Ultrasensitive detection of E. coli using bioinspired based platform†
Sawsan
Almohammed
ab,
Tristan
Nolan
c,
Niamh
Martin
c,
Wim G.
Meijer
c,
Brian J.
Rodriguez
 *ab and
James H.
Rice
*ab and
James H.
Rice
 *a
*a
aSchool of Physics, University College Dublin, Belfield, Dublin, D04 V1W8, Ireland. E-mail: james.rice@ucd.ie
bConway Institute of Biomolecular and Biomedical Research, University College Dublin, Belfield, Dublin, D04 V1W8, Ireland
cSchool of Biomolecular and Biomedical Science, University College Dublin, Belfield, Dublin, D04 V1W8, Ireland
First published on 20th November 2024
Abstract
Bacterial infections are a leading cause of mortality worldwide, underscoring the urgent need for effective detection methods. This study introduces a novel approach that combines surface-enhanced Raman spectroscopy (SERS) with an electro-optic technique for bacterial detection. The method utilizes a metal–semiconductor substrate that, when activated by an external electric field, significantly amplifies the SERS signal intensity. We validated this approach through a proof-of-concept study, demonstrating that the SERS signal of Gram-negative Escherichia coli can be enhanced tenfold by applying an electric field, confirming the method's efficacy for bacterial detection. Our findings highlight the potential of this rapid, label-free biosensor for pathogen detection with near single-cell sensitivity.
Introduction
Detecting and identifying pathogens is crucial for medicine, food safety, public health, security, and environmental quality control. Bacterial infections are a major global health issue, causing 6.7 million deaths annually and representing 8.7% of global healthcare costs, amounting to $33 billion.1,2 In Europe alone, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths each year are linked to antibiotic-resistant bacterial infections.3 The World Health Organization has highlighted that contaminated water can transmit communicable diseases such as diarrhea, cholera, dysentery, typhoid, and guinea worm infection.4 Timely and accurate pathogen detection is critical, as delays or inaccuracies can lead to serious illness and mortality.
000 deaths each year are linked to antibiotic-resistant bacterial infections.3 The World Health Organization has highlighted that contaminated water can transmit communicable diseases such as diarrhea, cholera, dysentery, typhoid, and guinea worm infection.4 Timely and accurate pathogen detection is critical, as delays or inaccuracies can lead to serious illness and mortality.
The detection of bacterial pathogens like Pseudomonas aeruginosa and Escherichia coli is of great interest in both life sciences and environmental research due to the potential harm caused by microbial contamination.5 Identifying the specific species is particularly important because bacteria within the same genus can vary significantly in pathogenicity.6 The current gold standard for bacterial detection relies on cell culture,7 which can take over 24 hours for analysis.2,8 While Polymerase Chain Reaction (PCR) is a faster alternative, it still typically requires hours to yield results.2,8
Surface-enhanced Raman spectroscopy (SERS) offers a promising alternative for bacterial detection with high specificity.9 By analyzing Raman spectra, SERS can differentiate between bacterial species and strains. For instance, SERS has been shown to accurately identify pathogenic and non-pathogenic species of Legionella and Mycobacteria.3,10 It has also successfully identified Staphylococcus aureus and Pseudomonas aeruginosa from blood samples with accuracies of 97.5% and 97.3%, respectively.11,12 In another study, SERS detected a variety of bacteria with an overall accuracy of 97.4%, and distinguished between pathogenic and non-pathogenic E. coli strains with 99.7% accuracy. These findings underscore the potential of SERS as a rapid and precise method for identifying pathogenic and non-pathogenic bacterial species.
SERS sensitivity is highly dependent on the design of the substrate, and various SERS-active substrate strategies have been developed. For efficient bacteria detection, as a three-dimensional (3D) substrates are considered optimal.13,14 Additionally, using safe and sustainable materials like peptides in SERS substrate design is preferred.15–18 Peptide nanotube-metal nanoparticle composites have proven effective in enabling SERS detection. Research has shown that applying an external electric field to these composites can enhance the platform's sensitivity by more than an order of magnitude, allowing for the detection of analytes such as porphyrin, amino nitrophenol, and rhodamine B at concentrations as low as 10−15 M.16,19
Inspired by these findings, we utilized a new bacterial detection method combining SERS with an electro-optic technique. The methodology employs a metal–semiconductor substrate that, when activated by an external electric field, significantly enhances the SERS signal intensity level. We show that using our approach the SERS signal level from E. coli bacteria can be boosted tenfold with the application of an electric field, confirming the method's effectiveness for bacterial detection. This work extends upon research that have developed synergistic systems that integrate multiple enhancement mechanisms, such as electromagnetic and electrochemical enhancements.20 Studies have shown that combining noble metal nanoparticles with piezoelectric polymers boosts SERS performance.20,21 Firstly, the piezoelectric properties of the polymer matrix enable the generation of electric charges under mechanical stress, leading to amplified SERS signals and improved detection sensitivity. Secondly, the polymer's chemical stability ensures the long-term durability of the substrate, while its compatibility with functionalization allows for further enhancement by incorporating metal nanoparticles or molecules with specific binding properties. This approach generates an electrical potential through an applied pressure or strain, converting it into stored energy.20 This design increased SERS signals up to 10 times. Combining piezoelectric active peptide nanotubes with silver nanoparticles has been used to demonstrate more than an order of magnitude increase in the SERS following the application of strain.21 This enhancement in SERS was attributed to charge transfer between the peptides and the silver nanoparticles following the application of strain to the peptides.
Results and discussion
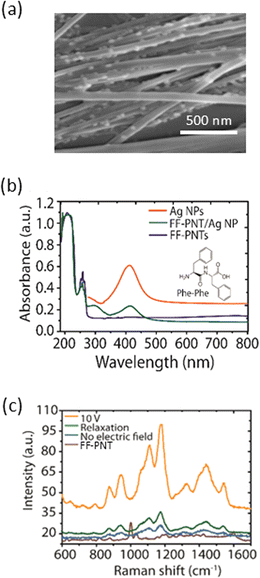
Our studies use a substrate design based on diphenylalanine peptide nanotube semiconductors (FF-PNT) and plasmon-active nanomaterials (Ag nanoparticles; Ag NPs). These materials are formed between two gold electrodes, as outlined schematically in Fig. 1(a). The gold electrodes on the Ag-FF substrate enable an electric field to be applied across the FF nanotubes. This substrate design was made following a previously reported method outlined in (ESI Fig. S1†).22,23Scanning electron microscopy (SEM) imaging was applied (Fig. 1(a)) to characterise the sample. The SEM images show that the FF-PNT/Ag NP substrate consists of Ag NPs clearly located along the FF-PNT nanotubes. SEM imaging also shows that the FF-PNTs are aligned between the electrodes (ESI Fig. S2 and S3†). Optical absorption spectra (Fig. 1(b)) show that for FF-PNT/Ag NP a red-shift in the nanoparticle's absorption band of ∼16 nm occurs relative to the nanoparticles alone.14 This redshift is attributed to amino acid carboxylate groups of the FF-PNT strongly binding to the surface of the metal Ag NPs.
It has been shown that applying an external electric field, generated by applying a DC voltage across an FF-PNT/Ag NP substrate, can enhance the SERS signal by more than 10 times.19 To illustrate this effect, we used glucose as a probe molecule. Fig. 1(c) demonstrates the impact of the electric field on the SERS spectra of glucose on the FF-PNT/Ag NP substrate. The SERS spectrum for glucose shows strong bands at 1060, 1125 and 1366 cm−1 in agreement with the literature.24 When the electric field is applied, the SERS spectra of glucose exhibit a pronounced strengthening (eight-fold) in SERS signal intensity (orange spectrum in Fig. 1(c)). Once the electric field is removed (green spectrum in Fig. 1(c)), the SERS intensity returns to its original level. The application of voltages between 10 to 30 V was seen as effective in enhancing the SERS signal (ESI Fig. S4†).
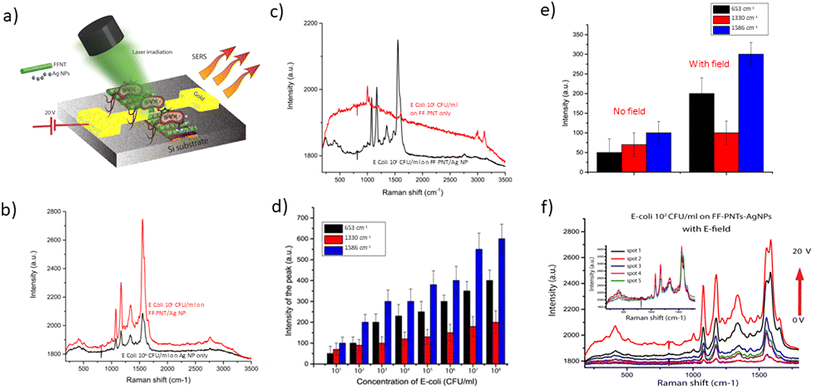
We then turned to assess if the substrate could enhance the SERS spectra of bacteria as well as simple molecules. To do this we studied E. coli bacteria as a proof of concept. The bacteria were deposited on the FF-PNT/Ag NP substrate between electrodes (schematically outlined in Fig. 2(a)). The substrate was prepared with E. coli at a concentration ranging between 108–101 CFU ml−1 in Ringer's saline solution.17
Before the application of an electric field, we assessed the efficiency of the peptide-silver nanoparticle substrate design relative to the use of silver nanoparticles only. It is known that SERS sensitivity is heavily influenced by the design of the substrate. FF-PNTs are quasi-3D surface structures creating a large surface area relative to a flat glass or silicon substrate surface. Such 3D-like surfaces are reported to be efficient for bacterial detection.13,14 For example, it has been shown that a hybrid composite formed from silver nanoparticle decorated bacterial nano cellulose served as a 3D SERS substrate that enabled nano-molar detection sensitivity.13 Our substrate design possesses a similar topography to substrates formed from cellulose fibre-like structures.13,25,26 Additionally, FF-PNTs possess water contact angles of greater than 80° depending on factors such as nanotube orientation and surface roughness.15 Such a high contact angle reflects the high hydrophobicity of the substrate. Hydrophobic surfaces are reported to enhance the SERS sensitivity of the substrate.13 To confirm this we compared silicon and FF-PNTs substrates. We studied E. coli on Ag NPs prepared on FF-PNTs or silicon. The SERS Raman bands for E. coli are outlined in Table S1 (ESI).† The SERS signal for FF-PNT/Ag NP substrate is higher than for Ag NPs on silicon (Fig. 2(b) and S5 (ESI)†) or on the peptide only (Fig. 2(c)). The stronger SERS signal from the peptide-based substrate is due to its higher surface area and high hydrophobicity of the substrate.
The FF-PNT/Ag NP substrate then was prepared with E. coli at a range of concentrations and the SERS recorded. A plot of E. coli concentration vs. SERS signal intensity for three different Raman peaks, before an electric field was applied, shows that a SERS signal was detected for bacteria at a concentration as low as 101 CFU ml−1 (Fig. 2(d)).
To further improve and enhance the detection of bacteria and to identify the bands associated with E. coli in a reproducible manner, we investigated using an externally applied DC electric field. Applying the electric field resulted in an enhanced signal-to-noise that improves the detection sensitivity for SERS detection (Fig. 2(e and f)). Following the application of an electric field a strong enhancement in the SERS peak-to-peak signal was seen, with a 10-fold enhancement seen (Fig. 2(e)). The significance of this can be seen with the applied electric field enabling the observation of weak Raman peaks for the bacteria. Following the application of an electric field the peak-to-peak SERS Raman bands are enhanced about ten-fold (Fig. 2(e and f)). The SERS spectra before and after the applied electric field show SERS bands for E. coli at around 1330 cm−1 (CH2/CH3 wagging mode in purine bases of nucleic acids), 1462 cm−1 (CH2/CH3 deformation of proteins and lipids), and 1665 cm−1 (amine I), which agree with what has been reported in the literature (Table S1 ESI†).27,28 Reproducibility tests were performed from 5 random locations on the template. A variation of <7% in SERS intensity was observed, indicating high reproducibility of SERS from the template. Following this work on E. coli in water, we demonstrated the wider applicability of the methodology to detect biomolecules. We undertook proof of principle studies to detect DNA base pairs and bacteria in blood (Fig. S6 & S7, ESI†) to demonstrate that the substrate can detect a range of bio-relevant systems.
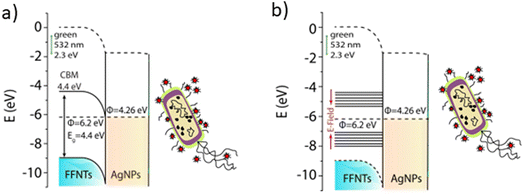
The mechanism for how the electric field enhances the bacterial detection sensitivity via boosting the SERS signal levels is through electro-optical synergy14 that modifies the density of states of the peptide nanotubes from a semiconductor to a metal. This increases charge transfer from the peptide nanotubes to the metal nanoparticles, generating hot electrons (outlined schematically in Fig. 3). Applying an electric field to the FF-PNT/Ag NP substrate allows the FF density of states to be tuned from a semiconductor to a metal, enabling effective charge transfer from the nanotube to the metal nanoparticles.14 This results in an enhancement in the state density of hot electrons. As a result of charge transfer an increase in charge on the metal nanoparticle enhances the surface plasmon resonance overlap with the Raman excitation laser resulting in a stronger SERS signal. The effect is optimized through the physical alignment of the FF-PNTs, since the inherent electric dipoles of the FF-PNTs are then aligned and maximally responsive to the applied electric field (a cooperative effect). An alternative explanation for the observed effects can be assigned to electric field induced polarization effects. An applied electric field can induce molecular reorientation in polar analytes, altering their scattering cross-sections. Studies have demonstrated that low frequency oscillating electric fields can significantly influence the SERS responses of thiophenol.29 In this study the authors reported that a low frequency oscillating electric fields (ranging from 5 mHz to 1 kHz) influences SERS peak intensities and affects specific vibrational modes of the analyte.
In conclusion, we show that electro-optical based method using a microfabricated chip formed using self-assembled peptide nanotubes can boost the SERS signal from bacteria samples. We demonstrate that by applying an external electric field to our microfabricated chip substrate the SERS signal from Gram-negative Escherichia coli can be boosted by over an order of magnitude.
Data availability
Data from this study is available in a data repository. Research Repository UCD https://www.researchrepository.ucd.ie. Access conditions: available upon request. Description of data: the data id stored as image formats and spreadsheets (Origin or Excel formats).Conflicts of interest
There are no conflicts to declare.References
- E. Witkowska, K. Niciński, D. Korsak, T. Szymborski and A. Kamińska, Sources of Variability in SERS Spectra of Bacteria: Comprehensive Analysis of Interactions between Selected Bacteria and Plasmonic Nanostructures, Anal. Bioanal. Chem., 2019, 411(10), 2001–2017, DOI:10.1007/s00216-019-01609-4.
- F. Moghtader, A. Tomak, H. M. Zareie and E. Piskin, Bacterial Detection Using Bacteriophages and Gold Nanorods by Following Time-Dependent Changes in Raman Spectral Signals, Artif. Cells Nanomed. Biotechnol., 2018, 46(sup2), 122–130, DOI:10.1080/21691401.2018.1452251.
- N. Xiao, C. Wang and C. Yu, A Self-Referencing Detection of Microorganisms Using Surface Enhanced Raman Scattering Nanoprobes in a Test-in-a-Tube Platform, Biosensors, 2013, 3(3), 312–326, DOI:10.3390/bios3030312.
- N. E. Dina, A. M. R. Gherman, V. Chiş, C. Sârbu, A. Wieser, D. Bauer and C. Haisch, Characterization of Clinically Relevant Fungi via SERS Fingerprinting Assisted by Novel Chemometric Models, Anal. Chem., 2018, 90(4), 2484–2492, DOI:10.1021/acs.analchem.7b03124.
- C. S. Ho, N. Jean, C. A. Hogan, L. Blackmon, S. S. Jeffrey, M. Holodniy, N. Banaei, A. A. E. Saleh, S. Ermon and J. Dionne, Rapid Identification of Pathogenic Bacteria Using Raman Spectroscopy and Deep Learning, Nat. Commun., 2019, 10(1), 4927, DOI:10.1038/s41467-019-12898-9.
- C. Wang, F. Madiyar, C. Yu and J. Li, Detection of Extremely Low Concentration Waterborne Pathogen Using a Multiplexing Self-Referencing SERS Microfluidic Biosensor, J. Biol. Eng., 2017, 11(1), 1–11, DOI:10.1186/s13036-017-0051-x.
- A. C. G. Foddai and I. R. Grant, Methods for Detection of Viable Foodborne Pathogens: Current State-of-Art and Future Prospects, Appl. Microbiol. Biotechnol., 2020, 104(10), 4281–4288, DOI:10.1007/s00253-020-10542-x.
- R. Weiss, M. Palatinszky, M. Wagner, R. Niessner, M. Elsner, M. Seidel and N. P. Ivleva, Surface-Enhanced Raman Spectroscopy of Microorganisms: Limitations and Applicability on the Single-Cell Level, Analyst, 2019, 144(3), 943–953, 10.1039/c8an02177e.
- J. F. M. Almarashi, N. Kapel, T. S. Wilkinson and H. H. Telle, Raman Spectroscopy of Bacterial Species and Strains Cultivated under Reproducible Conditions, Spectrosc., 2012, 27(5–6), 361–365, DOI:10.1155/2012/540490.
- Y.-F. Huang, D.-Y. Wu, H.-P. Zhu, L.-B. Zhao, G.-K. Liu, B. Ren and Z.-Q. Tian, Surface-Enhanced Raman Spectroscopic Study of p-Aminothiophenol, Phys. Chem. Chem. Phys., 2012, 14(24), 8485, 10.1039/c2cp40558j.
- M. Usman, J. W. Tang, F. Li, J. X. Lai, Q. H. Liu, W. Liu and L. Wang, Recent Advances in Surface Enhanced Raman Spectroscopy for Bacterial Pathogen Identifications, J. Adv. Res., 2023, 91–107, DOI:10.1016/j.jare.2022.11.010.
- A. Pistiki, M. Salbreiter, S. Sultan, P. Rösch and J. Popp, Application of Raman Spectroscopy in the Hospital Environment, Transl. Biophotonics, 2022, 1–13, DOI:10.1002/tbio.202200011.
- D. Huo, B. Chen, G. Meng, Z. Huang, M. Li and Y. Lei, Ag-nanoparticles@ bacterial nanocellulose as a 3D flexible and robust surface-enhanced Raman scattering substrate, ACS Appl. Mater. Interfaces, 2020, 12(45), 50713–50720 CrossRef.
- X. Zhou, Z. Hu, D. Yang, S. Xie, Z. Jiang, R. Niessner, C. Haisch, H. Zhou and P. Sun, Bacteria detection: from powerful SERS to its advanced compatible techniques, Adv. Sci., 2020, 7(23), 2001739, 10.1039/c7an00106a.
- S. Almohammed, S. Fedele, B. J. Rodriguez and J. H. Rice, Aligned Diphenylalanine Nanotube-Silver Nanoparticle Templates for High-Sensitivity Surface-Enhanced Raman Scattering, J. Raman Spectrosc., 2017, 1–9, DOI:10.1002/jrs.5254.
- S. Almohammed, A. Fularz, B. J. Rodriguez and J. H. Rice, Electric Field-Driven Catalytic Activity Using a Bioinspired Peptide and Titanium Dioxide Semiconductor Composite with Metal Nanoparticles, J. Phys. Chem. C, 2020, 124, 26874–26880, DOI:10.1021/acs.jpcc.0c08824.
- S. Almohammed, B. J. Rodriguez and J. H. Rice, Nucleobase Sensing Using Highly-Sensitive Surface-Enhanced Raman Spectroscopy Templates Comprising Organic Semiconductor Peptide Nanotubes and Metal Nanoparticles, Sens. Bio-Sens. Res., 2019, 24, 100287, DOI:10.1016/j.sbsr.2019.100287.
- S. Almohammed, M. Alruwaili, E. G. Reynaud, G. Redmond, J. H. Rice and B. J. Rodriguez, 3D-Printed Peptide-Hydrogel Nanoparticle Composites for Surface-Enhanced Raman Spectroscopy Sensing, ACS Appl. Nano Mater., 2019, 9b00940, DOI:10.1021/acsanm.9b00940.
- S. Almohammed, S. Tade Barwich, A. K. Mitchell, B. J. Rodriguez and J. H. Rice, Enhanced Photocatalysis and Biomolecular Sensing with Field-Activated Nanotube-Nanoparticle Templates, Nat. Commun., 2019, 10(1), 2496, DOI:10.1038/s41467-019-10393-9.
- Y. Xu, Z. Li, Y. Liao, J. Wang, T. Zhang, X. Liu and Y. Zhang, Unveiling the Dual-Enhancing Mechanisms of Kinetically Controlled Silver Nanoparticles on Piezoelectric PVDF Nanofibers for Optimized SERS Performance, ACS Sens., 2024, 9(2), 849–859 CrossRef PubMed.
- S. Almohammed, A. Fularz, F. Zhang, D. Alvarez-Ruiz, F. Bello, D. D. O'Regan, B. J. Rodriguez and J. H. Rice, Flexing piezoelectric Diphenylalanine–plasmonic metal nanocomposites to increase SERS signal strength, ACS Appl. Mater. Interfaces, 2020, 12(43), 48874–48881 CrossRef PubMed.
- S. Almohammed, A. Fularz, M. B. Kanoun, S. Goumri-Said, A. Aljaafari, B. J. Rodriguez and J. H. Rice, Structural Transition-Induced Raman Enhancement in Bioinspired Diphenylalanine Peptide Nanotubes, ACS Appl. Mater. Interfaces, 2022, 14(10), 12504–12514, DOI:10.1021/acsami.1c22770.
- S. Almohammed, A. Thampi, A. Bazaid, F. Zhang, S. Moreno, K. Keogh, M. Minary-Jolandan, J. H. Rice and B. J. Rodriguez, Energy Harvesting with Peptide Nanotube-Graphene Oxide Flexible Substrates Prepared with Electric Field and Wettability Assisted Self-Assembly, J. Appl. Phys., 2020, 128(11), 115101, DOI:10.1063/5.0017899.
- X. Sun, Glucose detection through surface-enhanced Raman spectroscopy: A review, Anal. Chim. Acta, 2022, 1026, 339226 CrossRef PubMed.
- A. Fularz, S. Almohammed and J. H. Rice, Metal-free cellulose-based platforms for biomolecule fluorescence signal enhancement, ACS Sustain. Chem. Eng., 2021, 10(1), 508–520 CrossRef.
- A. Fularz, S. Almohammed and J. H. Rice, SERS enhancement of porphyrin-type molecules on metal-free cellulose-based substrates, ACS Sustain. Chem. Eng., 2021,(49), 16808–16819 CrossRef.
- H. Zhou, D. Yang, N. P. Ivleva, N. E. Mircescu, R. Niessner and C. Haisch, SERS Detection of Bacteria in Water by in Situ Coating with Ag Nanoparticles, Anal. Chem., 2014, 86(3), 1525–1533, DOI:10.1021/ac402935p.
- Y. Zhao, Z. Zhang, Y. Ning, P. Miao, Z. Li and H. Wang, Simultaneous quantitative analysis of Escherichia coli, Staphylococcus aureus and Salmonella typhimurium using surface-enhanced Raman spectroscopy coupled with partial least squares regression and artificial neural networks, Spectrochim. Acta, Part A, 2023, 293, 122510 CrossRef PubMed.
- N. V. Nar, K. Kalantar-Zadeh and A. Mitchell, Influence of electric field on SERS: frequency effects, intensity changes, and susceptible bonds, J. Am. Chem. Soc., 2012, 134(10), 4646–4653 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay01677g |
| This journal is © The Royal Society of Chemistry 2025 |