Open Access Article
Open Access ArticleEnvironmental risk assessment of the use of zinc oxide medicated feeds for weaning piglets in the UK
Adam
Peters
 *a,
Graham
Merrington
a,
Ken
Stapleton
b and
Stephen
Lofts
*a,
Graham
Merrington
a,
Ken
Stapleton
b and
Stephen
Lofts
 c
c
aWCA Environment Ltd, Faringdon, Oxfordshire, UK. E-mail: Adam.Peters@wca-consulting.com
bChanelle Pharma, Loughrea, Co. Galway, Ireland
cCEH UK, Lancaster Environment Centre, Lancaster University, Lancaster, UK
First published on 20th March 2024
Abstract
Concerns over environmental impacts resulting from the use of zinc oxide containing medicines for weaning piglets led to the withdrawal of the authorisations for these products in the EU. In order to better understand these issues more detailed assessments were conducted for the UK, taking account of the fate of zinc in the environment and its bioavailability to ecological receptors. Four regional scenarios covered the main pig farming areas in the UK and the emission scenario was based on current agricultural practices in the UK. The fate and transport of zinc in the environment was modelled using the Intermediate Dynamic Model for Metals, and the toxicity of zinc in the environment was assessed based on current UK regulatory practices. The model takes account of historic additions of metals to the soils to calculate current and future metal levels in the environment. Whilst three of the four regional scenarios predicted a marginal risk, or no risk, to soils after 50 years of use one of the scenarios indicated a risk to surface waters prior to the use of zinc oxide medicated treatments for weaning piglets, and risks to local soils within 10 years of use. Further site-specific assessments were conducted for this region and one of the other regions, based on site specific emission scenarios, soil and surface waters characteristics. These two site-specific assessments revealed that the modelling results were accurate or conservative depending on the assumptions made about historic inputs of metals to agricultural soils from manure spreading, and that the regional scenario that resulted in significant predicted risks to surface waters did not reflect the actual conditions at the local pig farming sites considered. Comparisons between measured concentrations of copper and zinc at pig farming sites suggest that historic agricultural inputs have been an important source of these metals to agricultural soils at some sites. The limited data available for validation suggest that the IDMM is able to provide accurate predictions of metal levels in both soils and surface waters, but that there is significant uncertainty associated with historic inputs of metals to the soils.
Environmental significanceThis manuscript addresses the use of zinc-based medications that have been used in pig rearing and in recent years have been subject to restrictions at a European level due, at least in part, to concerns about the potential effects on the environment. This study uses the best currently available science, taking account of the fate and transport of zinc in the environment, and the bioavailability of zinc in soils, surface waters, and sediments, to assess the potential risks posed by this use of zinc over extended time periods. The study also includes site-specific assessments, including some monitoring of the levels of zinc at the sites, to evaluate the reliability of the model predictions. |
Background
Zinc oxide has been widely used as a medicine for the treatment of diarrhoea in weaning piglets.1 In 2017, the European Commission (EC) decided to withdraw the marketing authorisations of veterinary medicines containing zinc oxide for oral administration to food-producing animals by 26 June 2022. The concerns that led to this measure being taken were related to accumulation of zinc in the environment, and antimicrobial resistance.2,3 The UK has also taken similar steps to reduce the use of zinc oxide as a medicinal feed supplement, although zinc oxide products that were already in the supply chain can continue to be used until their end of shelf life. Alternatives to the use of zinc oxide to prevent diarrhoea in weaner piglets have been investigated in response to these measures.4,5Zinc oxide can also be used as a feed supplement for pigs and other animals to prevent mineral deficiencies. Consequently, measured information on the levels of zinc in the environment around agricultural facilities will reflect not only the medicinal uses, but also nutritional uses, as well as zinc from other sources such as galvanised steel, atmospheric deposition, and from natural sources. The majority of zinc that is provided to pigs through their diet is subsequently released into the environment via their manure. The relationship between zinc intake, dietary content, and faecal excretion in pigs has been reviewed previously.6 The study concluded that the excretion of zinc in manure is linearly related to the dietary intake rate of zinc, with no evident effect of different forms of zinc in the diet on the proportion of zinc excreted.
Given the high level of excretion of dietary zinc by pigs reducing their zinc intake may be desirable as a means of reducing the emission of trace metals into the environment from agricultural practices. However, some studies7,8 recommend that targeted treatments of pharmacological doses of zinc to pigs, for sows during the last 30 days of gestation, and for piglets one to two weeks post weaning, can result in improved animal health and performance. However, restricting zinc intakes at other times, in order to minimise any potential harm to the environment that occurs through the excretion of zinc, may be advisable because only a limited proportion of the dose is retained by the pigs.
Generic risk assessments of the potential risks posed by zinc from piglet medicine uses have been performed based on regionally relevant scenarios for local soils and surface waters for areas in the UK where pig farming is important. These risk assessments were conducted using a version of the Intermediate Dynamic Model for Metals (IDMM9) to predict the fate and transport of zinc added to surface soils from the spreading of pig manure.
Description of the Intermediate Dynamic Model for Metals
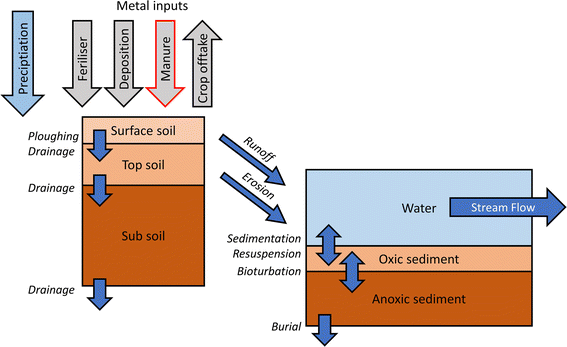
The IDMM is a model developed initially to allow the calculation of long-term metal accumulation in, and leaching from, soils.10,11 The model has subsequently been developed to simulate both long-term dynamics of metals within soil profiles and receiving waterbodies,12 with an emphasis on performing long term simulations to support exposure and risk assessment of metals added either intentionally or incidentally to agricultural soils.As used in this work, the model comprises one or more soil profiles, each of which contains a defined number of soil layers. The soil simulation operates on an annual timestep. Metal entering the uppermost soil layer can be retained by binding to the soil solids, or move either vertically to the next layer or laterally to surface water. Vertical metal movement occurs as dissolved metal in percolating porewater. Lateral movement can occur either as dissolved metal in runoff or bound to eroding soil particles. Metal removal in crop offtake can also be included.
In each soil layer, metal can be present in multiple forms: (i) labile (geochemically active) metal, comprising dissolved metal in the porewater and metal adsorbed to the soil solids; (ii) aged, comprising metal ‘fixed’ within the soil solids, and (iii) mineral, comprising relatively strongly fixed metal. All metal entering the soil profile is assumed to be in labile form. Within each layer, labile metal partitions between porewater and adsorbed forms according to chemical equilibrium principles. Partitioning calculations are performed by combining an empirical Freundlich-type expression10 and WHAM/Model VI.13 The Freundlich-type expression is used to compute the free metal ion concentration in the porewater as a function of the adsorbed pool, and WHAM/Model VI is used to compute the dissolved metal concentration in the porewater from the free ion concentration. Distribution of metal among the labile, aged and mineral pools is done according to a kinetic reaction schema.11
Loss of metal through erosion is simulated by specifying an annual mass of fine soil lost from each soil layer, which may be zero. It has been observed14 that metal contents of eroded soil are elevated above the bulk soil concentration, likely due to preferential mobilisation of fine soil fractions richer in organic matter and adsorbed. To account for this, an enrichment factor is applied to bulk soil contents of labile and aged metal, and organic matter, to provide contents in eroded soil materials.
Water volumes in drainage and/or runoff from each soil layer are specified as annual time series. Variables such as porewater pH, soil organic matter content, dissolved organic carbon (DOC) in drainage and runoff and erosion losses, are similarly specified. The soil layer moisture content is assumed to be constant from year to year.
The soil model is initialised for a pre-industrial or ‘pristine’ year for which all metal inputs are assumed to be natural (atmospheric deposition, mineral weathering), input and output metal fluxes in each layer are equal, and the kinetic relationships among the labile, aged and mineral pools in each layer are at equilibrium. This allows computation of the concentrations of all metal forms in each soil layer at steady state. The model is then run dynamically forward from this point in time.
The surface water component of the model runs on a daily time step. It comprises a single fully mixed water column and a two-layer sediment compartment, representing a first order stream or ditch. The width and length of the reach are fixed. Water and eroded soil material, with associated metal, enter the water column from baseflow and soil leaching. The baseflow volume is constant and fixed according to catchment characteristics. The daily water volume and eroded soil and metal fluxes are computed as a daily fraction of the annual leaching, using a fixed daily runoff pattern which is applied across all years. Eroded soil particles in the water column form suspended particulate matter in the water column which may settle to the bed and resuspend. Sediment deposition is simulated using a fixed sediment velocity approach. Resuspension is computed using a threshold approach whereby below a threshold discharge no resuspension occurs, and above the threshold the mass of resuspended sediment R (kg per day) is given by
| R = A(Q − Qthr)μ |
Labile metal partitions between the solution phase and suspended sediment in the water column using a partition coefficient (Kd, L kg−1) calculated using WHAM/Model VI and assuming particulate organic matter to comprise the active binding phase of suspended sediment. A Kd is computed for the first day of each simulation year and then used for all days in that year. Eroded aged and mineral metal is treated as inert; it remains associated to the sediment whether in the water column or the bottom layers.
The bottom sediment is simulated as two layers each of fixed porosity. The depth of the lower layer is fixed while the depth of the upper layer may vary due to sediment movement, but may not exceed a fixed maximum depth. Water column sediment exchanges with the top layer via deposition and resuspension, and sediment may be lost from the top layer due to bedload movement. Where the changes in total layer depth due to these processes result in a depth greater than the maximum defined depth, part of the lower layer is permanently lost (burial) and part of the upper layer moved into the lower layer to maintain its fixed depth. Sediment may also be exchanged between the layers due to bioturbation, using a fixed bioturbation rate constant (kg2 s−1).
A fixed concentration of acid-volatile sulphide (AVS) (mol g−1 sediment) may be specified for the lower (anoxic) sediment layer. Labile metal in this layer reacts with the AVS to form sulphide-bound metal. In the case of single metal simulation, if the concentration of labile metal (mol g−1 sediment) is smaller than the AVS concentration, all the labile metal becomes sulphide-bound. If the labile metal exceeds the AVS then the concentration of labile metal becoming sulphide-bound equals the AVS concentration. When sulphide-bound metal leaves the lower layer due to bioturbation or resuspension, the proportion returning to labile form on each time step is given by a fixed proportion αS. If αS = 1, sulphide-bound metal immediately returns to labile form, while if αS = 0 sulphide-bound metal remains in that form permanently.
A simplistic representation of the model structure, in terms of the metal fluxes and key processes, is shown in Fig. 1.
The first version of the IDMM was applied to assess the soil and surface water loadings of copper and zinc from animal feed additives used in agriculture.9 Some limitations to the model were noted, particularly the assessment of potential risks to surface waters and their associated sediments. The model has subsequently been modified further, principally with the aim of making the surface water compartment, and its associated sediment, more realistic, particularly the use of a two-compartment sediment model as recommended previously.15
Generic regional risk assessments
Generic risk assessments were conducted for four regional scenarios that were all based on a common exposure scenario but used locally relevant information on the soil conditions, climate, soil properties, and local surface water characteristics. The exposure route is assumed to be the spreading of manure from indoor pig rearing facilities. The regional environmental scenarios were selected on the basis of these areas having significant level of pig farming, including intensive pig farming sites that are regulated under IPPC (Integrated Pollution Prevention and Control) regulations. The regions identified were East (e.g. Lincolnshire and Norfolk), North East (e.g. North and East Yorkshire), Midlands (e.g. Shropshire and Staffordshire), and Scotland (e.g. Aberdeenshire). The soil and surface water conditions were selected from data in the GEMAS data set,16 and represent the actual conditions in the vicinity of major pig rearing facilities in each area. The derivation of the generic exposure scenario is explained below.17 The scenarios modelled are intended to be realistic but conservative, and are therefore expected to represent the worst case conditions that are likely to be encountered as a result of current agricultural practices.Breeding sows were assumed to have 2.3 litters of 13 piglets per year (29.9 piglets per sow per year). The piglets were assumed to weigh 12.5 kg each for the period over which the zinc oxide treatment would be used, and the total weight of treated piglets per sow per year was therefore 374 kg. The piglets were assumed to be treated with a dose of either 55 (low) or 90 (high) mg zinc per kg body weight per day for a period of 21 days. The different dosing rates are due to the fact that the zinc oxide treatment is dosed into medicated feeds, so the dose that each piglet receives depends upon the amount that they eat. The low dose is considered to be equivalent to the typical expected feeding rate, whereas the high dose is considered to be the maximum quantity of medicated feed that may be consumed. Modelling scenarios were based on the worst case dosing rate of 90 mg zinc per kg body weight per day for a period of 21 days.
The application rate of zinc to the soils was calculated based on an assumed equivalent stocking density that would not exceed the acceptable nitrogenloading rate for the soils of 170 kg (N) per hectare per year. This assumed that each sow and litter produced 16 kg of N per year, and the stocking density was therefore equivalent to 10.6 sows per hectare per year. Expressing the Zinc oxide treatment used as the metal only leads to a zinc loading of between 3.7 (low dose) and 6.0 (high dose) kg ha−1 a−1. Total zinc usage per year, for the treatment of post-weaning piglets, is estimated to be between 430 and 710 g of Zinc oxide per sow. Zinc oxide use was assumed to start in 2020, and the scenario calculations were performed for 50 years of use ending in 2070. The exposure assessment assumed an input of zinc from contemporary farming practices of 4.91 mmol Zn m−2 a−1 between 1950 and 2020. An addition of Cu was also assumed to occur alongside all additions of Zn. This addition of Cu in the assessment was included to ensure that there was competition for Zn complexation for formation of sulfides in sediments. Cu was added at a rate equivalent to 0.2 times the rate at which Zn was added, on a molar basis in order to provide competition for zinc binding with sulfide in sediments.
The key environmental properties and Predicted No Effect Concentration (PNEC) values for each compartment (soil, water, and sediment) that were assumed for each of the regional scenarios are summarised in Table 1. The soil properties were used to parameterise the IDMM scenarios, and were also used to calculate the sensitivity of the local soil communities to zinc.18 The surface water properties were used to assess the sensitivity of the local aquatic communities to zinc based on the pH, dissolved organic carbon (DOC), and calcium, and ambient background concentration (ABC) of zinc.19 The concentration of acid volatile sulfide (AVS) in sediment was used as an input parameter in the IDMM to characterise the local surface water sediments, and this influences the amount of labile zinc in the anoxic sediment layer.
| Medium | Parameter | Scotland | East | North East | Midlands |
|---|---|---|---|---|---|
| Soil | pH (in CaCl2) | 5.5 | 6.9 | 6.8 | 4.8 |
| Soil | pH (soil solution) | 6 | 7.4 | 7.1 | 5.4 |
| Soil | OM (%) | 3.6 | 3.6 | 9.5 | 4.1 |
| Soil | Texture | Sand | Loam | Loam | Sandy loam |
| Soil | Bulk density (kg m−3) | 1.35 | 1.51 | 1.35 | 1.2 |
| Soil | Clay (%) | 3 | 12 | 20 | 13 |
| Soil | Cation exchange capacity (meq. 100 g−1) | 11.7 | 18.8 | 25.7 | 17.1 |
| Soil | Zn ambient background concentration (mg kg−1) | 25.9 | 22.9 | 94.8 | 58.8 |
| Soil | Rainfall (mm a−1) | 747 | 659 | 651 | 701 |
| Soil | Zn PNEC (mg kg−1 total Zn) | 95.8 | 131.2 | 347.4 | 148.5 |
| Water | pH | 7.8 | 8.3 | 7.7 | 8.2 |
| Water | DOC (mg L−1) | 4.1 | 7.1 | 7.8 | 1.4 |
| Water | Calcium (mg L−1) | 10.2 | 161 | 24.2 | 100 |
| Water | ABC Zn (μg L−1) | 0.8 | 2.5 | 2.5 | 2.5 |
| Water | Zn PNEC (μg L−1 dissolved Zn) | 23.4 | 39.1 | 40.2 | 19.7 |
| Sediment | AVS (μmol g−1 dwt) | 17 | 18 | 13 | 84 |
| Sediment | Zn PNEC (mg kg−1 labile Zn) | 118 | 118 | 118 | 118 |
The effects assessment is based on the UK Environmental Quality Standard (EQS) for zinc for surface waters, and uses the Metal Bioavailability Assessment Tool to calculate the site specific EQS as a dissolved zinc concentration as a function of the local water chemistry conditions (https://www.wfduk.org/resources/rivers-lakes-metal-bioavailability-assessment-tool-m-bat). The effects assessment for soils is based on the generic soil screening value (PNEC) of 35.6 mg kg−1 derived by the Environment Agency,18 which requires account to be taken of both the soil ambient background concentration and bioavailability (https://www.gov.uk/government/publications/soil-screening-values-for-assessing-ecological-risk). This takes account of the local soil conditions including the soil pH, the percentage of organic matter (OM), clay content, and cation exchange capacity (CEC) in calculating the local soil PNEC.
The sediment effects assessment is the least well developed of the three major compartments and is based on the EU risk assessment performed under the Existing Substances Regulations, but also includes additional data now available from the laboratory and the field.20,21 The PNEC derived for sediment was derived following the guidelines set out under REACH,22 resulting in a sediment PNEC of 118 mg kg−1 and is based on the fraction of metal that is not associated with AVS in the whole sediment, taking account of the ambient background concentration of zinc. The PNEC for soil is assessed against the total zinc concentration in the upper 20 cm of soil, the PNEC for surface water is assessed against the dissolved zinc concentration, and the PNEC for sediment is assessed against the labile zinc concentration in the whole sediment.
Results of generic regional risk assessments
The local exposure concentrations for each compartment were calculated using the IDMM (V3), and are summarised in Table 2. Results from the IDMM simulations are provided for a number of different time intervals. The initial timepoint is 1950, which is intended to provide an indication of background concentrations prior to the addition of any zinc in manures. The years 2000, 2010, and 2020, provide an indication of the current ambient conditions predicted by the IDMM for each site. Considering these predictions collectively it may be possible to assess the validity of the model for each scenario. This provides a basis for interpreting and understanding the predictions in later years after 10, 20, 30, 40, or 50 years of zinc oxide use (i.e. until 2030, 2040, 2050, 2060, and 2070, respectively) based on the reasonable worst case emission scenario.| Scenario | Scotland | East | North East | Midlands | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medium | Soil | Water | Sediment | Soil | Water | Sediment | Soil | Water | Sediment | Soil | Water | Sediment |
| Year | mg kg−1 | μg l−1 | mg kg−1 | mg kg−1 | μg l−1 | mg kg−1 | mg kg−1 | μg l−1 | mg kg−1 | mg kg−1 | μg l−1 | mg kg−1 |
| 1950 | 31.5 | 1.67 | 0.01 | 24.8 | 0.09 | 0.33 | 33.9 | 0.10 | 0.16 | 68.2 | 0.01 | 0.11 |
| 2000 | 68.4 | 53.7 | 0.28 | 55.5 | 0.33 | 5.47 | 61.9 | 0.16 | 2.84 | 100.2 | 0.15 | 9.14 |
| 2010 | 75.9 | 64.0 | 0.33 | 62.4 | 0.38 | 6.50 | 68.4 | 0.18 | 3.40 | 106.3 | 0.18 | 10.5 |
| 2020 | 84.1 | 73.7 | 0.37 | 69.8 | 0.44 | 7.50 | 75.5 | 0.20 | 3.95 | 112.6 | 0.21 | 11.6 |
| 2030 | 99.0 | 99.3 | 0.47 | 82.8 | 0.55 | 9.70 | 87.9 | 0.23 | 5.15 | 125.2 | 0.29 | 15.2 |
| 2040 | 113.4 | 120.8 | 0.55 | 95.7 | 0.66 | 11.6 | 100.3 | 0.27 | 6.20 | 137 | 0.36 | 17.8 |
| 2050 | 127.5 | 140.9 | 0.62 | 108.6 | 0.77 | 13.5 | 112.7 | 0.30 | 7.23 | 147.9 | 0.42 | 20.1 |
| 2060 | 141.3 | 159.6 | 0.69 | 121.5 | 0.87 | 15.3 | 125.0 | 0.33 | 8.21 | 158.1 | 0.49 | 22.0 |
| 2070 | 154.7 | 177.1 | 0.75 | 134.3 | 0.97 | 17.0 | 137.3 | 0.36 | 9.17 | 167.6 | 0.56 | 23.7 |
The results of the IDMM modelling for the four regional scenarios indicate two different situations. For the Midlands, East, and North East scenarios there are no risks predicted in any of the environmental compartments prior to when the use of zinc oxide medication for piglets is assumed to start, with some limited risks to the soil compartment predicted after approximately 30 years for the Midlands scenario and within 50 years for the Eastern scenario. However, risks to local surface waters are predicted for the Scottish scenario by the year 2000 due to contemporary agricultural practices which increase considerably due to the assumed zinc oxide use, and risks are predicted for the local soil within 10 years of the assumed zinc oxide use starting. No risks to sediments were predicted for any of the scenarios.
It is normal for generic risk assessments to be conducted on a worst-case basis, and then potentially refined if any risks are predicted. There are several potential sources of conservatism in this assessment that could result in the assumed loadings of zinc to the soil as a result of the use of zinc oxide medication being greater than actually occurs in practice. No additional nitrogen input from piglets was assumed, this would reduce the rate at which manure could be spread on land, which would reduce the loading of zinc applied. The treatment period was assumed to be three weeks, rather than two, based on the maximum rather than the 14 day treatment period noted in the product authorisations for the UK. The weight of the piglets was assumed to be 12.5 kg for the whole of the treatment period. The feeding rate, and therefore the dosing rate of the medication in the feed, is higher than the typical feeding rates. Although none of these individual factors is expected to be conservative by more than a factor of 1.64, collectively they suggest that the emission scenario may overestimate the typical loading rates by a factor of 3.7 times.
An important area of uncertainty is the assumptions that are made about the emission of zinc to agricultural soils due to contemporary farming practices, especially given that the surface waters for the Scottish scenario are predicted to be at risk due to zinc emissions before the use of zinc oxide medication was assumed to start. Unfortunately, there is insufficient information available to enable this issue to be refined significantly either on a site-specific or generic basis.
There is a high level of uncertainty associated with the Scottish scenario. Not only does this scenario indicate significant risks for both sols and surface waters, but there are risks predicted for surface waters prior to the assumed use of zinc oxide medicated feeds. Furthermore, there are no indications of widespread failures of the EQS for zinc at rural locations away from mining areas within in this region (https://www.sepa.org.uk/data-visualisation/water-classification-hub/). The uncertainty associated with the historic agricultural practices and apparently unrealistically conservative prediction of risks to surface waters prompted a similar risk assessment for a specific site in the region.
Site-specific risk assessments
In order to better understand the potential risks to the environment from the use of zinc oxide medication for weaning piglets a more detailed site-specific assessment was performed for two of the scenarios based on farms that use this medication. The Scottish scenario is of particular concern since risks for surface water were predicted to occur prior to the assumed use of zinc as a veterinary medicine. A more detailed assessment of the Scottish scenario is required to properly understand any potential risks that may arise from this particular use of zinc oxide. The Eastern England scenario is also considered appropriate for further investigation because this is a particularly important region for pig farming, although this scenario did not result in any predicted risks in the previous assessment. The site-specific assessments involved sampling of the soils and local surface waters for one site in each of these regions, as well as collecting site-specific information about the use of zinc oxide medication for piglets from three relevant sites in Scotland. Soil and surface water sampling was conducted to facilitate refining the IDMM inputs for the regional scenarios and to enable validation of the predicted metal concentrations in the local soils and surface waters.A range of exposure scenarios have been used for the calculations based on the feeding regimes for weaning piglets that were reported by the farms for the use of medicated feeds containing zinc oxide at a concentration of 0.125% (1.25 kg of zinc oxide per tonne of feed, equivalent to 1.0 kg of Zn per tonne of feed). These feeding regimes are based on a specified ration of medicated feed that is allocated to each weaning piglet during the treatment period, and range from 2 to 7 kg of medicated feed per piglet for the feeding regimes that are currently used by farms for which specific information was provided. An additional scenario was also included for higher levels of zinc oxide use to represent the increased doses that may be required in the absence of phytase feed supplements, this is based on approximately 13 kg of medicated feed per piglet. The feeding regimes assumed and the associated resulting zinc application rates due to the spreading of manures and slurries are summarised in Table 3.
| Medicated feed per piglet (kg) | Total dose of zinc oxide per piglet (g) | Zinc application rate (mol m−2 a−1) |
|---|---|---|
| 2 | 2.5 | 5.72 × 10−4 |
| 7 | 8.8 | 2.07 × 10−3 |
| 13 | 16.3 | 4.00 × 10−3 |
| 26 | 32.6 | 8.00 × 10−3 |
In practice the equivalent stocking densities in terms of the number of piglets per hectare that the manure or slurry is spread over are very similar for all of the farms that reported information about these factors. Consequently, the relationship between the ration of medicated feed allocated per piglet and the zinc application rates is consistent across the different scenarios. This may be related to limits on manure and slurry spreading based on the loading of nutrients such as nitrate. The lowest reported dosing rate of 2 kg of medicated feed per piglet is only relevant to the Scottish site-specific assessment, as no other sites reported similarly low dosing rates.
In order to provide an indication of the likely metal exposures for each of the sites under reference conditions with no copper or zinc added as a result of manure spreading the calculations were also performed for each site without any metal inputs from either historic or contemporary manure applications, but with all other conditions remaining the same as for the simulation scenarios. Metal inputs were still considered from geogenic (erosion) sources, atmospheric deposition, and fertiliser additions.
Ten soil samples were collected from the two sites for which site-specific assessments were performed, one in Scotland and one in the East of England. The samples were collected from fields that usually receive pig manure or slurry treatments on an annual basis, and samples were collected prior to the application of manure or slurry in the year when sampling was conducted. Samples were collected at two depths at each of the sampled locations, with the upper sample from the surface to 5 cm depth, and the lower sample collected between 5 and 15 cm depth.
Soils were characterised for particle size, texture, bulk density, cation exchange capacity, organic matter content, pH, phosphorus, nitrate, total zinc, and total copper. Data for the two different soil layers were treated separately and the mean, standard deviation, and geometric mean of the ten replicate samples were calculated for each parameter in each soil layer. Where possible, local surface waters were also sampled for pH, dissolved organic carbon, calcium, zinc, and copper. Surface water sampling was only possible for the Scottish site.
For the IDMM parameterisation the organic matter content, clay content, bulk density, and the proportion of stones were used to set the site-specific soil characteristics for each soil layer. These properties were set for all soil layers with the exception of the sub-soil layer. Lower soil layers were assumed to have the same properties as the 5 to 15 cm depth samples because no site-specific data were collected for the lower soil layers.
For the calculation of the local soil PNEC the soil pH, organic matter content, clay content, and cation exchange capacity were used. Zinc background concentrations at the site were assumed to be negligible, although the soil PNEC for zinc is usually corrected for the local background concentration. The measured metal concentrations were used for validation purposes to compare against the predicted concentrations of the metals in the soil. Local surface waters were characterised for pH, dissolved organic carbon, calcium, dissolved zinc, and dissolved copper. The pH, dissolved organic carbon, and calcium concentration were used to calculate the local PNEC value for zinc, and the dissolved metal concentrations were used for validation of the predicted zinc exposures in the local surface waters.
Scotland
The site-specific soil conditions in the upper soil layer were modified to an organic matter content of 3.5% (from 3.6%), the clay content was increased to 7.8% (from 3.0%), the bulk density was increased to 1.66 g cm−3 (from 1.2 g cm−3), and the fraction of stones was increased to 12% (from 0%). The site-specific soil conditions in the lower soil layers were modified to an organic matter content of 3.5% (from 3.6%), the clay content was increased to 5.9% (from 3.0%), the bulk density was increased to 1.74 g cm−3 (from 1.2 g cm−3), and the fraction of stones was increased to 12.5% (from 0%).The generic Scottish scenario was developed for a sandy soil with a relatively low pH of 5.5, a cation exchange capacity of 11.7 meq. 100 g−1 and an organic matter content of 3.6%. This scenario previously had a site-specific PNEC for soil of 95.8 mg kg−1, taking account of a local ambient background concentration of zinc of 25.9 mg kg−1. The local site-specific scenario has been updated to a pH of 6.2, a cation exchange capacity of 9.0 meq. 100 g−1 and an organic matter content of 3.5%. This scenario now has a site-specific PNEC for soil of 108.2 mg kg−1, and this PNEC has not taken into account any local ambient background concentrations of zinc. The updated PNEC for soil is consistent with that previously used for the Scottish scenario.
The site-specific PNEC for surface water was 23.4 μg L−1, taking account of a local ABC for zinc of 0.81 μg L−1. This was based on a pH of 7.8, a DOC concentration of 4.05 mg L−1, and a calcium concentration of 10.2 mg L−1. The local surface water monitoring found the water chemistry conditions to have a pH of 7.8, a DOC concentration of 8.5 mg L−1, and a calcium concentration of 50.5 mg L−1. These updated water chemistry conditions result in a local PNEC for surface water of 57.9 μg L−1 dissolved zinc, without taking account of an ABC for zinc. The local surface water is therefore less sensitive than was previously assumed.
Eastern
For the Eastern scenario the site-specific soil conditions in the upper soil layer were modified to an organic matter content of 2.0% (from 3.6%), the clay content was increased to 17.2% (from 12.0%), the bulk density was increased to 1.78 g cm−3 (from 1.51 g cm−3), and the fraction of stones was increased to 39% (from 0%). The site-specific soil conditions in the lower soil layers were modified to an organic matter content of 2.0% (from 3.6%), the clay content was increased to 16.5% (from 12%), the bulk density was increased to 1.77 g cm−3 (from 1.51 g cm−3), and the fraction of stones was increased to 46% (from 0%). The site-specific soil conditions in the sub-soil layer were modified to increase the bulk density to 1.77 g cm−3 (from 1.71 g cm−3), and the fraction of stones was increased to 46% (from 0%).The Eastern scenario was developed for a sandy soil with a circumneutral pH of 6.9, a cation exchange capacity of 18.8 meq. 100 g−1 and an organic matter content of 3.6%. This scenario previously used a site-specific PNEC for soil of 131.2 mg kg−1, taking account of a local ambient background concentration of zinc of 22.9 mg kg−1. The local site-specific scenario was updated to a pH of 7.6, a cation exchange capacity of 10.7 meq. 100 g−1 and an organic matter content of 2.0%. This scenario now has a site-specific PNEC for soil of 132.7 mg kg−1, and this PNEC has not taken into account any local ambient background concentrations of zinc. The revised PNEC is consistent with the PNEC previously used for this scenario.
The site-specific PNEC for surface water was 39.1 μg L−1, taking account of a local ABC for zinc of 2.5 μg L−1. This was based on a pH of 8.3, a DOC concentration of 1.4 mg L−1, and a calcium concentration of 161 mg L−1. There are no surface waters in the vicinity of the sampled site and so it was not possible to collect water samples from this location. Therefore, the existing scenario parameters have been retained for the purposes of the assessment for both stream and ditch scenarios.
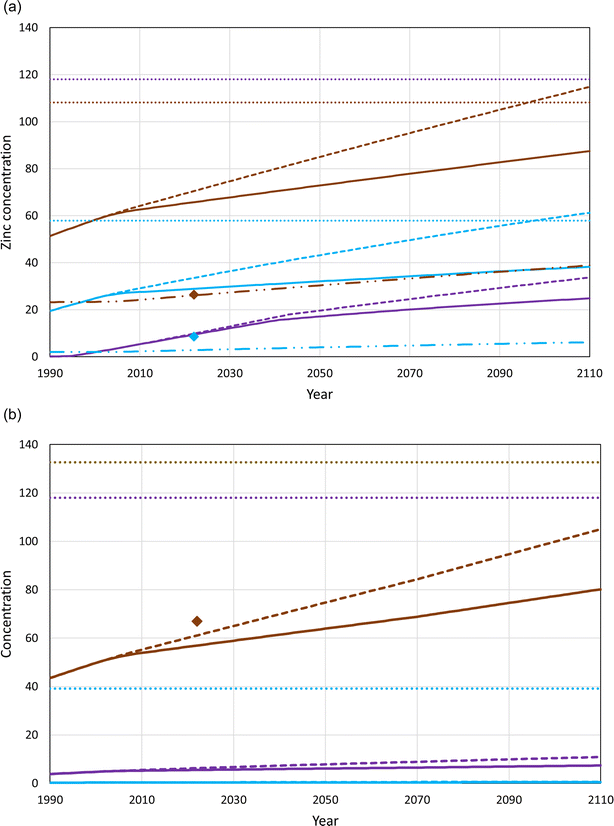
The results of the modelling of the site-specific risk assessments are shown in Fig. 2 for both the Scottish and Eastern scenarios and both the 7 and 13 kg of medicated feed per piglet exposure scenarios, which cover typical dosing rates and possible elevated dosing rates that may be used on farms where phytase treatments are not routinely used. The 2 kg of medicated feed per piglet exposure scenario is shown only for the Scottish scenario, and is only shown for a scenario in which there was assumed to be no input of zinc to soils from historic agricultural manure spreading. The Scottish scenario results in predicted risks to both soils and surface waters occurring after approximately 90 years of treatment for the 13 kg of medicated feed per piglet exposure scenario. There are no predicted risks to either soils or surface waters for the Eastern scenario within the 100 year treatment period of the modelled simulation.
Discussion
The present study focuses on UK farming practices and uses generic scenarios and specific sites from the UK to assess the potential risks posed for the environment by the use of zinc oxide based treatments for weaning piglets. However, it is likely that there are similar pig farming practices throughout many parts of Europe, and possibly also outside Europe. The environmental conditions in other regions may be different, but the approach followed here could be applied to pig farming practices in any location given sufficient information about the local agricultural practices and environment.Whilst there is evidence of benefits for pig production from the use of mineral supplements23 this also needs to be balanced against the potential for environmental contamination due to the limited proportion of the mineral supplement that is retained by the pigs. It is this need to balance the conflicting requirements of improved agricultural productivity and reduced environmental contamination that has caused some authors to recommend targeted mineral treatments at specific growth stages.7 The present study assumed that all of the zinc consumed would be excreted, and whilst this is approximately correct, based on reported results,6 it is slightly conservative and will lead to a small overestimation of zinc loadings to soils from this source because some zinc (<20%) is retained by the piglets.
Validation of the IDMM
The IDMM has previously been applied9 to predict the labile metal pools in UK upland soils and metal pools and concentrations in soils and waters of a small Swiss agricultural catchment. It was shown that labile metal pools in soils and metal concentrations in surface waters could be predicted for upland catchments in the UK. It was also demonstrated that existing data for the Swiss catchment could be used to predict soil metal pools and metal concentrations in drainage water and compared them against a limited number of measurements.For the UK upland sites,10 where soils are typically rather thin layers over bedrock, a simplified soil profile comprising a single layer was used. Good agreement between the predicted and measured labile metal pools in soils and concentrations in surface waters was seen, with most pools and concentrations being predicted to within a factor of three. For the Swiss catchment the IDMM provided good estimates of both copper in mineral soil and its drainage water, and zinc concentrations in mineral and organic soils and their drainage waters.9 Copper concentrations in organic soil and its drainage water were however underestimated. The authors concluded that due to the measured concentrations being not greatly higher than the upper end of the range of predicted concentrations, the likelihood of heterogenous inputs across the catchment, and uncertainties in the cumulative inputs of copper, that the predictions were reasonable.
A separate study11 tested the ability of the IDMM to predict current concentrations of copper, cadmium, and zinc in surface soils (upper 20 cm) for a reservoir catchment in China from estimates of historic natural and anthropogenic inputs. No significant differences between were found between the measured and IDMM predicted total and EDTA extractable metal concentrations for the single available sampling time. The model successfully simulated the influence of metal fixation (aging) in controlling the size of the labile (EDTA-extractable) pools of the metals.
The IDMM has also been used to successfully model labile metal concentrations resulting from four different soil treatments (untreated, farmyard manure, sewage sludge, and compost) in a long term field experiment.24 As the experiment comprised multiple adjacent plots subject to ploughing, it was necessary to incorporate lateral soil mixing into the model. Labile metal concentrations in the surface soil resulting from sewage sludge additions were not well predicted by the IDMM for the early stages of the experiment (prior to about 1990) based on literature information about the concentrations of metals in Swiss sewage sludges. An approach based on setting metal concentrations in sludge to fit the initial measurements in soil provided a considerably improved fit to the data. The prediction of labile (EDTA extractable) concentrations of copper, cadmium, lead, and zinc in the soils for all treatments were good following the modifications mentioned above to accommodate lateral mixing due to ploughing and metal inputs in sewage sludge. The IDMM was able to represent the observed trends in metal concentrations, e.g. an initial increase during the early stages of treatment followed by a (near) steady-state for the farmyard manure and compost treatments, and a rapid increase followed by a steady decline for the sewage sludge treatment, based on measurements covering a period of approximately 40 years. This study did not consider the release of metals from the soil into local surface waters, or the accumulation of metals in deeper soil.
Collectively these studies demonstrate that IDMM is reasonably successful at predicting concentrations of labile metals in soils in response to external inputs, and that the greatest source of uncertainty in predictions is due to a lack of knowledge on historic metal input rates. Validation of the approach for surface waters is even more challenging due to the paucity of available data, although where application has been possible the model again provides reasonable agreement with observations.
Comparison of site-specific scenarios against regional scenarios
The calculations for the updated Scotland scenario resulted in contemporary soil zinc concentrations of approximately 65 mg kg−1 based on the typical dosing rate of 7 kg of medicated feed per piglet. The measured concentrations of zinc in the soil are lower than predicted, but are within a factor of three of the measured concentrations at the sampled site based on the assumptions used. This suggests that the IDMM predictions are reasonably good but could be refined, and that this scenario is somewhat conservative. It is likely that the assumptions made about historic inputs of zinc in animal manures are a key source of uncertainty in this assessment. However, without independent information about the actual metal concentrations in the manures and the spreading rates, or equivalent information that would enable the rate of zinc addition to the soil to be robustly calculated, it is not possible to reliably adjust this aspect of the calculations other than to assume a scenario with no historic agricultural manure spreading for comparison against the measured data. This approach is highly uncertain given that measured data is only available for a single time point. However, the validation efforts do suggest that the assumptions made about the historic loadings of zinc to agricultural soils from animal manures may result in an overestimation of the total loading of zinc in at least some agricultural soils.The lowest exposure scenario that relates to actual usage levels on operating farms, of 2 kg of medicated feed per piglet, results in an assumption that there is a lower input of zinc currently than there was due to more unrestricted use of zinc as a feed supplement and spreading of manures and slurries. In practice this could potentially underestimate the actual loading of zinc to the soils due to additional inputs of zinc from other sources that are not accounted for in this assessment, such as nutritional supplements. Although nutritional supplements are likely to be used at considerably lower levels than the use of zinc oxide as a veterinary medicine, they may be used more routinely and therefore account for a significant additional loading on an annual basis. Pig feeds that have not been supplemented with zinc will also contain some zinc due to the natural background levels of zinc in the ingredients.
Conversely, the assumptions made in the modelling scenarios about historic agricultural practices may significantly overestimate the contemporary concentrations of zinc in some agricultural soils. For example, the measured concentrations of zinc in the site-specific Scottish assessment are lower than the predicted concentrations for both the soil and surface water for exposure scenarios that assume inputs of zinc from historic agricultural practices, although the concentration in the soil is consistent with both the results of the reference scenario calculations for this site, the closest reported measured zinc concentration reported by the National Soil Map of Scotland, and an exposure scenario that assumes no historic agricultural manure spreading and a site specific zinc oxide dosing regimen for piglets.
It is likely that the assumptions made about historic inputs of zinc in animal manures are a key source of uncertainty in this assessment. However, without independent information about the actual metal concentrations in the manures and the spreading rates, or equivalent information that would enable to rate of zinc additions to the soil to be calculated, it is not possible to definitively identify this as the cause of the over estimation of contemporary zinc exposures at this site.
The calculations for the updated Eastern scenario resulted in contemporary predicted soil zinc concentrations of approximately 60 mg kg−1. The measured concentrations of zinc in the soil are very close to the predicted concentrations at the sampled site. This suggests that the IDMM predictions are good and for this scenario does not indicate any clear need for refinement. However, given that there is only a single timepoint against which comparisons between measured and predicted concentrations can be made, and that there is uncertainty about both the historic additions of zinc to soils from agricultural practices, the year in which the use of zinc oxide based medicated feeds for piglets started, and the importance of other sources of zinc both from existing agricultural practices and from other local anthropogenic activities, there is clearly scope for an improved understanding of zinc exposures from contemporary agricultural practices.
Reference calculations for scenarios
The calculations for the Scotland reference scenario resulted in relatively consistent concentrations of both copper and zinc in the soil, surface water, and sediment throughout the relevant time period (1970 to 2110). The predicted soil concentrations are 29 mg kg−1 for zinc and 8 mg kg−1 for copper, with surface water concentrations predicted to be 1.7 and 0.9 μg L−1 for zinc and copper respectively in stream water, and labile concentrations in sediments predicted to be 0.02 and 0.09 mg kg−1 for zinc and copper respectively. The predicted concentrations of both copper and zinc in the soil are both very similar to the measured concentrations at the sampled site, and also to the closest reported measured zinc concentration reported by the National Soil Map of Scotland of 27.8 mg kg−1. This suggests that local farming practices at this site have not resulted in topsoil zinc concentrations being significantly elevated above ambient background levels for the region.The calculations for the Eastern reference scenario also resulted in relatively consistent concentrations of both copper and zinc in the soil, surface water, and sediment throughout the relevant time period (1970 to 2110). The predicted soil concentrations are 24 mg kg−1 for zinc and 6 mg kg−1 for copper, with surface water concentrations predicted to be 0.7 and 0.6 μg L−1 for zinc and copper respectively in ditches and 0.1 and 0.06 μg L−1 for zinc and copper respectively in stream water. Labile concentrations in ditch sediments are predicted to be 0.3 and 0.1 mg kg−1 for zinc and copper respectively, and 0.5 and 0.2 mg kg−1 for zinc and copper respectively in stream sediments.
The predicted concentrations of both copper and zinc in the soil for the reference scenario are approximately a factor of three-fold lower than the measured concentrations at the sampled site. This suggests that agricultural practices are likely to have resulted in elevated levels of both copper and zinc in the soils of this region.
Overestimation of the levels of zinc in soils in the IDMM calculations could potentially be due to uncertainties associated with assumed historic applications of zinc in manure, particularly between 1970 and 2000, when high levels of additions of both copper and zinc were assumed to be added to agricultural soils due to the use of these metals in feed supplements. The calculations conducted for the Scottish scenario that assumed no historic agricultural input of zinc from manure spreading, and a low site-specific zinc oxide dosing rate for piglets, provide a high level of consistency with the measured concentrations in both soils and surface waters for the site.
Conclusions
The IDMM is able to provide acceptable predictions of the levels of zinc in agricultural soils, although there is significant uncertainty associated with the historic loadings of metals to the soils that are required as model inputs. Based on the output from the IDMM and site-specific sampling conducted at one site in each region (Scotland and East England), the use of zinc oxide medicated feeds for weaning piglets does not pose any risks to agricultural soils or local surface waters in the short to medium term (i.e. within a few decades) based on the reasonable worst case use, but could in the longer term. Understanding the true risk of zinc to agricultural soils and local surface waters would require consideration of all other possible sources of zinc, although the available validation data suggests that these additional inputs may not be important to the overall risk. More extensive sampling, both within the region(s) and temporally, would also present a fuller picture against which to compare the IDMM results.Author contributions
Adam Peters designed the study, carried out the calculations and drafted the manuscript. Graham Merrington developed the regional scenarios and contributed to the effects assessment. Ken Stapleton supervised the work. Stephen Lofts provided advice on modelling and detailed information about the model used and its validation. All authors discussed the results and contributed to the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Grace Webster of GW Pig Consultants for advice on the use of treatments in the UK and the identification of sites for site-specific assessments. The authors would like to thank Nial O'Brien of the Veterinary Medicines Directorate for the provision of information and advice on the study, and for reviewing an early draft of the manuscript.References
- H. Poulsen, Zinc oxide for weanling piglets, Acta Agric. Scand., Sect. A, 1995, 45, 159–167 CAS.
- J. Jensen, N. Kyvsgaard, A. Battisti and K. Baptiste, Environmental and public health related risk of veterinary zinc in pig production - Using Denmark as an example, Environ. Int., 2018, 114, 181–190 CrossRef CAS PubMed.
- M. Imseng, M. Wiggenhauser, M. Muller, A. Keller, E. Frossard, W. Wilcke and M. Bigalke, The fate of Zn in agricultural soils: a stable isotope approach to anthropogenic impact, soil formation, and soil-plant cycling, Environ. Sci. Technol., 2019, 53, 4140–4149 CrossRef CAS PubMed.
- G. Satessa, N. Kjeldsen, M. Mansouryar, H. Hansen, J. Bache and M. Nielsen, Effects of alternative feed additives to medicinal zinc oxide on productivity, diarrhoea incidence and gut development in weaned piglets, Animal, 2020, 14, 1638–1646 CrossRef CAS PubMed.
- H. Oh, M. Kim, W. Yun, J. Lee, J. An, Y. Kim, M. Kim, H. Kim and J. Cho, Effect of nano zinc oxide or chelated zinc as alternatives to medical zinc oxide on growth performance, faecal scores, nutrient digestibility, blood profiles and faecal Escherichia coli and Lactobacillus concentrations in weaned piglets, Ital. J. Anim. Sci., 2022, 21, 708–716 CrossRef CAS.
- S. Hansen, J. Norgaard, T. Woyengo and T. Nielsen, The relationship between zinc intake, dietary content, and fecal excretion in pigs, Livest. Sci., 2023, 271, 105228 CrossRef.
- G. Shurson, P. Urriola and Y. Hung, Too Much of a Good Thing: Rethinking Feed Formulation and Feeding Practices for Zinc in Swine Diets to Achieve One Health and Environmental Sustainability, Animals, 2022, 12, 3374 CrossRef PubMed.
- K. Blaabjerg and H. Poulsen, The use of zinc and copper in pig production, DCA – Nationalt Center for Jordbrug og Fødevarer, Aarhus, Denmark, 2017, https://pure.au.dk/ws/files/116535883/2017_05_01_Pi Search PubMed.
- S. C. Monteiro, S. Lofts and A. B. A. Boxall, Pre-assessment of environmental impact of zinc and copper used in animal Nutrition, Report to the European Food Safety Authority, 2010, DOI:10.2903/sp.efsa.2010.EN-74.
- S. Lofts, E. Tipping, A. J. Lawlor and L. Shotbolt, An intermediate complexity dynamic model for predicting accumulation of atmospherically-deposited metals (Ni, Cu, Zn, Cd, Pb) in catchment soils: 1400 to present, Environ. Pollut., 2013, 180, 236–245 CrossRef CAS PubMed.
- L. Xu, S. Lofts and Y. Lu, Terrestrial ecosystem health under long-term metal inputs: modeling and risk assessment, Ecosyst. Health Sustain., 2016, 2(5), e01214, DOI:10.1002/ehs2.1214.
- S. Lofts, and L. Walker, IDMM-ag v3: An improved model for prediction of metal concentrations in soils and receiving waters of agricultural catchments, Report to European Copper Association and International Zinc Association, Natural Environment Research Council, Swindon, UK, 2016, 57 pp Search PubMed.
- E. Tipping, Humic Ion-Binding Model VI: An Improved Description of the Interactions of Protons and Metal Ions with Humic Substances, Aquat. Geochem., 1998, 4, 3–48 CrossRef CAS.
- J. N. Quinton and J. A. Catt, Enrichment of heavy metals in sediment resulting from soil erosion on agricultural fields, Environ. Sci. Technol., 2007, 41, 3495–3500 CrossRef CAS PubMed.
- A. Peters, W. Adams, M. Diamond, W. Davison, D. Di Toro, P. Doyle, D. Mackay, J. Nriagu, C. Ptacek, E. Tipping and H. Waeterschoot, Integrated approach for hazard assessment of metals and inorganic substances: the Unit World Model approach, Chapter in Assessing the Hazard of Metals and Inorganic Metals Substances in Aquatic and Terrestrial Systems, SETAC CRC Press, Boca Raton, FL, 2003, 146 pp Search PubMed.
- Chemistry of Europe's Agricultural Soils – Part A: Methodology and Interpretation of the GEMAS Data Set, Geologisches Jahrbuch (Reihe B 102), ed. C. Reimann, M. Birke, A. Demetriades, P. Filzmoser and P. O'Connor, Schweizerbarth, Hannover, 2014, 528 pp Search PubMed.
- Animal and Plant Health Agency (APHA) Livestock Demographic Data Group: Pig Population Report, DEFRA, London, UK, 2019, http://apha.defra.gov.uk/documents/surveillance/diseases/lddg-pop-report-pig2019.pdf Search PubMed.
- Environment Agency, Soil Screening Values for Assessing Ecological Risk, Bristol, UK, 2017, https://www.gov.uk/government/publications/soil-screening-values-for-assessing-ecological-risk Search PubMed.
- UK Technical Advisory Group (UKTAG) 2014, Rivers & Lakes - Metal Bioavailability Assessment Tool (M-BAT), SNIFFER, Edinburgh, UK, 2014, https://www.wfduk.org/resources/rivers-lakes-metal-bioavailability-assessment-tool-m-bat Search PubMed.
- A. G. Burton, L. T. H. Nguyen, C. Janssen, R. Baudo, R. McWilliam, B. Bossuyt, M. Beltrami and A. Green, Field validation of sediment zinc toxicity, Environ. Toxicol. Chem., 2005, 24(3), 541–553 CrossRef PubMed.
- W. P. T. Norwood, T. Watson-Leung and D. Milani, Impacts of Zn-Spiked Sediments on Four Invertebrates: Implications for the Canadian Sediment Quality Guideline, Environment Canada, 2009 Search PubMed.
- European Chemicals Agency (ECHA), Guidance on Information Requirements and Chemical Safety Assessment Chapter R.10: Characterisation of Dose [Concentration]-Response for Environment, Helsinki, Finland, 2008 Search PubMed.
- C. Mille, E. Burrough, B. Kerr, W. Schweer and N. Gabler, Dietary Pharmacological Zinc and Copper Enhances Voluntary Feed Intake of Nursery Pigs, Front. Anim. Sci., 2022, 3, 874284, DOI:10.3389/fanim.2022.874284.
- C. Cagnarini, S. Lofts, L. D'Acqui, J. Mayer, R. Gruter, S. Tandy, R. Schulin, B. Costerousse, S. Orlandini and G. Renella, Modelling of long-term Zn, Cu, Cd and Pb dynamics from soils fertilised with organic amendments, Soil, 2021, 7, 107–123 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |