Efficient circularly polarized luminescence from zero-dimensional terbium- and europium-based hybrid metal halides†
Yan
Zhang
a,
Yi
Wei
*a,
Chen
Li
a,
Yuxuan
Wang
a,
Yulian
Liu
a,
Meiying
He
a,
Zhishan
Luo
a,
Xiaoyong
Chang
a,
Xiaojun
Kuang
 b and
Zewei
Quan
b and
Zewei
Quan
 *a
*a
aDepartment of Chemistry, Southern University of Science and Technology (SUSTech), Shenzhen, Guangdong 518055, China. E-mail: quanzw@sustech.edu.cn; weiy3@sustech.edu.cn
bCollege of Materials Science and Engineering, Guilin University of Technology, Guilin 541004, China
First published on 29th November 2024
Abstract
Zero-dimensional (0D) chiral hybrid metal halides (HMHs) with narrow-band circularly polarized luminescence (CPL) show considerable promise in three-dimensional displays. In this work, 0D (S/R-3MOR)3TbCl6 and (S/R-3MOR)3EuCl6 (abbreviated as S/R-TbCl, S/R-EuCl) enantiomers with characteristic rare-earth ion emissions are synthesized. S/R-TbCl and S/R-EuCl exhibit narrow-band green and red emissions with high photoluminescence quantum yields of (85–91)% and (48–52)%, respectively. These materials present distinct CPL signals with dissymmetry factors up to ±0.006 and ±0.009 for S/R-TbCl and S/R-EuCl, respectively. These chiroptical properties confer the potential for their applications in circularly polarized light-emitting diodes for future displays.
Zero-dimensional (0D) chiral organic–inorganic hybrid metal halides (HMHs) have become indispensable due to their excellent optical properties, demonstrating exceptional performances in circularly polarized luminescence (CPL).1,2 As a result of the strong electron–phonon coupling and soft crystal lattice, 0D chiral HMHs exhibit highly efficient broad-band self-trapped exciton (STE) emissions, making them promising CPL-active candidates for advanced illumination applications.3,4 In comparison, narrow-band emissive materials hold great potential in high-resolution displays on account of high color purity.5,6 As current studies mainly focus on the ns2 metal cations, exploring novel luminescent centers in 0D chiral HMHs with narrow-band emissions is in high demand.
Owing to the unique d–f and f–f transitions, rare-earth-based luminescent materials display outstanding optical properties including high photoluminescence quantum yields (PLQYs), narrow emission peaks and high color purities, showing great potential in narrow-band light-emitting diodes (LEDs) for displays.7,8 Despite recent progress on the rare-earth-based HMHs,9–12 Tb- and Eu-based chiral HMHs with obvious CPL are rarely investigated.
In this work, two pairs of chiral 0D enantiomorphic (S/R-3MOR)3TbCl6 and (S/R-3MOR)3EuCl6 (S/R-3MOR = (S/R)-3-methylmorpholine, abbreviated as S/R-TbCl, S/R-EuCl) HMHs are synthesized by a facile solvent-evaporation method. S/R-TbCl and S/R-EuCl exhibit green and red narrow-band emissions with high PLQYs of (85–91)% for S/R-TbCl, and (48–52)% for S/R-EuCl, respectively. They also show mirror-image CPL signals with dissymmetry factor (glum) values of ±0.006 and ±0.009 for S/R-TbCl and S/R-EuCl, respectively. Based on the excellent chiroptical performances, green and red emissive circularly polarized LEDs (CP-LEDs) with polarization degrees of ±0.1% and ±0.3% are prepared by using S/R-TbCl and S/R-EuCl enantiomers, demonstrating their potential as CP-LED light sources for three-dimensional (3D) displays.
Two pairs of S/R-TbCl and S/R-EuCl enantiomers are synthesized through a solvent evaporation method. Generally, protonated chiral ligands are mixed stoichiometrically with TbCl3·6H2O/EuCl3·6H2O salts in HCl solutions, and the precursor solutions are evaporated at 110 °C to obtain S/R-TbCl and S/R-EuCl single crystals (details can be found in the Experimental section, ESI†). Single-crystal X-ray diffraction (SC-XRD) analyses reveal that S/R-TbCl and S/R-EuCl crystallize in the monoclinic P21 space group. Each Tb3+ or Eu3+ ion is coordinated with six Cl− ions to form [TbCl6]3− and [EuCl6]3− octahedra. The inorganic [TbCl6]3− and [EuCl6]3− octahedra are fully isolated by chiral organic cations to form 0D structures, as demonstrated in Fig. 1. The detailed crystallographic data are listed in Tables S1 and S2 (ESI†). The powder X-ray diffraction (PXRD) patterns align well with the simulated ones (Fig. S1 and S2, ESI†), confirming the high phase purity of the as-synthesized samples. X-ray photoelectron spectroscopy (XPS) examines the valence state of Tb/Eu ions in S/R-TbCl and S/R-EuCl (Fig. S3, ESI†). The binding energies of 1277.4 and 1243.0 eV, corresponding to 3d3/2 and 3d5/2 of Tb, are in accordance with the theoretical values of Tb3+. The presence of a satellite peak at 1241.2 eV is ascribed to the deexcitation process concurrent with core ionization.13 Comparably, the binding energies of 1164.0 and 1134.2 eV coincide with 3d3/2 and 3d5/2 of Eu, proving the existence of Eu3+.14 The above results demonstrate that the oxidative states of Tb and Eu are consistent with those of precursor salts with a +3 oxidation state.
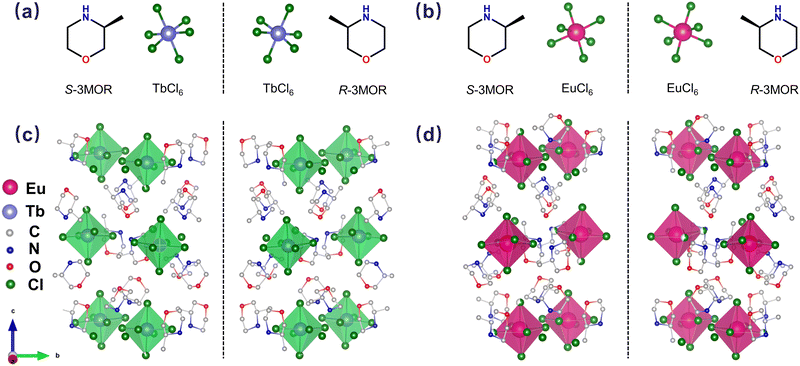 | ||
| Fig. 1 (a) and (b) Structural models of S/R-3MOR, TbCl6 and EuCl6. (c) and (d) Crystal structure of S/R-TbCl and S/R-EuCl. Hydrogen atoms and bonds are omitted for clarity. | ||
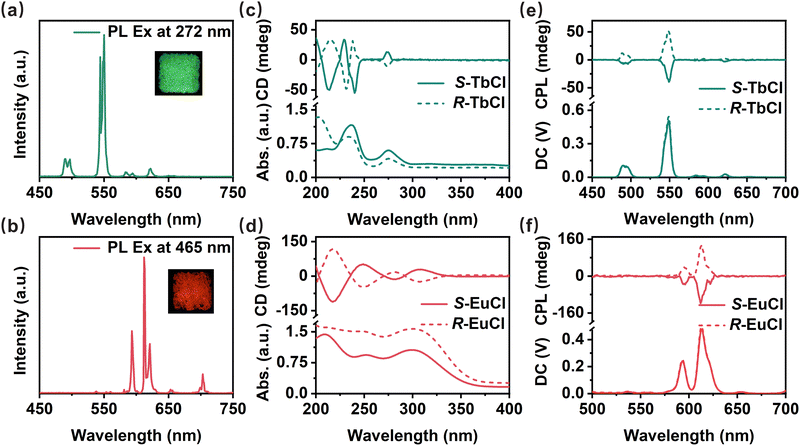
Photoluminescence (PL) measurements are investigated to elucidate the optical properties of S/R-TbCl and S/R-EuCl. S/R-TbCl and S/R-EuCl exhibit multi-narrow-band emissions, consistent with previous reports about Tb3+- and Eu3+-based phosphors. Under the excitation of 272 nm, S/R-TbCl powders display bright green multi-emissions peaking at 489, 550 and 621 nm, corresponding to 5D4 → 7FJ (J = 3, 4 and 6) transitions of Tb3+ ions (Fig. 2a and S4).15,16 Among these emissions, the green emission at 550 nm plays a dominant role with a narrow full-width at half-maximum (FWHM) of 8.1 ± 0.2 nm. The CIE chromaticity coordinates for both S-TbCl and R-TbCl are calculated to be (0.33, 0.62), which corresponds to the color purity of 92.3% (Fig. S5, ESI†). The average PL lifetimes of S-TbCl and R-TbCl monitored at 550 nm are measured to be 4.62 and 4.61 ms (Fig. S6, ESI†), respectively, in agreement with other reported Tb3+-based phosphors. In addition, S/R-TbCl crystals exhibit high PLQYs of 91.2% and 85.1% at room temperature (RT), respectively (Fig. S7, ESI†). Similarly, upon 465 nm excitation, S/R-EuCl powders exhibit brilliant red multi-emissions at 593, 612, 621, 653 and 703 nm, corresponding to 5D0 → 7FJ (J = 1–4) transitions of Eu3+ ions (Fig. 2b and Fig. S8, ESI†).17 The main peak at 612 nm shows an extremely narrow FWHM of 2.2 ± 0.2 nm. The CIE chromaticity coordinates for both S-EuCl and R-EuCl are (0.64, 0.35), showing a color purity of 98.0% (Fig. S9, ESI†). The PL lifetimes of S/R-EuCl monitored at 612 nm are measured to be 2.27 and 2.25 ms, respectively (Fig. S10, ESI†). The microsecond PL lifetimes are consistent with those values in the reported Eu3+-based phosphors. The PLQYs of S/R-EuCl are measured to be 52.0% and 48.5% at RT, respectively (Fig. S11, ESI†). Moreover, analogous spectra are obtained under different excitation wavelengths, further proving the excitation independence of these materials (Fig. S12, ESI†). All of the results indicate that the characteristic emissions of rare-earth ions are well preserved in S/R-TbCl and S/R-EuCl. Although the lifetimes and CIE coordinates of the synthesized S/R-TbCl and S/R-EuCl align with those of previously reported Tb/Eu-based systems, the significantly higher PLQYs of these materials suggest their strong potential for advanced optoelectronic applications (Table S3, ESI†).
Considering their chiral structures and bright green/red emissions, circular dichroism (CD) and CPL spectra are measured to investigate the chiroptical properties of S/R-TbCl and S/R-EuCl. Fig. 2c and d depict the absorption and CD spectra of S/R-TbCl and S/R-EuCl. The absorption bands of S/R-TbCl appear from 200 to 300 nm. The CD spectra show intense Cotton effects and strong mirror-image signals from 200 to 280 nm. For S/R-EuCl, the absorption bands exhibit continuous signals from 200 to 350 nm, and the corresponding CD spectra exhibit mirror symmetry in the same region. Compared with the CD spectra of the chiral organic ligands that only show signals below 225 nm (Fig. S13, ESI†), it is reasonable to conclude that the CD signals from 225 to 280 nm for S/R-TbCl and 225 to 350 nm for S/R-EuCl originate from inorganic [TbCl6]3− and [EuCl6]3− units, suggesting the successful chirality induction from organic ligands to inorganic octahedra. In general, the degree of CPL emission of chiral fluorescent molecules is quantified by dissymmetry factor (glum), glum = 2 × (IL − IR)/(IL + IR), where IL and IR represent the intensities of left- and right-handed CPL emissions, respectively.18 Based on the CPL spectra illustrated in Fig. 2e and f, both S/R-TbCl and S/R-EuCl exhibit strong and sharp CPL signals, well matching with their PL spectra. The CPL signals of both materials exhibit the same signs instead of the typical alternating behavior seen in lanthanide CPL spectra, which arises from different electronic transitions and selection rules for magnetic dipole (MD) and electric dipole (ED) transitions. In Ln-based chiral HMHs, the organic ligands are completely separated from the inorganic framework. The strength and uniformity of the chiral environment, along with the dominance of MD transitions are different from those in Ln-MOFs and Ln-complexes. This may suppress the expected alternating pattern, leading to similar CPL signals for Tb- and Eu-based chiral HMHs. However, further validation is required. Furthermore, the individual LnCl63− units result in a low molar extinction coefficient (ε < 10 L mol−1 cm−1) due to forbidden 4f–4f transitions of Ln3+ ions. By contrast, Ln-MOFs and Ln-complexes can achieve high extinction coefficients up to 1.26 × 104 L mol−1 cm−1) through the antenna effect.19,20 The largest glum values of S/R-TbCl peaked at 550 nm are ±0.006 and those of S/R-EuCl peaked at 612 nm are ±0.009, respectively (Fig. S14, ESI†), demonstrating their great potential as narrow-band CPL light sources for 3D displays. Using distinct organic molecules to alter the strength and distribution of hydrogen bonds between chiral and luminous components, or modifying metal/halogen dopant ion concentration may further improve the glum values.
Due to the high working temperature of LED devices, thermogravimetry analysis (TGA) and temperature-dependent PL spectroscopy are conducted to verify the stability of S/R-TbCl and S/R-EuCl. As demonstrated in Fig. S15 (ESI†), TGA results reveal that the decomposition temperatures of S/R-TbCl and S/R-EuCl are as high as 200 °C, indicating that these materials will not decompose on the LED devices (working temperature below 150 °C). The normalized temperature-dependent PL spectra of S-TbCl and S-EuCl show that the PL shape and peak positions remain unchanged as the temperature rises from 80 to 440 K (Fig. S16a and b, ESI†). The PL intensity of S-TbCl gradually decreases with increased temperature from 80 to 300 K, while further increasing the temperature leads to a sharp improvement in PL intensity until 440 K. The PL intensity of S-TbCl undergoes a rapid deterioration over 440 K, which arises from thermal-activated defects in S-TbCl (Fig. S16c, ESI†).21 As for S-EuCl, a sharp increase in PL intensity occurs from 80 to 180 K, and the PL intensity of S-EuCl gradually decreases with further increasing temperature (Fig. S16d, ESI†). Despite the PL quenching effect over 180 K, the integrated PL intensity of S-EuCl at 380 K (working temperature for LEDs) still preserves 62% of the intensity at 300 K. The above results demonstrate that S-TbCl and S-EuCl present significant structural and PL stabilities, which is beneficial to further application in CP-LED devices.
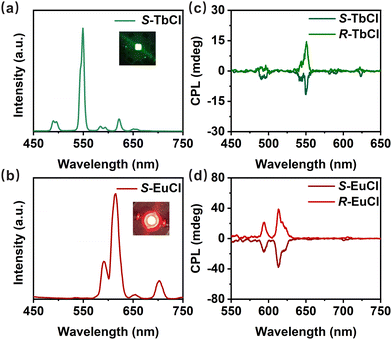
Inspired by their excellent chiroptical performances and PL thermal stabilities, green- and red-emitting CP-LED devices are fabricated by coating the S/R-TbCl and S/R-EuCl powders onto commercial UV-LED chips. Considering the discrepancy of the excitation wavelengths of S/R-TbCl and S/R-EuCl, 275 and 395 nm commercial UV chips are used to manufacture the CP-LED devices, respectively. PL spectra of Tb-based green emissive (Fig. 3a) and Eu-based red emissive devices (Fig. 3b) are recorded at a driving voltage of 3 V and a driving current of 20 mA, in agreement with PL spectra of S/R-TbCl and S/R-EuCl. The insets show the photographs of the working CP-LED devices, presenting bright green and red emissions, respectively. The CPL properties of the as-prepared devices are also measured under the same conditions, and the results are illustrated in Fig. 3c and d. Mirror symmetric CPL signals are observed from both green and red emissive devices with prominent peaks at 548 and 612 nm, respectively. The measured polarization degrees are around ±0.1% for Tb-based CP-LEDs and ±0.3% for Eu-based CP-LEDs (Fig. S17, ESI†). The dissymmetry degrees of CP-LEDs are slightly decreased with respect to those of S/R-TbCl and S/R-EuCl powders. This discrepancy mainly arises from the varied temperatures during the tests, because high temperatures often deteriorate CPL performances. In addition, internal emission from the back electrode can also cause the degradation of the CPL performance.22 The above results clearly demonstrate the feasible application of S/R-TbCl and S/R-EuCl in CP-LEDs.
In summary, two enantiomeric pairs of terbium- and europium-based chiral HMHs are successfully synthesized. The narrow-band PL spectra are in accordance with features of electronic transitions in rare-earth ions, with high color purities of 92.3% for S/R-TbCl and 98.0% for S/R-EuCl. S/R-TbCl exhibits brilliant green emissions peaking at 550 nm with high PLQYs of (85–91)% and glum values of ±0.006, while S/R-EuCl displays strong red emissions peaking at 612 nm with PLQYs of (48–52)% and glum values of ±0.009. The fabricated CP-LED devices exhibit obvious CPL signals, with polarization degrees of ±0.1% and ±0.3% for Tb- and Eu-based CP-LED devices. The observed chiroptical properties indicate that these materials show potential as a CPL light source for 3D displays.
This work was supported by the National Natural Science Foundation of China (NSFC) (22375084 and 52302179), and the Shenzhen Science and Technology Innovation Committee (RCJC202106091044441068, RCBS20221008093335082, KCXFZ20211020174805008).
Data availability
The data supporting this article are included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Lu, Z. V. Vardeny and M. C. Beard, Nat. Rev. Chem., 2022, 6, 470–485 CrossRef.
- D.-Y. Liu, H.-Y. Li, R.-P. Han, H.-L. Liu and S.-Q. Zang, Angew. Chem., Int. Ed., 2023, 62, e202307875 CrossRef CAS PubMed.
- Y. Liu, Z. Luo, Y. Wei, C. Li, Y. Chen, X. He, X. Chang and Z. Quan, Angew. Chem., Int. Ed., 2023, 62, e202306821 CrossRef CAS.
- Y. Wei, C. Li, Y. Li, Z. Luo, X. Wu, Y. Liu, L. Zhang, X. He, W. Wang and Z. Quan, Angew. Chem., Int. Ed., 2022, 61, e202212685 CrossRef CAS.
- M. Zhao, Q. Zhang and Z. Xia, Mater. Today, 2020, 40, 246 CrossRef CAS.
- K. Han, J. Jin, X. Zhou, Y. Duan, M. V. Kovalenko and Z. Xia, Adv. Mater., 2024, 36, 2313247 CrossRef CAS.
- Y. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao and Z. Tang, Matter, 2022, 5, 837–875 CrossRef CAS.
- Y. Zhong, Z. Wu, Y. Zhang, B. Dong and X. Bai, InfoMat, 2023, 5, e12392 CrossRef CAS.
- H. Zhao, Q. Wang, Z. Wen, H. Sun, S. Ji, X. Meng, R. Zhang, J. Jiang, Z. Tang and F. Liu, Angew. Chem., Int. Ed., 2023, 62, e202316336 CrossRef CAS.
- Q. Wang, T. Bai, S. Ji, H. Zhao, X. Meng, R. Zhang, J. Jiang and F. Liu, Adv. Funct. Mater., 2023, 33, 2303399 CrossRef CAS.
- Y. Wang, X. Yang, C. Liu, Z. Liu, Q. Fang, F. Bai, S. Wang, X. Hou, B. Feng, B. Chen and B. Zou, Angew. Chem., Int. Ed., 2022, 61, e202210836 CrossRef CAS PubMed.
- J.-X. Zhang, D. Hou, J.-Y. Li, H. Lin, R. Huang, Y. Zhang and J. Hao, J. Phys. Chem. C, 2020, 124, 19774–19780 CrossRef CAS.
- Z. Wan, D. Xu, W. She, F. Xie, Y. Feng, J. Yang, G. Liu and X. Tong, Ceram. Int., 2024, 50, 9499–9509 CrossRef CAS.
- M. Lee, H. Chung, S. V. Hong, H. Y. Woo, J.-Y. Chae, T. Y. Yoon, B. T. Diroll and T. Paik, Nanoscale, 2023, 15, 1513 RSC.
- H. Guo, Y. Chen, L. Wang, Q. Shi, C. E. Cui, P. Huang and J. Qiao, Inorg. Chem. Front., 2024, 11, 799 RSC.
- W. Zhou, Y. Yu, P. Han, C. Li, T. Wu, Z. Ding, R. Liu, R. Zhang, C. Luo, H. Li, K. Zhao, K. Han and R. Lu, Adv. Mater., 2024, 36, 2302140 CrossRef CAS.
- D. Cortecchia, W. Mróz, G. Folpini, T. Borzda, L. Leoncino, A. L. Alvarado-Leaños, E. M. Speller and A. Petrozza, Chem. Mater., 2021, 33, 2289 CrossRef CAS PubMed.
- J. Ma, H. Wang and D. Li, Adv. Mater., 2021, 33, 2008785 CrossRef CAS PubMed.
- Y. Hasegawa, Y. Kitagawa and T. Nakanishi, NPG Asia Mater., 2018, 10, 52–70 CrossRef CAS.
- B. Zhang, T. Xiao, C. Liu, Q. Li, Y. Zhu, M. Tang, C. Du and M. Song, Inorg. Chem., 2013, 52, 13332–13340 CrossRef CAS PubMed.
- T. Lu, Z. Ma, C. Du, Y. Fang, H. Wu, Y. Jiang, L. Wang, L. Dai, H. Jia, W. Liu and H. Chen, Sci. Rep., 2014, 4, 6131 CrossRef CAS.
- F. Zinna, U. Giovanella and L. D. Bari, Adv. Mater., 2015, 27, 1791–1795 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The crystal structures, detailed experimental procedures and characterizations. CCDC 2364010 and 2364011 for S/R-TbCl, 2364012 and 2364013 for S/R-EuCl, respectively. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc05206d |
| This journal is © The Royal Society of Chemistry 2025 |