Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unravelling microbiome changes in aerobic granular sludge saline wastewater treatment using a slow stepwise salt increase strategy†
Ana M. S.
Paulo
 *a,
Oihane
Salazar
a,
Joana
Costa
b,
Daniela P.
Mesquita
bc,
Eugénio C.
Ferreira
*a,
Oihane
Salazar
a,
Joana
Costa
b,
Daniela P.
Mesquita
bc,
Eugénio C.
Ferreira
 bc,
Paula M. L.
Castro
a and
Catarina L.
Amorim
bc,
Paula M. L.
Castro
a and
Catarina L.
Amorim
 a
a
aUniversidade Católica Portuguesa, CBQF – Centro de Biotecnologia e Química Fina – Laboratório Associado, Escola Superior de Biotecnologia, Rua Diogo Botelho 1327, 4169-005 Porto, Portugal. E-mail: apaulo@ucp.pt
bCEB – Centre of Biological Engineering, Universidade do Minho, Campus de Gualtar, 4710-057 Braga, Portugal
cLABBELS – Associate Laboratory, Braga/Guimarães, Portugal
First published on 21st October 2024
Abstract
Saline wastewaters mainly result from various industrial activities. In response to water shortage, seawater is increasingly utilized for diverse purposes, leading to an increased production of saline wastewater. The presence of salts in wastewater frequently impairs the efficiency of biological wastewater treatment technologies. Among these, aerobic granular sludge (AGS) has emerged as the most effective aerobic biological treatment process for treating saline wastewater, primarily due to the high biomass aggregation and self-protection afforded by granules. In this study, the AGS biomass was acclimated to saline wastewater through a slow stepwise salt increment strategy over a period of ca. 250 days, from 0 to 14 g NaCl L−1. This acclimation strategy facilitated stable and efficient removal of carbon (>90%), phosphorus (>95%), and ammonium (>98%), without nitrite accumulation in the effluent. Notably, it was observed that the increase in extracellular polymeric substance (EPS) content was concomitant with the enrichment in EPS-producing bacteria, in the AGS biomass. Other salt tolerant bacteria were also enriched in the biomass, particularly those from the Lysobacter and Rhodocyclus bacterial genera, related to nutrient removal and AGS stability. Besides, the high nutrient removal performance was corroborated by the identification of bacteria responsible for these processes. Thus, by employing a slow stepwise increase of wastewater salinity, the AGS process successfully adapted by maintaining the metabolic diversity necessary for various biological removal processes. This study underscores the microbial selection and plasticity inherent in AGS processes, highlighting their significant potential for upgrading saline wastewater treatment.
Environmental significanceThe increased production of saline wastewater poses several challenges to wastewater treatment processes. In recent years, the granular sludge technology has emerged as a robust process for the treatment of wastewater, owing to its remarkable efficiency and capability to deal with wastewater-related stressors, including salts. Nevertheless, the granular sludge process adaptation to salinity is still poorly understood, especially at the microbiome level. Thus, this study aims to provide a comprehensive evaluation of the system performance during adaptation to high salinity through a slow salt stepwise addition strategy. By focusing on the community composition and ecosystem functioning, we strive to ascertain on how communities restructure and which taxa are key to maintaining systems functionality under stress. These new insights will contribute to the advancement of the granular sludge technology and foster the understanding on the microbiome adaptation and resilience, which is essential for optimizing and managing the treatment systems. |
Introduction
Population growth and climate changes are affecting water resources all over the world. Earth contains 97.5% of seawater, unfit for human consumption as well as for agriculture and food production. In addition, it is estimated that 50% of the world's population lives less than 100 km from the coastline, a number that is expected to increase due to the aggravation of social and economic differences between the coast and more deserted inland regions.1 To reduce the pressure on freshwater usage, several coastal cities have started to use seawater for diverse purposes such as fire control or even roads and toilet flushing.2 Besides this, saline wastewater is produced by several industrial activities such as seafood processing, vegetable pickling, leather tanning, petroleum refining, and the aquaculture industry, among others.1,3 Therefore, saline waste streams generated by municipalities and industries that ended up in wastewater treatment plants (WWTP) are expected to increase. However, high salinity can inhibit the microbial activity of biological processes such as those typically found in WWTPs.4,5 Aerobic granular sludge (AGS) is an innovative technology used for wastewater treatment, with several advantages compared to the conventional activated sludge systems, such as faster biomass settling within the reactor tank before effluent discharge, simultaneous carbon, nitrogen and phosphorus removal, and higher microbial protection towards toxic and inhibitory compounds.6,7 All these advantages have increased the interest in applying AGS for saline wastewater treatment. Although NaCl is the main salt present in seawater and in different types of industrial wastewater streams, NaCl has been shown to have a different impact in AGS processes compared to the mixture of other relevant salts present in the seawater.8 Nevertheless, the instability caused by high salinity primarily affects sensitive microbial groups, namely nitrifiers and phosphate-accumulating bacteria (PAO), highly relevant for the good nutrient's removal performance.9 Among these, nitrite-oxidising bacteria (NOB) are the most sensitive, followed by PAO and ammonia-oxidising bacteria (AOB).4,10 In fact, nitrite accumulation in effluents discharged by AGS processes treating saline wastewater was often reported,4,10,11 eventually affecting phosphate accumulation and removal.4,12 This inhibition of biological activity started to occur at salt concentrations above 10 g L−1 or at increased salinity levels.3,10Different strategies can enhance the long-term performance and stability of AGS during the treatment of saline wastewater. Although halophilic microorganisms can be added into the AGS reactor, the AGS biomass presents a high microbial diversity which allows selecting for the most salt tolerant bacterial groups, through acclimation to saline wastewater.11 Besides this, the microbial response to stress caused by salt can promote a higher secretion of extracellular polymeric substances (EPSs). Since EPS production is at the basis of AGS biomass formation and structural stability,13 it is crucial to promote a balanced EPS production in the AGS system. This will keep granules' physical integrity, determinant for the success of the process, while protecting the microorganisms responsible for nutrient removal. Most research using AGS for treating saline wastewater has been performed by using biomass non-adapted to salt and by performing a fast transition from low to high salt concentrations, often resulting in poor removal efficiency processes.3,10,11 One strategy to overcome AGS instability problems, related either to nutrient removal or biomass stability, is to perform a stepwise increase in salt content allowing the AGS biomass to gradually adapt to the stress caused by salt. This was successfully applied by Bassin et al.,14 achieving good nitrification and biomass stability with salinity increase up to 20 g NaCl L−1. Li et al.,9 applied a slow salt increase when treating saline wastewater at low temperature (12 °C) which resulted in effective carbon and nutrient removal as well as biomass stability, but biomass disintegration occurred at a salt concentration of 14 g NaCl L−1. Wang et al.3 tested different conditions for evaluating AGS adaptation to saline wastewater treatment at lower dissolved oxygen concentrations, finding that gradual adaptation to salt improved the overall system efficiency and stability. Amancio Frutuoso et al.15 performed a gradual salt increase up to 10 g NaCl L−1 and, despite obtaining good nitrification and phosphorus removal, the AGS biomass collapsed above 7.5 g NaCl L−1. Although Bassin et al.14 focused on nitrifiers present in the AGS biomass during gradual salt adaptation, no information was provided regarding PAO. Wang et al.3 evaluated nitrifiers, PAOs and GAOs dynamics under the different tested conditions. In this study, the AGS process lost PAO activity during adaptation to salt at low dissolved oxygen concentrations. Amancio Frutuoso et al.15 and Li et al.9 went further in AGS microbial dynamics, by identifying a greater diversity of bacteria and their putative functional roles throughout the salt acclimation process. However, these studies also revealed performance failure during gradual AGS salt adaptation. Although all these studies suggest that a slow salt adaptation can be a good strategy for reaching a more stable saline AGS wastewater treatment, the continuous addition of salt might lead to biomass disintegration and/or nutrient decreased removal, often causing an irreversible impact on phosphate removal. Therefore, it remains crucial to continue exploring the specific impact of salt on the AGS microbiome and process performance, while minimizing the uncertainties surrounding the response of AGS processes to overlapping stress factors commonly encountered in real conditions. In this study we aimed to evaluate how and to what extent a salt adaptation strategy by slow stepwise feeding alters the microbiome composition and function with the objective of keeping the efficiency and stability of the AGS treatment. For that, over 250 days of operation, a very slow transition from 0 to 14 g NaCl L−1 in wastewater salt level was applied, at an increment often lower than 2 g NaCl L−1. The AGS reactor performance in terms of carbon, nitrogen and phosphorus removal as well as the granules' features concerning their morphology and EPS production was assessed, while identifying the main bacteriome changes occurring during AGS adaptation to saline wastewater.
Materials and methods
AGS reactor setup and operation
An AGS-SBR (sequencing batch reactor) with a working volume of 2.5 L was operated in four successive treatment cycles of 6 h per day, each including 60 minutes of static plug flow anaerobic feeding, 292 minutes of aeration, 3 minutes of settling and 5 minutes of effluent withdrawal, according to Paulo et al.12 The AGS used as inoculum was collected from another lab-scale reactor that had been previously exposed to NaCl concentrations up to 2.5 g L−1, on average.16 The reactor was fed with a synthetic wastewater consisting of ca. 632 ± 56 mg L−1 of sodium acetate (686 ± 61 mg O2 L−1; 258 ± 23 mg TOC L−1). The medium also contained 18.3 ± 2.6 mg PO43−–P L−1 and 40.2 ± 5.9 mg NH4+–N L−1 and was prepared similarly to previous studies.17 Most of the chloride containing compounds present in the feed composition were replaced by compounds with the required cation and an alternative anion, in their molecular formula, for a better monitoring of the salinity level. The pH of the feed varied from 7 to 7.5. A slow stepwise addition of NaCl to wastewater was performed along reactor operation, divided into 9 operational phases, according to the NaCl concentration in the wastewater (Table 1). In phase I, NaCl was absent from the wastewater. Thereafter, in each operational phase the NaCl concentration in the wastewater increased gradually by 1–2 g L−1 of NaCl except for phases II and IX (ca. 2.7 and 2.4 g L−1, respectively). Initially, the salt increase steps were applied during shorter periods of time but, due to the cumulative salts in the feed, higher salt contents were applied in the later phases and the exposure period was extended to guarantee microbial adaptation and avoid system failure. The pH of the AGS process was monitored over time but not controlled.| Phase | Day | NaCl concentration (g L−1) | Duration (days) |
|---|---|---|---|
| I | 0–7 | 0 | 8 |
| II | 8–21 | 2.7 ± 0.1 | 13 |
| I | 22–35 | 4.3 ± 0.3 | 15 |
| IV | 36–64 | 5.9 ± 0.6 | 29 |
| V | 65–90 | 6.8 ± 0.4 | 26 |
| VI | 91–124 | 8.0 ± 0.5 | 34 |
| VII | 125–168 | 9.7 ± 0.9 | 44 |
| VIII | 169–203 | 11.6 ± 0.5 | 35 |
| IX | 204–249 | 14.1 ± 0.6 | 44 |
Analytical methods
The AGS process performance was evaluated by measuring organic carbon and nutrient removal from wastewater. For this, bulk liquid reactor samples were collected at the beginning (in) and at the end of the aeration period (outlet), when reactor bulk liquid was completely mixed, guaranteeing a representative sampling. Suspended solids were removed by using syringe nylon membrane filters (0.45 μm pore-size) and the filtrate was used for the subsequent analysis. A Total Organic Carbon (TOC) Analyzer (Shimadzu, Japan) was used for evaluating organic carbon removal from wastewater. The concentrations of ammonia, nitrate, nitrite, phosphorus, and chloride were measured using appropriate photometric test kits (Spectroquant®, Merck Millipore, USA), following the manufacturer's instructions. The chloride concentration was used as an indirect measurement of NaCl present in the wastewater. Total suspended solid (TSS) and volatile suspended solid (VSS) concentrations in the outlet were quantified according to standard methods.18 To monitor potential process disturbances, the pH in the outlet was measured on sampling days.EPS extraction and composition
AGS samples were collected for EPS quantification and biochemical analysis. EPSs were extracted from the granules using a sodium carbonate (Na2CO3) aqueous solution, heat and constant mixing, as described by Felz et al.19 Proteins, polysaccharides and humic acid like substances were quantified using colorimetric-based methods.20–22Quantitative image analysis
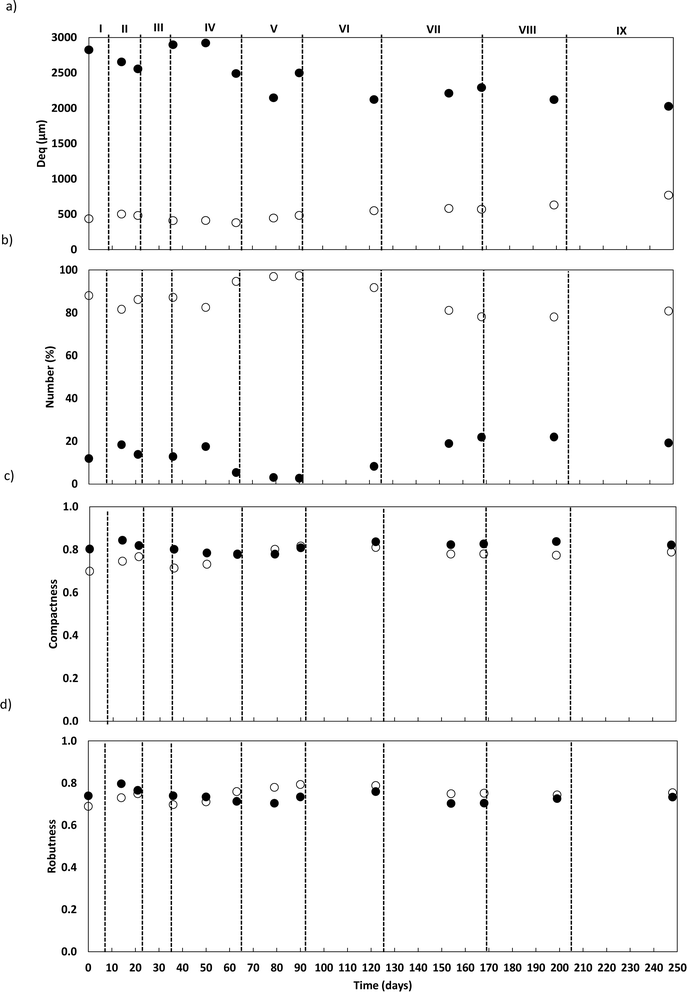
AGS samples were collected in duplicate during reactor operation and preserved according to Paulo et al.12 Structural characteristics of the granules were followed by quantitative image analysis (QIA), for describing size and morphological parameters variations, namely the equivalent diameter (Deq), number (%Nb), convexity (Conv), robustness (Rob) and roundness (round) of the granules, according to Costa et al.23 Two granular biomass size classes were considered based on the Deq: small granules (SG) (Deq lower than 1500 μm) and large granules (LG) (Deq above 1500 μm).AGS microbial community analysis
Statistical analysis
Results from EPS biochemical composition (carbohydrates, proteins, humic acids, PN/PS ratio and total EPS) were statistically analysed by one-way ANOVA. Significant differences between the means were determined by Tukey's post hoc test using the SPSS program (SPSS Inc., Chicago, IL Version 24.0).Results
AGS reactor removal performance
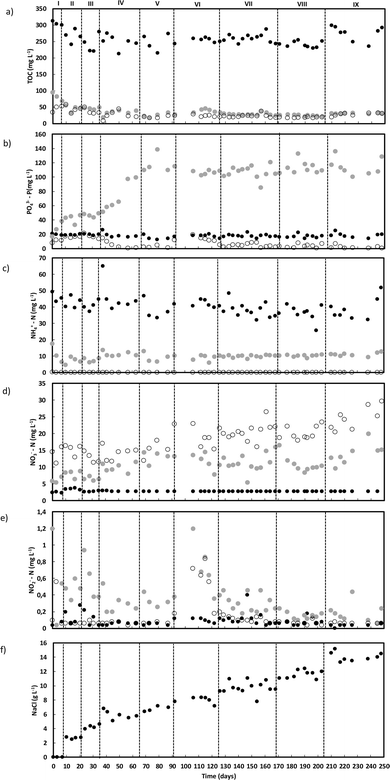
The wastewater used to feed the reactor had an average concentration of 632 ± 56 mg L−1 of sodium acetate (258 ± 23 mg TOC L−1; 686 ± 61 mg O2 L−1) (Fig. 1a), and an estimated OLR of 1.8 ± 0.2 g O2 L−1 day−1 was applied throughout the operation. Despite the unstable carbon removal observed during the first 50 days of operation (till the middle of phase IV), ranging from 78 to 89%, overall, carbon removal reached 90 ± 4%, resulting in a final effluent often containing less than 60 mg O2 L−1, below the legal discharge limit (125 mg O2 L−1). Most of the carbon was removed during the anaerobic feeding period, with initial instability matching the adaptation of the phosphate removal process. Before day 50 (middle phase IV), 50 to 60% of phosphate was removed by the end of each cycle. From phase V onwards, phosphate accumulation stabilized until the end of AGS operation, reaching an average value of 111.1 ± 10.1 mg L−1 with up to 99% of phosphate removal (Fig. 1b).Ammonium removal reached values close to 100% throughout the AGS reactor operation (Fig. 1c). Considering the amount of ammonium expected in the reactor bulk liquid after the anaerobic feeding period, part of the ammonium content was possibly assimilated by the AGS biomass. During phases I, II and III (day 35), about 20 to 25% of the ammonium in the wastewater was assimilated by the AGS decreasing to values between 15 and 20% until the middle of phase VI (day 150), and even further (between 15% and 10%) until the end of operation. Complete nitrification was reached throughout the operation, with nitrate as the main product in the outlet (Fig. 1d). The lowest concentrations of nitrate in both the outlet and in the bulk liquid after the anaerobic feeding period were achieved until the end of phase III (day 35), increasing thereafter until the end of reactor operation, possibly due to an increased ammonium availability during the aerated period. Nitrite did not accumulate in the reactor outlet at the end of each treatment cycle (Fig. 1e). The amount of nitrite present in the bulk reactor after the anaerobic period showed more variability until the end of phase VIII, exhibiting a decreasing trend until the end of operation. The pH in the reactor outlet was always between 8 and 9 (Fig. S1†), indicating the potential completion of the nitrification process. In addition, the lack of abrupt oscillations on the pH values indicate that the performance of the reactor was stable during most of its operation.
Granular biomass dynamic properties
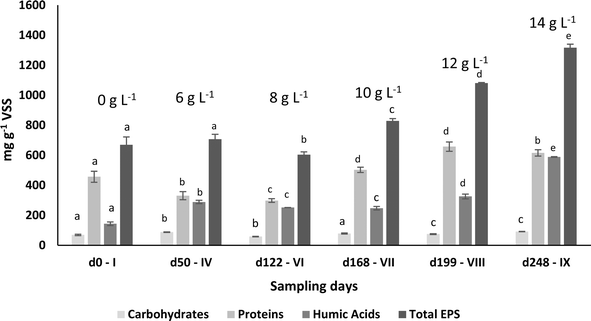
| Sampling day – phase | Proteins (mg gVSS−1 ± SD) | Carbohydrates (mg gVSS−1 ± SD) | Humic acids (mg gVSS−1 ± SD) | PN/PS | Total EPS (mg gVSS−1 ± SD) |
|---|---|---|---|---|---|
| a PN/PS – protein – polysaccharide (carbohydrate) ratio. The results are expressed as average ± standard deviation. Superscript letters represent the statistical analysis. Means with different letters in the same column differed significantly according to Tukey's post hoc test (P < 0.05). | |||||
| d0 – I | 456.7 ± 36.7a | 69.3 ± 4.8a | 143.8 ± 11.6a | 6.6 ± 0.4a | 669.8 ± 51.3a |
| d50 – IV | 331.0 ± 27.0b | 87.8 ± 2.0b | 288.7 ± 11.2b | 3.8 ± 0.4b | 707.4 ± 32.4a |
| d122 – VI | 298.3 ± 12.0b | 58.6 ± 1.7c | 251.9 ± 0.4c | 5.1 ± 0.2c | 603.9 ± 18.9b |
| d168 – VII | 502.3 ± 17.6a | 78.9 ± 3.4d | 247.7 ± 11.8c | 6.4 ± 0.4a | 828.9 ± 15.1c |
| d199 – VIII | 657.8 ± 31.0c | 75.1 ± 2.8d | 325.7 ± 16.1d | 8.8 ± 0.7d | 1080.4 ± 4.3d |
| d248 – IX | 615.2 ± 21.3c | 91.8 ± 0.8b | 588.4 ± 2.8e | 6.7 ± 0.3a | 1315.6 ± 23.5e |
The granules were also morphologically characterized based on compactness and robustness (Fig. 3c and d). In general, LG exhibited compact and elongated structures with regular boundaries across all phases. In contrast, SG became more compact and elongated with more regular boundaries along the operation, though with greater variability.
AGS bed-height and solid washout
The amount and structure of the AGS biomass changed along the operation. At the start of the process, the AGS was mostly composed of granular biomass, with a bed height of 20 cm, which decreased during the first operational phases (Fig. 4a). Simultaneously, accumulation of suspended biomass on the top of the granular biomass started to be observed after day 65 (end of phase V), persisting until day 110 (middle of phase VII). During this same period, the granular biomass bed height decreased until ca. 7 cm. Between days 110 and 205 (end of phase VIII), the AGS bed height increased, reaching ca. 22.5 cm, and concomitantly the suspended biomass fraction decreased and remained like that until the end of operation.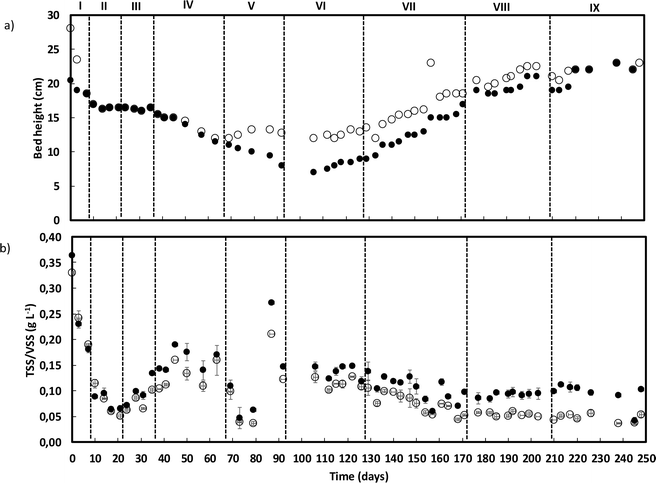 | ||
| Fig. 4 AGS bed height (a) (● – granular; ○ – granular + suspended biomass) and outlet TSS (●) and VSS (○) concentrations (b) along reactor operation. | ||
The concentration of TSS in the outlet was variable until the end of phase V but stabilized towards the end of reactor operation (Fig. 4b). The highest concentration of TSS in the outlet was observed throughout phase IV (max. 190 mg TSS L−1). From phase V onwards, the outlet TSS concentration tended to decrease and during the two last operational phases (VIII and IX), the TSS concentration was, on average, 100 mg TSS L−1. The VSS concentration in the outlet followed the trend of TSS, and the percentage of VSS decreased until the end of operation, from ca. 100 to 80% (day 150, phase VII), to ca. 65% (phase VIII) and finally to ca. 53% (phase IX).
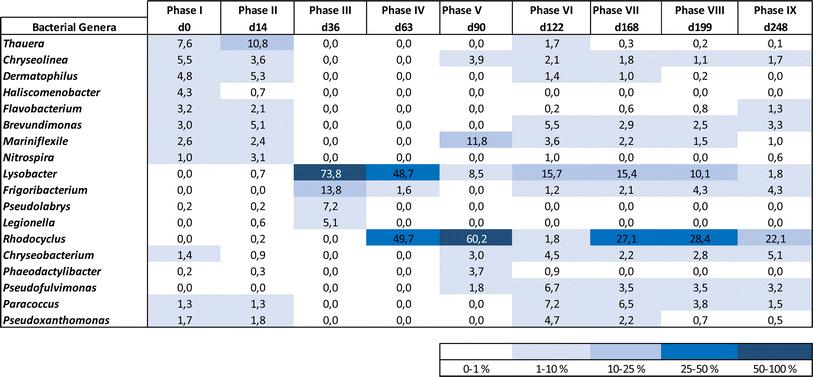
AGS microbiome diversity and compositional dynamics under small stepwise salt increments
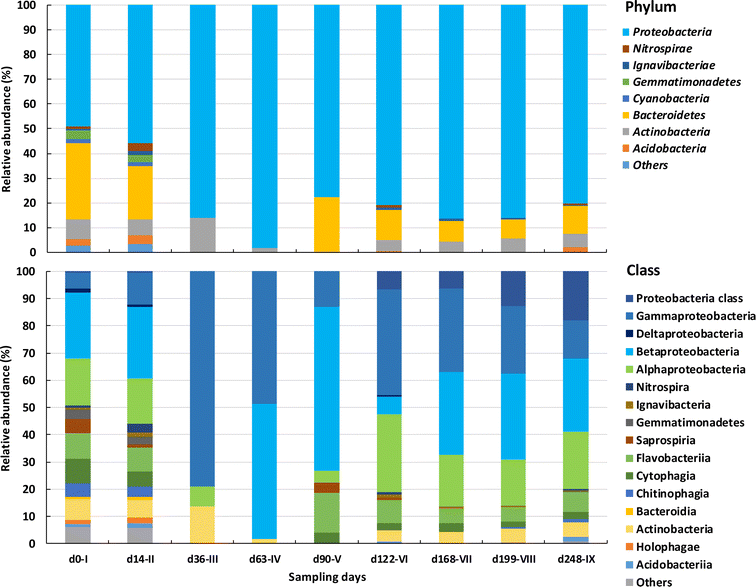
The diversity of bacterial classes identified in the AGS biomass decreased substantially between the initial phases and the following ones, with some classes being enriched and others reduced (Fig. 5b). Proteobacteria were initially composed of Alpha, Beta, Delta and Gammaproteobacteria classes. Deltaproteobacteria, presented the lowest relative abundance and lost in number to other bacterial classes, such as Gammaproteobacteria. Initially less abundant (6%), members of the Gammaproteobacteria class increased in relative abundance during phases III and IV (between 49 and 100%) and became dominant until the end of operation. On the other hand, both Beta and Alphaproteobacteria were present at high relative abundance at the beginning of AGS operation (ca. 25 and 17%, respectively) and by the end of operation their relative abundance was like the one present in the inoculum (27 and 21%, respectively). While Alphaproteobacteria relative abundance was kept stable over time, Betaproteobacteria abundance greatly varied, reaching a high relative abundance by the end of phase V (60%) which was then lost. Bacteria belonging to the Proteobacteria which were not further classified, increased along reactor operation from 0.4 (phase I) to 17.9% (phase IX).
Bacteroidetes diversity was also higher during phases I and II, compared to the next phases. Cytophagia and Flavobacteriia bacterial classes remained in the biomass with higher relative abundancies (>2 and 5%, respectively) when compared to Chitinophagia and Saprospiria, which decreased in numbers after phase IV (between 0 and 1.2%).
Acidobacteriia and Holophagae bacterial classes, from Acidobacteria phylum, were identified during phases I and II but were no longer detected during most of the following operational phases. Only the Actinobacteria bacterial class was identified within Actinobacteria phylum. Despite some variation, this bacterial class presented a similar relative abundance in the AGS biomass at the beginning and end of operation (7.5 and 5.2%, respectively). Meanwhile, Gemmatimonadetes were only detected during phases I and II while Ignavibacteriae were identified along reactor operation (between 0.3 and 1.7%) as well as Nitrospirae (from 0.6 to 4.6%).
Discussion
The acclimation of the AGS process to a slow salt stepwise increase resulted in a high and stable carbon, ammonium and phosphate removal performance, associated with gradual changes of the microbiome at a structural and taxonomic level. During the acclimation process, the microbiome of the AGS biomass changed through the selection of more adapted bacterial genera, some known as halotolerant. The low salt increments combined with their application for shorter periods of time during the initial acclimation period, might also have promoted a faster microbial composition adaptation. Nevertheless, a stable bacteriome composition was achieved when treating wastewater containing the highest tested salt concentrations, likely due to the longer adaptation periods. The greatest fluctuations in reactor performance as well as in the AGS microbiome were observed while treating wastewater with a salinity increase up to ca. 6 g NaCl L−1. The initial variability in the carbon removal efficiency was concomitant with the period of greater instability in phosphate removal, which occurred during the first 50 days of operation. After that, both carbon and phosphate removal reached their maximum efficiency, and remained consistent till the end of operation. Corroborating previous studies, salinity increase did not affect carbon removal.11,12,26 Several bacteria associated with carbon uptake were identified in the AGS biomass, namely denitrifiers and putative EPS producers. These last ones, well represented in the biomass microbiome by different taxa, also included bacteria with denitrifying capacity (e.g., Denitromonas, Thauera and Paracoccus).Phosphate removal improved during salt acclimation. The lower PAO activity observed during initial salt load is not expected to be associated with a salt inhibitory effect, since PAO activity improved and stabilized despite the salinity increase up to 14 g NaCl L−1. In an AGS process treating saline wastewater, the PAO activity was affected at 11 g L−1 of NaCl, being outcompeted by GAO,10. The salt step increase of 11 g L−1 applied (from 0 to 11 g L−1) might not have allowed the adaptation of the AGS biomass to salt. After the beginning of the reactor operation and before the last operational phase, the applied salt increment was gradual and often lower than 2 g NaCl L−1. This might have allowed for a better adaptation of PAO, which were identified in the AGS biomass from the start until the end of the process, with a relative abundance up to 2.4%. During phase IV, the high abundance of Rhodocyclus, a PAO-related microorganism, matched the increase of phosphate accumulation and its subsequent removal during the aerated stage of the treatment cycle (Fig. 1b). The Rhodocyclaceae family was also enriched in AGS salt-supplemented reactors, adapted to salt stepwise increase, although the Rhodocyclus genus was not further identified.15 Although Rhodocyclus is not typically classified as a PAO, it is known to accumulate substantial amounts of phosphate and has been identified in biological processes associated with phosphate removal.27 Also, this genus capability to thrive in saline environments was previously reported.28 The possibility of Rhodocyclus to tolerate high salinity and to produce EPSs,29 might have favoured its enrichment in the AGS biomass, contributing to an increased level of phosphate accumulation by the biomass. Considering that phosphorus removal during saline wastewater treatment, even under gradual salt increase, might depend on the type of PAOs present in the inoculum,3 a high diversity of PAOs identified in the AGS biomass might have allowed a better adaptation to increased salinity, and outcompete GAOs, whose capacity to deal with salt stress is higher when compared to PAOs.3
At the beginning and before reaching its highest activity, PAO might have been outcompeted by PHA accumulating bacteria and/or by denitrifying bacteria, since GAO were only identified towards the latter phases of operation. Thauera and Plasticicumulans (not shown) are putative PHA accumulating microorganisms30,31 which were identified in the AGS biomass during the initial phases. Lysobacter is among the most abundant denitrifying bacteria identified in the AGS biomass. This bacterium might have been involved in different processes during granules disintegration and granulation, contributing for AGS stability. Lysobacter is a bacterium related to carbon uptake and incomplete denitrification in AGS processes, associated with EPS production and to enhanced AGS stability, being also known for its lytic activity on other microorganisms.32–34 Despite the lower numbers in the microbiome, some bacterial genera identified as putative denitrifiers increased their relative abundance with increased wastewater salinity, namely Nitratireductor, Pseudofulvimonas, Hydrogenophaga, and Denitromonas, while Azoarcus was present in the reactor biomass throughout the entire operation. With increased salinity, Thauera lost its dominance to other bacteria, such as Paracoccus. Thauera might be halotolerant bacteria since members of this genus were found to be dominant in an AGS system treating saline wastewater (up to 10 g NaCl L−1).35 However, in the present study, Thauera might have been outcompeted by bacteria with similar functional roles in nitrogen removal but also by EPS producing bacteria, such as Paracoccus, but also by Chryseobacterium or Pseudoxanthomonas, all putative EPS producers.12Nitratireductor, Denitromonas and Paracoccus were identified in an AGS process treating hypersaline wastewater (90 g NaCl L−1).36Azoarcus and Pseudofulvimonas were identified in an AGS reactor treating wastewater with increased salt concentration (up to 20 g L−1).37Azoarcus was also enriched in an AGS process subjected to slow salt increase up to 10 g NaCl L−1, under low temperature.9Hydrogenophaga is an autotrophic denitrifier previously identified in a AGS process treating shale gas flowback saline water.38 The relative abundance of Chryseobacterium increased throughout the operation, and Pseudoxanthomonas persisted in the process without significant changes in numbers. Scarce information is available on Chryseobacterium or Pseudoxanthomonas salt tolerance in AGS or other aerobic treatment processes, but our results indicate that these bacteria might be able to thrive in saline environments. On the other hand, Flavobacterium decreased along the salt acclimation period. Flavobacterium was found to be affected by salinity increase in wastewater treated by an AGS process37 and decreased in abundance during the granulation of an AGS process treating saline wastewater at low temperature.9 Despite decreasing in relative abundance with increasing salt concentrations, Phaeodactylibacter, a denitrifying bacterium, was identified in a biofilm reactor treating hypersaline wastewater (>100 g NaCl L−1).39
Nitrification was not affected during salt acclimation, with AOB (e.g., Nitrosomonas) and NOB (e.g., Nitrospira) being identified in the AGS biomass throughout the operation. Nitrosomonas was the only AOB identified in this study and the fact that this bacterium was enriched in an AGS process adapted to gradual salinity increase,9 indicates that Nitrosomonas can thrive in AGS processes subjected to salt stress. Although several studies have shown that nitrification occurring in an AGS process is not expected to be affected by salt content bellow 30 g L−1 of NaCl,10 there are also several studies showing contradictory results. In a study using AGS for treating a mixture of synthetic wastewater and seawater, nitrite accumulation occurred when wastewater contained 20 to 100% of seawater (between 7 and 35 g L−1 of salinity).1 Besides Nitrospira, Nitrobacter was also detected in the AGS biomass after nitrite levels increase in the outlet. Possibly Nitrobacter has shown to be better adapted to higher nitrite concentrations compared to Nitrospira.14Devosia, a nitrifier bacteria observed in the microbiome at the end of salt acclimation, was a dominant bacterial genus in an AGS process treating saline wastewater containing aromatic compounds, where the salt content reached up to 29 g L−1.40
Defluviicoccus, a GAO, was identified in the biomass after exposure to the highest salt concentrations, thus validating previous observations related to GAOs high salt tolerance.10,39 Despite this, GAO presence in the microbiome did not affect phosphate accumulation and removal efficiency at a salt concentration of 14 g L−1, different from the observations made by Wang et al.3 when testing a salt concentration of 15 g L−1. The slow salt increase might have avoided nitrite accumulation and its known detrimental effect on PAO activity.4,10 In fact, Pronk et al.4 have shown that when NOB are not inhibited, PAO can deal with NaCl concentrations up to 21 g L−1 of NaCl.
Mariniflexile, a bacterium commonly found in saline water, and Frigoribacterium, a psychrophilic bacterial genus, were identified in the AGS biomass throughout its gradual acclimation to high salt levels. This indicates the ability of both bacteria genera to thrive under saline conditions.
On the other hand, the intolerance to salt might have resulted in the disappearance of some bacterial genera, namely of Candidatus Kuenenia (Anammox) and Candidatus Accumulibacter (PAO) that were present in the inoculum but were not further identified in the AGS microbiome along operation.
The granule's structure presented a dynamic behaviour. Despite the slow salt increments, TSS monitoring confirmed the partial degranulation of the AGS biomass, mostly during the first three months of operation, which was followed by a fast granulation and formation of stable bigger granules. The adaptation to a different wastewater composition together with a salinity increase (above 4 g NaCl L−1) might have promoted a greater biomass washout. But the continuous slow salt increase and the enrichment of Rhodocyclus and Lysobacter, both associated with EPS production and AGS stability, could have contributed for the fast recovery and granulation of the biomass. The EPS concentration in the granules increased with higher salt concentration, which is in accordance with previous studies showing that increased salinity can stimulate EPS production,13 and result in a higher AGS stability. In fact, salinity, associated with the presence of Na+ but also of Ca2+ and Mg2+ ions in wastewater can improve the physical strength of AGS, being this related to EPS production and its effect in the granulation process, as observed in a study performed by Li et al.1 Different from our results, concentrations higher than 10 g NaCl L−1 led to AGS disintegration and process failure.9 In this different study, a sharp salinity increment (ca. 10 g L−1 of NaCl) might have promoted the granule's destabilization. Regarding EPS composition, polysaccharides concentration in the EPSs was found not to be as relevant as the EPS protein content. Polysaccharides are known to form the backbone of the AGS granular structure41 and proteins are believed to be important for AGS integrity, associated with a higher surface hydrophobicity and reduced negative charge on the surface, being secreted as a protective mechanism to resist salinity.1 Therefore, an increased protein content in the EPS of the granules was expected with salt increase. The increased concentration of humic acids in the AGS granules is not as commonly determined as protein concentration. However, the presence of humic substances in the EPS matrix is also considered to play a key role in the ionic binding of particles alongside proteins.42 The fact that humic acid concentration increased with salt concentration, can also be an indication of a response to the osmotic stress induced to the AGS system, possibly also contributing to the AGS performance stability and granule integrity. The EPS production might have contributed to granules physical integrity, as suggested by QIA results, which demonstrated stable values for both granule robustness and compactness.
In this study, a slow and gradual salt stepwise increase up to 14 g NaCl L−1 was shown to keep the stability of the AGS biomass while keeping the system performance, in terms of carbon and phosphate removal as well regarding nitrification. Although these results contribute to a general understanding that a gradual and possibly longer period of adaptation of the AGS processes to high salinity wastewater can be a good strategy for reaching process stability, it is not possible to establish a general rule. AGS stability might be more challenging under less optimal operational conditions such as those in a real scenario. Despite applying a gradual salt increase, Li et al.9 were not able to keep a stable AGS process for treating saline wastewater with 14 g NaCl L−1 possibly due to the low temperature (12 °C). In a different study also working with acclimation to gradual salinity increase,3 it was only after the increase of the dissolved oxygen concentration from 2.5 to 8 mg L−1 that an AGS process was able to recover its nitrification activity. However, in this later case, this was not sufficient to recover the PAO activity, determinant for process stability. While Bassin et al.14 achieved good nitrification rates by applying a gradual salt increase, no results were shown concerning phosphate removal or PAO presence in the AGS microbiome. On the other hand, Pronk et al.,4 noticed a much lower phosphate removal rate when increasing salt concentration up to 13 g L−1, as well as a lower accumulation of phosphate in the reactor at this concentration. Wang et al.3 also tested the effect of a gradual salt increase, while applying a lower DO level. The stress caused by both salt and low DO concentration affected PAO activity irreversibly, while nitrifiers were able to recover when higher DO levels were applied. Nitrifiers seem to have the capacity to recover faster from the different stress conditions, being more difficult for PAO to recover their activity. Therefore, there is an indication that a gradual salt increase can allow a better AGS adaptation to salt if it does not affect PAO activity. Although AGS processes have been designed to promote complete nitrogen removal through denitrification, the removal of nitrate from the reactor bulk liquid was not observed. As denitrifiers are facultative aerobes, abundant in the AGS biomass, this was possibly due to carbon and nitrate transfer limitations, to the inner layers of the granule. Carbon uptake was probably performed mostly for anaerobic processes associated with phosphate uptake and EPS production.
Although additional strategies could still be tested, such as evaluating the effects of higher salinity levels on granules structural stability and test real wastewater with similar salt concentrations, these results can be applied to saline wastewater resulting from very diverse types of industrial processes.26,43 Despite this, there are challenges associated with the application of this strategy to a scaled-up system. For example, the first months of operation of a full scale AGS reactor treating saline wastewater would need to be dedicated to the adaptation to wastewater with incremental salt concentrations, in a controlled industrial setting. This would represent consuming more time and resources during the first operational phases but seems to guarantee a better reactor performance in the longer time span, avoiding future operational problems. Considering that AGS wastewater treatment processes are cost effective, this would reduce the need for an expensive tertiary treatment before wastewater discharge. On the other hand, if the WWTP is located nearby the coastline, the treated water can reach the required quality to be discharged in the sea. Nevertheless, if the treated wastewater is discharged into freshwater streams, it can cause fluctuations in their salinity levels and as such a desalination treatment should be considered.
Conclusions
The slow salt adaptation of AGS allowed for stable and efficient removal of carbon, phosphorus, and ammonium by promoting the gradual adaptation of the microbiome. No significant nitrite accumulation in the effluent was observed, indicating that AGS nitrifying bacteria effectively managed salinity levels up to 14 g NaCl L−1. Phosphate removal reached its highest efficiency during salt acclimation, coinciding with the enrichment of Rhodocyclus bacterial genus.The dynamic response of the AGS microbiome to the slow salt increase led to an enrichment of halotolerant species possessing diverse metabolic capabilities. The application of slow salt increments proved crucial for maintaining high stability in AGS removal performance, as this strategy favoured the persistence of key microbial players, namely related to phosphate removal. This approach can be highly valuable for treating industrial wastewater containing salts in their composition.
Data availability
Data supporting this article are available upon request from the authors. Metagenomic data are publicly available in the NCBI database at https://www.ncbi.nlm.nih.gov/sra with PRJNA1129501.Author contributions
Ana M. S. Paulo: conceptualization, investigation, formal analysis, writing – original draft. Oihane Salazar: formal analysis. Joana Costa: software, formal analysis. Daniela P. Mesquita: software, writing – review & editing. Eugénio C. Ferreira: supervision, writing – review & editing. Paula M. L. Castro: supervision, writing – review & editing. Catarina L. Amorim: conceptualization, supervision, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financed by FCT through the project GReAT-PTDC/BTA-BTA/29970/2017 (POCI-01-0145-FEDER-029970). The authors thank the CBQF scientific collaboration under the FCT project UIDB/50016/2020. The authors thank the FCT under the scope of the strategic funding of UIDB/04469/2020 unit. Daniela P. Mesquita acknowledges FCT funding under CEEC INST 2ed (CEECINST/00018/2021/CP2806/CT0004; DOI: https://doi.org/10.54499/CEECINST/00018/2021/CP2806/CT0004).References
- X. Li, J. Luo, G. Guo, H. R. Mackey, T. Hao and G. Chen, Seawater-based wastewater accelerates development of aerobic granular sludge: A laboratory proof-of-concept, Water Res., 2017, 115, 210–219, DOI:10.1016/j.watres.2017.03.002.
- Y. Zhao, X. Zhuang, S. Ahmad, S. Sung and S. Q. Ni, Biotreatment of high-salinity wastewater: current methods and future directions, World J. Microbiol. Biotechnol., 2020, 36(3), 1–11, DOI:10.1007/s11274-020-02815-4.
- Z. Wang, M. C. M. van Loosdrecht and P. E. Saikaly, Gradual adaptation to salt and dissolved oxygen: Strategies to minimize adverse effect of salinity on aerobic granular sludge, Water Res., 2017, 124, 702–712, DOI:10.1016/j.watres.2017.08.026.
- M. Pronk, J. P. Bassin, M. K. De Kreuk, R. Kleerebezem and M. C. M. van Loosdrecht, Evaluating the main and side effects of high salinity on aerobic granular sludge, Appl. Microbiol. Biotechnol., 2014, 98(3), 1339–1348, DOI:10.1007/s00253-013-4912-z.
- A. Xu, D. Yu, Y. Qiu, G. Chen, Y. Tian and Y. Wang, A novel process of salt tolerance partial denitrification and anammox (ST-PDA) for treating saline wastewater, Bioresour. Technol., 2022, 345, 126472, DOI:10.1016/j.biortech.2021.126472.
- M. K. de Kreuk and M. C. van Loosdrecht, Formation of aerobic granules with domestic sewage, J. Environ. Eng., 2006, 132(6) DOI:10.1061/(ASCE)0733-9372(2006)132:6(694).
- M. K. H. Winkler, C. Meunier, O. Henriet, J. Mahillon, M. E. Suárez-Ojeda and M. G. Del, et al., An integrative review of granular sludge for the biological removal of nutrients and recalcitrant organic matter from wastewater, Chem. Eng. J., 2018, 336, 489–502, DOI:10.1016/j.cej.2017.12.026.
- D. R. de Graaff, M. C. M. van Loosdrecht and M. Pronk, Biological phosphorus removal in seawater-adapted aerobic granular sludge, Water Res., 2020, 172, 115531, DOI:10.1016/j.watres.2020.115531.
- J. Li, Z. Ma, M. Gao, Y. Wang, Z. Yang and H. Xu, et al., Enhanced aerobic granulation at low temperature by stepwise increasing of salinity, Sci. Total Environ., 2020, 722, 137660, DOI:10.1016/j.scitotenv.2020.137660.
- J. P. Bassin, M. Pronk, G. Muyzer, R. Kleerebezem, M. Dezotti and M. C. M. van Loosdrecht, Effect of elevated salt concentrations on the aerobic granular sludge process: Linking microbial activity with microbial community structure, Appl. Environ. Microbiol., 2011, 77(22), 7942–7953 CrossRef CAS PubMed.
- S. F. Corsino, M. Capodici, C. Morici, M. Torregrossa and G. Viviani, Simultaneous nitritation-denitritation for the treatment of high-strength nitrogen in hypersaline wastewater by aerobic granular sludge, Water Res., 2016, 88, 329–336 CrossRef CAS PubMed.
- A. M. S. Paulo, C. L. Amorim, J. Costa, D. P. Mesquita, E. C. Ferreira and P. M. L. Castro, Long-term stability of a non-adapted aerobic granular sludge process treating fish canning wastewater associated to EPS producers in the core microbiome, Sci. Total Environ., 2021, 756, 144007, DOI:10.1016/j.scitotenv.2020.144007.
- S. F. Corsino, R. Campo, G. Di Bella, M. Torregrossa and G. Viviani, Cultivation of granular sludge with hypersaline oily wastewater, Int. Biodeterior. Biodegrad., 2015, 105, 192–202, DOI:10.1016/j.ibiod.2015.09.009.
- J. P. Bassin, R. Kleerebezem, G. Muyzer, A. S. Rosado, M. C. M. Van Loosdrecht and M. Dezotti, Effect of different salt adaptation strategies on the microbial diversity, activity, and settling of nitrifying sludge in sequencing batch reactors, Appl. Microbiol. Biotechnol., 2012, 93(3), 1281–1294, DOI:10.1007/s00253-011-3428-7.
- F. K. Amancio Frutuoso, A. Ferreira dos Santos, L. L. da Silva França, A. R. Mendes Barros and A. Bezerra dos Santos, Influence of salt addition to stimulating biopolymers production in aerobic granular sludge systems, Chemosphere, 2023, 311, 137006, DOI:10.1016/j.chemosphere.2022.137006.
- A. M. S. Paulo, C. L. Amorim, J. Costa, D. P. Mesquita, E. C. Ferreira and P. M. L. Castro, High carbon load in food processing industrial wastewater is a driver for metabolic competition in aerobic granular sludge, Front. Environ. Sci., 2021, 9, 1–15, DOI:10.3389/fenvs.2021.735607.
- C. L. Amorim, A. S. Maia, R. B. R. Mesquita, A. O. S. S. Rangel, M. C. M. van Loosdrecht and M. E. Tiritan, et al., Performance of aerobic granular sludge in a sequencing batch bioreactor exposed to ofloxacin, norfloxacin and ciprofloxacin, Water Res., 2014, 50, 101–113, DOI:10.1016/j.watres.2013.10.043.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington (DC), 1998 Search PubMed.
- S. Felz, S. Al-Zuhairy, O. A. Aarstad, M. C. M. van Loosdrecht and Y. M. Lin, Extraction of structural extracellular polymeric substances from aerobic granular sludge, J. Visualized Exp., 2016, 115, 1–8, DOI:10.3791/54534.
- O. H. Lowry, N. J. Rosebrough, R. J. Randall and A. Lewis, Protein Measurement with the Folin Phenol Reagent, J. Biol. Chem., 1951, 193(2), 265–275 CrossRef CAS PubMed , https://pubmed.ncbi.nlm.nih.gov/14907713/.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Colorimetric Method for Determination of sugars and Related Substances, Anal. Chem., 1956, 28(3), 350–356, DOI:10.1021/ac60111a017.
- B. Frølund, T. Griebe and P. H. Nielsen, Enzymatic activity in the activated-sludge floc matrix, Appl. Microbiol. Biotechnol., 1995, 43(4), 755–761, DOI:10.1007/BF00164784.
- J. G. Costa, A. M. S. Paulo, C. L. Amorim, A. L. Amaral, P. M. L. Castro and E. C. Ferreira, et al., Quantitative image analysis as a robust tool to assess effluent quality from an aerobic granular sludge system treating industrial wastewater, Chemosphere, 2022, 291, 132773, DOI:10.1016/j.chemosphere.2021.132773.
- S. Turner, K. M. Pryer, V. P. W. Miao and J. D. Palmer, Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis, J. Eukaryotic Microbiol., 1999, 46(4), 327–338, DOI:10.1111/j.1550-7408.1999.tb04612.x.
- V. Kisand, R. Cuadros and J. Wikner, Phylogeny of culturable estuarine bacteria catabolizing riverine organic matter in the northern Baltic Sea, Appl. Environ. Microbiol., 2002, 68(1), 379–388, DOI:10.1128/AEM.68.1.379-388.2002.
- P. F. Carrera, R. Campo, R. Méndez, G. Di Bella, J. L. Campos and A. Mosquera-Corral, et al., Does the feeding strategy enhance the aerobic granular sludge stability treating saline effluents?, Chemosphere, 2019, 226, 865–873, DOI:10.1016/j.chemosphere.2019.03.127.
- M. Beer, H. M. Stratton, P. C. Griffiths and R. J. Seviour, Which are the polyphosphate accumulating organisms in full-scale activated sludge enhanced biological phosphate removal systems in Australia?, J. Appl. Microbiol., 2006, 100(2), 233–243, DOI:10.1111/j.1365-2672.2005.02784.x.
- W. Li, X. Lv, J. Ruan, M. Yu, Y. B. Song and J. Yu, et al., Variations in soil bacterial composition and diversity in newly formed coastal wetlands, Front. Microbiol., 2019, 10, 1–9, DOI:10.3389/fmicb.2018.03256.
- B. Zhang, M. Ji, Z. Qiu, H. Liu, J. Wang and J. Li, Microbial population dynamics during sludge granulation in an anaerobic-aerobic biological phosphorus removal system, Bioresour. Technol., 2011, 102(3), 2474–2480, DOI:10.1016/j.biortech.2010.11.017.
- Y. Jiang, D. Sorokin, R. Kleerebezem, G. Muyzer and M. van Loosdrecht, Plasticicumulans acidivorans gen. nov., sp. nov., a polyhydroxyalkanoate-accumulating gammaproteobacterium from a sequencing-batch bioreactor, Int. J. Syst. Evol. Microbiol., 2011, 61(9), 2314–2319, DOI:10.1099/ijs.0.021410-0.
- M. Oshiki, M. Onuki, H. Satoh and T. Mino, PHA-accumulating microorganisms in full-scale wastewater treatment plants, Water Sci. Technol., 2008, 58(1), 13–20, DOI:10.2166/wst.2008.652.
- I. de Bruijn, X. Cheng, V. de Jager, R. G. Expósito, J. Watrous and N. Patel, et al., Comparative genomics and metabolic profiling of the genus Lysobacter, BMC Genomics, 2015, 1–16, DOI:10.1186/s12864-015-2191-z.
- J. Luo, T. Hao, L. Wei, H. R. Mackey, Z. Lin and G. H. Chen, Impact of influent COD/N ratio on disintegration of aerobic granular sludge, Water Res., 2014, 62, 127–135, DOI:10.1016/j.watres.2014.05.037.
- Y. Yin, F. Liu, L. Wang and J. Sun, Overcoming the instability of aerobic granular sludge under nitrogen deficiency through shortening settling time, Bioresour. Technol., 2019, 289, 121620, DOI:10.1016/j.biortech.2019.121620.
- L. Lei, J. C. Yao, Y. D. Liu and W. Li, Performance, sludge characteristics and microbial community in a salt-tolerant aerobic granular SBR by seeding anaerobic granular sludge, Int. Biodeterior. Biodegrad., 2021, 163, 105258, DOI:10.1016/j.ibiod.2021.105258.
- J. Yao, W. Li, D. Ou, L. Lei, M. Asif and Y. Liu, Performance and granular characteristics of salt-tolerant aerobic granular reactors response to multiple hypersaline wastewater, Chemosphere, 2021, 265, 129170, DOI:10.1016/j.chemosphere.2020.129170.
- H. Wang, L. Guo, X. Ren, M. Gao, C. Jin and Y. Zhao, et al., Enhanced aerobic granular sludge by static magnetic field to treat saline wastewater via simultaneous partial nitrification and denitrification (SPND) process, Bioresour. Technol., 2022, 350, 126891, DOI:10.1016/j.biortech.2022.126891.
- J. Liang, Q. Wang, Q. X. Li, L. Jiang, J. Kong and M. Ke, et al., Aerobic sludge granulation in shale gas flowback water treatment : Assessment of the bacterial community dynamics and modeling of bioreactor performance using artificial neural network, Bioresour. Technol., 2020, 313, 123687, DOI:10.1016/j.biortech.2020.123687.
- J. Wang, J. Zhou, Y. Wang, Y. Wen, L. He and Q. He, Efficient nitrogen removal in a modified sequencing batch biofilm reactor treating hypersaline mustard tuber wastewater: The potential multiple pathways and key microorganisms, Water Res., 2020, 177, 115734, DOI:10.1016/j.watres.2020.115734.
- C. Ramos, M. E. Suárez-ojeda and J. Carrera, Long-term impact of salinity on the performance and microbial population of an aerobic granular reactor treating a high-strength aromatic wastewater, Bioresour. Technol., 2015, 198, 844–851, DOI:10.1016/j.biortech.2015.09.084.
- S. S. Adav and D. J. Lee, Extraction of extracellular polymeric substances from aerobic granule with compact interior structure, J. Hazard. Mater., 2008, 154(1–3), 1120–1126, DOI:10.1016/j.jhazmat.2007.11.058.
- S. Felz, H. Kleikamp, J. Zlopasa, M. C. M. van Loosdrecht and Y. Lin, Impact of metal ions on structural EPS hydrogels from aerobic granular sludge, Biofilm, 2020, 2, 100011, DOI:10.1016/j.bioflm.2019.100011.
- A. Srivastava, V. K. Parida, A. Majumder, B. Gupta and A. K. Gupta, Treatment of saline wastewater using physicochemical, biological, and hybrid processes: Insights into inhibition mechanisms, treatment efficiencies and performance enhancement, J. Environ. Chem. Eng., 2021, 9(4), 105775, DOI:10.1016/j.jece.2021.105775.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4va00248b |
| This journal is © The Royal Society of Chemistry 2024 |