Open Access Article
Open Access ArticleBioinspired flavin analogues as organic electrode materials for supercapacitor applications†
Dipayan
Mondal
,
Ishita
Naskar
,
Melepurath
Deepa
 * and
Ashutosh Kumar
Mishra
* and
Ashutosh Kumar
Mishra
 *
*
Department of Chemistry, Indian Institute of Technology-Hyderabad, Kandi-502284, India. E-mail: akm@chy.iith.ac.in; mdeepa@chy.iith.ac.in
First published on 5th June 2024
Abstract
With the increasing interest in incorporating redox-active organic molecules as potential materials in energy storage systems, we envisaged a chemical design of a naturally occurring redox-active flavin moiety. Herein, we report the fabrication and characterization of asymmetric supercapacitors (ASCs) based on modified flavins as cathode materials. Notably, subtle chemical modification with the incorporation of a carboxylic functionality around the flavin core (cFl) was found to impart superior ion-storage properties compared to a simple flavin derivative (Fl). As determined, the specific capacitance (SC) for cFl and Fl as individual electrodes was found to be 170 and 62 F g−1, respectively, whereas in a two electrode ASC with activated carbon serving as the anode, the SC was found to be 107 and 29 F g−1, respectively, at a current density of 1.25 A g−1. With better cycling stability (retaining 87% of its initial SC in the case of cFl) and significantly higher energy density (38 W h kg−1 for cFl) as compared to most of the known organic material-based electrodes, the modified flavin derivatives serve as better organic electrode alternatives for practical energy storage applications.
1. Introduction
Design and development of novel materials for advanced energy storage applications is much needed to cater to the rising demand from electric vehicles, portable electronic products, etc.1 For instance, though supercapacitors are better suited as energy storage devices owing to their high-power densities, long-term cycling stabilities, and faster rechargeability,2 their lower energy densities severely restrict their widespread usage.3 Numerous materials have been tried and tested for supercapacitor applications, including nanocarbons,4 transition metal oxides,5 and conducting polymers6 such as polythiophene and polyaniline.4 However, their energy densities are still about an order of magnitude lower than those of commercial batteries,7 which fuels the search for novel materials with better energy density, along with durability and fast rechargeability.Recently, redox-active organic materials owing to their excellent electrochemical reversibility offer an exciting alternative to the existing materials for energy storage applications.8 Notably, significantly higher specific capacitances (SCs) were reported for these organic materials as compared to porous carbon materials, plausibly due to the multi-electron faradaic reaction capability of such redox-active organic entities.9 Interestingly, the incorporation of hetero-atom and/or organic functionalities within the structural framework has been reported to significantly improve the SC, possibly due to the availability of multiple redox sites and increased wettability between the electrode and electrolyte.10–13 Moreover, structural modulation around the core organic skeleton can be rationally engineered to incorporate multiple active sites resulting in enhanced faradaic redox reactions for better efficiency. Consequently, the demand for novel design strategies for developing suitable redox-active organic molecules as electroactive materials is witnessing a surge.14,15
In this context, naturally occurring flavin is known for its redox-active behaviour and is also known to play a pivotal role in various biochemical phenomena, including mitochondrial respiratory chain, signal transduction and DNA repair together with enzymatic chemical transformations.16–21 Inspired by the redox activities of the flavin entity, modified flavin analogues such as sodium salts of flavin mononucleotides or alloxazine, a tautomeric analogue of isoalloxazine, has recently been investigated as electrolytic materials for the fabrication of redox flow batteries.22,23 Barring a couple of reports, further modification around the flavin core with the incorporation of suitable functionality to fine-tune the functional behaviour remains largely unexplored.
Interested in designing novel analogues around the flavin core skeleton for various applications such as catalytic transformations, bio-imaging, sensing, and metal coordinated polymers,24–34 we designed and synthesized a novel flavin analogue as an electroactive material for supercapacitor application. The incorporation of carboxyl functionality into the flavin structural framework was envisioned to potentially improve the surface wettability of the electrode and/or enhance ionic conductivity at the electrode–electrolyte interface. The carboxyl functionalized flavin analogue viz. 3-(2,4-dioxo-3,4-dihydrobenzo[g]pteridin-10(2H)-yl) propanoic acid (cFl) was synthesized using a slightly modified procedure.35 A simple flavin without the carboxyl functionality viz. 10-propylbenzo[g]pteridine-2,4(3H,10H)-dione (Fl) was also used (previously synthesized from our group)29 as a control to ascertain the role of the carboxyl functionality. In-depth investigations evaluating the electrochemical nature of the synthesized flavin analogues and their applicability for supercapacitors were performed using cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), cycling stability, conductivity, and resistance measurements.
2. Result and discussion
2.1. Synthesis of flavin analogues
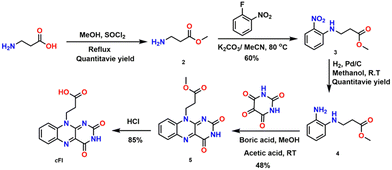
As shown in Scheme 1, the synthesis of cFl was achieved by protecting the carboxyl group of β-alanine followed by the nucleophilic aromatic substitution using fluoro-nitrobenzene to give the corresponding nitro derivative 3. Further reduction of 3 resulted in the monofunctionalized diaminobenzene derivative 4, which was used without purification for the condensation reaction with alloxan monohydrate in the presence of boric acid in acetic acid/methanol solution to give the isoalloxazine ester derivative 5. Simple deprotection of the ester derivative resulted in a yield of 85% for our target compound cFl. A similar synthetic protocol as reported earlier by our group was used to synthesize Fl.29 Standard characterization techniques were used to characterize the final compounds and the details are given in the supporting information section.2.2. Physical characterization
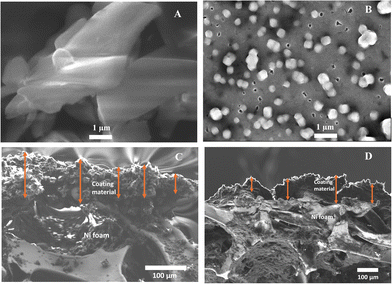
To investigate the morphological topography of the flavin analogues (cFl and Fl), the field emission scanning electron microscopy (FE-SEM) technique was used. Sample preparation involved preparing an aliquot solution containing 0.05 mM cFl or Fl and drop-casting it onto the silicon wafer surface for SEM imaging studies. As shown in Fig. 1A and Fig. S8A (ESI†), the SEM micrograph of the cFl analogue reveals molten agglomerates-like textured features with varying shapes and sized particles. Contrarily, the SEM image of Fl exhibits a completely different morphology with cube-like particles on the silicon wafer surface as shown in Fig. 1B and Fig. S8B (ESI†). Further, the thickness of the cFl and Fl based working electrodes was measured and found to be 87.2 μm and 111.6 μm, respectively (Fig. 1C and D).Further X-ray photoelectron spectroscopy (XPS) studies were performed for cFl and Fl and the data are shown in Fig. 2. The XPS survey spectrum displays the presence of multiple peaks at 282, 397 and 529 eV corresponding to the C 1s, N 1s and O 1s core levels in both the cases. Furthermore, while the deconvoluted C 1s core level spectra of cFl and Fl display three peaks at 284.2, 285.5, 287.8 and 284.2, 285.2, 287.7 eV, and these peaks correspond to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N/C–O and C
C, C–N/C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively, of the isoalloxazine ring common to both compounds. An additional peak in the deconvoluted spectra of cFl was observed at 288 eV, which is due to the carboxyl group present in cFl. Moreover, the deconvoluted N 1s core level spectrum of cFl and Fl shows two peaks individually at 399.3, 400.3 and 399.2, 400.2 eV which are attributed to the C–N
O, respectively, of the isoalloxazine ring common to both compounds. An additional peak in the deconvoluted spectra of cFl was observed at 288 eV, which is due to the carboxyl group present in cFl. Moreover, the deconvoluted N 1s core level spectrum of cFl and Fl shows two peaks individually at 399.3, 400.3 and 399.2, 400.2 eV which are attributed to the C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–H bonds, respectively.36
C and N–H bonds, respectively.36
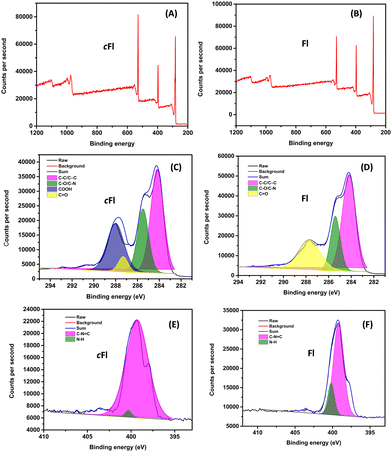 | ||
| Fig. 2 (A) and (B) XPS core spectra of cFl and Fl. The deconvoluted C 1s (C) and (D) and N 1s (E) and (F) core level spectra of cFl and Fl. | ||
The composite of cFl and carbon black (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
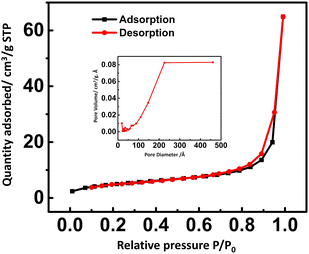
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) was further investigated using N2 adsorption/desorption isotherms (BET study) and the Barrett−Joyner−Halenda (BJH) analysis to understand the mesoporous nature. As shown in Fig. 3, cFl and carbon black composite exhibit a hysteresis loop of type H4 within the typical IV isotherm. Pore size and pore diameter were found to be 17.8091 m2 g−1 and 22.55 nm respectively. The carbon black helps in increasing the pore size as well as pore dimensions of cFl and carbon black composite.
10) was further investigated using N2 adsorption/desorption isotherms (BET study) and the Barrett−Joyner−Halenda (BJH) analysis to understand the mesoporous nature. As shown in Fig. 3, cFl and carbon black composite exhibit a hysteresis loop of type H4 within the typical IV isotherm. Pore size and pore diameter were found to be 17.8091 m2 g−1 and 22.55 nm respectively. The carbon black helps in increasing the pore size as well as pore dimensions of cFl and carbon black composite.
Further, the BET study and BJH analysis of the individual cFl and Fl (without mixing carbon black) was also performed. While the cFl entity displays a pore size of 5.13 m2 g−1 and a pore diameter of 11.27 nm, Fl exhibited a pore size of 3.77 m2 g−1 and a pore diameter of 12.73 nm. While in general, both the materials exhibit a mesoporous nature with pore dimensions of 2–50 nm, as shown in Fig. S9 (ESI†), a subtle difference in the pore size dimension was obtained for cFl and Fl.
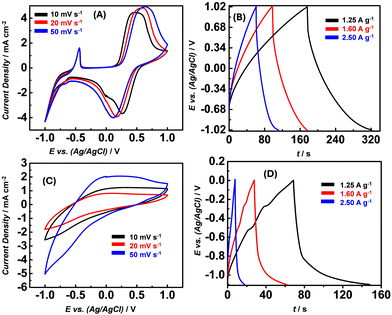
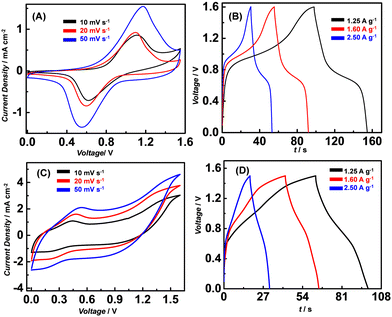
The cyclic voltammetry curves for cFl and Fl recorded at the scan rates of 10, 20 and 50 mV s−1 are shown in Fig. 4A and C. While for cFl the CV curves reveal broad oxidation peaks and comparatively sharper reduction peaks even at all scan rates, almost featureless CV profiles were observed for Fl. The observed peaks can be typically assigned to the redox reactions involving the flavin entity. While the peak current remains almost the same with a change in the scan rate, a noticeable shift in the peak potential was observed. With the increase in the scan rate, the cathodic peak was found to shift to the positive side while the anodic peak shifted to the negative side indicative of a diffusion-controlled electron transfer process.
To account for the observed difference in CV plots, although both cFl and Fl have similar redox cores, CV plots of cFl and Fl alone were recorded in an aqueous medium (tris buffer, pH = 7.4). As shown in Fig. S13 (ESI†), both flavin analogues display similar profiles. Plausibly, the varying interaction due to the presence/absence of carboxyl functionality with that of the PEO-KOH gel electrolyte and/or carbon black and PVdF (polyvinylidene fluoride) used for fabrication purposes might account for the observed difference in the CV profiles.
In addition to the electrochemical characterization, the GCD curves for cFl and Fl at three different charge/discharge current densities were recorded in the three-electrode configuration. As shown in Fig. 4B and D, the GCD curves for cFl and Fl display slight deviation from the ideal triangular response with slightly bent curves observed at low voltages during charging and at higher voltages during the discharge process. This observation is suggestive of a shorter charging time needed while a longer time is required for the discharging process. Interestingly, a sharp difference in the peak shape and size is observed in the GCD curves for cFl and Fl, displaying a prominent bent curve in the case of Fl when compared to that in cFl. The comparably longer discharge time in the case of cFl indicates slightly better charge storage properties when compared to Fl. Fl also shows a much larger Ohmic drop compared to cFl. This aspect is of paramount importance, especially in view of practical applications, which require a low Ohmic drop.
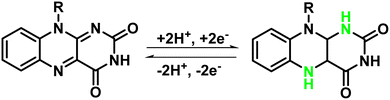
Further, the specific capacitance (SC) was calculated from the GCD curves using the standard equation (SC = I × Δt/m × ΔV, where I is the charge–discharge current in Amperes, m is the mass of the active material in grams, and ΔV is the voltage window of operation and Δt is the discharge time in seconds). SCs as determined for cFl and Fl in the three-electron setups were found to be 170 F g−1 and 62 F g−1, respectively, at a current density of 1.25 A g−1. Interestingly, the SC was found to decrease with the increase in the current density as shown with the GCD plot with varying current density (Fig. 4B and D). As determined from the Csp values calculated at different current densities, the effect seems to be more prominent in the case of cFl, as compared to that in Fl, with the changes in the Csp values from 170 to 166 F g−1 and 62 to 15 F g−1, respectively, when the current density is increased from 1.25 to 2.5 A g−1. A plausible faradaic reaction of the flavin units in PEO-KOH gel electrolyte is illustrated in Scheme 2, where the isoalloxazine entity undergoes two proton and two electron transfer to attain fully oxidized or fully reduced form.
All the CV curves for cFl//AC recorded at different scan rates displayed well-defined redox peaks in the anodic and cathodic branches. The slight change in the potential value (ΔE) with the change in the scan rate is indicative of its quasi-reversible nature. Furthermore, the GCD curves recorded in the voltage window of 0–1.6 V at different current densities in the range of 1.25 to 2.50 A g−1 as shown in Fig. 5A and B, which reveal comparatively slower current recharge and faster current discharge with a plateau-like response implying the involvement of faradaic contribution to the charge storage process.
Contrarily, the CV curves for the Fl//AC electrode system when recorded at different scan rates in the potential window of 0 to 1.6 V, display quasi-leaf-like shapes with a broad oxidation peak and broad reduction wave, indicative of charge storage being dominated by the electrical double layer (EDL) formation. Furthermore, the GCD curves recorded at different current densities in the range of 1.25 to 2.50 A g−1 further corroborate the observed CV curves with a moderately curved potential decay profile, over the 1.6 V potential domain, as shown in Fig. 5C and D. Both these observations are suggestive of the underlying EDLC process for charge storage at the electrode–electrolyte interface.37
Further, SC was calculated using the method mentioned above from the GCD curves at the different current densities ranging from 1.25 to 2.5 A g−1. Interestingly, cFl//AC ASC delivered higher SC values of (107 to 103 F g−1) as compared to much lower values of (29–22 F g−1) for Fl//AC ASC. A similar observation was made in the case of the electrode system. The proposed structural variation in cFl compared to Fl seems to play an important role in deciding the capacitance properties. While the major redox behaviour can be attributed to the core flavin entity, the presence of carboxylic functionality in the case of cFl, possibly decreases the electrode surface resistivity as well as increases the surface wettability for the physisorption of the electrolytic ions on the electrode, thereby enhancing the ionic conductivity at the electrode–electrolyte interfaces.37
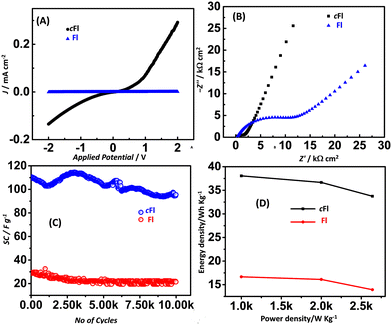
Additionally, the linear sweep voltammetry (LSV) plots were recorded to evaluate the electrochemical stability window of the cFl and Fl electrode materials and are shown in Fig. 6A. A potential range of −2 V to +2 V and a scan rate of 10 mV s−1 at 25 °C, were used for the measurements and the slopes of the resulting I–V curves gave the electrical conductance of cFl-and Fl-based electrodes directly, and the values were found to be 170 and 0.8 mS cm−1, respectively.
Electrochemical impedance spectra were recorded to further understand the charge transfer and transport behaviour of the cFl//AC and Fl//AC ASCs. As shown in Fig. 6B, an AC bias of 20 mV was applied over a frequency range of 1 MHz to 0.01 Hz, and the data were fitted into the [R(RC)W] circuit. The fitted parameters are summarized in Table 1. As shown in Fig. 6B, the Nyquist plots for cFl and Fl-based electrodes display a straight line with a linear rise in the low-frequency region in the case of cFl, which is indicative of significant capacitive behaviour with low diffusion resistance. Contrarily, the Fl-based electrode displayed a skewed semicircle followed by an inclined line, indicating a higher equivalent series resistance and a lower capacitive behaviour. Also, the charge transfer resistance (Rct) was found to be lower for cFl/AC (1.40 Ω cm2) as compared to that for Fl//AC (11.69 Ω cm2) ASCs. This observation further supports that the cation and anion accumulation from the KOH-PEO gel electrolyte at the electroactive sites of the cFl-based electrode are more facile when compared to the Fl-based electrode.
| Device | R s (Ω cm2) | R ct (Ω cm2) | C dl (F cm−2) |
|---|---|---|---|
| cFl//AC | 0.40 | 1.40 | 1.1 × 10−3 |
| Fl//AC | 0.23 | 11.69 | 2.8 × 10−4 |
Furthermore, to ascertain the stability of cFl and Fl electrode materials for their practical usage as electrochemical supercapacitors, the cycling stabilities of cFl//AC and Fl//AC were investigated at a current density of 5 A g−1 for 2500 cycles. Interestingly, a clear distinction between the cFl/Fl-based ASCs was observed, where cFl as the electrode displayed excellent electrochemical stability retaining about 94% of its initial SC as compared to the 71% retention capability displayed by the Fl//AC ASC (Fig. S10, ESI†). Furthermore, the number of cycles was increased to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles for both materials to observe the loss of cycling performance. Notably, only a 7% loss of cycling performance was observed for cFl (cycling stability = 87%), whereas a 5% loss of cycling performance was observed for Fl (cycling stability = 66%) (Fig. 6C), when compared to the cycling stability after 2500 cycles. The observed cycling stability for cFl-based ASC was observed to be among the best of the organic molecule-based electrode materials with close to 90% retention capability after 10
000 cycles for both materials to observe the loss of cycling performance. Notably, only a 7% loss of cycling performance was observed for cFl (cycling stability = 87%), whereas a 5% loss of cycling performance was observed for Fl (cycling stability = 66%) (Fig. 6C), when compared to the cycling stability after 2500 cycles. The observed cycling stability for cFl-based ASC was observed to be among the best of the organic molecule-based electrode materials with close to 90% retention capability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.38
000 cycles.38
Furthermore, the Ragone plots were plotted for cFl//AC and Fl//AC ASCs to evaluate the energy and power densities of the cells. As shown in Fig. 6D, the power density versus energy density plot for cFl and Fl-based ASCs, the maximum energy density was found to be ∼38 W h kg−1, for the cFl-based system while the comparably lower energy density of ∼10 W h kg−1 was observed in the case of Fl-based ASC at a given power density of 1 kW kg−1. Notably, the energy density of ∼34 W h kg−1 was retained for cFl//AC ASC as compared to ∼7 W h kg−1 for Fl//AC ASC when the power density was increased to 2.6 kW kg−1.
To further determine the electrochemical process and capacitive and diffusive components of cFl//AC, the following equations were used.
| i(V) = icap + idiff = k1ν + k2ν1/2 | (3) |
| i(V)/ν1/2 = k1ν1/2 + k2 | (4) |
The capacitive and diffusive contributions for the cFl//AC electrode in percentages are shown in Fig. 7A. The advantage of the capacitive effect is more significant when the scan rate changes from higher to lower values, with 91% of capacitive and 9% of diffusive contributions at 10 mV s−1. The effect of the capacitance control gradually decreases when the scanning rate increases, and the proportion of ion diffusion control increases. The CV plot illustrating the total contribution (diffusive and capacitive) and the diffusive contribution in the shaded curve are shown in Fig. 7B. The constants k1 and k2 were calculated from the slope and the intercept of a linear plot of i(V)/ν1/2versus ν1/2.
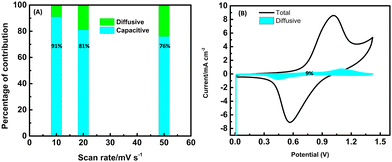 | ||
| Fig. 7 (A) Capacitive and diffusion control percentage with different scan rates of the cFl//AC electrode. (B) Proportion of surface control at 10 mV s−1. | ||
Notably, much better electrochemical performance was reported with transition metals oxides/sulfides-based electrode materials when compared with the cFl-based material.39–45 However, the cFl-based material demonstrates a comparable result with reported organic-based supercapacitors. A comparison of energy density value for the cFl//AC with the reported organic supercapacitor-based electrodes is given in Table S1 (ESI†).46–52
Furthermore, to verify the practicability of the cFl electrode material for its practical behavior, three cFl-based asymmetrical ASC cells were connected in a series. The fully charged 3S assembly consisting of the cFl-based electrodes was observed to illuminate a green light emitting diode (LED) for approximately 35 s; thereby supporting the usage of the flavin-based material for ASCs (Fig. 8).
3. Conclusions
In conclusion, molecular design around the naturally occurring redox-active flavin core skeleton was envisaged, synthesized, and evaluated for energy storage applications. Subtle chemical modification incorporating a carboxyl functionality within the flavin core (cFl) was synthesized and compared with a simple flavin (Fl) analogue as efficient electrode material. As observed, significant variation in the electrochemical behavior was observed between the two flavin analogues as electrodes with comparably better efficiency in the case of cFl. In a three-electrode setup, SCs of 170 F g−1 and 62 F g−1 were achieved for cFl and Fl, respectively, at a current density of 1.25 A g−1. The practical applicability of the flavin as electrode materials was evaluated by fabricating cFl//AC and Fl//AC ASCs and KOH-PEO gel electrolytes, which revealed the SC of 107 F g−1 and 29 F g−1, respectively. In addition, better cyclic stability was observed for cFl (87% retention), while the energy densities of 38 W h kg−1 in the case of cFl and 10 W h kg−1 in the case of Fl were obtained at a power density of 1 kW kg−1. Moreover, impedance and LSV analysis further support the chemically modified flavin analogue cFl-based electrode material as a potential candidate for energy storage devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. M. thanks PMRF (MHRD) for the scholarship. I. N. thankful to CSIR-India for a scholarship. A. K. M. thanks SERBIndia for the (CRG/2022/006706), for the research funding. All authors thank the Indian Institute of Technology (IIT)-Hyderabad for providing infrastructure and instrument facilities.References
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CAS.
- C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang and J. Zhang, Chem. Soc. Rev., 2015, 44, 7484–7539 RSC.
- J. Armenta, C. Núñez, N. Visair and I. Lázaro, J. Power Sources, 2015, 284, 452–458 CrossRef CAS.
- S. Saha, P. Samanta, N. C. Murmu and T. Kuila, J. Energy Storage, 2018, 17, 181–202 CrossRef.
- Nanomaterials in Advanced Batteries and Supercapacitors, Transition metal oxides as supercapacitor materials. ed K. I. Ozoemena and S. Chen, Springer, New York, 2016, 317–344 Search PubMed.
- Q. Meng, K. Cai, Y. Chen and L. Chen, Nano Energy, 2017, 36, 268–285 CrossRef CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- P. Poizot, J. Gaubicher, S. Renault, L. Dubois, Y. Liang and Y. Yao, Chem. Rev., 2020, 120, 6490–6557 CrossRef CAS PubMed.
- T. R. Kuo, L. Y. Lin, S. Kubendhiran, Y. C. Li, R. J. Chung and S. Yougbaré, J. Energy Storage, 2022, 53, 105085 CrossRef.
- C. Largeot, C. Portet, J. Chmiola, P. L. Taberna, Y. Gogotsi and P. Simon, J. Am. Chem. Soc., 2008, 130, 2730–2731 CrossRef CAS PubMed.
- L. Z. Fan, S. Qiao, W. Song, M. Wu, X. He and X. Qu, Electrochim. Acta, 2013, 105, 299–304 CrossRef CAS.
- X. Ma, W. Zhou, D. Mo, J. Hou and J. Xu, Electrochim. Acta, 2015, 176, 1302–1312 CrossRef CAS.
- M. D. Najafi, E. Kowsari, H. R. Naderi, A. Chinnappan, S. Ramakrishna, A. Ehsani and A. Shokravi, Compos. Sci. Technol., 2021, 211, 108844 CrossRef CAS.
- X. Chen, X. Feng, B. Ren, L. Jiang, H. Shu, X. Yang, Z. Chen, X. Sun, E. Liu and P. Gao, Nano-Micro Lett., 2021, 13, 1–6 CrossRef CAS PubMed.
- F. Ma, X. Wang, Z. Hu, L. Hou, Y. Yang, Z. Li, Y. He and H. Zhu, Energy Fuels, 2020, 34, 13079–13088 CrossRef CAS.
- A. M. Edwards, Methods Mol. Biol., 2014, 1146, 3–13 CrossRef CAS PubMed.
- S. Ghisla and D. E. Edmondson, Flavin Coenzymes, John Wiley & Sons, Ltd, Hoboken, NJ, 2001, pp. 1–9 Search PubMed.
- A. Sancar, Chem. Rev., 2003, 103, 2203–2238 CrossRef CAS PubMed.
- I. Chaves, R. Pokorny, M. Byrdin, N. Hoang, T. Ritz, K. Essen, L. O. Brettel, G. T. van der Horst, A. Batschauer and M. Ahmad, Annu. Rev. Plant Biol., 2011, 62, 327–364 CrossRef PubMed.
- A. Lukacs, P. J. Tonge and S. R. Meech, Acc. Chem. Res., 2022, 55, 402–414 CrossRef CAS PubMed.
- T. E. Swartz, S. B. Corchnoy, J. M. Christie, J. W. Lewis, I. Szundi, W. R. Briggs and R. A. Bogomolni, J. Biol. Chem., 2001, 276, 36493–36500 CrossRef CAS PubMed.
- A. Orita, M. G. Verde, M. Sakai and Y. S. Meng, Nat. Commun., 2016, 7, 1–8 Search PubMed.
- K. Lin, R. Gómez-Bombarelli, E. S. Beh, L. Tong, Q. Chen, A. Valle, A. Aspuru-Guzik, M. J. Aziz and R. G. Gordon, Nat. Energy, 2016, 1, 1–8 Search PubMed.
- M. V. Mouli, D. Mondal, K. Kumari, S. K. Singh and A. K. Mishra, Org. Biomol. Chem., 2023, 21, 3311–3316 RSC.
- M. V. Mouli, S. Katyal and A. K. Mishra, Synlett, 2023, 829–834 CAS.
- M. V. Mouli and A. K. Mishra, RSC Adv., 2022, 12, 3990–3995 RSC.
- M. V. Mouli and A. K. Mishra, Dyes Pigm., 2023, 212, 111148 CrossRef CAS.
- M. V. Mouli and A. K. Mishra, Org. Biomol. Chem., 2023, 21, 5622–5628 RSC.
- M. V. Mouli and A. K. Mishra, CrystEngComm, 2022, 24, 2221–2225 RSC.
- M. V. Mouli, H. G. Agrawal, T. Maddeshiya, A. Tamrakar, S. R. Tripathy, M. D. Pandey and A. K. Mishra, Luminescence, 2023, 38, 1206–1214 CrossRef CAS PubMed.
- M. V. Mouli, H. G. Agrawal, M. Kumar and A. K. Mishra, Luminescence, 2023, 38, 1185–1191 CrossRef CAS PubMed.
- M. V. Mouli and A. K. Mishra, ChemistrySelect, 2022, 7, e202202126 CrossRef.
- H. G. Agrawal, S. Khatun, A. K. Rengan and A. K. Mishra, Polyhedron, 2023, 243, 116536 CrossRef.
- H. G. Agrawal, P. S. Giri, P. Meena, S. N. Rath and A. K. Mishra, ACS Med. Chem. Lett., 2023, 14(12), 1857–1862 CrossRef CAS PubMed.
- M. V. Mouli and A. K. Mishra, J. Chem. Sci., 2022, 134, 59 CrossRef CAS.
- F. Ma, Z. Hu, L. Jiao, X. Wang, Y. He, Y. Yang and Z. Li, ACS Appl. Energy Mater., 2021, 4, 5493–5503 CrossRef CAS.
- J. Shen, A. Liu, Y. Tu, G. Foo, C. Yeo, M. B. Chan-Park, R. Jiang and Y. Chen, Energy Environ. Sci., 2011, 4, 4220–4229 RSC.
- N. An, Z. Hu, H. Wu, Y. Yang, Z. Lei and W. Dong, J. Mater. Chem. A, 2017, 5, 25420–25430 RSC.
- H. Y. Jung, S. I. Kim, J. Kim, Y. J. Kim, H. Hong, J. Yun and W. Ryu, J. Mater. Chem. A, 2023, 11, 20608–20622 RSC.
- T. Zhu, Z. He, Z. An, R. Xu, Y. Li, R. Zhe, H. E. Wang and H. Pang, Sci. China Mater., 2023, 66, 2216–2226 CrossRef CAS.
- P. S. Chauhan, S. Kumar, A. Mondal, P. Sharma, M. N. Parekh, V. Panwar, A. M. Rao and A. Misra, J. Mater. Chem. A., 2023, 11, 95–107 RSC.
- T. Zhu, J. Pan, Z. An, R. Zhe, Q. Ou and H. E. Wang, J. Mater. Chem. A, 2022, 10, 20375–20385 RSC.
- Y. Zang, H. Luo, H. Zhang and H. Xue, ACS Appl. Energy Mater., 2021, 4, 1189–1198 CrossRef CAS.
- Y. Ren, T. Zhu, Y. Liu, Q. Liu and Q. Yan, Small, 2021, 17, 2008047 CrossRef CAS PubMed.
- S. Thareja and A. Kumar, ACS Sustainable Chem. Eng., 2021, 9, 2338–2347 CrossRef CAS.
- L. Jiao, Z. Hu, F. Ma, Y. He, Q. Zhou, L. Xiao, L. Lv and Y. Yang, J. Energy Storage, 2022, 52, 104777 CrossRef.
- F. Ma, Z. Hu, L. Jiao, X. Wang, Y. He, Y. Yang and Z. Li, ACS Appl. Energy Mater., 2021, 4, 5493–5503 CrossRef CAS.
- H. Peng, R. Zhao, J. Liang, S. Wang, F. Wang, J. Zhou, G. Ma and Z. Lei, ACS Appl. Mater. Interfaces, 2018, 10, 37125 CrossRef CAS PubMed.
- F. Ma, Z. Hu, L. Jiao, X. Wang, Y. Yang, Z. Li and Y. He, Adv. Mater. Interfaces, 2021, 8, 2002161 CrossRef CAS.
- F. Ma, X. Wang, Z. Hu, L. Hou, Y. Yang, Z. Li, Y. He and H. Zhu, Energ Fuel., 2020, 34, 13079–13088 CrossRef CAS.
- G. Lv, X. Dai, Y. Qiao, G. Ren, H. Fan, Y. Liu and Y. Chen, J. Chem. Eng., 2023, 470, 144068 CrossRef CAS.
- Z. Song, D. Zhu, L. Li, T. Chen, H. Duan, Z. Wang, Y. Lv, W. Xiong, M. Liu and L. Gan, J. Mater. Chem A, 2019, 7, 1177–1186 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental details including synthetic procedure and characterization and photophysical properties. See DOI: https://doi.org/10.1039/d4ya00001c |
| This journal is © The Royal Society of Chemistry 2024 |