Open Access Article
Open Access ArticleA quick and effective strategy for the synthesis of Ti3C2Txvia electrochemical method†
Shrabani
De
a,
Sourav
Acharya
a,
Satyanarayan
Sahoo
 b and
Ganesh Chandra
Nayak
b and
Ganesh Chandra
Nayak
 *a
*a
aDepartment of Chemistry and Chemical Biology, Indian Institute of Technology (ISM), Dhanbad-826004, Jharkhand, India. E-mail: gcnayak@iitism.ac.in
bP. G. Department of Chemistry, Berhampur University, Odisha 760007, India
First published on 19th March 2024
Abstract
A quick and potent electrochemical etching of Ti3AlC2 at room temperature is developed. Studies reveal the purity of Ti3C2Tx without any formation of TiO2 or over-etching of MAX phase. The delaminated MXene was studied as all-solid-state flexible supercapacitor which performed better than the conventionally synthesized one.
MXenes are an interesting class of layered 2D transition metal carbides, nitrides, and/or carbonitrides discovered in 2011, hold unique combination of fascinating properties like hydrophilicity, high electrical conductivity, stability, light weight, and porosity.1,2 They are generally described by Mn+1XnTx (n = 1–4; M is transition metals; X is C and/or N; Tx is surface terminal groups including –F, –OH, –O, and/or –Cl).3 Generally, MXenes are synthesized by selective elimination of A atoms from hexagonal layered MAX phase (Mn+1AXn; A is mostly group 13 or 14 element) materials.4 Among different synthesized MXenes, titanium carbide (Ti3C2Tx) and its composites have been gaining huge interest in wide range of areas including energy conversion and storage, catalysis, sensing, adsorption, field effect transistors, electromagnetic interference shielding, water purification, separation, lubrication, and photothermal conversion.5–9
Previous studies have shown that chemical etching is the most common method to prepare Ti3C2Tx from Ti3AlC2.1 The mechanism of chemical etching is dependent on the higher chemical reactivity of ‘M–A’ bond than ‘M–C’ bond and this is basically an electrochemical method.10 Each surface active spots involves in both cathodic and anodic reaction during a usual chemical etching process. In case of electrochemical etching, the cathodic and anodic sites are isolated, and the etching reactions are operated by the bias.11 According to previous studies, various electrolytes like HCl, NaCl, H2SO4, HNO3, NH4Cl, NaOH, and FeCl3 have been used for the electrochemical etching of MAX phases including Ti2AlC, V2AlC, Cr2AlC, and Ti3AlC2.2 But, the atomically thin A layer elimination was not successful may be due to kinetic problems.12 MAX phases either get over etched like both A and M layers get eliminated resulting carbide derived carbon, or only the M layer get etched resulting an AX like structure (for Ti3SnC2 and Ti2SC).13,14 In previous studies, compacted MAX phase block has been electrochemically etched and the etching reaction preferentially occurred on the surface.2 It is usually difficult to obtain pure MXene sheets with complete etching which are generally obtained from chemical methods. But, chemical etching processes suffer from longer synthesis time like 2 to 3 days to obtain completely etched product often requiring higher temperatures.15
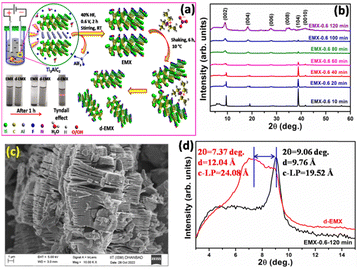
To overcome these limitations, in this study, MAX phase (Ti3AlC2) has been electrochemically etched by dispersing in the electrolyte (40% HF) with two platinum strips as electrodes. In contrast with the previously reported electrochemical etching methods, complete etching is obtained. Also, this process is much quicker than the commonly followed chemical etching methods. The completely etched MXene (EMX) is obtained within just 2 hours at room temperature and the quality of MXene sheets are comparable with the same obtained via chemical etching without any sheet breaking or formation of TiO2. Moreover, the obtained Ti3C2Tx is delaminated (named as d-EMX) using ionic liquid [(Et3HN)+(NO3)−] and its capacitive performance as well as electrocatalytic activity is studied.
Electrochemical etching is carried out in a two-electrode set up keeping platinum strips as both positive and negative electrodes in HF electrolyte (Fig. 1a). The formation of AlF3 during the etching in HF is generally a slow process. The reaction of Al with fluorine ions gets triggered in presence of applied potential which leads to a fast overall etching of Ti3C2Tx.16 The overall electrochemical etching reaction is described as follows:17 A comparison of previously reported electrochemical etching methods with this study are included in Table S1 (ESI†).
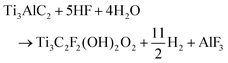 | (1) |
The etching time of the prepared Ti3C2Tx is optimized using X-ray diffraction (XRD) analysis by varying the time from 10 to 120 minutes (Fig. 1b). The diffraction peak at 2θ = 38.9° associated with (104) plane of Al6 reduces with increasing time and completely disappears after 120 min (2 hours). Further, the absence of TiO2 plane in XRD, confirms no over-etching of the EMX sheets. The applied voltage was changed from 0.4 to 0.8 V, and no such change in the XRD pattern is observed (Fig. S1a, ESI†). The process is also useful to process large batches of MAX phase to prepare pure Ti3C2Tx within 2 hours which is observed from XRD analysis (Fig. S1c, ESI†). Also, at the same reaction condition without the applied voltage, MAX phase cannot be completely etched (Fig. S1c, ESI†). The field emission scanning electron microscopy (FESEM) analysis (Fig. S2, S4 and S6, ESI†) shows distinct elimination of Al layer and formation of thin EMX sheets (Fig. 1c) with increasing etching time up to 2 hours. The disappearance of Al plane observed from XRD is well supported by the energy dispersive X-ray spectroscopy (EDS) analysis (Fig. S1d, S3, S5 and S7, ESI†) where the Al content gradually decreases with increasing etching time. From XRD, FESEM, and EDS analysis, it is confirmed that the complete etching is obtained within 2 hours with overall layer separation and low Al%.
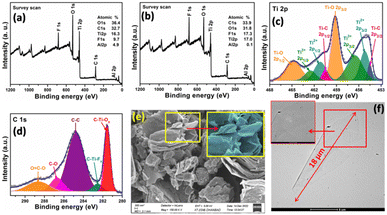
Additionally, X-ray photoemission spectroscopy (XPS) (Fig. 2a and b) supports the above fact that the atomic percentage of aluminium present in EMX sample obtained after 2 hour of etching (EMX-0.6–120 min) is much lower than the EMX sample obtained after 20 minute of etching (EMX-0.6–20 min). Regarding the deconvoluted XPS analysis of EMX-0.6–120 min sample, its Ti 2p spectrum (Fig. 2c) shows four doublets of Ti 2p3/2 and Ti 2p1/2 with doublet gap of 5.9 eV and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 area ratio, in accordance with prior XPS reports of pure Ti3C2Tx.18,19 The peaks of Ti 2p3/2 for Ti–C, Ti2+, Ti3+, and Ti–O bond are observed at 454.5, 455.2, 456.5, and 459.2 eV, respectively. On the other hand, the peaks of Ti 2p1/2 for Ti–C, Ti2+, Ti3+, and Ti–O bond are observed at 460.5, 461.1, 462.5, and 464.7 eV, respectively.18–20 In Fig. 2d, the core level C 1s XPS spectrum displays characteristic peaks at 281.5, 282.5, 284.8, 286.7, 287.6, and 288.6 eV associated with C–Ti–Ox, C–Ti–Fx, C–C, C–O, C
1 area ratio, in accordance with prior XPS reports of pure Ti3C2Tx.18,19 The peaks of Ti 2p3/2 for Ti–C, Ti2+, Ti3+, and Ti–O bond are observed at 454.5, 455.2, 456.5, and 459.2 eV, respectively. On the other hand, the peaks of Ti 2p1/2 for Ti–C, Ti2+, Ti3+, and Ti–O bond are observed at 460.5, 461.1, 462.5, and 464.7 eV, respectively.18–20 In Fig. 2d, the core level C 1s XPS spectrum displays characteristic peaks at 281.5, 282.5, 284.8, 286.7, 287.6, and 288.6 eV associated with C–Ti–Ox, C–Ti–Fx, C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O
O, and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O bonds, respectively.21,22 Here, the presence of Ti–O, C–Ti–O, C–Ti–F, and C–O bonds indicate the surface functionality of Ti3C2Tx. It is observed that the peak positions for Ti 2p and C 1s in EMX-0.6–60 min (Fig. S9a and b, ESI†) and EMX-0.6–100 min (Fig. S9c and d, ESI†) are same as EMX-0.6–120 min. Thus, there is no indication of over etching or oxidation of Ti with increasing the etching time. As the Al layer gets removed from the MAX phase, the increment in surface functionality with increasing the etching time is indicated by increasing the peak area of C–Ti–Ox and C–Ti–Fx in C 1s spectrum. For the Raman spectrum (Fig. S1b, ESI†), the two peaks at 400, and 620 cm−1 are assigned to Ti–C vibrations.23 The D and G bands at 1346, and 1567 cm−1, respectively represent the molecular defects and in-plane sp2 carbon atoms and the ID/IG ratio (0.78) reveals the ratio of disordered and amorphous carbon present in Ti3C2Tx structure.23
C–O bonds, respectively.21,22 Here, the presence of Ti–O, C–Ti–O, C–Ti–F, and C–O bonds indicate the surface functionality of Ti3C2Tx. It is observed that the peak positions for Ti 2p and C 1s in EMX-0.6–60 min (Fig. S9a and b, ESI†) and EMX-0.6–100 min (Fig. S9c and d, ESI†) are same as EMX-0.6–120 min. Thus, there is no indication of over etching or oxidation of Ti with increasing the etching time. As the Al layer gets removed from the MAX phase, the increment in surface functionality with increasing the etching time is indicated by increasing the peak area of C–Ti–Ox and C–Ti–Fx in C 1s spectrum. For the Raman spectrum (Fig. S1b, ESI†), the two peaks at 400, and 620 cm−1 are assigned to Ti–C vibrations.23 The D and G bands at 1346, and 1567 cm−1, respectively represent the molecular defects and in-plane sp2 carbon atoms and the ID/IG ratio (0.78) reveals the ratio of disordered and amorphous carbon present in Ti3C2Tx structure.23
The synthesized EMX-0.6–120 min is further delaminated with ionic liquid [(Et3HN)+(NO3)−] for the first time. The ionic liquid is recovered after delamination and can be used further. After the addition of EMX into the ionic liquid, bulky (Et3HN)+ ions interact with the negatively charged EMX surface due to the presence of –F, –OH, –O functional groups. Further, shaking promotes delamination due to the insertion of bulky (Et3HN)+ ions inside overall EMX layers. The d-EMX suspension remains quite stable differently from the EMX (EMX-0.6–120 min) dispersion which is not stable more than 1 hour (Fig. 1a). Delamination of the sheets of EMX-0.6–120 min sample is supported by XRD analysis (Fig. 1d). The left shifting and broadening of (002) diffraction plane can be observed from 2θ = 9.06° of EMX to 2θ = 7.37° of d-EMX with increment of d-spacing and c-lattice parameter (c-LP) of 2.28 Å and 4.56 Å, respectively. The insertion of bulky (Et3HN)+ group inside the stacked EMX layers disrupted the ordered stacking of the sheets resulting delaminated disordered sheets for d-EMX observed from the FESEM analysis (Fig. 2e). The d-EMX sheets can be clearly observed from Transmission Electron Microscopy (TEM) analysis (Fig. 2f). The transparent d-EMX sheets confirm the delamination using ionic liquid. The TEM elemental mapping exhibits the presence of C, Ti, F, and O (Fig. S8a–e, ESI†) which confirms the Ti3C2Tx structure. The presence of bigger d-EMX sheets with diagonal length of ∼18 μm indicates that shaking did not break the sheet during delamination which is much effective and useful method than the general delamination of MXene using sonication method.
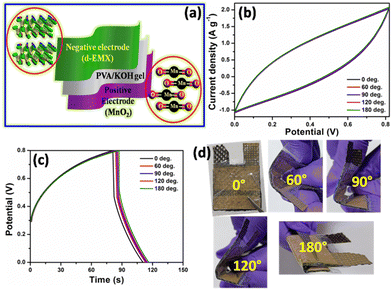
After confirming the compositional, structural, and morphological properties, the electrochemical performance of d-EMX is studied by assembling all-solid-state flexible asymmetric supercapacitor device (ASSASC). The electrode preparation process is included in ESI† (Electrochemical study). As it is an established fact that Ti3C2Tx is a good anode material,24 we checked the electrochemical performance of d-EMX (Fig. S8f, ESI†) in three-electrode system via cyclic voltammetry (CV) analysis in both positive and negative voltage window using 1 M KOH electrolyte and much higher performance can be observed in negative voltage range indicating d-EMX a better anode material than cathode. Therefore, we use d-EMX as negative electrode and MnO2 (purchased from SRL, India) which is a commercial cathode material is used as positive electrode for the two-electrode system. The device study was performed within a potential range of 0.0 to 0.8 V using PVA–KOH as separator as well as gel electrolyte. The PVA–KOH gel was first painted over the electrodes with the dimension of 2 cm × 3 cm and dried to assemble ASSASC device. Fig. 3(a) schematically represents the components of the ASSASC device. The active mass of the loaded electrode material is 10 mg. The ASSASC device exhibited a quasi rectangular CV curve (Fig. S10a, ESI†) at scan rates from 10 to 100 mV s−1 which indicates capacitive behavior of the sample. Similar trend is followed by the GCD analysis at different current densities from 1 to 3 A g−1 (Fig. S10b, ESI†). The device delivered specific capacitance, energy density, and power density of 162 F g−1, 14.4 W h kg−1, and 800 W kg−1, respectively at 1 A g−1 current density. Notably, such high specific capacitance is surpasses those of the priorly reported chemically or electrochemically etched Ti3C2Tx materials (Table S2, ESI†). The high specific capacitance is attributed to the proper etching without any oxidation of Ti atoms as well as effective delamination with few layered Ti3C2Tx sheets.
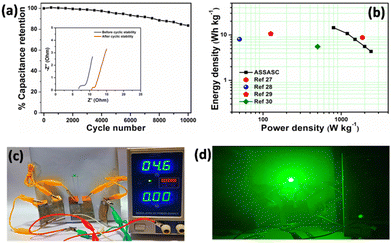
Fig. 3b and c shows the CV analysis at 10 mV s−1 scan rate and galvanostatic charge–discharge (GCD) analysis at 1.5 A g−1, respectively, at different bending angles like 0°, 60°, 90°, 120°, and 180° (Fig. 3d). A good flexibility and electrochemical stability is established for the assembled device as there is no obvious change can be observed from the CV and GCD curves under various bending states. The ASSASC device also exhibited long term cyclic life with 83.6% specific capacitance retention up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (Fig. 4a). Inset of Fig. 4a shows the Nyquist plot before and after the cyclic stability study. The pore blockage and the degradation of the PVA–KOH separator during continuous charge–discharge lead to the increment in solution resistance and thus the decrease in cyclic life. However, decrease in charge transfer resistance after the cyclic stability study indicates that there is increased amount of electrolyte access on the surface of the electrode.25,26 FESEM image (Fig. S10c, ESI†) reveals that the structural morphology of d-EMX remains quite stable after the stability study. Further, the Ragone plot (Fig. 4b) revealed that the energy density of our device is much higher than that of the previously reported MXene based devices (+//−) including Ti3C2Tx/N-doped carbon foam//Ti3C2Tx/N-doped carbon foam (8.75 W h kg−1),27 reduced graphene oxide//Ti3C2Tx (8 W h kg−1),28 Ti3C2Tx/carbon fibre//Ti3C2Tx/carbon fibre (10.6 W h kg−1),29 and Ti3C2Tx//activated carbon (5.5 W h kg−1).30 From these characteristic electrochemical performances, it can be concluded that d-EMX can be used as an efficient anode material for supercapacitor application.
000 cycles (Fig. 4a). Inset of Fig. 4a shows the Nyquist plot before and after the cyclic stability study. The pore blockage and the degradation of the PVA–KOH separator during continuous charge–discharge lead to the increment in solution resistance and thus the decrease in cyclic life. However, decrease in charge transfer resistance after the cyclic stability study indicates that there is increased amount of electrolyte access on the surface of the electrode.25,26 FESEM image (Fig. S10c, ESI†) reveals that the structural morphology of d-EMX remains quite stable after the stability study. Further, the Ragone plot (Fig. 4b) revealed that the energy density of our device is much higher than that of the previously reported MXene based devices (+//−) including Ti3C2Tx/N-doped carbon foam//Ti3C2Tx/N-doped carbon foam (8.75 W h kg−1),27 reduced graphene oxide//Ti3C2Tx (8 W h kg−1),28 Ti3C2Tx/carbon fibre//Ti3C2Tx/carbon fibre (10.6 W h kg−1),29 and Ti3C2Tx//activated carbon (5.5 W h kg−1).30 From these characteristic electrochemical performances, it can be concluded that d-EMX can be used as an efficient anode material for supercapacitor application.
The practical applicability of the assembled ASSASC device is further studied by connecting three devices in series and Fig. 4c shows the charging set-up. The assembled devices are able to brightly glow a 3 V green LED shown in Fig. 4d. Fig. S10e and f (ESI†) show that bending does not affect the practical applicability of the devices.
MXenes are increasingly appealing for their interesting electrocatalytic property.31,32 Here, the bifunctional electrocatalytic property of d-EMX is also studied as full water-splitting catalyst. OER and HER activities of the sample are carried out in 1 M KOH and 1 M H2SO4 electrolyte, respectively. The LSV curves for OER and HER at different scan rates (5, 10, and 50 mV s−1) are shown in Fig. S11a and b (ESI†). The LSV study of d-EMX at 50 mV s−1 (Fig. S11a, ESI†) exhibits a low onset potential of 1.45 V vs. RHE for OER performance. In case of HER study, the LSV curve of d-EMX at 50 mV s−1 (Fig. S11b, ESI†) shows a low onset potential of −0.19 V vs. RHE. These results are much superior than that of previously reported MXene based electrocatalysts (Table S3, ESI†). These catalytic performances suggest that d-EMX can be an efficient bifunctional electrocatalyst for overall water–splitting.
In summary, we have demonstrated a quick strategy to synthesize Ti3C2Tx from Ti3AlC2via electrochemical etching method. By optimizing the applied voltage and time Ti3C2Tx can be synthesized only in 2 hours without any over-etching or oxidation of Ti atoms. The obtained MXene was successfully delaminated to few layered sheets with bigger sheet length using [(Et3HN)+(NO3)−] ionic liquid for the first time via shaking. The delaminated MXene delivered superior specific capacitance of 162 F g−1 at 1 A g−1 while assembled as all-solid-state asymmetric supercapacitor. The fabricated device was highly flexible with no obvious change in electrochemical performance under different bending angles. Additionally, the electrocatalytic performance of the delaminated MXene was also studied as bifunctional electrocatalyst for over-all water splitting and it exhibited good electrocatalytic activity in OER and HER. Therefore, this work paves a way to rapid bulk synthesis of MXene for commercial energy storage and conversion applications.
The IIT (ISM), Dhanbad provided research funds (Project No: IIT(ISM)/2023-2024/1001/INSTITUTE), a lab, central research facilities, and fellowship, which the authors gratefully acknowledge.
Conflicts of interest
There are no conflicts to declare.Notes and references
- W. Sun, S. Shah, Y. Chen, Z. Tan, H. Gao, T. Habib, M. Radovic and M. Green, J. Mater. Chem. A, 2017, 5, 21663–21668 RSC.
- S. Yang, P. Zhang, F. Wang, A. G. Ricciardulli, M. R. Lohe, P. W. Blom and X. Feng, Angew. Chem., 2018, 130, 15717–15721 CrossRef.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 1–17 Search PubMed.
- A. VahidMohammadi, A. Hadjikhani, S. Shahbazmohamadi and M. Beidaghi, ACS Nano, 2017, 11, 11135–11144 CrossRef CAS PubMed.
- S.-Y. Pang, Y.-T. Wong, S. Yuan, Y. Liu, M.-K. Tsang, Z. Yang, H. Huang, W.-T. Wong and J. Hao, J. Am. Chem. Soc., 2019, 141, 9610–9616 CrossRef CAS PubMed.
- C. Cui, M. Hu, C. Zhang, R. Cheng, J. Yang and X. Wang, Chem. Commun., 2018, 54, 8132–8135 RSC.
- M. Zhu, Y. Huang, Q. Deng, J. Zhou, Z. Pei, Q. Xue, Y. Huang, Z. Wang, H. Li and Q. Huang, Adv. Energy Mater., 2016, 6, 1600969 CrossRef.
- L. Huang, L. Ding, J. Caro and H. Wang, Angew. Chem., Int. Ed., 2023, e202311138 CAS.
- L. Huang, L. Ding and H. Wang, Small Sci., 2021, 1, 2100013 CrossRef CAS.
- P. Srivastava, A. Mishra, H. Mizuseki, K.-R. Lee and A. K. Singh, ACS Appl. Mater. Interfaces, 2016, 8, 24256–24264 CrossRef CAS.
- V. Naguib and M. Mochalin, Adv. Mater., 2014, 26, 992 CrossRef PubMed.
- T. Li, L. Yao, Q. Liu, J. Gu, R. Luo, J. Li, X. Yan, W. Wang, P. Liu and B. Chen, Angew. Chem., Int. Ed., 2018, 57, 6115–6119 CrossRef CAS PubMed.
- M. R. Lukatskaya, J. Halim, B. Dyatkin, M. Naguib, Y. S. Buranova, M. W. Barsoum and Y. Gogotsi, Angew. Chem., 2014, 126, 4977–4980 CrossRef.
- M. Q. Zhao, M. Sedran, Z. Ling, M. R. Lukatskaya, O. Mashtalir, M. Ghidiu, B. Dyatkin, D. J. Tallman, T. Djenizian and M. W. Barsoum, Angew. Chem., 2015, 127, 4892–4896 CrossRef.
- M. Benchakar, L. Loupias, C. Garnero, T. Bilyk, C. Morais, C. Canaff, N. Guignard, S. Morisset, H. Pazniak and S. Hurand, Appl. Surf. Sci., 2020, 530, 147209 CrossRef CAS.
- X. Zhang and T. Devine, J. Electrochem. Soc., 2006, 153, B375 CrossRef CAS.
- S. De, S. Acharya, S. Sahoo and G. C. Nayak, Mater. Chem. Front., 2021, 5, 7134–7169 RSC.
- W. Y. Chen, X. Jiang, S.-N. Lai, D. Peroulis and L. Stanciu, Nat. Commun., 2020, 11, 1–10 CrossRef.
- R. B. Rakhi, B. Ahmed, M. N. Hedhili, D. H. Anjum and H. N. Alshareef, Chem. Mater., 2015, 27, 5314–5323 CrossRef CAS.
- Y. T. Liu, P. Zhang, N. Sun, B. Anasori, Q. Z. Zhu, H. Liu, Y. Gogotsi and B. Xu, Adv. Mater., 2018, 30, 1707334 CrossRef.
- X. Li, X. Yin, M. Han, C. Song, H. Xu, Z. Hou, L. Zhang and L. Cheng, J. Mater. Chem. C, 2017, 5, 4068–4074 RSC.
- R. P. Raj, P. Ragupathy and S. Mohan, J. Mater. Chem. A, 2015, 3, 24338–24348 RSC.
- S. Shen, T. Ke, K. Rajavel, K. Yang and D. Lin, Small, 2020, 16, 2002433 CrossRef CAS PubMed.
- S. De, C. K. Maity, S. Sahoo and G. C. Nayak, ACS Appl. Energy Mater., 2021, 4, 3712–3723 CrossRef CAS.
- Y. Wang, L. Liu, Y. Wang, J. Qu, Y. Chen and J. Song, ACS Nano, 2023, 17, 21761–21770 CrossRef PubMed.
- Y. Wang, J. Song and W. Y. Wong, Angew. Chem., 2023, 135, e202218343 CrossRef.
- L. Sun, G. Song, Y. Sun, Q. Fu and C. Pan, ACS Appl. Mater. Interfaces, 2020, 12, 44777–44788 CrossRef CAS PubMed.
- A. M. Navarro-Suárez, K. L. Van Aken, T. Mathis, T. Makaryan, J. Yan, J. Carretero-González, T. Rojo and Y. Gogotsi, Electrochim. Acta, 2018, 259, 752–761 CrossRef.
- L. Sun, Q. Fu and C. Pan, J. Hazard. Mater., 2021, 410, 124565 CrossRef CAS PubMed.
- T.-H. Chang, T. Zhang, H. Yang, K. Li, Y. Tian, J. Y. Lee and P.-Y. Chen, ACS Nano, 2018, 12, 8048–8059 CrossRef CAS PubMed.
- A. Liu, X. Liang, X. Ren, W. Guan, M. Gao, Y. Yang, Q. Yang, L. Gao, Y. Li and T. Ma, Adv. Funct. Mater., 2020, 30, 2003437 CrossRef CAS.
- L. Xiu, Z. Wang, M. Yu, X. Wu and J. Qiu, ACS Nano, 2018, 12, 8017–8028 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, characterization techniques, experimental procedures, XRD, FESEM, EDS, mapping of Ti3C2Tx, electrochemical performance of assembled supercapacitor device, and electrocatalytic activity of delaminated Ti3C2Tx for OER and HER. See DOI: https://doi.org/10.1039/d3ya00489a |
| This journal is © The Royal Society of Chemistry 2024 |