused by an imbalance between angiogenic and angioinhibitory factors, which leads to disturbed regulation of angiogenesis.122 Furthermore, and besides a disequilibrium of growth factors in the wound microenvironment, which also impairs balanced immune cell circulation, the lack of energy, a driving parameter in all phases of wound healing, is affected.123 By this, the progress during the healing phases is blocked.
There are, in particular, infections of the wound that can result in an amputation of limbs.124 Already here, Na-polyP has proven to achieve beneficial results.23 In addition, it is reported here that bacteria become wrapped in the coacervate gel, preventing at least their overproliferation (Fig. 7). Moreover, an enhanced physiologic metabolism of eukaryotic MSC is observed. Considering these data, it appeared to be indicated to first investigate the effect of the Na-polyP-based wound-healing hydrogel in an animal study conducted on genetically engineered diabetic mice (db/db).75 After that, a successful clinical proof-of-concept study has been undertaken.23,29
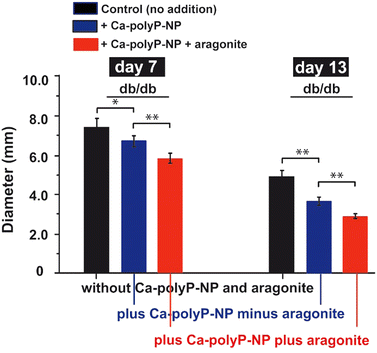
In order to find out whether the co-treatment of chronic wounds with Ca-polyP-NP and aragonite has an additional effect on wound healing compared to Ca-polyP-NP alone, an additional animal study was carried out using db/db mice, which show delayed wound healing. Experimental wounds (diameter 8 mm) were set in the interscapular region of the diabetic mice. The wounds were either left untreated or treated with either Ca-polyP-NP alone or with Ca-polyP-NP together with crushed aragonite, as described in the “Experimental section”. The results showed that topical administration of polyP alone results in a significant reduction in wound diameter after a healing period of 7 d and 13 d compared to the untreated controls (Fig. 9), confirming previous results.75 However, the effect of polyP on wound healing was significantly accelerated when the wounds were co-treated with Ca-polyP-NP and aragonite. After a healing period of 13 days, the diameter of the wounds treated with both components decreased to 78% of the wound diameter measured for Ca-polyP-NP alone and to 59% compared to wounds in the untreated animal group.
The dysregulation and imbalance of regeneration-promoting factors cause a reduction in angiogenesis, which is accompanied by a reduction of granulation tissue formation due to a nutrient and oxygen deficiency.125 The granulation tissue is the decisive “germination” zone that develops after skin injury and from which the angiogenic processes take place, such as microvascular hyperpermeability, local degradation of the basement membrane, migration and sprouting of newly formed cells, giving rise to the formation of new blood vessels; finally, the reconstruction of the basement membrane occurs.126
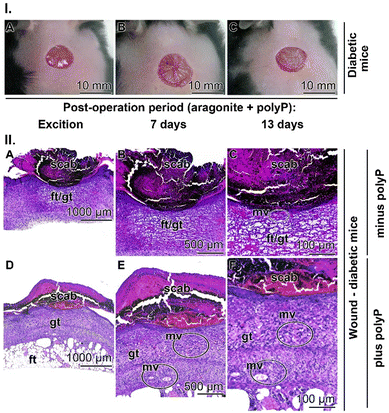
To investigate the effects of Ca-polyP-NP on granulation tissue and microvessel formation, regenerating wounds of the db/db mice (Fig. 10(I)-A–C) were sliced and then stained with hematoxylin–eosin. In the diabetic control animals not treated with polyP, the regenerating wound was sealed with a scab, the hard, dried blood clot over the wound (Fig. 10(II)-A–C). The slices shown are taken from animals 13 d after setting the injury. In controls, not treated with polyP, the stained sections through the epidermis and the dermis show a voluminous hypodermis (connective tissue) composed of fat droplets. Basically, no granulation tissue can be discerned and therefore only very few microvessels are present. This histological observation fits well with previous findings reporting an increase in the fat layer within the connective tissue in diabetic patients.127 Due to disturbed skin metabolism, diabetic patients often experience itchy and dry skin with the consequence that the patients are advised to apply skin-friendly cleansers and skin moisturizers.128
A completely different histological picture can be seen in tissues slices from diabetic mice treated with polyP (Fig. 10(II)-D–F)). In these specimens it is immediately visible that the granulation tissue layer is very, very bulky, providing space and cell resources (endothelial cells) for extensive and dynamic microvessel development. From there, a controlled remodelling of the epidermis and dermis can start.129
4. Conclusion
It is an inherent property of coacervation that the microscale droplets formed during liquid–liquid phase separation are in a dynamic state, even during the apparent equilibrium of the phases. During this process, the liquid droplets are tightly packed together, influencing the interactions, free energy patterns and dynamic properties of the molecular constituents.130 Liquid–liquid phase separations underlying aqueous two-phase systems are the basis for a number of biologically relevant processes, such as membrane biocondensation or supramolecular functional organization,131 allowing complex biochemical reactions in a confined space.132,133 In addition, they contribute to the understanding of the viscoelastic state of living organized systems.134 Focusing on the topic here, liquid–liquid phase separation, coacervation, is also a key factor in wound healing,135,136 in addition to the organization and migration of macrophages during tissue homeostasis, as well as the controlled supply of cytokines that suppress destructive immunity.137 Even more, the development of those regulatory macrophages is promoted that trigger tissue remodeling and wound healing, including angiogenesis.138The distinguished feature of the system reported here is that the previously published polyP-controlled coacervation, liquid–liquid phase separation, attracts MSC and activates them to actively migrate in the polyP coacervate matrix.18 Coupled with the coacervation process of polyP is the effect of the polymer to attract and scavenge Ca2+ from the labile crystalline aragonite phase and to include the carbonate anion into the dense phase of the formed Ca-polyP coacervate (Fig. 1). Vigorous flow areas certainly occur during the Ca2+ flux as reported for Ca2+/polyaspartate-rich coacervates.139 In this microenvironment, upon separation of Ca2+ from carbonate, a preferential surrounding for strong mineral deposition is found; calcium phosphate is formed at the expense of calcium carbonate. Even more, in the coacervate, continuous reactions (dynamic phase separations) occur that do not reach a thermodynamic equilibrium. Such nonequilibrium thermodynamics mimics biological systems, which are always in disequilibrium. Disequilibria between anabolic and catabolic reactions are the driving forces for biological systems.
Liquid–liquid phase separation processes, when broken down, are the onset of diseases such as amyloid formation in Alzheimer's disease. Direct exposure of neuronal cells to the amyloid-β fragment Aß25-35 does not cause apoptosis; only after preincubation of the peptide for 5 d, cell toxicity can be seen.140 Under physiological conditions, the cells exploit precautionary measures such as deaggregation caused by heat shock proteins that facilitate or prevent refolding.141 Another driving force to keep the (bio)chemical reactions alive is ATP.132,142 After enzymatic hydrolysis of the phosphoric acid anhydride bond in ATP, energy is released that is required for chemical bond formation or bond breakage in coupled reactions. Since ATP is formed after enzymatic hydrolysis of polyP,12 especially in the extracellular environment, the results described in the present contribution adds two crucial aspects for stimulating regeneration processes in humans. First, the two interconnected coacervation circuits; Na-polyP with aragonite (Ca-carbonate) liquid–liquid phase separations and, second, ATP fueling for the regenerating areas as long as polyP is present at the repair site. It is the dynamics of the bicomponent liquid–liquid phase separation/coacervation system that accelerates wound regeneration by the present formulation, including tube formation and increased MSC proliferation as shown here or proposed based on the asymmetric cluster vibrations provoked by intrinsic non-isotropic ATP consumption.143
Future experiments will follow to contribute to the understanding of the bicomponent coacervation reported here in other aspects of skin disorders, such as thermoregulatory sweating during hypohidrosis or anhidrosis, which is likewise primarily due to an impaired ATP homeostasis, based on dysfunction of Na,K-ATPase activity.144 Other promising potential applications would be psoriasis, a disease related to wound healing145 and characterized by a switch of keratinocytes from growth to differentiation due to a dysregulation of N-methyl-D-aspartate receptor (NMDAR) in these cells. Since memantine is a blocker of NMDAR,146 a curative approach could be the encapsulation of this drug in polyP-based core–shell NP.147
Author contributions
Werner E. G. Müller: conceptualization, data curation, formal analysis, original draft, writing – review and editing. Meik Neufurth: methodology, writing. Xiaohong Wang: data curation, writing, investigation, strategic focus. Shunfeng Wang: data curation, methodology, nanoparticle preparation, coacervation studies. Hadrian Schepler: human studies; histology. Rafael Muñoz-Espí: spectroscopic methodology, data analysis. Hiroshi Ushijima: data curation, writing of methods. Heinz C. Schröder: conceptualization, discussion, corrections, supervisions.Data availability
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.Conflicts of interest
The authors declare no competing interest.Acknowledgements
We thank Prof. Dr Vesna Erakovic Haber and Dr Dinko Relkovic (both at Fidelta Ltd., Zagreb; Croatia) for important contributions with the animal wound healing studies during the EU integrated project BlueGenics (grant no. 311848; 2012–2016). Furthermore we appreciate the contributions of Ms Kerstin Bahr, Institute of Functional and Clinical Anatomy, University Medical Center of the Johannes Gutenberg University, Mainz (Germany) for continuous support. Finally, we sincerely express our thanks to Mr Gunnar Glasser (Department of Physical Chemistry of Polymers, Max Planck Institute for Polymer Research, Mainz; Germany) for continuous and very expert support for the SEM analyses. W. E. G. M. is the holder of an ERC Advanced Investigator Grant (grant number 268476). In addition, W. E. G. M. has obtained three ERC-PoC grants (Si-Bone-PoC, grant no. 324564; MorphoVES-PoC, grant no. 662486; and ArthroDUR, grant no. 767234). Further support came from the BMBF (Chinese-German cooperation in industry-led collaborative project “SKIN-ENERGY” – FKZ 13GW0403B). Finally, this work was supported by grants from the International Human Frontier Science Program and the BiomaTiCS research initiative of the University Medical Center, Mainz.References
- N. M. Vecin and R. S. Kirsner, Front. Med., 2023, 10, 1154567 CrossRef PubMed.
- A. J. Singer and R. A. Clark, N. Engl. J. Med., 1999, 341, 738–746 CrossRef CAS PubMed.
- A. V. Nguyen and A. M. Soulika, Int. J. Mol. Sci., 2019, 20, 1811 CrossRef CAS PubMed.
- A. Sreedhar, L. Aguilera-Aguirre and K. K. Singh, Cell Death Dis., 2020, 11, 444 CrossRef CAS PubMed.
- F. Lipman, Adv. Enzymol. Relat. Subj. Biochem., 1941, 1, 99–162 Search PubMed.
- A. Trautmann, Sci. Signaling, 2009, 26, pe6 Search PubMed.
- L. L. Feng, Y. Q. Cai, M. C. Zhu, L. J. Xing and X. Wang, Cancer Cell Int., 2020, 20, 110 CrossRef CAS PubMed.
- Inorganic Polyphosphates – Biochemistry, Biology, Biotechnology, ed. H. C. Schröder and W. E. G. Müller, Springer Press, Heidelberg, 1999 Search PubMed.
- I. S. Kulaev, V. Vagabov and T. Kulakovskaya, The Biochemistry of Inorganic Polyphosphates, John Wiley, Chichester, 2004 Search PubMed.
- N. N. Rao, M. R. Gómez-García and A. Kornberg, Annu. Rev. Biochem., 2009, 78, 605–647 CrossRef CAS PubMed.
- T. L. Lindahl, S. Ramström, N. Boknäs and L. Faxälv, Biochem. Soc. Trans., 2016, 44, 35–39 CrossRef CAS PubMed.
- W. E. G. Müller, H. C. Schröder and X. H. Wang, Chem. Rev., 2019, 119, 12337–12374 CrossRef PubMed.
- H. Holmsen and H. J. Weiss, Annu. Rev. Med., 1979, 30, 119–134 CrossRef CAS PubMed.
- M. H. Fukami, C. A. Dangelmaier, J. S. Bauer and H. Holmsen, Biochem. J., 1980, 192, 99–105 CrossRef CAS PubMed.
- W. E. G. Müller, S. F. Wang, M. Neufurth, M. Kokkinopoulou, Q. L. Feng, H. C. Schröder and X. H. Wang, J. Cell Sci., 2017, 130, 2747–2756 CrossRef PubMed.
- Y. Akosah, J. Yang and E. Pavlov, Biochem. Soc. Trans., 2024, 52, 671–679 CrossRef CAS PubMed.
- A. Y. Baev, P. R. Angelova and A. Y. Abramov, Biochem. J., 2020, 477, 1515–1524 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, M. Neufurth, M. Ackermann, R. Muñoz-Espí, I. Lieberwirth, G. Glasser, H. C. Schröder and X. H. Wang, Small, 2018, 14, 1801170 CrossRef PubMed.
- W. E. G. Müller, M. Neufurth, I. Lieberwirth, S. F. Wang, H. C. Schröder and X. H. Wang, Mater. Today Bio, 2022, 16, 100404 CrossRef PubMed.
- D. W. R. Balkenende, S. M. Winkler and P. B. Messersmith, Eur. Polym. J., 2019, 116, 134–143 CrossRef CAS PubMed.
- A. N. Singh and A. Yethiraj, J. Phys. Chem. B, 2021, 125, 3023–3031 CrossRef CAS PubMed.
- S. Chen, Q. Guo and J. Yu, Aggregate, 2022, 3, e293 CrossRef CAS.
- W. E. G. Müller, H. Schepler, M. Neufurth, S. F. Wang, V. Ferrucci, M. Zollo, R. W. Tan, H. C. Schröder and X. H. Wang, J. Mater. Sci. Technol., 2023, 135, 170–185 CrossRef.
- E. Tolba, W. E. G. Müller, B. M. A. El-Hady, M. Neufurth, F. Wurm, S. F. Wang, H. C. Schröder and X. H. Wang, J. Mater. Chem. B, 2016, 4, 376–386 RSC.
- X. Li, X. Yang, X. Liu, W. He, Q. Huang, S. Li and Q. Feng, Prog. Nat. Sci.: Mater. Int., 2018, 28, 598–608 CrossRef CAS.
- X. H. Wang, H. C. Schröder and W. E. G. Müller, J. Mater. Chem. B, 2018, 6, 2385–2412 RSC.
- W. E. G. Müller, M. Neufurth, S. F. Wang, H. C. Schröder and X. H. Wang, Small, 2024, 2309528 CrossRef PubMed.
- Y. Wang, M. Li, P. Li, H. Teng, D. Fan, W. Du and Z. Guo, BioMed Res. Int., 2019, 2019, 5141204 Search PubMed.
- H. Schepler, M. Neufurth, S. F. Wang, Z. She, H. C. Schröder, X. H. Wang and W. E. G. Müller, Theranostics, 2022, 12, 18–34 CrossRef CAS PubMed.
- A. M. Moe, A. E. Golding and W. M. Bement, Semin. Cell Dev. Biol., 2015, 45, 18–23 CrossRef CAS PubMed.
- S. F. Wang, M. Neufurth, H. Schepler, R. Tan, Z. She, B. Al-Nawas, X. H. Wang, H. C. Schröder and W. E. G. Müller, Pharmaceutics, 2023, 15, 494 CrossRef CAS PubMed.
- C. Navarro-Requena, S. Pérez-Amodio, O. Castaño and E. Engel, Nanotechnology, 2018, 29, 395102 CrossRef PubMed.
- T. Subramaniam, M. B. Fauzi, Y. Lokanathan and J. X. Law, Int. J. Mol. Sci., 2021, 22, 6486 CrossRef CAS PubMed.
- J. Liang, D. Kang, Y. Wang, Y. Yu, J. Fan and E. Takashi, PLoS One, 2015, 10, e0117106 CrossRef PubMed.
- O. Sulpis, E. Jeansson, A. Dinauer and S. K. Lauvset, Nat. Geosci., 2021, 14, 423–428 CrossRef CAS.
- V. Lauth, M. Maas and K. Rezwan, J. Mater. Chem. B, 2014, 2, 7725–7731 RSC.
- L. Addadi, D. Joester, F. Nudelman and S. Weiner, Chemistry, 2006, 12, 980–987 CrossRef CAS PubMed.
- L. Qiao, Q. L. Feng and S. Lu, Cryst. Growth Des., 2008, 8, 1509–1514 CrossRef CAS.
- J. Sun and B. Bhushan, RSC Adv., 2012, 2, 7617–7632 RSC.
- J. Pei, Y. Wang, X. Zou, H. Ruan, C. Tang, J. Liao, G. Si and P. Sun, Front. Bioeng. Biotechnol., 2021, 9, 649665 CrossRef PubMed.
- C. A. Schmidt, E. Tambutté, A. A. Venn, Z. Zou, C. Castillo Alvarez, L. S. Devriendt, H. A. Bechtel, C. A. Stifler, S. Anglemyer, C. P. Breit, C. L. Foust, A. Hopanchuk, C. N. Klaus, I. J. Kohler, I. M. LeCloux, J. Mezera, M. R. Patton, A. Purisch, V. Quach, J. S. Sengkhammee and P. U. P. A. Gilbert, Nat. Commun., 2024, 15, 1812 CrossRef CAS PubMed.
- F. Vallés, Controversiarum Medicarum et Philosophicarum libri decem, Marnius & Aubrius, Hanoviae, 1606, p. 368 Search PubMed.
- X. J. Loh, D. J. Young, H. Guo, L. Tang, Y. Wu, G. Zhang, C. Tang and H. Ruan, Materials, 2021, 14, 2797 CrossRef CAS PubMed.
- M. Liu, J. Tao, H. Guo, L. Tang, G. Zhang, C. Tang, H. Zhou, Y. Wu, H. Ruan and X. J. Loh, Materials, 2021, 14, 4458 CrossRef CAS PubMed.
- E. Lopez, A. Le Faou, S. Borzeix and S. Berland, Tissue Cell, 2000, 32, 95–101 CrossRef CAS PubMed.
- D. W. Green, H. J. Kwon and H. S. Jung, Mol. Cells, 2015, 38, 267–272 CrossRef CAS PubMed.
- F. Müller, N. J. Mutch, W. A. Schenk, S. A. Smith, L. Esterl, H. M. Spronk, S. Schmidbauer, W. A. Gahl, J. H. Morrissey and T. Renné, Cell, 2009, 139, 1143–1156 CrossRef PubMed.
- L. Faxälv, N. Boknäs, J. O. Ström, P. Tengvall, E. Theodorsson, S. Ramström and T. L. Lindahl, Blood, 2013, 122, 3818–3824 CrossRef PubMed.
- B. Lorenz and H. C. Schröder, Biochim. Biophys. Acta, 2001, 1547, 254–261 CrossRef CAS PubMed.
- S. A. Smith, S. H. Choi, R. Davis-Harrison, J. Huyck, J. Boettcher, C. M. Rienstra and J. H. Morrissey, Blood, 2011, 117, 3477 CAS.
- J. J. Verhoef, A. D. Barendrecht, K. F. Nickel, K. Dijkxhoorn, E. Kenne, L. Labberton, O. J. McCarty, R. Schiffelers, H. F. Heijnen, A. P. Hendrickx, H. Schellekens, M. H. Fens, S. de Maat, T. Renné and C. Maas, Blood, 2017, 129, 1707–1717 CrossRef CAS PubMed.
- W. L. Dean, World J. Biol. Chem., 2010, 1, 265–270 CrossRef PubMed.
- R. Docampo and S. N. Moreno, Cell Calcium, 2011, 50, 113–119 CrossRef CAS PubMed.
- S. Omelon, A. Baer, T. Coyle, R. M. Pilliar, R. Kandel and M. Grynpas, Mater. Res. Bull., 2008, 43, 68–80 CrossRef CAS.
- Y. C. Li, C. R. Chen and T. H. Young, Pharm. Biol., 2013, 51, 289–297 CrossRef PubMed.
- L. M. Harwood, L. C. Hodgkinson, J. K. Sutherland and P. Towers, Can. J. Chem., 1984, 62, 1922–1925 CrossRef CAS.
- V. P. Kutyshenko, M. Molchanov, P. Beskaravayny, V. N. Uversky and M. A. Timchenko, PLoS One, 2011, 6, e28824 CrossRef CAS PubMed.
- N. Ferrara, Oncologist, 2004, 9(Suppl. 1), 2–10 CrossRef CAS PubMed.
- Y. Zhang, Y. Xu, J. Ma, X. Pang and M. Dong, Sci. Rep., 2017, 7, 40524 CrossRef CAS PubMed.
- J. Gim, A. Koch, L. M. Otter, B. H. Savitzky, S. Erland, L. A. Estroff, D. E. Jacob and R. Hovden, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2107477118 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, H. C. Schröder, S. F. Wang, G. Glaßer, R. Muñoz-Espí, T. Link and X. H. Wang, Mater. Lett., 2015, 148, 166 CrossRef.
- W. E. G. Müller, M. Ackermann, S. F. Wang, M. Neufurth, R. Muñoz-Espí, Q. L. Feng, H. C. Schröder and X. H. Wang, Cell. Mol. Life Sci., 2018, 75, 21–32 CrossRef PubMed.
- D. Xie, D. Ju, C. Speyer, D. Gorski and M. A. Kosir, J. Visualized Exp., 2016, 114, e54074 Search PubMed.
- M. Neufurth, S. F. Wang, H. C. Schröder, B. Al-Nawas, X. H. Wang and W. E. G. Müller, Biofabrication, 2022, 14, 015016 CrossRef PubMed.
- W. E. G. Müller, M. Neufurth, S. F. Wang, H. C. Schröder and X. H. Wang, Cancers, 2021, 13, 750 CrossRef PubMed.
- M. Xu, D. J. McCanna and J. G. Sivak, J. Pharmacol. Toxicol. Methods, 2015, 71, 1–7 CrossRef CAS PubMed.
- W. E. G. Müller, S. Engel, X. H. Wang, S. E. Wolf, W. Tremel, N. L. Thakur, A. Krasko, M. Divekar and H. C. Schröder, Biomaterials, 2008, 29, 771–779 CrossRef PubMed.
- A. Giacomini, M. Ackermann, M. Belleri, D. Coltrini, B. Nico, D. Ribatti, M. A. Konerding, M. Presta and M. Righi, Angiogenesis, 2015, 18, 499–510 CrossRef CAS PubMed.
- J. Campbell, Curr. Protoc. Chem. Biol., 2011, 3, 100115 Search PubMed.
- M. Wiens, X. H. Wang, U. Schloßmacher, I. Lieberwirth, G. Glasser, H. Ushijima, H. C. Schröder and W. E. G. Müller, Calcif. Tissue Int., 2010, 87, 513–524 CrossRef CAS PubMed.
- M. Wiens, X. H. Wang, H. C. Schröder, U. Kolb, U. Schloßmacher, H. Ushijima and W. E. G. Müller, Biomaterials, 2010, 31, 7716–7725 CrossRef CAS PubMed.
- S. M. Manohar, A. A. Padgaonkar, A. Jalota-Badhwar, V. Sonawane, M. J. Rathos, S. Kumar and K. S. Joshi, BMC Cancer, 2011, 11, 338 CrossRef CAS PubMed.
- R. D. Barber, D. W. Harmer, R. A. Coleman and B. J. Clark, Physiol. Genomics, 2005, 21, 389–395 CrossRef CAS PubMed.
- M. W. Pfaffl, Nucleic Acids Res., 2001, 29, 2002–2007 CrossRef PubMed.
- W. E. G. Müller, D. Relkovic, M. Ackermann, S. F. Wang, M. Neufurth, A. Paravic-Radicevic, H. Ushijima, H. C. Schröder and X. H. Wang, Polymers, 2017, 9, 300 CrossRef PubMed.
- R. D. Lillie, Histopathologic Technic and Practical Histochemistry, McGraw-Hill Book Co., New York, USA, 3rd edn, 1965 Search PubMed.
- S. van der Burgh, A. de Keizer and M. A. Stuart, Langmuir, 2004, 20, 1073–1084 CrossRef CAS PubMed.
- E. Ortega-Rivas, P. Juliano and H. Yan, Food powders: Physical properties, processing, and functionality, Springer Science & Business Media, New York, 2006 Search PubMed.
- Y. P. Timilsena, T. O. Akanbi, N. Khalid, B. Adhikari and C. J. Barrow, Int. J. Biol. Macromol., 2019, 121, 1276–1286 CrossRef CAS PubMed.
- T. R. Dargaville, B. L. Farrugia, J. A. Broadbent, S. Pace, Z. Upton and N. H. Voelcker, Biosens. Bioelectron., 2013, 41, 30–42 CrossRef CAS PubMed.
- H. H. Leveen, G. Falk, B. Borek, C. Diaz, Y. Lynfield, B. J. Wynkoop, G. A. Mabunda, J. L. Rubricius and G. C. Christoudias, Ann. Surg., 1973, 178, 745–753 CrossRef CAS PubMed.
- Y. Chen, K. Tao, W. Ji, V. B. Kumar, S. Rencus-Lazar and E. Gazit, Mater. Today, 2022, 60, 106–127 CrossRef CAS.
- N. Neville, K. Lehotsky, Z. Yang, K. A. Klupt, A. Denoncourt, M. Downey and Z. Jia, Cell Rep., 2023, 42, 113082 CrossRef CAS PubMed.
- J. H. E. Cartwright, A. G. Checa and C. I. Sainz-Díaz, ACS Nano, 2020, 14, 9277–9281 CrossRef CAS PubMed.
- L. E. Murr and D. A. Ramirez, JOM, 2012, 64, 469–474 CrossRef CAS.
- L. A. Casella, E. Griesshaber, X. Yin, A. Ziegler, V. Mavromatis, D. Müller, A. C. Ritter, D. Hippler, E. M. Harper and M. Dietzel, Biogeosciences, 2017, 14, 1461–1492 CrossRef CAS.
- B. Gao and K. M. Poduska, Solids, 2022, 3, 684–696 CrossRef CAS.
- B. Gao, K. M. Poduska, S. Kababya and A. Schmidt, J. Am. Chem. Soc., 2023, 145, 25938–25941 CrossRef CAS PubMed.
- K. J. Davis, P. M. Dove and J. J. De Yoreo, Science, 2000, 290, 1134–1137 CrossRef CAS PubMed.
- W. E. G. Müller, M. Neufurth, J. Huang, K. Wang, Q. L. Feng, H. C. Schröder, B. Diehl-Seifert, R. Muñoz-Espí and X. H. Wang, ChemBioChem, 2015, 16, 1323–1332 CrossRef PubMed.
- B. Hinz, Curr. Res. Transl. Med., 2016, 64, 171–177 CAS.
- A. Khoshmanesh, P. L. Cook and B. R. Wood, Analyst, 2012, 137, 3704–3709 RSC.
- C. G. Kontoyannis and N. V. Vagenas, Analyst, 2000, 125, 251–255 RSC.
- I. R. Sweeney, M. Miraftab and G. Collyer, Int. Wound J., 2012, 9, 601–612 CrossRef PubMed.
- T. Subramaniam, M. B. Fauzi, Y. Lokanathan and J. X. Law, Int. J. Mol. Sci., 2021, 22, 6486 CrossRef CAS PubMed.
- V. K. Shukla, D. Shukla, S. K. Tiwary, S. Agrawal and A. Rastogi, J. Wound Care, 2007, 16, 291–294 CrossRef CAS PubMed.
- J. Harvey, K. T. Mellody, N. Cullum, R. E. B. Watson and J. Dumville, Wound Repair Regen., 2022, 30, 317–333 CrossRef PubMed.
- B. J. Tighe and A. Mann, in Woodhead Publishing Series in Biomaterials – Advanced Wound Repair Therapies, ed. D. Farrar, Elsevier, Ansterdam, 2011, pp. 321–357 Search PubMed.
- E. E. Tudoroiu, C. E. Dinu-Pirvu, M. G. Albu Kaya, L. Popa, V. Anuta, R. M. Prisada and M. V. Ghica, Pharmaceuticals, 2021, 14, 1215 CrossRef CAS PubMed.
- H. Hennings, D. Michael, C. Cheng, P. Steinert, K. Holbrook and S. H. Yuspa, Cell, 1980, 19, 245–254 CrossRef CAS PubMed.
- J. J. Grzesiak and M. D. Pierschbacher, J. Clin. Invest., 1995, 95, 227–233 CrossRef CAS PubMed.
- T. B. McEwan, R. A. Sophocleous, P. Cuthbertson, K. J. Mansfield, M. L. Sanderson-Smith and R. Sluyter, Life Sci., 2021, 283, 119850 CrossRef CAS PubMed.
- M. Dosch, J. Gerber, F. Jebbawi and G. Beldi, Int. J. Mol. Sci., 2018, 19, 1222 CrossRef PubMed.
- Y. Mo, H. Sarojini, R. Wan, Q. Zhang, J. Wang, S. Eichenberger, G. J. Kotwal and S. Chien, Front. Pharmacol., 2020, 10, 1502 CrossRef PubMed.
- W. E. G. Müller, E. Tolba, Q. L. Feng, H. C. Schröder, J. S. Markl, M. Kokkinopoulou and X. H. Wang, J. Cell Sci., 2015, 128, 2202–2207 CrossRef PubMed.
- T. Ushiki, T. Mochizuki, K. Suzuki, M. Kamimura, H. Ishiguro, T. Suwabe and T. Kawase, Int. J. Mol. Sci., 2022, 23, 11293 CrossRef CAS PubMed.
- M. Guitart-Mampel, P. Urquiza, F. Carnevale Neto, J. R. Anderson, V. Hambardikar, E. R. Scoma, G. E. Merrihew, L. Wang, M. J. MacCoss, D. Raftery, M. J. Peffers and M. E. Solesio, Front. Cell Dev. Biol., 2022, 10, 833127 CrossRef PubMed.
- B. Nocek, S. Kochinyan, M. Proudfoot, G. Brown, E. Evdokimova, J. Osipiuk, A. M. Edwards, A. Savchenko, A. Joachimiak and A. F. Yakunin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17730–17735 CrossRef CAS PubMed.
- A. M. Goodwin, Microvasc. Res., 2007, 74, 172–183 CrossRef CAS PubMed.
- M. Rodrigues, N. Kosaric, C. A. Bonham and G. C. Gurtner, Physiol. Rev., 2019, 99, 665–706 CrossRef CAS PubMed.
- S. Maxson, E. A. Lopez, D. Yoo, A. Danilkovitch-Miagkova and M. A. Leroux, Stem Cells Transl. Med., 2012, 1, 142–149 CrossRef CAS PubMed.
- A. L. Byrd, Y. Belkaid and J. A. Segre, Nat. Rev. Microbiol., 2018, 16, 143–155 CrossRef CAS PubMed.
- S. Carmona-Cruz, L. Orozco-Covarrubias and M. Sáez-de-Ocariz, Front. Cell. Infect. Microbiol., 2022, 12, 834135 CrossRef CAS PubMed.
- J. D. Rembe, C. Fromm-Dornieden, J. Böhm and E. K. Stuermer, Wound Repair Regen., 2018, 26, 27–35 CrossRef PubMed.
- J. Roewe, G. Stavrides, M. Strueve, A. Sharma, F. Marini, A. Mann, S. A. Smith, Z. Kaya, B. Strobl, M. Mueller, C. Reinhardt, J. H. Morrissey and M. Bosmann, Nat. Commun., 2020, 11, 4035 CrossRef CAS PubMed.
- J. P. Woodley, D. W. Lambert and I. O. Asencio, Tissue Eng., Part B, 2022, 28, 569–578 CrossRef PubMed.
- R. L. Splitt and K. A. DeMali, Biol. Cell., 2023, 115, e202200108 CrossRef PubMed.
- M. A. Fardin, O. M. Rossier, P. Rangamani, P. D. Avigan, N. C. Gauthier, W. Vonnegut, A. Mathur, J. Hone, R. Iyengar and M. P. Sheetz, Soft Matter, 2010, 6, 4788–4799 RSC.
- Q. Zhao, D. W. Lee, B. K. Ahn, S. Seo, Y. Kaufman, J. N. Israelachvili and J. H. Waite, Nat. Mater., 2016, 15, 407–412 CrossRef CAS PubMed.
- S. H. Woo, Y. J. Mo, Y. I. Lee, J. H. Park, D. Hwang, T. J. Park, H. Y. Kang, S. C. Park and Y. S. Lee, J. Invest. Dermatol., 2023, 143, 2295–2310 CrossRef CAS PubMed.
- Y. Gouriou, M. R. Alam, Z. Harhous, C. Crola Da Silva, D. B. Baetz, S. Badawi, E. Lefai, J. Rieusset, A. Durand, R. Harisseh, A. Gharib, M. Ovize and G. Bidaux, Cells, 2020, 9, 2542 CrossRef CAS PubMed.
- S. Rana, S. D. Burke and S. A. Karumanchi, Am. J. Obstet. Gynecol., 2022, 226, S1019–S1034 CrossRef CAS PubMed.
- J. Y. Ji, D. Y. Ren and Y. Z. Weng, Int. J. Nanomed., 2022, 17, 3163–3176 CrossRef PubMed.
- K. Shankhdhar, Adv. Skin Wound Care, 2021, 34, 1–4 CrossRef PubMed.
- J. Li, Y. P. Zhang and R. S. Kirsner, Microsc. Res. Tech., 2003, 60, 107–114 CrossRef CAS PubMed.
- M. Marx, R. A. Perlmutter and J. A. Madri, J. Clin. Invest., 1994, 93, 131–139 CrossRef CAS PubMed.
- K. McLellan, J. S. Petrofsky, G. Bains, G. Zimmerman, M. Prowse and S. Lee, Med. Eng. Phys., 2009, 31, 165–172 CrossRef PubMed.
- R. S. Kirsner, G. Yosipovitch, S. Hu, A. Andriessen, J. R. Hanft, P. J. Kim, L. Lavery, L. Meneghini and L. C. Ruotsi, J. Drugs Dermatol., 2019, 18, 1211–1217 CAS.
- M. Jiang, X. Jiang, H. Li, C. Zhang, Z. Zhang, C. Wu, J. Zhang, J. Hu and J. Zhang, Front. Immunol., 2023, 14, 1136098 CrossRef CAS PubMed.
- S. Guseva, V. Schnapka, W. Adamski, D. Maurin, R. W. H. Ruigrok, N. Salvi and M. Blackledge, J. Am. Chem. Soc., 2023, 145, 10548–10563 CrossRef CAS PubMed.
- H. Obayashi, R. Wakabayashi, N. Kamiya and M. Goto, Chem. Commun., 2023, 59, 414–417 RSC.
- A. A. Hyman, C. A. Weber and F. Jülicher, Annu. Rev. Cell Dev. Biol., 2014, 30, 39–58 CrossRef CAS PubMed.
- S. F. Banani, H. O. Lee, A. A. Hyman and M. K. Rosen, Nat. Rev. Mol. Cell Biol., 2017, 18, 285–298 CrossRef CAS PubMed.
- J. Berry, C. P. Brangwynne and M. Haataja, Rep. Prog. Phys., 2018, 81, 046601 CrossRef PubMed.
- Y. Wen and J. Ma, Front. Immunol., 2022, 13, 986589 CrossRef CAS PubMed.
- E. W. Hester, S. Carney, V. Shah, A. Arnheim, B. Patel, D. Di Carlo and A. L. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2306467120 CrossRef CAS PubMed.
- E. Cammarota, C. Soriani, R. Taub, F. Morgan, J. Sakai, S. L. Veatch, C. E. Bryant and P. Cicuta, J. R. Soc., Interface, 2020, 17, 20190803 CrossRef CAS PubMed.
- S. Chen, J. Yang, Y. Wei and X. Wei, Cell Mol. Immunol., 2020, 17, 36–49 CrossRef CAS PubMed.
- A. T. Rowland, D. N. Cacace, N. Pulati, M. L. Gulley and C. D. Keating, Chem. Mater., 2019, 31(24), 10243–10255 CrossRef CAS.
- W. E. G. Müller, F. J. Romero, S. Perović, G. Pergande and P. Pialoglou, J. Brain Res., 1996, 37, 575–577 Search PubMed.
- D. Zwicker, M. Decker, S. Jaensch, A. A. Hyman and F. Jülicher, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E2636–E2645 CrossRef CAS PubMed.
- C. F. Lee, C. P. Brangwynne, J. Gharakhani, A. A. Hyman and F. Jülicher, Phys. Rev. Lett., 2013, 111, 088101 CrossRef PubMed.
- S. K. Vogel, C. Wölfer, D. A. Ramirez-Diaz, R. J. Flassig, K. Sundmacher and P. Schwille, Cells, 2020, 9, 1432 CrossRef CAS PubMed.
- B. Yao, J. Xie, N. Liu, T. Hu, W. Song, S. Huang and X. Fu, Cell Death Dis., 2019, 10, 272 CrossRef PubMed.
- V. B. Morhenn, T. E. Nelson and D. L. Gruol, J. Dermatol. Sci., 2013, 72, 87–92 CrossRef CAS PubMed.
- W. E. G. Müller, H. C. Schröder, H. Ushijima, J. Dapper and J. Bormann, Eur. J. Pharmacol., 1992, 226, 209–214 CrossRef PubMed.
- W. E. G. Müller, E. Tolba, S. F. Wang, M. Neufurth, I. Lieberwirth, M. Ackermann, H. C. Schröder and X. H. Wang, Sci. Rep., 2020, 10, 17147 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2024 |