Linear phenolic polymers with amide stabilized catechol moieties†
Jie
Wang
a,
Rui
Wang
a,
Yan
Gao
ab,
Baoxia
Wang
a,
Shuqi
Dong
*b and
Liang
Yuan
 *a
*a
aAnhui Provincial Engineering Center for High Performance Biobased Nylon, School of Materials and Chemistry, Anhui Agricultural University, Hefei, Anhui 230036, P. R. China. E-mail: yuanliang2020@ahau.edu.cn
bInstitute of Plant Nutrition, Resources and Environment, Beijing Academy of Agriculture and Forestry Sciences, Beijing 100097, P. R. China. E-mail: sqdong77@163.com
First published on 21st November 2024
Abstract
Phenolic polymers with catechol groups easily undergo oxidative crosslinking. Through the incorporation of pendant amide groups, the thermal stability of linear phenolic polymers with catechol moieties was significantly improved with maintained antioxidation performance and solubility in THF after storing at 80 °C for 96 hours.
Over 8000 phenolic compounds have been identified from nature, and they have exhibited a wide range of functions including pigments, antimicrobial, antioxidation, anti-cancer as well as surface coatings.1–3 Due to the activation effect of the phenolic –OH groups, phenolic compounds, especially those with two or three phenolic –OH groups on one aromatic ring (dopamine, catechin, urushiol, gallic acid, tannic acid, etc.), easily undergo oxidation and crosslinking to generate complicated molecules and networks.4–6 The crosslinking mechanism generally involves the formation of a quinone intermediate and subsequent coupling reactions (radical coupling, Michael addition, Schiff base formation, etc.).4 However, the exact structures of the resulting materials are complicated and have not yet been elucidated clearly.
With the advancement in synthetic polymer chemistry, linear phenolic polymers with repetitive phenolic groups and well-defined structures have been designed and synthesized, offering great diversity towards different applications.7–10 For example, styrene copolymers with one, two, three, four and five phenolic –OH groups on each styrene monomer unit were separately prepared, and these phenolic copolymers exhibited increasing underwater adhesion with more –OH groups on an aromatic ring.11 Through the copolymerization of sulphur dioxide and eugenol, linear phenolic polymers were developed as degradable fertilizer coating materials.12 We recently reported the polycondensation of aldehyde-functionalized phenolic compounds and polymercaptans to produce linear phenolic polymers, exhibiting different levels of antioxidation and surface adhesion properties depending on the monomer structures (Scheme 1A).13,14 Due to their susceptibility to oxidation, we noticed stability issues for polymers with pendant catechol groups, such as discoloration and precipitation from solution, darkening and crosslinking of the bulk materials.15 Medeina and coworkers observed similar stability concerns for copolymers with pendant catechol groups (Scheme 1B).16 As far as we know, limited attention has been paid to addressing the stability issue of catechol-containing phenolic polymers. Facile preparation of phenolic polymers with stabilized catechol functionality demands innovative polymerization methods.
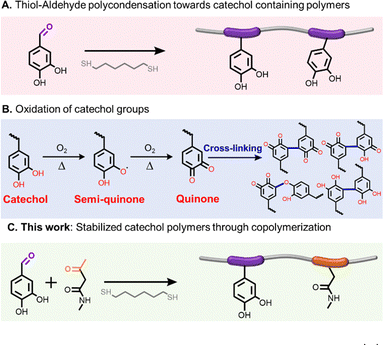 | ||
| Scheme 1 (A) Preparation of phenolic polymers with pendant catechol groups; (B) oxidation of catechol groups; (C) preparation of polymers with amide stabilized catechol groups. | ||
Herein, we communicate the incorporation of amide-containing ketones as a comonomer into the “thiol-aldehyde” copolymerization system, affording linear polymers with coexisting catechol and amide groups, and report their enhanced storage stability (Scheme 1C).
As shown in Fig. 1A, we examined the tri-component copolymerization between 3,4-dihydroxybenzene (A), amide ketone (K) and 1,6-hexanedithiol (T). The ratio between (A + K):T was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the ratio of A
1, and the ratio of A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T was examined at 9
T was examined at 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 8
10, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 7
10, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 6
10, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 5
10, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Catalysed by BF3·2H2O, the conversion of the amide ketone monomer was calculated through 1H NMR, referencing to the dithioacetal proton at 5.0 ppm, or to the methyl and methylene protons next to the ketone group, since the ketone group will be converted to dithioketal after the polymerization. It needs to be mentioned that the aldehyde monomer A was consumed quickly, reaching 100% conversion within 1 hour, while the amide ketone monomers (K1, K2, K3) were polymerized much slower. At each testing ratio, no complete conversion of the ketone monomers was observed (Fig. S1–S3 ESI,† and Table 1). However, with higher feeding ratio of amide ketone monomer, its conversion also increased. For example, over 80% of K2 and K3 were consumed when the ratio of A
10. Catalysed by BF3·2H2O, the conversion of the amide ketone monomer was calculated through 1H NMR, referencing to the dithioacetal proton at 5.0 ppm, or to the methyl and methylene protons next to the ketone group, since the ketone group will be converted to dithioketal after the polymerization. It needs to be mentioned that the aldehyde monomer A was consumed quickly, reaching 100% conversion within 1 hour, while the amide ketone monomers (K1, K2, K3) were polymerized much slower. At each testing ratio, no complete conversion of the ketone monomers was observed (Fig. S1–S3 ESI,† and Table 1). However, with higher feeding ratio of amide ketone monomer, its conversion also increased. For example, over 80% of K2 and K3 were consumed when the ratio of A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T was 5
T was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, while around 30% of the ketone monomers were converted when the ratio of A
10, while around 30% of the ketone monomers were converted when the ratio of A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T was 9
T was 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The lower reactivity of amide ketones when compared with A was further confirmed through its copolymerization with T. When T
10. The lower reactivity of amide ketones when compared with A was further confirmed through its copolymerization with T. When T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K was kept at 1
K was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, only 35.9% of K1 was converted after 1 hour, and 59% was reacted even after 10 days. K2 and K3 were found to polymerize faster than K1, since 42% of K2 and 59% of K3 were converted after 1 hour and 100% conversion were obtained for both monomers after 10 days (Fig. S4–S6, ESI†). These observations indicate the lower reactivity of the amide ketone monomers as compared to the aldehyde monomer A. The feeding monomer ratio of K to A was kept below 5
1, only 35.9% of K1 was converted after 1 hour, and 59% was reacted even after 10 days. K2 and K3 were found to polymerize faster than K1, since 42% of K2 and 59% of K3 were converted after 1 hour and 100% conversion were obtained for both monomers after 10 days (Fig. S4–S6, ESI†). These observations indicate the lower reactivity of the amide ketone monomers as compared to the aldehyde monomer A. The feeding monomer ratio of K to A was kept below 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, since the catechol group is our functionality of interest. When K3:A was increased to 8
5, since the catechol group is our functionality of interest. When K3:A was increased to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 9
2 or 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the incorporation ratio of K3 can reach 97% (Fig. S3, ESI†).
1, the incorporation ratio of K3 can reach 97% (Fig. S3, ESI†).
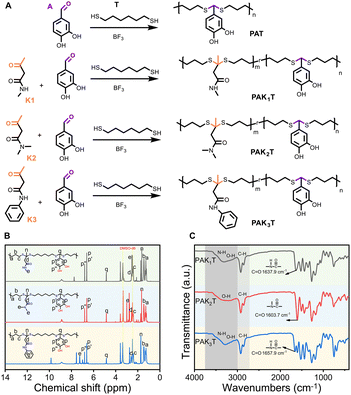 | ||
Fig. 1 (A) Preparation of catechol-containing polymers; (B) 1H NMR spectra of amide-containing catechol polymers; (C) FT-IR spectra of amide-containing catechol polymers obtained from A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T = 5 T = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. 10. | ||
Feeding ratio of K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T T |
Conversion of the amide monomer | ||
|---|---|---|---|
| PAK1T (%) | PAK2T | PAK3T (%) | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
30.3 | Insoluble | 31.8 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
33.0 | 22.0% | 34.0 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
46.7 | 26.0% | 81.6 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
59.0 | 45.8% | 82.7 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
59.0 | 83.6% | 83.0 |
The tricomponent copolymers from A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K
K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T = 5
T = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were precipitated from water to remove the catalyst, solvent and remaining monomers, and freeze dried to remove any residual water to have PAK1T, PAK2T, and PAK3T. The prepared copolymers were subjected to 1H NMR analysis (Fig. 1B). The peak at 9.67 ppm corresponding to the aldehyde group (–CHO) of monomer A disappeared in the copolymers, and the aromatic protons on monomer A at 6.89 ppm, 7.21 ppm, and 7.24 ppm, respectively, were shifted to 6.62 ppm and 6.81 ppm after the polymerization. The methylene protons (–CH2–) denoted as a, b, and c of monomer T remain largely unchanged after polymerization. Upon copolymerization with the amide monomer K1, the methyl group (–CH3) adjacent to the carbonyl group shifted from 2.01 ppm to 1.64 ppm, while the methylene group (–CH2–) next to the amide group shifted from 3.41 ppm to 2.54 ppm (Fig. 1B). Similar trends are observed in polymers PAK2T and PAK3T. FT-IR spectra of all three copolymers show strong –OH peaks between 3000–3600 cm−1 and the carbonyl stretching vibration peaks on the amide groups in the range of 1600–1660 cm−1, depending on the amide monomer structures (Fig. 1C). Both 1H NMR and FT-IR results confirm the successful synthesis of linear catechol polymers with amide groups. By introducing multi-functional polymercaptans, it is theoretically possible to produce non-linear structured phenolic polymers.
10 were precipitated from water to remove the catalyst, solvent and remaining monomers, and freeze dried to remove any residual water to have PAK1T, PAK2T, and PAK3T. The prepared copolymers were subjected to 1H NMR analysis (Fig. 1B). The peak at 9.67 ppm corresponding to the aldehyde group (–CHO) of monomer A disappeared in the copolymers, and the aromatic protons on monomer A at 6.89 ppm, 7.21 ppm, and 7.24 ppm, respectively, were shifted to 6.62 ppm and 6.81 ppm after the polymerization. The methylene protons (–CH2–) denoted as a, b, and c of monomer T remain largely unchanged after polymerization. Upon copolymerization with the amide monomer K1, the methyl group (–CH3) adjacent to the carbonyl group shifted from 2.01 ppm to 1.64 ppm, while the methylene group (–CH2–) next to the amide group shifted from 3.41 ppm to 2.54 ppm (Fig. 1B). Similar trends are observed in polymers PAK2T and PAK3T. FT-IR spectra of all three copolymers show strong –OH peaks between 3000–3600 cm−1 and the carbonyl stretching vibration peaks on the amide groups in the range of 1600–1660 cm−1, depending on the amide monomer structures (Fig. 1C). Both 1H NMR and FT-IR results confirm the successful synthesis of linear catechol polymers with amide groups. By introducing multi-functional polymercaptans, it is theoretically possible to produce non-linear structured phenolic polymers.
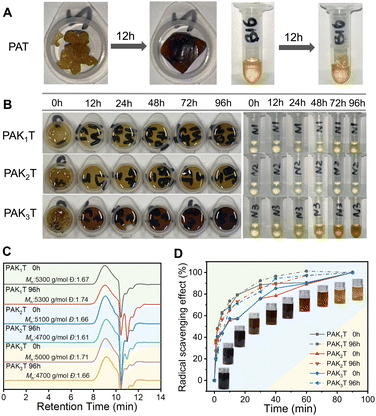
The choice of catalyst and purification process were important to obtain the product. When catalysed by ZrCl4, the polymer PAT turned to be completely black after the polymerization (Fig. S7, ESI†). When BF3·2H2O was used, the PAT crude solution showed slightly darker colour over the light brown solution of PAKT copolymers (Fig. S8, ESI†). It is suggested to precipitate the polymer product from unconcentrated solution to remove the catalyst and unreacted monomer. If the catalyst BF3 was left with the product for too long, the concentrated solution would turn to be dark colour and became insoluble (Fig. 2A). So, it is critical to separate the polymer product from the catalyst timely after the polymerization to have a light colour product (Fig. 2A). GPC analysis of PAK1T, PAK2T and PAK3T shows very similar molecular weights of 5 kg mol−1 and a polydispersity index of 1.7 (Fig. 2B).
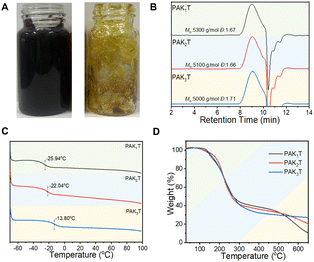 | ||
| Fig. 2 (A) Photo of PAT with the catalyst (left) and without the catalyst (right), (B) GPC analysis, (C) DSC analysis and (D) TGA analysis of the purified amide containing copolymers. | ||
The copolymers are all sticky and elastic with glass transition temperatures of −25.9 °C, −22.0 °C, and −13.8 °C for PAK1T, PAK2T and PAK3T, respectively (Fig. 2C). In contrast, the copolymer PAT shows a Tg of −1.8 °C, and the incorporation of amide ketone monomers reduced its Tg. With decreasing pendant amide groups from K3 to K2 and K1, Tg of the copolymers gradually decreased due to increased polymer backbone mobility. The thermal stability of the copolymers was compared by TGA analysis. As shown in Fig. 2D, the Td5 of these amide copolymers were determined to be 150 °C, 167 °C and 156 °C, and Td50 is about 260 °C, respectively. Td5 of PAT was tested to be 198 °C. Thus, the copolymerization with amide monomers reduced its thermal stability.
As we discussed earlier, phenolic polymers with catechol groups were unstable due to oxidation. We noticed that polymer PAT gradually lost its solubility after being left in the open air at room temperature. In the current work, the amide containing copolymers maintained good solubility in THF even after several months. Thus, we plan to set up a method to illustrate the influence of amide groups on the polymer stability.
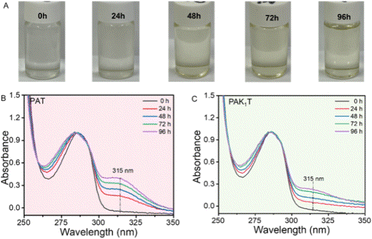
Polymers PAT, PAK1T, PAK2T and PAK3T were put into a Petri-dish (3 cm in diameter) and covered with a lid before being left in an oven of 80 °C (Fig. 3). Samples were taken at 12 h, 24 h, 48 h, 72 h and 96 h for solubility testing, 1H NMR analysis, GPC, DSC testing, and DPPH antioxidation performance evaluation. As shown in Fig. 3A, PAT lost its solubility in THF after 12 h, showing serious darkening. The amide-containing copolymers were still easily soluble even after being kept at 80 °C for 96 h, although a minor discoloration of the samples was observed (Fig. 3B). GPC analysis of the samples indicated similar molecular weight and distribution, indicating no significant coupling reactions in an atmospheric environment and 80 °C (Fig. 3C). 1H NMR tracking of the samples during the thermal stability test shows that the phenolic hydroxyl groups (9.0 ppm) and the proton on the thioacetal group (5.0 ppm) were both stable throughout the test (Fig. S9–S11, ESI†). The shape of the catechol –OH groups also became better separated, probably due to better hydrogen bonding interactions promoted by thermal treatment.17 DSC analysis of these polymers indicates that with the extension of thermal treatment, their glass transition temperatures gradually increased (Fig. S12, ESI†) at different levels (5–10 °C).
The impact of thermal storage on the antioxidation performance of the copolymers was compared through DPPH analysis in THF solution (Fig. S13–S15, ESI†).18 For the untreated samples, the free radical scavenging efficiency of PAK1T was higher than that of PAK2T and PAK3T. It took only 2.4 minutes for PAK1T to clear 50% of DPPH free radicals, while PAK2T and PAK3T needed 7.6 minutes and 7.1 minutes, respectively. Thus, the amide containing copolymers show good antioxidation properties. The samples after storing at 80 °C for 96 hours were similarly evaluated by DPPH assay. We noticed that the free radical scavenging efficiency of the three copolymers was slightly improved, possibly because better hydrogen bond interactions were induced by high temperature (Fig. 3D).
Besides the thermal stability test in the bulk state, we prepared polymer solution in DMF at a concentration of 2.0 mg mL−1, and kept them in an 80 °C oven. The solutions were taken out for colour observation and UV-Vis test after 12, 24, 48, 72 and 96 hours. A photo of the PAK1T solution is shown in Fig. 4A, and we observed yellowing of the solution. UV-vis absorption of the solution was tracked. A peak at 285 nm was assigned to the catechol group.19 An apparent shoulder peak at 300–325 nm was observed for the polymer solution of PAT, due to the oxidation of the catechol group (Fig. 4B). Thus, their UV-vis spectra were normalized by the peak at 285 nm. The absorption of PAK1T (Fig. 4C) and PAK2T solutions (Fig. S16, ESI†) at 300–325 nm was negligible before the storage test, and only increased to be below 0.3 after 96 hours of testing. However, the PAK3T solution shows a stronger absorption at 300–325 nm even before the storage test, which increased to be almost 0.5 after the storage test (Fig. S16, ESI†), indicating that PAK3T was relatively less stable than PAK1T and PAK2T in solution. In fact, during the bulk storage test, PAK3T showed the worst colour change, while PAK1T and PAK2T exhibited slight colour change.
The mechanism for the enhanced thermal storage stability of the catechol polymers in the presence of amide functionality is still unclear. We propose that the enhanced thermal stability is due to the hydrogen bonding-assisted stabilization of the catechol group or the phenolic hydroxyl radical.20 As shown in Scheme 1B, it is critical to stabilize the catechol group or the more reactive radical and lower its reactivity towards crosslinking. The introduced amide groups have –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, N, and N–H to form hydrogen bonding with the phenolic OH groups or the O· radical centre.21,22 This could help explain why PAK3T is less stable than PAK1T and PAK2T, as the phenyl ring might exhibit steric hindrance for efficient H-bonding.23 Also, –N⋯HO– hydrogen bonding is supposed to be stronger over –O⋯HO-hydrogen bonding due to the reduced electronegativity of N over O.24 Previously, high-precision DFT calculations have also proved the H-bonding stabilization effect of phenolic compounds or radicals.20
O, N, and N–H to form hydrogen bonding with the phenolic OH groups or the O· radical centre.21,22 This could help explain why PAK3T is less stable than PAK1T and PAK2T, as the phenyl ring might exhibit steric hindrance for efficient H-bonding.23 Also, –N⋯HO– hydrogen bonding is supposed to be stronger over –O⋯HO-hydrogen bonding due to the reduced electronegativity of N over O.24 Previously, high-precision DFT calculations have also proved the H-bonding stabilization effect of phenolic compounds or radicals.20
Overall, a tricomponent copolymerization system was developed to reach macromolecular antioxidants with catechol groups. The copolymerization behaviour of amide-containing ketone monomers was studied and the incorporation of amide functionalities was helpful to improve the thermal stability of catechol groups. The copolymers maintained good solubility, molecular weight and antioxidation performance after being kept at 80 °C for 4 days. The current study provides a facile strategy towards stabilized phenolic polymers, which show great potential in applications as adhesives and antioxidants with further optimizations over molecular weights and structural motifs.
This work was supported by the National Natural Science Foundation of China (Grant no. 52373096, 32202614), Natural Science Foundation of Anhui Province (Grant no. 2208085MC69, 2108085MC109), and Anhui Agricultural University.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- B. R. Albuquerque, S. A. Heleno, M. B. P. P. Oliveira, L. Barros and I. C. F. R. Ferreira, Food Funct., 2021, 12, 14–29 RSC.
- M. Takó, E. B. Kerekes, C. Zambrano, A. Kotogán, T. Papp, J. Krisch and C. Vágvölgyi, Antioxidants, 2020, 9, 165 CrossRef.
- X. Chen, W. Lan and J. Xie, Food Chem., 2024, 440, 138198 CrossRef CAS.
- H. Choi and K. Lee, Appl. Sci., 2022, 12, 11626 CrossRef CAS.
- G. P. Maier, C. M. Bernt and A. Butler, Biomater. Sci., 2018, 6, 332–339 RSC.
- X. Yao, X. Zheng, J. Zhang and K. Cai, RSC Adv., 2016, 6, 76473–76481 RSC.
- J. Brito, H. Hlushko, A. Abbott, A. Aliakseyeu, R. Hlushko and S. A. Sukhishvili, ACS Appl. Mater. Interfaces, 2021, 13, 41372–41395 CrossRef CAS.
- R. Hlushko, J. F. Ankner and S. A. Sukhishvili, Macromolecules, 2020, 53, 1033–1042 CrossRef CAS.
- R. Hlushko, H. Hlushko and S. A. Sukhishvili, Polym. Chem., 2018, 9, 506–516 RSC.
- J. Wang, S. Huang, K. Yan, J. Shi, S. Shi, Y. Jin and L. Yuan, React. Funct. Polym., 2023, 191, 105671 CrossRef CAS.
- B. Cheng, J. Yu, T. Arisawa, K. Hayashi, J. J. Richardson, Y. Shibuta and H. Ejima, Nat. Commun., 2022, 13, 1892 CrossRef CAS.
- L. Liu, Y. Ni, Y. Zhi, W. Zhao, M. Pudukudy, Q. Jia, S. Shan, K. Zhang and X. Li, Macromolecules, 2020, 53, 936–945 CrossRef CAS.
- Y. Jin, C. Hu, J. Wang, Y. Ding, J. Shi, Z. Wang, S. Xu and L. Yuan, Angew. Chem., 2023, 135, e202305677 CrossRef.
- J. Wang, W. Gao, Y. Jin, W. Tian, Y. Zhang, C. Hu, B. Wang, S. Dong and L. Yuan, Carbohydr. Polym., 2024, 339, 122246 CrossRef CAS.
- R. Pinnataip and B. P. Lee, ACS Omega, 2021, 6, 5113–5118 CrossRef CAS PubMed.
- M. Steponaviciute, V. Klimkevicius and R. Makuska, Mater. Today Commun., 2020, 25, 101262 CrossRef CAS.
- P. Charisiadis, V. G. Kontogianni, C. G. Tsiafoulis, A. G. Tzakos, M. Siskos and I. P. Gerothanassis, Molecules, 2024, 19, 13643–13682 CrossRef PubMed.
- I. Gulcin and S. H. Alwasel, Processes, 2023, 11, 2248 CrossRef CAS.
- A. Scalzone, M. A. Bonifacio, S. Cometa, F. Cucinotta, E. De Giglio, A. M. Ferreira and P. Gentile, Front. Bioeng. Biotechnol., 2020, 8, 712 CrossRef PubMed.
- Y. Chen, H. Xiao, J. Zheng and G. Liang, PLoS One, 2015, 10(3), e0121276 CrossRef.
- R. K. Castellano, Y. Li, E. A. Homan, A. J. Lampkins, I. V. Marín and A. Khalil, Eur. J. Org. Chem., 2012, 4483–4492 CrossRef CAS.
- H. Zhao, S. Tang, X. Xu and L. Du, Int. J. Mol. Sci., 2017, 18(1), 4 CrossRef.
- A. Nowok, M. Dulski, J. Grelska, A. Z. Szeremeta, K. Jurkiewicz, K. Grzybowska, M. Musiał and S. Pawlus, J. Phys. Chem. Lett., 2021, 12, 2142–2147 CrossRef CAS.
- P. I. Nagy, Int. J. Mol. Sci., 2014, 15(11), 19562–19633 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, 1H NMR for monomer conversion calculation, UV-Vis spectra for antioxidation test, photo of the polymerized product before purification, DSC of samples after thermal storage tests. See DOI: https://doi.org/10.1039/d4cc05066e |
| This journal is © The Royal Society of Chemistry 2025 |