Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pesticides and transformation products in surface waters of western Montérégie, Canada: occurrence, spatial distribution and ecotoxicological risks†
Xiameng
Feng
 a,
Zhen
Liu
a,
Zhen
Liu
 b,
Sung
Vo Duy
a,
Gabriel
Munoz
a,
Lise
Parent
c and
Sébastien
Sauvé
b,
Sung
Vo Duy
a,
Gabriel
Munoz
a,
Lise
Parent
c and
Sébastien
Sauvé
 *a
*a
aDepartment of Chemistry, Université de Montréal, Montréal, QC H2V 0B3, Canada. E-mail: sebastien.sauve@umontreal.ca
bIndustrial Chair on Drinking Water, Department of Civil, Mining and Geological Engineering, Polytechnique Montréal, Montréal, QC H3T 1J4, Canada
cDepartment of Science and Technology, Université TÉLUQ, Montréal, QC H2S 3L5, Canada
First published on 29th April 2024
Abstract
The objective of this study was to investigate the occurrence, spatial distribution, and ecotoxicological risk of pesticides and transformation products in surface waters of western Montérégie (Quebec, Canada). A total of 29 samples were collected from 11 rivers during the summers of 2019 and 2021, and the samples were analyzed for 48 pesticides and 8 transformation products. The downstream data were used to assess the ecotoxicological risks based on Quebec's acute or chronic aquatic life criterion (AALC or CALC). Overall, 9 herbicides (glyphosate, S-metolachlor, 2,4-D, metribuzin, atrazine, MCPA, prometryn, dimethenamid, simazine), 3 insecticides (clothianidin, imidacloprid, chlorantraniliprole), and 4 fungicides (azoxystrobin, fluxapyroxad, tebuconazole, carbendazim) were detected at all sampling sites, demonstrating their widespread use in western Montérégie. Glyphosate (87–4095 ng L−1), S-metolachlor (6–2519 ng L−1), and 2,4-D (6–1094 ng L−1) were identified as the most abundant pesticides in surface water. Furthermore, 6 pesticide transformation products (metolachlor ESA, AMPA, metolachlor OA, desethylatrazine, atrazine-2-hydroxy, desisopropylatrazine) were detected at all sampling sites. The concentration of transformation products accounted for 51% on average of the total concentration, demonstrating the abundance of transformation products in surface waters. Neonicotinoids exhibited the highest ecotoxicological risk in the surface water samples with an average CALC risk quotient of 28 for 2019 and 12 for 2021, respectively. The present study offers insights into pesticides occurrence and their ecological impacts on surface waters of western Montérégie and allows for supporting future pesticide management and ecotoxicological risk mitigation strategies.
Environmental significanceThe widespread occurrence of pesticides in surface waters has caused a global concern over their detrimental impact on non-target organisms and human health. Although a large number of studies have investigated the occurrence of pesticides in surface waters, much less is known about the occurrence of their transformation products. In this study, we investigated the occurrence and spatial distribution of 48 pesticides and 8 transformation products in surface waters of western Montérégie, one of the most important agricultural hub in Quebec, Canada. We found that transformation products were omnipresent and accounted for 51% on average of the total concentration. This study demonstrates the urgent need to assess the ecotoxicological risk of not only the parent pesticide compounds but also their transformation products. |
1 Introduction
The presence of synthetic pesticides in waters has been reported worldwide owing to the increasing use of pesticides1 and the development of analytical chemistry.2–7 Following their application in agricultural, domestic, and municipal use, pesticides can be transported to surface waters where they are often found at trace concentrations from low ng L−1 to high μg L−1 levels, also depending on the river/creek dilution capability. Furthermore, pesticides can generate transformation products owing to physical, chemical and biological processes in the environment.8–10 An increasing number of studies have revealed the detrimental impact of pesticides and their transformation products on aquatic organisms, including algae,11,12 zooplankton,13 and fish.14,15 Some studies also indicated that pesticides and transformation products exposure through long-term food and water consumption can cause sublethal toxicity to mammals, such as reproductive toxicity, genotoxicity, and carcinogenicity.16–19 Although pesticides have been well studied in the past, much less is known about the occurrence of their transformation products in the environment.In North America, herbicides are the most widely used pesticides, among which glyphosate is the most used active ingredient since 2001, followed by atrazine and S-metolachlor.20 In Quebec, glyphosate has experienced a continuous rise in sales since 2000 due to its increased use for genetically modified corn and soybean crops,21 from 500 tons (based on active ingredients) in 2000 to 1878 tons in 2019.22 In contrast, atrazine, another widely used herbicide, has witnessed a substantial decrease of 96% in sales in Quebec since the implementation of the requirement for agronomic justification in 2018,23 and sales of alternative herbicides to atrazine, including simazine, S-metolachlor, 2,4-D, and dicamba, have experienced noticeable growth from 2017 to 2020.23 Neonicotinoids insecticides have been reported to be extensively present in surface waters across Canada due to their continuous uses.24–26 A chronic aquatic life criterion (CALC) of 8.3 ng L−1 has hence been adopted by the Quebec Ministry of the Environment for either individual neonicotinoid or their sum to protect aquatic organisms in surface waters.27 The occurrence of chlorantraniliprole, a neonicotinoids substitute, has been recently reported in Canadian waters,24,26,28 even though its sales are still relatively low. For instance, according to Quebec's pesticides sales report of 2020, chlorantraniliprole sales were one order of magnitude lower than those of neonicotinoids.23 Despite fluctuations in the sales of individual pesticides, the total annual sales of pesticides in Quebec have remained consistent (>4 million kg of active ingredients) since 2012.23 This demonstrates an ongoing and substantial input of synthetic pesticides into the environment. Even though the concentration of individual pesticides in surface waters may still be low or even below their guidelines, one should note that the co-occurrence of pesticides can be more toxic due to synergistic or additive effects.29 Furthermore, many of their transformation products exhibit equal or greater persistence/toxicity in the environment, such as desethylatrazine (DEA) and metolachlor ethanesulfonic acid (metolachlor ESA).30,31 It is therefore essential to monitor the occurrence of pesticides and their transformation products in waters and assess the potential risks they pose to ecosystems and human health.
Montérégie is an administrative region located on the south bank of the St. Lawrence River in southwestern Quebec. Spanning an area of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 176 km2 and accommodating over 1.5 million people, it is known for being the most significant agricultural hub in Quebec. The region has a diverse range of farms, covering nearly a quarter of Quebec's agricultural land. Montérégie dominates in grain corn and soybean production, contributing 62% and 48% respectively to Quebec's output. Additionally, it also represents a significant share of Quebec's processing vegetable production, with 78% of the total output.32 It is also the major contributor to apple production in Quebec, accounting for 65% of the total yield.32 Based on a 2019 report on pesticides sales in Quebec,22 the sole Montérégie region accounted for 34% of the users who have been prescribed the top five pesticides with the highest potential risks (i.e., atrazine, chlorpyrifos, clothianidin, imidacloprid, and thiamethoxam). Despite the intensive use of pesticides and previous characterization reports for other areas in Quebec,25,33 the occurrence, distribution and ecotoxicological risk to aquatic organisms of pesticides and their transformation products remain essentially unknown in the surface waters of western Montérégie, where smaller streams and rivers are located compared to central and eastern Montérégie.
176 km2 and accommodating over 1.5 million people, it is known for being the most significant agricultural hub in Quebec. The region has a diverse range of farms, covering nearly a quarter of Quebec's agricultural land. Montérégie dominates in grain corn and soybean production, contributing 62% and 48% respectively to Quebec's output. Additionally, it also represents a significant share of Quebec's processing vegetable production, with 78% of the total output.32 It is also the major contributor to apple production in Quebec, accounting for 65% of the total yield.32 Based on a 2019 report on pesticides sales in Quebec,22 the sole Montérégie region accounted for 34% of the users who have been prescribed the top five pesticides with the highest potential risks (i.e., atrazine, chlorpyrifos, clothianidin, imidacloprid, and thiamethoxam). Despite the intensive use of pesticides and previous characterization reports for other areas in Quebec,25,33 the occurrence, distribution and ecotoxicological risk to aquatic organisms of pesticides and their transformation products remain essentially unknown in the surface waters of western Montérégie, where smaller streams and rivers are located compared to central and eastern Montérégie.
The main objective of the present study is to investigate the presence and spatial distribution of pesticides and their transformation products in surface waters of western Montérégie. Forty-eight commonly used pesticides (including herbicides, insecticides, and fungicides) and eight transformation products were investigated in five main watersheds of western Montérégie (including the Saint-Louis River, the Châteauguay River, the Saint-Régis River, the Tortue River, and the Saint-Jacques River) for two summer seasons (2019 and 2021). Furthermore, ecotoxicological risks to aquatic organisms were assessed by comparing the highest concentrations of individual compounds with Quebec's acute aquatic life criterion (AALC) and the chronic aquatic life criterion (CALC). This study provides much-needed data on the state of pesticide and transformation product contamination status in the western Montérégie region and highlights the need to rationalize the management of pesticides during the agricultural cycle.
2 Methods
2.1 Standards and reagents
In the present study, 56 compounds were selected for the investigation, including 27 herbicides, 12 insecticides, 9 fungicides, and 8 transformation products. Further information about the pesticide treatment method and the mode of action can be found in Table S1.† High-purity (≥95%) certified chemical standards and isotope-labelled internal standards were purchased from Sigma Aldrich (St. Louis, MO, USA), Toronto Research Chemicals Inc. (North York, ON, Canada), Cambridge Isotope Laboratories, Inc. (Andover, MA, U.S.A.) or Santa Cruz Biotechnology (Dallas, TX, USA). Details on chemical standards, isotope-labelled internal standards, solvents, and other materials are given in ESI (Tables S2–S4†).2.2 Study sites and sample collection
The geographical distribution of sampling sites is presented in Fig. 1. The western Montérégie study area is situated in southwestern Quebec and is bounded to the north by the island of Montreal, separated by the St. Lawrence River. To the south, it shares a border with the United States. Covering a total area of 4165 km2, with 3713 km2 being terrestrial and as of 2007, the region had a population of approximately 401![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133.34 It features a humid continental climate, with an average temperature of 7.1 °C. Precipitation is highest during the summer months, with an annual average rainfall of 944 mm.35 The majority of the territory (3155 km2) is dedicated to agricultural use, with large farms (crop or animal farming) occupying 2344.3 km2 (equivalent to 63.1%).36 The predominant crops cultivated in this region include corn and soybean.37 The Châteauguay, the Saint-Louis, the Saint-Jacques, the Tortue, and the Saint-Régis rivers constitute the five major watersheds in western Montérégie. Detailed information on each watershed is provided in Table S5.† The drainage water from the crop fields flows into these tributaries before ultimately reaching the St. Lawrence River.
133.34 It features a humid continental climate, with an average temperature of 7.1 °C. Precipitation is highest during the summer months, with an annual average rainfall of 944 mm.35 The majority of the territory (3155 km2) is dedicated to agricultural use, with large farms (crop or animal farming) occupying 2344.3 km2 (equivalent to 63.1%).36 The predominant crops cultivated in this region include corn and soybean.37 The Châteauguay, the Saint-Louis, the Saint-Jacques, the Tortue, and the Saint-Régis rivers constitute the five major watersheds in western Montérégie. Detailed information on each watershed is provided in Table S5.† The drainage water from the crop fields flows into these tributaries before ultimately reaching the St. Lawrence River.
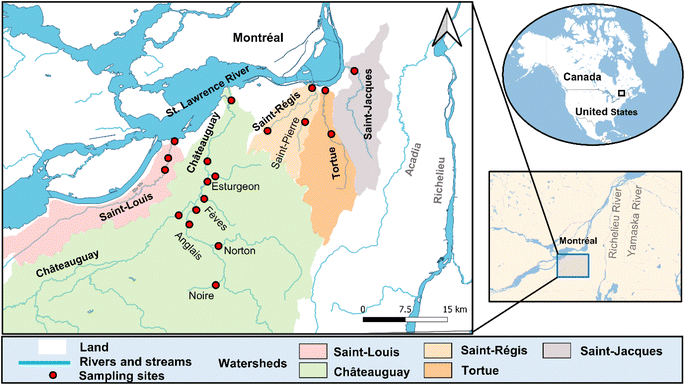 | ||
| Fig. 1 Map of the study area and sampling sites (see also Table S5† for watershed size and cropland occupation, and Table S6† for sampling coordinates). | ||
Sampling campaigns were carried out following the first heavy rainfall in the July of 2019 and 2021 across five watersheds located in the west of Montérégie (i.e. the Saint-Louis watershed, the Châteauguay watershed, the Saint-Régis watershed, the Tortue watershed, and the Saint-Jacques watershed). The reasons for such sampling timing are two-fold: first, pesticides are intensively applied from late April to late June in Québec,38 and the highest pesticide levels in surface water are most likely to occur following the rainwater runoffs in July39 (Precipitation data are presented in Fig. S1†). Second, rivers were at their lowest flowrates during the year (i.e., dry season) in summer (Fig. S2†). Therefore, minimal dilution effects were anticipated for pesticides and hence increased the likelihood of detecting the peak concentration of pesticides and their transformation products in surface water. A total of 29 samples were collected from 11 rivers, including 19 samples from 2019 and 10 samples from 2021. In 2019, sampling was conducted at both upstream locations (in 5 main rivers) and downstream locations (in all 11 rivers). Higher pesticides concentrations were expected downstream compared to the upstream areas. This is mainly because most rivers pass through agricultural zones. In 2021, sampling was only conducted downstream for the assessment of ecotoxicological risks to protect the aquatic organisms. Field blank samples were also collected in parallel. For all samples, a pre-rinsed amber glass bottle (1 L) was filled to the brim, sealed, and transported to the laboratory. The sample was filtered on a 0.45 μm regenerated cellulose filter to remove particles and then stored at 4 °C until analysis. Regenerated cellulose filters were selected for the present study because of their higher recoveries compared to other types of filters (Table S7†).
2.3 Sample preparation and analysis
Sample preparation procedures were adapted from previous studies.25,28 Three distinct sample preparation methods were employed based on the target compounds and are described in the following subsections. Full details on the instrument acquisition methods, including chromatographic columns, chromatographic gradients and mobile phases, source parameters, and high-resolution mass spectrometry settings (UHPLC-HRMS Orbitrap Q-Exactive, Thermo Scientific, Waltham, MA, U.S.A.) are provided in ESI (Tables S8–S10†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v) water: methanol mixture, ultrasonicated for 5 min and vortexed for 0.5 min to ensure the complete redissolution of analytes. Finally, the sample was filtered through 0.2 μm regenerated cellulose filter and submitted to UHPLC-HRMS analysis (injection volume: 50 μL).
20 (v/v) water: methanol mixture, ultrasonicated for 5 min and vortexed for 0.5 min to ensure the complete redissolution of analytes. Finally, the sample was filtered through 0.2 μm regenerated cellulose filter and submitted to UHPLC-HRMS analysis (injection volume: 50 μL).
2.4 Quality assurance and quality control
Method detection limits were in the range of 0.001–5 ng L−1 (Table S13†). Matrix-matched calibration curves were constructed in a blank surface water matrix from the Saint-Maurice River and had suitable determination coefficients (R2 > 0.9952) (Table S11†). The accuracy of continued calibration verification (CCV) standards injected after every 10 samples was in the range of 74–112% (Table S11†). Whole-method accuracy was evaluated by adding certified standards and isotope-labelled internal standards to a surface water matrix (n = 5) prior to the extraction and analysis steps. The accuracies of all targeted analytes were in the range of 89–120% (Table S11†). Intraday (n = 5) and interday (n = 15) precisions (% RSD) were in the range of 1–12 and 2–18%, respectively.The absolute extraction efficiencies of methods without internal standards correction were evaluated with a separate set of experiments. Most target analytes showed absolute extraction efficiencies in the range of 61–118% except for dinotefuran (51%) and chlorpyrifos (131%) (Table S12†). However, these two compounds showed acceptable method accuracies when using surrogate internal standards correction.
Field blanks and method blanks were performed by filling amber glass bottles (1 L) with HPLC-grade water on the sampling site and in the laboratory, respectively. Blank water samples were spiked with internal standards and prepared in the same way as field surface water samples. Blanks remained free of target pesticides with a few exceptions (11 pesticides with detected concentration ranges of 0.01–0.55 ng L−1 in blanks). Blank concentrations were subtracted from the field samples in the corresponding batch to avoid reporting false positives.
2.5 Cumulative concentrations
Cumulative concentrations were calculated for each river for each year by adding up the concentrations of each pesticide and/or transformation product (TP) detected downstream (eqn (1)).| Cumulative concentrations = ∑Cpesticide + ∑CTP | (1) |
2.6 Ecotoxicological risk assessment
We calculated the potential environmental impacts using an ecotoxicological risk quotient (RQ) calculated according to eqn (2).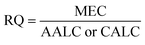 | (2) |
2.7 Statistical analysis and GIS
Due to the non-normal distribution of the data set, median values were used to evaluate the occurrence of pesticides. The other data were reported as averages. Non-detect data and data falling below the calculated method detection limit were treated as 0 for summary statistics. Significance tests were conducted using Wilcoxon rank-sum test with a significance level of p < 0.05. The Quantum GIS software (QGIS version 3.22.5) was used as a geographic information system and base maps were retrieved from MapTiler Planet.403 Results and discussion
3.1 Occurrence of pesticides in surface water
The detection frequencies, minimum, maximum, median, and mean concentrations of targeted pesticides are summarized in Table 1. Note that only downstream data for both 2019 and 2021 were included in Table 1 as the downstream section exhibited generally higher pesticide concentration compared to the upstream section. The upstream results of 2019 are presented in Table S15.† Overall, 44 out of 48 pesticides and all 8 transformation products were detected in surface water. Specifically, 24 out of 27 herbicides, 11 out of 12 insecticides, and all 9 fungicides were detected. Their occurrence is discussed in detail in the following subsections.| MDL | Summer 2019 | Summer 2021 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DF (%) | Min (ng L−1) | Max (ng L−1) | Median (ng L−1) | Mean (ng L−1) | DF (%) | Min (ng L−1) | Max (ng L−1) | Median (ng L−1) | Mean (ng L−1) | ||
| Herbicides | |||||||||||
| Glyphosate | 2 | 100 | 87 | 4095 | 681 | 1137 | 100 | 134 | 3261 | 744 | 1085 |
| (S)-Metolachlor | 0.08 | 100 | 36 | 2519 | 858 | 906 | 100 | 6 | 685 | 122 | 190 |
| 2,4-D | 0.1 | 100 | 6 | 189 | 53 | 69 | 100 | 6 | 1094 | 84 | 211 |
| Metribuzin | 0.37 | 100 | 28 | 203 | 92 | 96 | 100 | 9 | 157 | 38 | 58 |
| Atrazine | 0.001 | 100 | 12 | 426 | 74 | 132 | 100 | 5 | 49 | 24 | 25 |
| Bentazon | 0.3 | 100 | 3 | 574 | 46 | 139 | 80 | 8 | 213 | 34 | 65 |
| MCPA | 0.05 | 100 | 3 | 530 | 24 | 85 | 100 | 2 | 1627 | 30 | 191 |
| Mesotrione | 0.328 | 100 | 6 | 256 | 23 | 63 | 50 | 2 | 26 | 9 | 13 |
| Prometryn | 0.05 | 100 | 0.1 | 178 | 26 | 47 | 100 | 1 | 407 | 41 | 109 |
| Dimethenamid | 0.034 | 100 | 3 | 174 | 15 | 40 | 100 | 1 | 218 | 6 | 36 |
| Simazine | 0.02 | 100 | 0.4 | 19 | 2 | 4 | 100 | 0.5 | 8 | 1 | 2 |
| Flumetsulam | 0.032 | 100 | 0.2 | 21 | 3 | 5 | 60 | 0.2 | 9 | 0.4 | 2 |
| Cyanazine | 4.5 | 91 | 9 | 44 | 17 | 21 | 80 | 5 | 28 | 15 | 14 |
| Fomesafen | 0.05 | 91 | 2.6 | 45 | 7 | 12 | 100 | 1 | 294 | 11 | 41 |
| Linuron | 0.01 | 91 | 0.02 | 553 | 4 | 71 | 100 | 0.3 | 1492 | 93 | 245 |
| Mecoprop | 0.05 | 91 | 0.1 | 7 | 1 | 2 | 90 | 1 | 135 | 20 | 35 |
| Topramezone | 0.5 | 91 | 2 | 4 | 2 | 3 | 30 | 1 | 3 | 1 | 2 |
| Pendimethalin | 0.5 | 73 | 4 | 18 | 7 | 9 | 60 | 1 | 24 | 2 | 6 |
| Bromoxynil | 0.026 | 73 | 0.1 | 3 | 0.3 | 1 | 90 | 0.4 | 2 | 1 | 1 |
| Imazethapyr | 0.01 | 64 | 43 | 43 | 43 | 43 | 90 | 2 | 36 | 8 | 10 |
| Dicamba | 5 | 45 | 7 | 67 | 27 | 33 | 60 | 7 | 65 | 19 | 25 |
| Hexazinone | 0.07 | 18 | 0.2 | 3 | 1.7 | 2 | — | — | — | — | — |
| Nicosulfuron | 0.05 | 9 | 21 | 21 | 21 | 21 | 30 | 0.1 | 8 | 0.4 | 3 |
| Glufosinate | 0.6 | — | — | — | — | — | 50 | 8 | 14 | 12 | 11 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Insecticides | |||||||||||
| Clothianidin | 0.1 | 100 | 11 | 265 | 32 | 65 | 100 | 4 | 36 | 11 | 14 |
| Imidacloprid | 0.015 | 100 | 1 | 161 | 23 | 57 | 100 | 1 | 157 | 10 | 40 |
| Chlorantraniliprole | 0.1 | 100 | 2 | 45 | 6 | 10 | 100 | 4 | 103 | 25 | 39 |
| Thiamethoxam | 0.01 | 91 | 2 | 514 | 22 | 123 | 100 | 0.5 | 94 | 23 | 31 |
| Acetamiprid | 0.01 | 64 | 0.07 | 5 | 0.1 | 1 | 80 | 0.02 | 1 | 0.1 | 0.2 |
| Chlorpyrifos | 1.2 | 64 | 3 | 6 | 3 | 4 | 20 | 1 | 1 | 1 | 1 |
| Flonicamid | 0.31 | 27 | 1 | 19 | 5 | 8 | 90 | 1 | 5 | 1 | 2 |
| Nitenpyram | 0.046 | 27 | 0.1 | 0.2 | 0.1 | 0.1 | 20 | 0.2 | 0.2 | 0.2 | 0.2 |
| Thiacloprid | 0.05 | 27 | 0.1 | 1 | 0.2 | 0.4 | 50 | 0.1 | 0.4 | 0.1 | 0.2 |
| Carbaryl | 0.025 | — | — | — | — | — | 30 | 1 | 2 | 2 | 2 |
| Dinotefuran | 0.018 | — | — | — | — | — | 10 | 0.3 | 0.3 | 0.3 | 0.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Fungicides | |||||||||||
| Azoxystrobin | 0.02 | 100 | 0.1 | 25 | 5 | 7 | 100 | 0.1 | 186 | 19 | 51 |
| Propiconazole | 0.19 | 100 | 1 | 11 | 2 | 3 | 90 | 1 | 29 | 3 | 6 |
| Fluxapyroxad | 0.01 | 100 | 1 | 4 | 1 | 2 | 100 | 0.1 | 19 | 3 | 6 |
| Tebuconazole | 0.018 | 100 | 0.5 | 7 | 1 | 2 | 100 | 0.3 | 81 | 1 | 9 |
| Carbendazim | 0.015 | 100 | 0.1 | 8 | 0.7 | 2 | 100 | 0.3 | 17 | 2 | 5 |
| Metconazole | 0.05 | 73 | 0.1 | 1 | 0.2 | 0.3 | 30 | 0.2 | 0.2 | 0.2 | 0.2 |
| Pyrimethanil | 0.01 | 82 | 0.01 | 1 | 0.03 | 0.2 | 100 | 0.1 | 12 | 1 | 2 |
| Fluazinam | 0.1 | 9 | 0.2 | 0.2 | 0.2 | 0.2 | 60 | 0.2 | 2 | 1 | 1 |
| Chlorothalonil | 0.5 | 9 | 1 | 1 | 1 | 1 | — | — | — | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Transformation products | |||||||||||
| Metolachlor ethanesulfonic acid | 0.007 | 100 | 447 | 2489 | 1248 | 1287 | 100 | 180 | 2070 | 604 | 734 |
| AMPA | 2 | 100 | 153 | 5836 | 934 | 1564 | 100 | 162 | 4902 | 647 | 1452 |
| Metolachlor oxanilic acid | 0.008 | 100 | 143 | 944 | 447 | 500 | 100 | 82 | 598 | 259 | 274 |
| Desethylatrazine | 0.13 | 100 | 16 | 60 | 35 | 35 | 100 | 12 | 37 | 23 | 23 |
| Atrazine-2-hydroxy | 0.02 | 100 | 9 | 52 | 28 | 31 | 100 | 8 | 47 | 21 | 24 |
| Desisopropylatrazine | 0.058 | 100 | 5 | 23 | 12 | 13 | 100 | 4 | 21 | 10 | 11 |
| Desnitro-imidacloprid | 0.024 | — | — | — | — | — | 90 | 0.4 | 10 | 2 | 3 |
| 3,5,6-Trichloro-2-pyridinol | 0.2 | — | — | — | — | — | 10 | 0.2 | 0.2 | 0.2 | 0.2 |
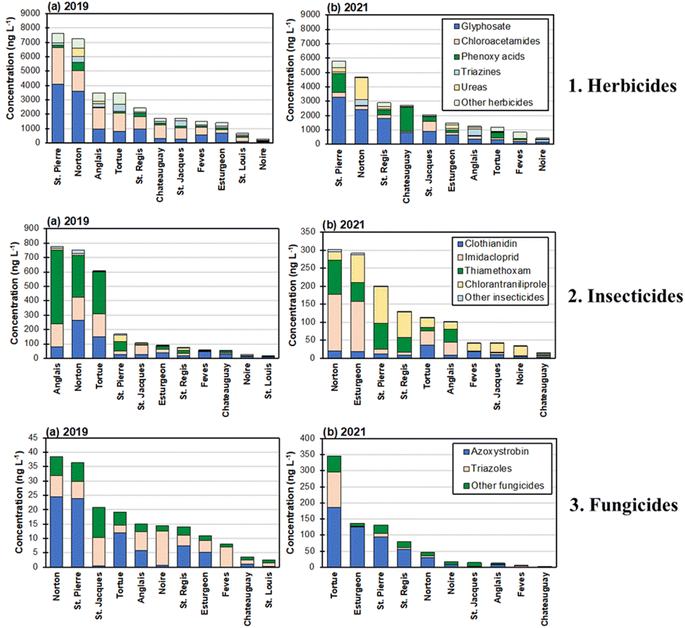
In terms of concentrations levels, S-metolachlor was the most abundant herbicide in surface water in 2019 with a median concentration of 858 ng L−1, followed by glyphosate (median: 681 ng L−1), metribuzin (median: 92 ng L−1), atrazine (median: 74 ng L−1), and 2,4-D (median: 53 ng L−1). In 2021, glyphosate was the most abundant herbicide in surface water with a median concentration of 744 ng L−1, followed by S-metolachlor (median: 122 ng L−1), linuron (median: 93 ng L−1), 2,4-D (median: 84 ng L−1), and prometryn (median: 41 ng L−1). S-metolachlor, glyphosate, and 2,4-D were among the most abundant herbicides in surface water in both sampling campaigns (2019 and 2021). These results agree with sales records where S-metolachlor and glyphosate appear among the most sold pesticides in Quebec (Details on sales scale of each pesticide in Quebec for 2018, 2019, and 2020 are given in Table S16†). In particular, 2,4-D, a potential atrazine substitute, had a constantly growing sales volume from 2017 to 2020.22,23,41 A significant increase in concentration from 2019 to 2021 was observed for linuron (median: 4 ng L−1versus 93 ng L−1) (Wilcoxon-test, p < 0.05), which may be attributed to its increasing use in Quebec as a replacement for glyphosate and atrazine.23 Finally, the median concentration of atrazine decreased from 74 ng L−1 in 2019 to 24 ng L−1 in 2021, possibly because atrazine is being phased out from the Quebec market in recent years.22,23,41
With respect to the concentrations of transformation products, metolachlor ethanesulfonic acid (ESA) was the most abundant one (median: 1248 ng L−1) in 2019 among all the investigated herbicide transformation products, followed by AMPA (median: 934 ng L−1) and metolachlor oxanilic acid (OA) (median: 447 ng L−1). In 2021, AMPA exhibited the highest concentration (median: 647 ng L−1), followed by metolachlor ESA (median: 604 ng L−1) and metolachlor OA (median: 259 ng L−1). Given the fact that AMPA is the primary transformation product of glyphosate and metolachlor ESA/metolachlor OA were transformation products of S-metolachlor,42,43 the high concentrations of these three transformation products align with detections of the parent compounds glyphosate and S-metolachlor at high levels. In fact, metolachlor ESA even had a higher concentration than its parent compound (i.e., S-metolachlor) in both 2019 and 2021. Such a result may be attributed to the higher persistence and low retention factor of the ESA transformation product in soils,30 enabling its transportation through soil erosion and surface runoff, as well as its replenishment from groundwater to surface water during low-flow periods in the river.
Transformation products of atrazine (i.e., desethylatrazine, atrazine-2-hydroxy, desisopropylatrazine) remained the lowest in terms of median concentration (10–35 ng L−1) among all the investigated herbicide transformation products in both 2019 and 2021. However, the summed median concentration of the transformation products is higher than that of atrazine for both 2019 (80 ng L−1versus 74 ng L−1) and 2021 (63 ng L−1versus 24 ng L−1). This could be explained by the equal or higher persistence of these metabolites than atrazine in surface water and soil.31,44,45
The concentration of insecticides was noticeably lower than that of herbicides. Clothianidin was the most abundant in terms of median concentration (32 ng L−1) in 2019, followed by imidacloprid (23 ng L−1), thiamethoxam (22 ng L−1), chlorantraniliprole (6 ng L−1), and flonicamid (5 ng L−1). In 2021, chlorantraniliprole was found at the highest median concentration (25 ng L−1), followed by thiamethoxam (23 ng L−1), clothianidin (11 ng L−1), imidacloprid (10 ng L−1), and carbaryl (2 ng L−1). Three neonicotinoid insecticides (i.e., clothianidin, thiamethoxam, imidacloprid) were among the most abundant insecticides for both 2019 and 2021, a result that agrees with their widespread usage in Quebec.22,23,33,41,46 Furthermore, a significant increase in concentration was observed for chlorantraniliprole from 2019 to 2021 (Wilcoxon-test, p < 0.05). This is because the use of chlorantraniliprole is on the rise in Quebec as a substitute for neonicotinoid pesticides.21
3.2 Spatial distribution of pesticides in surface water
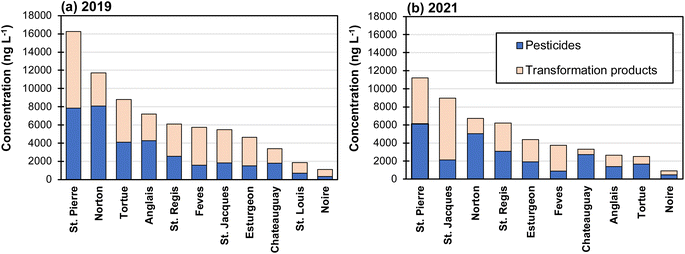
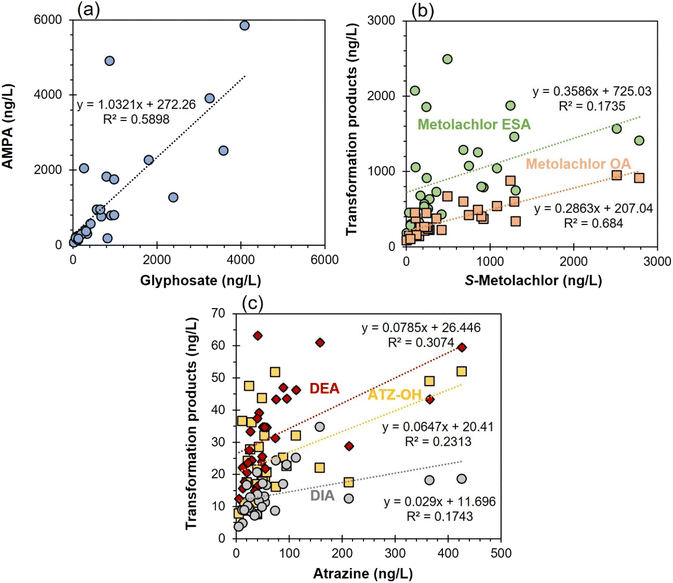
To assess the spatial distribution of pesticides in the surface water of western Montérégie, the cumulative concentrations of all the investigated pesticides and transformation products are illustrated against the investigated rivers in Fig. 2. Note that only downstream data were included in this analysis because the cumulative concentration was generally shown to be higher in the downstream section compared to the upstream section (Fig. S3†). The results demonstrate that the St. Pierre River was the most contaminated river in both 2019 and 2021. In contrast, the Noire River constantly showed the lowest cumulative concentrations during both sampling campaigns. The different contamination levels in the rivers were mainly due to the vegetation type around the watershed: the St. Pierre River is mainly adjacent to cropland21 while the Noire River is principally surrounded by forest according to the land use of the Châteauguay watershed.47 Furthermore, the results demonstrated that transformation products represent a significant proportion of cumulative concentrations. For example, the cumulative concentration of the 8 transformation products accounted for on average 51% of the total concentration of the 56 investigated compounds. Including only parent compounds and neglecting transformation products could therefore lead to severely underestimating the total load of pesticides in surface water.To assess the distribution of herbicide transformation products in the surface water of western Montérégie, the concentrations of transformation products at all the sampling sites were presented against their parent compounds in Fig. 4. Generally, the concentrations of the investigated transformation products are proportional to the concentrations of their parent compounds (R2: 0.17–0.68). Among them, the correlation coefficients between S-metolachlor and metolachlor OA, and between glyphosate and AMPA, are 0.6840 and 0.5898, respectively, whereas the correlations between atrazine and DIA, and between S-metolachlor and metolachlor ESA, are only 0.1743 and 0.1735, respectively. The weak correlation between the latter two transformation products and their parent molecules suggests that other factors may be influencing the concentrations of these transformation products, such as soil affinity, migration dynamics, the presence of groundwater recharge etc. Furthermore, the concentrations of AMPA, metolachlor ESA, and metolachlor OA were higher than the concentrations of their parent compounds (i.e., glyphosate and S-metolachlor). For example, the concentration of AMPA in the St. Jacques River was much higher than that of glyphosate in both 2019 (approximately 8 times higher) and 2021 (approximately 6 times higher) (Fig. S4†). This is because the sampling site for the St. Jacques River was close to urban areas and AMPA could also originate from municipal discharge that contained phosphonic acids commonly found in some detergents.49 A similar result was also observed for the Fèves River and the Esturgeon River, where the concentration of metolachlor ESA was much higher than that of S-metolachlor in both 2019 and 2021 (Fig. S5†). This can be attributed to the recharge of metolachlor ESA from groundwater to surface water during periods of low flowrate in these two tributaries within the Châteauguay watershed.30 Metolachlor ESA exhibited higher concentrations compared to metolachlor OA, and desethylatrazine (DEA) showed higher concentrations than atrazine-2-hydroxy (ATZ-OH) and desisopropylatrazine (DIA) (Wilcoxon-test, p < 0.05). This may be due to the higher resistance to degradation of metolachlor ESA50 and the higher leachability of DEA in soil drainage systems.51
3.3 Comparison with past studies in Québec and other provinces of Canada
In comparison with a previous study conducted by the Ministry of Environment of Quebec,21 where researchers investigated the occurrence of pesticides in central and eastern Montérégie, the sampling sites in the present study are mainly located in western Montérégie. Contrasting results were observed with regard to the occurrence and spatial distribution of pesticides in these regions. For example, some pesticides, such as glyphosate, AMPA, S-metolachlor, atrazine, clothianidin, and imidacloprid showed higher concentrations in the present study than that of Giroux et al.21 (approximately 1–4 times). On the other hand, some pesticides, such as bentazon, mesotrione, chlorantraniliprole and thiamethoxam showed higher concentrations in central and eastern Montérégie than that in the present study (approximately 2–21 times). Furthermore, Montiel-León et al.25 investigated the occurrence of pesticides in 15 tributaries of the St. Lawrence River. They observed higher levels of pesticides in south shore tributaries (Yamaska River) than north shore tributaries (e.g., Du Loup River, Yamachiche River, and Saint-Maurice River), corroborating the severe pesticide contamination in the region of Montérégie. Concentrations of some pesticides (e.g., glyphosate, AMPA, atrazine) in the present study are 5–56 times higher than those in the St. Lawrence River25 and 1–955 times higher than those in St. Lawrence Estuary and Gulf.28 This is because of the much higher water flow of the St. Lawrence River compared to the small streams/rivers studied in the present study. The role of river flow on pesticide discharge is further discussed in Section 3.4.The neonicotinoid insecticides (e.g., clothianidin, imidacloprid, and thiamethoxam) have also been detected in other Canadian waters. For example, concentrations of neonicotinoids (clothianidin, imidacloprid, and thiamethoxam) were reported to range from <0.2 ng L−1 to 10 μg L−1 (ref. 53) and from 0.2 ng L−1 to 838 ng L−1 (ref. 24) for southern Ontario and maritime region, respectively. By contrast, the concentrations in western Montérégie were found to be lower (0.5 ng L−1 to 514 ng L−1). Chlorantraniliprole has also been reported in other provinces of Canada.54 Past studies found that the concentration of chlorantraniliprole is at 2–155 ng L−1 in the Maritime region24 and 0.545 μg L−1 (median) in southwestern Ontario,26 both higher than the level found in the present study (2–103 ng L−1). Considering neonicotinoids are currently being substituted with chlorantraniliprole, close monitoring of the occurrence of chlorantraniliprole in waters should be warranted.
3.4 Implications for the pesticide discharge to the St. Lawrence River
To estimate the contribution of pesticide loading in the St. Lawrence River by the watersheds of western Montérégie, we evaluated the discharge of all the investigated pesticides and transformation products from five tributaries of western Montérégie using the downstream data of 2019 and 2021 (Table 2). The flow for each river was estimated using historical data or modelling data (Section S12†). Overall, the Châteauguay River was the main contributor to the pesticide discharge to the St. Lawrence River with a range of 47–515 kg pesticides per month. In contrast, the St. Régis, St. Jacques and Tortue Rivers had similar and lowest contributions with an average discharge of 2 kg pesticides/month.| Rivers | Loading (kg per month) | |||
|---|---|---|---|---|
| Min | Max | Median | Mean | |
| Châteauguay | 47 | 515 | 67 | 84 |
| Saint-Louis | 10 | 16 | 13 | 13 |
| Saint-Régis | 2 | 5 | 2 | 2 |
| Saint-Jacques | 1 | 7 | 1 | 2 |
| Tortue | 1 | 12 | 1 | 2 |
3.5 Ecotoxicological risk assessment
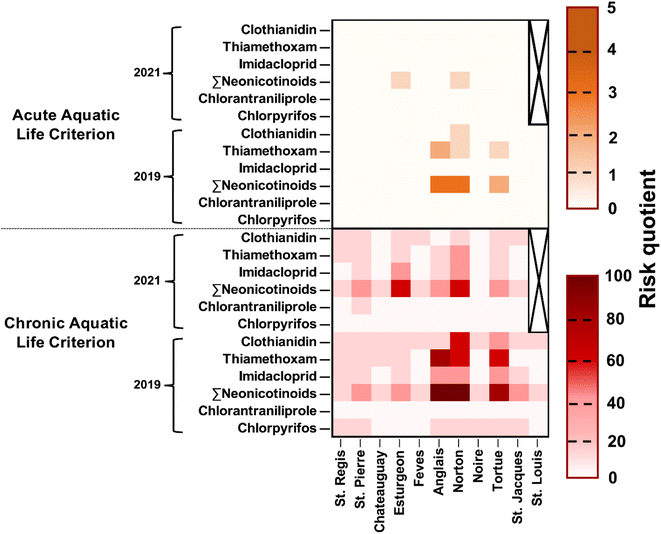
To assess the ecotoxicological risks imposed by the pesticides present in surface waters of western Montérégie, the risk quotients of clothianidin, thiamethoxam, imidacloprid, total neonicotinoids, chlorantraniliprole, and chlorpyrifos are presented in Fig. 5 for all the investigated rivers. The assessment for the other pesticides was omitted due to the lack of AALC and CALC values or their low risks (RQ < 1). It should be noted that the criterion for atrazine covers both atrazine itself and its transformation products. However, the provincial guidelines do not specify criteria for other transformation products. Results show that total neonicotinoids exhibited the highest ecotoxicological risk in the surface water of western Montérégie with an average CALC (criterion: 8.3 ng L−1) risk quotient of 28 and 12 for 2019 and 2021, respectively. The concentration of total neonicotinoids even surpassed the AALC criterion (200 ng L−1) at some sampling sites with the average AALC risk quotient of 3 and 1 for 2019 and 2021, respectively. The high ecotoxicological risk of total neonicotinoids is mainly due to three neonicotinoid insecticides: clothianidin, thiamethoxam, and imidacloprid. Notably, thiamethoxam has a significantly higher average risk quotient in 2019, with values of 21 and 2 based on CALC and AALC, respectively. In contrast, calculated risk quotients of chlorantraniliprole (criterion: 100 ng L−1) and chlorpyrifos (criterion: 2 ng L−1) were much lower. For instance, the average risk quotient for chlorantraniliprole was found to be 0.02 in 2019 and 1 in 2021 based on CALC.Regarding the spatial distribution of the ecotoxicological risk, the Norton River (i.e., Anglais's upstream river) and Anglais River were the rivers with the highest risks with an average CALC risk quotient of 27 and 25, respectively. The Anglais's mainstream river (i.e., Châteauguay) showed a lower risk compared to itself, an observation mainly resulting from the dilution effect. Finally, the Noire River was identified as the lowest in terms of ecotoxicological risk in the region of western Montérégie with an average CALC risk quotient of 1 and 0.4 for 2019 and 2021, respectively.
4 Conclusions
The present study investigated the occurrence and spatial distribution of pesticides and their transformation products in surface waters of western Montérégie, and for the first time, we assessed the ecotoxicological risks to the aquatic organisms originating from these pesticides. Overall, 9 herbicides (glyphosate, S-metolachlor, 2,4-D, metribuzin, atrazine, MCPA, prometryn, dimethenamid, simazine), 3 insecticides (clothianidin, imidacloprid, chlorantraniliprole), 4 fungicides (azoxystrobin, fluxapyroxad, tebuconazole, carbendazim), and 6 pesticide transformation products (metolachlor ESA, AMPA, metolachlor OA, desethylatrazine, atrazine-2-hydroxy, desisopropylatrazine) were the most detected compounds (detection frequency: 100%). Glyphosate (87–4095 ng L−1), S-metolachlor (6–2519 ng L−1), and 2,4-D (6–1094 ng L−1) were the most abundant pesticides in surface water. Although the concentrations of transformation products were proportional to the concentrations of their parent compounds, we found that transformation products accounted for 51% (on average) of the total concentration. This result reveals the abundance of transformation products in surface waters and therefore the need to assess the ecotoxicological risk of transformation products. We recommend that water quality monitoring project should incorporate not only the parent pesticide compounds but also their transformation products, particularly those with higher concentrations or toxicity, such as metolachlor-ESA and DEA. Additionally, ecotoxicological data are much needed for transformation products to develop water quality criteria and protect aquatic organisms. Further research and action are warranted to mitigate the environmental risks associated with pesticide use and their transformation products to safeguard water quality and aquatic biodiversity.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Dr Dinh Quoc Tuc for his assistance in the laboratory. The authors also extend their gratitude to Yannick Arnold Nombre and Henri Bouchard-Marcotte for their help in surface water sampling. We also acknowledge the Collectif écosanté sur les pesticides, les politiques et les alternatives (CREPPA) - FDE project - Environment and Climate Change Canada (ECCC) (L. Vandelac and L. Parent) and the Réseau de recherche en écotoxicologie du Québec (EcotoQ) for the PhD scholarship awarded to Xiameng Feng.References
- Food and Agriculture Organization Corporate Statistical Database, 2022, https://www.fao.org/faostat/en/#data/RP/visualize Search PubMed.
- R. W. Becker, D. S. Araújo, C. Sirtori, N. P. Toyama, D. A. Tavares and G. A. Cordeiro, et al., Pesticides in surface water from Brazil and Paraguay cross-border region: Screening using LC-QTOF MS and correlation with land use and occupation through multivariate analysis, Microchem. J., 2021, 168, 106502 CrossRef CAS.
- J. da Silva Sousa, H. O. do Nascimento, H. de Oliveira Gomes and R. F. do Nascimento, Pesticide residues in groundwater and surface water: Recent advances in solid-phase extraction and solid-phase microextraction sample preparation methods for multiclass analysis by gas chromatography-mass spectrometry, Microchem. J., 2021, 168, 106359 CrossRef CAS.
- Z. Liu, M. Solliec, I. Papineau, K. M. Lompe, M. Mohseni and P. R. Bérubé, et al., Elucidating the removal of organic micropollutants on biological ion exchange resins, Sci. Total Environ., 2022, 808, 152137 CrossRef CAS PubMed.
- C. Moschet, I. Wittmer, J. Simovic, M. Junghans, A. Piazzoli and H. Singer, et al., How a complete pesticide screening changes the assessment of surface water quality, Environ. Sci. Technol., 2014, 48(10), 5423–5432 CrossRef CAS PubMed.
- J. Potapowicz, D. Lambropoulou, C. Nannou, K. Kozioł and Ż. Polkowska, Occurrences, sources, and transport of organochlorine pesticides in the aquatic environment of Antarctica, Sci. Total Environ., 2020, 735, 139475 CrossRef CAS.
- K. R. Solomon, D. Houghton and S. A. Harris, Nonagricultural and residential exposures to pesticides, Scand. J. Work, Environ. Health, 2005, 74–81 Search PubMed.
- J.-M. Bollag and S.-Y. Liu, Biological transformation processes of pesticides, Pesticides in the Soil Environment: Processes, Impacts and Modeling, 1990, vol. 2, pp. 169–211 Search PubMed.
- H. D. Burrows, J. Santaballa and S. Steenken, Reaction pathways and mechanisms of photodegradation of pesticides, J. Photochem. Photobiol., B, 2002, 67(2), 71–108 CrossRef CAS.
- K. Fenner, S. Canonica, L. P. Wackett and M. Elsner, Evaluating pesticide degradation in the environment: blind spots and emerging opportunities, science, 2013, 341(6147), 752–758 CrossRef CAS PubMed.
- F. I. Y. Mostafa and C. S. Helling, Impact of four pesticides on the growth and metabolic activities of two photosynthetic algae, J. Environ. Sci. Health, Part B, 2002, 37(5), 417–444 CrossRef PubMed.
- J. R. Stenstrom, J. Kreuger and W. Goedkoop, Pesticide mixture toxicity to algae in agricultural streams? Field observations and laboratory studies with in situ samples and reconstituted water, Ecotoxicol. Environ. Saf., 2021, 215, 112153 CrossRef.
- R. A. Relyea and J. T. Hoverman, Interactive effects of predators and a pesticide on aquatic communities, Oikos, 2008, 117(11), 1647–1658 CrossRef.
- A. Bhatnagar, A. S. Yadav and N. Cheema, Genotoxic effects of chlorpyrifos in freshwater fish Cirrhinus mrigala using micronucleus assay, Adv. Biol., 2016, 2016, 9276963 Search PubMed.
- K. H. Macneale, P. M. Kiffney and N. L. Scholz, Pesticides, aquatic food webs, and the conservation of Pacific salmon, Front. Ecol. Environ., 2010, 8(9), 475–482 CrossRef.
- E. Amenyogbe, J.-s. Huang, G. Chen and Z. Wang, An overview of the pesticides' impacts on fishes and humans, Int. J. Aquat. Biol., 2021, 9(1), 55–65 Search PubMed.
- A. Jabbar and S. Mallick, Pesticides and Environment Situation in Pakistan, Sustainable Development Policy Institute, 1994 Search PubMed.
- H. M. van der Werf, Assessing the impact of pesticides on the environment, Agric., Ecosyst. Environ., 1996, 60(2–3), 81–96 CrossRef CAS.
- T. C. Marrs, Toxicology of insecticides to mammals, Pest Manage. Sci., 2012, 68(10), 1332–1336 CrossRef CAS PubMed.
- A. Sharma, V. Kumar, B. Shahzad, M. Tanveer, G. P. S. Sidhu and N. Handa, et al., Worldwide pesticide usage and its impacts on ecosystem, SN Appl. Sci., 2019, 1(11), 1–16 Search PubMed.
- I. Giroux, Présence de pesticides dans l'eau au Québec: Portrait et tendances dans les zones de maïs et de soya – 2018 à 2020, Ministère de l'Environnement et de la Lutte contre les changements climatiques, Direction de la qualité des milieux aquatiques, Québec, 2022, vol. 71 Search PubMed.
- MELCC, Bilan des ventes de pesticides au Québec pour l'année 2019 (Québec), Ministère de l'Environnement et de la Lutte contre les changements climatiques, 2021 Search PubMed.
- MELCC, Bilan des ventes de pesticides au Québec pour l'année 2020 (Québec), Ministère de l'Environnement et de la Lutte contre les changements climatiques, 2022 Search PubMed.
- B. Lalonde and C. Garron, Temporal and Spatial Analysis of Surface Water Pesticide Occurrences in the Maritime Region of Canada, Arch. Environ. Contam. Toxicol., 2020, 79(1), 12–22 CrossRef CAS PubMed.
- J. M. Montiel-León, G. Munoz, S. V. Duy, D. T. Do, M.-A. Vaudreuil and K. Goeury, et al., Widespread occurrence and spatial distribution of glyphosate, atrazine, and neonicotinoids pesticides in the St. Lawrence and tributary rivers, Environ. Pollut., 2019, 250, 29–39 CrossRef.
- M. Raby, L. Lissemore, G. Kaltenecker, D. Beaton and R. S. Prosser, Characterizing the exposure of streams in southern Ontario to agricultural pesticides, Chemosphere, 2022, 294, 133769 CrossRef CAS.
- Critères de qualité de l'eau de surface, Ministère de l'Environnement et de la Lutte contre les changements climatiques, 2023, https://www.environnement.gouv.qc.ca/eau/criteres_eau/index.asp Search PubMed.
- J.-C. Picard, G. Munoz, S. V. Duy and S. Sauvé, Longitudinal and vertical variations of waterborne emerging contaminants in the St. Lawrence Estuary and Gulf during winter conditions, Sci. Total Environ., 2021, 777, 146073 CrossRef CAS.
- A. Kortenkamp and M. Faust, Regulate to reduce chemical mixture risk, Science, 2018, 361(6399), 224–226 CrossRef CAS PubMed.
- S. Huntscha, H. Singer, S. Canonica, R. P. Schwarzenbach and K. Fenner, Input Dynamics and Fate in Surface Water of the Herbicide Metolachlor and of its Highly Mobile Transformation Product Metolachlor ESA, Environ. Sci. Technol., 2008, 42(15), 5507–5513 CrossRef CAS PubMed.
- N. D. Jablonowski, A. Schäffer and P. Burauel, Still present after all these years: persistence plus potential toxicity raise questions about the use of atrazine, Environ. Sci. Pollut. Res., 2011, 18, 328–331 CrossRef CAS.
- MAPAQ, Plan stratégique de la table filière des légumes de transformation 2016-2019, Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec, 2016 Search PubMed.
- I. Giroux, Présence de pesticides dans l'eau de surface au Québec – Zones de vergers et de cultures maraîchères, 2013 à 2016, Ministère de l'Environnement et de la Lutte contre les changements climatiques, Direction de la qualité des milieux aquatiques, Québec, 2017, vol. 47 Search PubMed.
- ISQ, Regional Statistical Bulletin (Edition 2008) CRÉ-Vallée-Du-Haut-Saint-Laurent, Institut de la statistique du Québec, 2008 Search PubMed.
- Canada Weather Stats, Environment and Climate Change Canada, 2023, https://www.weatherstats.ca/?zoom=8;lat=46.81;long=-71.22 Search PubMed.
- C. Akkari, Adaptation of Agriculture to Climate Change in Quebec: the co-construction of Agricultural Policies in the RCM of Haut-Richelieu, 2015 Search PubMed.
- MAPAQ, Profil régional de l'industrie bioalimentaire au Québec - Estimations pour l'année 2021, Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec, 2021 Search PubMed.
- L. Tuduri, D. Champoux, C. Jolly, J. Côté and M. Bouchard, Preventing chemical risks of pesticide use among Québec apple growers: Status report and measures to improve personal protection, Institut de recherche Robert-Sauvé en santé et en sécurité du travail, 2018 Search PubMed.
- I. Giroux and L. Pelletier, Présence de pesticides dans l'eau au Québec: bilan dans quatre cours d'eau de zones en culture de maïs et de soya en 2008, 2009 et 2010, Ministère du Développement durable, de l'Environnement et des Parcs, Direction du suivi de l'état de l'environnement, Québec, 2012, vol. 46 Search PubMed.
- MapTiler Planet, https://www.maptiler.com/planet/ Search PubMed.
- MELCC, Bilan des ventes de pesticides au Québec pour l'année 2018 (Québec), Ministère de l'Environnement et de la Lutte contre les changements climatiques, 2020 Search PubMed.
- L. Rivard and E. M. Branch, Environmental fate of metolachlor, Chemistry, 2003, 51218(45), 42 Search PubMed.
- J. Schuette, Environmental fate of glyphosate, Environ. Monit. Pest Manage., 1998, 1(1), 1–13 Search PubMed.
- N. D. Jablonowski, S. Koeppchen, D. Hofmann, A. Schaeffer and P. Burauel, Spatial distribution and characterization of long-term aged 14C-labeled atrazine residues in soil, J. Agric. Food Chem., 2008, 56(20), 9548–9554 CrossRef CAS PubMed.
- L. Yang and Y. Zhang, Effects of atrazine and its two major derivatives on the photosynthetic physiology and carbon sequestration potential of a marine diatom, Ecotoxicol. Environ. Saf., 2020, 205, 111359 CrossRef CAS PubMed.
- I. Giroux, Présence de pesticides dans l'eau au Québec : Portrait et tendances dans les zones de maïs et de soya – 2015 à 2017, Ministère de l'Environnement et de la Lutte contre les changements climatiques, Direction de la qualité des milieux aquatiques, Québec, 2019 Search PubMed.
- MELCC, Atlas du bassin versant de la rivière Châteauguay, 2023 Search PubMed.
- R. Kruć-Fijałkowska, K. Dragon, D. Drożdżyński and J. Górski, Seasonal variation of pesticides in surface water and drinking water wells in the annual cycle in western Poland, and potential health risk assessment, Sci. Rep., 2022, 12(1), 3317 CrossRef PubMed.
- D. W. Kolpin, E. M. Thurman, E. A. Lee, M. T. Meyer, E. T. Furlong and S. T. Glassmeyer, Urban contributions of glyphosate and its degradate AMPA to streams in the United States, Sci. Total Environ., 2006, 354(2–3), 191–197 CrossRef CAS PubMed.
- P. Phillips, G. Wall, E. Thurman, D. Eckhardt and J. Vanhoesen, Metolachlor and its metabolites in tile drain and stream runoff in the Canajoharie Creek watershed, Environ. Sci. Technol., 1999, 33(20), 3531–3537 CrossRef CAS.
- H. Pionke and D. Glotfelty, Contamination of groundwater by atrazine and selected metabolites, Chemosphere, 1990, 21(6), 813–822 CrossRef CAS.
- D. Loser, K. Grillberger, M. G. Hinojosa, J. Blum, Y. Haufe and T. Danker, et al., Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling, Arch. Toxicol., 2021, 95, 3695–3716 CrossRef CAS PubMed.
- J. Struger, J. Grabuski, S. Cagampan, E. Sverko, D. McGoldrick and C. H. Marvin, Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada, Chemosphere, 2017, 169, 516–523 CrossRef CAS PubMed.
- J. Toth, M. Pineda and V. Yargeau, Fast and simplified quantitative multiresidue analytical method for pesticides in surface waters by UHPLC-MS/MS with online sample preparation, Chemosphere, 2023, 318, 137962 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3va00231d |
| This journal is © The Royal Society of Chemistry 2024 |