Analyte-initiated disassembly of electrochromic metal–organic framework-based nanocomposites for smart colorimetric sensing†
Min
Zhou‡
,
Shan
Huang‡
,
Pengcheng
Huang
 * and
Fang-Ying
Wu
* and
Fang-Ying
Wu
 *
*
School of Chemistry and Chemical Engineering, Nanchang University, Nanchang 330031, China. E-mail: pchuang@ncu.edu.cn; fywu@ncu.edu.cn
First published on 27th November 2024
Abstract
An electrochromic (EC) sensing strategy was presented through analyte-induced disassembly of an EC metal–organic framework-based nanocomposite. Carbon dots underwent charge reversion to separate from the entity stimulated by analytes, acting as a nanoswitch of the EC activity. We achieved colorimetric detection of glucose via the EC activity recovery mediated by released protons during glucose oxidation.
Development of simple yet effective analytical protocols is of great significance for diverse applications, especially in resource-limited circumstances. Among existing advanced analytical approaches, colorimetric assays have received enormous interest due to their low cost, operational simplicity, and practicality for point-of-care testing because of visual readout without costly or sophisticated instrumentation.1–3 For instance, noble-metal nanoparticle (NP)-based colorimetric assays have been broadly used for visual detection of various analytes owing to the integration of unique optical properties and versatile surface modification.2,4 However, this approach suffers from some drawbacks. First, tedious and complex conjugation processes are usually required during NP surface functionalization. Moreover, the dispersion/aggregation of NPs is susceptible to external environments. On the other hand, the nanozyme-based colorimetric strategy has also been well developed in the analytical field.5,6 Despite good sensing performances, many of them have several issues. For example, the color interference caused by nanozymes themselves is difficult to diminish because most nanozymes have deep colors, and besides, nanozymes mostly work in very acidic media, which may impair the accuracy owing to the mismatch with realistic sample conditions. Thus, it is still desirable to develop novel colorimetric methodologies to meet the practical demands, especially for complicated systems.
Recently, electrochromic (EC) materials, whose colors can be reversibly switched under applied potentials, have emerged as promising alternatives to conventional colorimetric probes.7,8 By merging the advantages of electrochemistry and colorimetry, EC sensors would provide possibilities to enhance sensing performances in terms of sensitivity, reliability, and convenience. Conventional EC materials such as metal oxides, Prussian blue and conjugated polymers have been utilized to construct EC sensing platforms.9–17 Unfortunately, their sensing abilities are only limited to finite analytes mainly because of the difficulty to impart specific recognition elements to their bulk structures.11 Furthermore, sophisticated sensor fabrication and relatively low sensitivity due to their unsatisfactory EC properties severely restrict efficient analytical applications.17
Over the last decade, metal–organic frameworks (MOFs) have been widely exploited as attractive candidates for EC materials.18–26 Notably, the intrinsic properties of MOFs including high porosity, large specific surface area, and good stability can largely promote EC activity. More importantly, MOFs possess composition and structural diversity and tuneability, which presents great opportunities to advance their recognition capacities by rational design, modification, and/or functionalization. For example, we recently reported the first example of an EC MOF-based visual sensor through facile surface modification to metal nodes of the framework.27 Clearly, these above features make EC MOFs particularly attractive to act as competitive colorimetric sensing platforms. But the study about EC MOFs for colorimetric assays is in its infancy, which is presumably because it is still difficult for specific analytes to effectively regulate the EC activities of MOFs. To this end, further exploration of new, simple strategies to endow EC MOFs with remarkable sensing performances is urgently demanded.
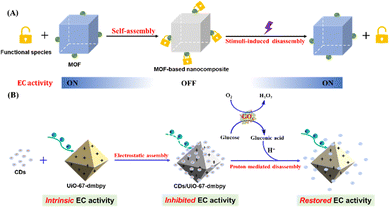
Nowadays MOF-based nanocomposites have garnered continuous attention since the combination of MOFs and exogenous species can not only overcome some inherent limitations of pure MOFs but also exhibit new functionalities through the synergy of two individual components.28 The distinct characteristics of these nanocomposites render them to be employed in a wider variety of application fields. However, EC MOF-based nanocomposites have not been reported. Herein, we present a straightforward EC sensing strategy based on the disassembly of the EC MOF-based nanocomposite in which additional species function as a switch to finely tune the EC activity of EC MOFs upon the stimulus of analytes (Scheme 1A). Specifically, the nanocomposite (denoted as CDs/UiO-67-dmbpy) is spontaneously assembled from an EC-active Zr-MOF, i.e., UiO-67-dmbpy, and carbon dots (CDs) mainly driven by electrostatic interaction. UiO-67-dmbpy is positively charged because of the viologen-like moiety, and undergoes a good EC behavior upon applied potential. This property makes UiO-67-dmbpy an ideal colorimetric reporter. The CDs bearing abundant carboxyl groups at the surface facilitate strong electrostatic binding with UiO-67-dmbpy, which substantially weakens the intrinsic EC activity of UiO-67-dmbpy due to the shielding effect of CDs to inhibit the interface electron transfer. Moreover, the CDs exhibit pH-sensitive responsiveness due to dangling carboxyl groups. Therefore, the incorporation of the proton would induce the charge reversion of CDs to trigger their dissociation from the UiO-67-dmbpy surface. Accompanied by the disassembly of the nanocomposite, the EC activity of UiO-67-dmbpy would be well recovered. Clearly, this switching mechanism based on the self-assembly and analyte-initiated disassembly of the EC MOF-based nanocomposite generates a new colorimetric approach with low technical demand and good sensing performances. As a proof of concept, we choose glucose as a model analyte to validate the feasibility of the presented EC sensing strategy (Scheme 1B). Glucose can be catalytically oxidized by glucose oxidase (GOx) to produce protons, which could mediate the disassembly of CDs/UiO-67-dmbpy and consequently restore the EC activity of UiO-67-dmbpy. This switchable EC behavior can well reflect the glucose amount by clear color changes observed via the naked eye as well as the smartphone, and the separation of electrochemical and optical signals ensures high sensitivity and accuracy. On the basis of this, the potential of this assay for glucose detection in human serum samples is well validated.
We first synthesized CDs and UiO-67-dmbpy according to previous reports, respectively.27,29 Transmission electron microscopy (TEM) images revealed that the mean size of CDs was about 3.6 nm (Fig. S1 and S2, ESI†). The powder X-ray diffraction (PXRD) pattern confirmed the successful preparation of UiO-67-dmbpy (Fig. S3A, ESI†). Zeta potential measurements (Fig. S3B, ESI†) showed that CDs were negatively charged and UiO-67-dmbpy is positively charged; the nanocomposite was thus readily fabricated via direct electrostatic assembly of CDs and UiO-67-dmbpy, as indicated by a considerable reduction in zeta potential of UiO-67-dmbpy. The PXRD pattern (Fig. S3A, ESI†) displayed that the nanocomposite exhibited almost identical diffraction peaks to those of pure UiO-67-dmbpy, indicative of the well-retained crystallinity. TEM images showed that CDs/UiO-67-dmbpy had an approximately octahedral geometry of UiO-67-dmbpy that was decorated with slightly aggregated CDs to produce a relatively rough surface (Fig. S3C and D, ESI†). N2 sorption isotherms (Fig. S3E, ESI†) illustrated that the BET surface area of CDs/UiO-67-dmbpy was much smaller than that of pure UiO-67-dmbpy, which is mainly due to the blockage of some pores by CDs. In addition, FT-IR spectra were compared between CDs/UiO-67-dmbpy and its two components. As depicted in Fig. S3F (ESI†), CDs/UiO-67-dmbpy exhibited similar absorption bands to pure UiO-67-dmbpy; and besides, new absorption peaks attributed to the stretching vibration of O–H centered at 3200 cm−1 and a vibrational absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1690 cm−1 appeared,29 implying the incorporation of CDs. The optical properties of CDs/UiO-67-dmbpy were further studied to confirm the efficient assembly of CDs and UiO-67-dmbpy. As shown in Fig. S4A (ESI†), in the nanocomposite, the intensities corresponding to the excitation and emission of CDs were both lower than those of free CDs, along with a slight shift of the peak position compared with CDs. The differences may result from electrostatic interaction between the two components. In the UV-vis absorption spectrum (Fig. S4B, ESI†), CDs/UiO-67-dmbpy exhibited characteristic absorption bands of the two components although the peak intensity of CDs ascribed to the π → π* electronic transition of the aromatic C
O at 1690 cm−1 appeared,29 implying the incorporation of CDs. The optical properties of CDs/UiO-67-dmbpy were further studied to confirm the efficient assembly of CDs and UiO-67-dmbpy. As shown in Fig. S4A (ESI†), in the nanocomposite, the intensities corresponding to the excitation and emission of CDs were both lower than those of free CDs, along with a slight shift of the peak position compared with CDs. The differences may result from electrostatic interaction between the two components. In the UV-vis absorption spectrum (Fig. S4B, ESI†), CDs/UiO-67-dmbpy exhibited characteristic absorption bands of the two components although the peak intensity of CDs ascribed to the π → π* electronic transition of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C was weakened. Taken together, these observations demonstrated the successful fabrication of the nanocomposite through self-assembly of CDs and UiO-67-dmbpy.
C was weakened. Taken together, these observations demonstrated the successful fabrication of the nanocomposite through self-assembly of CDs and UiO-67-dmbpy.
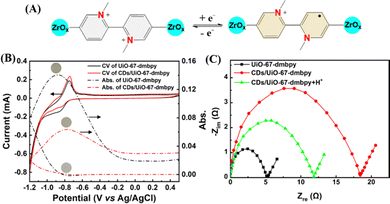
As reported previously by us,27 UiO-67-dmbpy is EC active because it contains the viologen-like linker (Fig. 1A). However, for CDs/UiO-67-dmbpy where CDs were anchored onto the surface of UiO-67-dmbpy, the EC activity was greatly hindered. As shown in Fig. 1B, the cathodic peak current of the nanocomposite became weaker than that of UiO-67-dmbpy; concurrently, its change in absorbance at 430 nm assigned to the monocationic radicals formed by the linker accepting electrons at the applied voltages also showed a significant decrease. This was also confirmed by a big difference in color change upon applying a negative voltage of −0.85 V (Fig. 1B, inset). It is seen that the CDs/UiO-67-dmbpy film did not show a noticeable color change from colorless to yellow compared with the UiO-67-dmbpy film. The electrochemical impedance spectroscopy (EIS) was also measured. As seen in Fig. 1C, after the assembly with CDs, the charge transfer resistance (Rct) of UiO-67-dmbpy underwent a marked increase, indicating an increased impedance. This was the result of strong binding of CDs around UiO-67-dmbpy to produce the steric hindrance leading to the inhibited electron transfer.13,27 Therefore, weakened EC activity of UiO-67-dmbpy was observed.
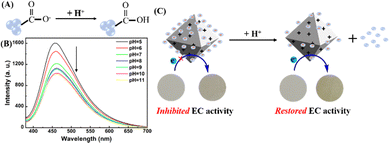
As demonstrated above, negatively-charged CDs possessed plenty of carboxyl groups, which would confer a pH-sensitive property onto CDs (Fig. 2A). As shown in Fig. 2B, as the pH value increased from 5 to 11, the emission intensity of CDs gradually decreased, and the emission peak had a slight redshift. The corresponding UV-vis absorption spectra also underwent an obvious pH-dependent change (Fig. S5, ESI†). Furthermore, the zeta potential of CDs dramatically transformed from a negative into a positive state with altering the pH value from basic to acidic (Fig. S6, ESI†), indicating the protonation/deprotonation of the surface groups of CDs. Clearly, pH-sensitive responsiveness of CDs led to substantial reversion of the surface charge under different pH values. Therefore, the injection of protons into the nanocomposite would attenuate the electrostatic attraction between CDs and UiO-67-dmbpy and eventually trigger the disassembly of CDs/UiO-67-dmbpy. Accordingly, the EC activity of UiO-67-dmbpy could be recovered. As illustrated in Fig. 2C, when a voltage of −0.85 V was applied to the nanocomposite film under an acidic medium, a clear color change from colorless to yellow was observed, which is very similar to the case of pure UiO-67-dmbpy. Moreover, the EIS measurement showed a sharp decrease of the impedance for the nanocomposite film (Fig. 1C). These results confirmed that the EC activity of UiO-67-dmbpy was switched on again after the disassembly of the nanocomposite facilitating electron transfer.
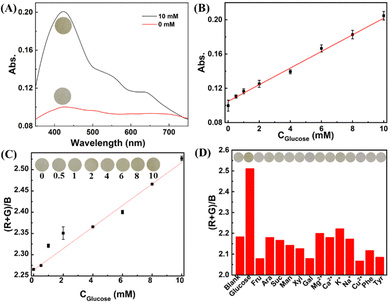
Based on the pH-sensitive EC activity of the nanocomposite, we studied its potential as a colorimetric probe to monitor enzymatic reactions associated with pH change. Specifically, we chose glucose as a model analyte because glucose oxidation catalyzed by GOx would release protons produced by the reaction product gluconic acid.30 Hence, glucose could ultimately lead to the recovery of the EC activity of UiO-67-dmbpy accompanied by visible color changes (Scheme 1B and Fig. 3A). Some experimental conditions including the mass ratio between CDs and UiO-67-dmbpy, applied negative voltage and time of applied voltage were first optimized (Fig. S7, ESI†). Then, we examined the sensing performances of CDs/UiO-67-dmbpy for glucose detection. As presented in Fig. S8 (ESI†), the EC response gradually increased with the continuous increase of glucose concentration in the solution containing GOx, followed by a vivid color change of the film from colorless to yellow. There is a good linear correlation between the absorbance at 430 nm and glucose concentration in the range of 0–10 mM (Fig. 3B). The limit of detection (LOD) was 0.08 mM. In addition, by using ImageJ software to convert each color of the photographs to digital RGB values, the (R + G)/B value was found to be correlated linearly with glucose concentration (Fig. 3C) with an LOD value of 0.17 mM. Assay selectivity was next evaluated. Fig. 3D manifests high selectivity of the nanocomposite toward glucose over potential interferents including metal ions, amino acids, and glucose analogs because only glucose could induce significant EC response and color change. Finally, the practicability of this EC sensing system was testified in pretreated human serum with the standard addition method by recovery experiments. As described in Table S1 (ESI†), satisfactory recoveries and relative standard deviations were acquired with both the spectrophotometric method and smartphone-based visual analysis, suggesting high accuracy and reproducibility in complicated biological samples. Besides, for increasing spiked concentrations of glucose, the yellowing process of the film became more obvious, which was visually discerned (Fig. S9, ESI†). Compared with existing colorimetric approaches for glucose (Table S2, ESI†), the presented assay is comparable or even more efficient in some aspects, for example, simple preparation and operation, as well as high reliability due to the split of the electrochemically driven source and intuitive optical readout. These results demonstrated that proton-mediated disassembly of CDs/UiO-67-dmbpy was successfully utilized for the colorimetric detection of glucose. Considering that many enzymes can catalytically produce protons, for example, acetylcholinesterase and acid phosphatase,14,31 this switching mechanism based on the EC MOF-based nanocomposite may be expanded to probe these enzymatic reactions efficiently.
In summary, we developed a simple colorimetric sensing strategy based on the disassembly of the EC MOF-based nanocomposite driven by analyte-activated specific reactions. Specifically, CDs electrostatically assembled onto the surface of UiO-67-dmbpy acted as a nanoswitch to subtly regulate the EC activity of UiO-67-dmbpy through proton-mediated dissociation caused by big charge reversion. This strategy offered a favorable paradigm for developing highly efficient colorimetric protocols taking advantage of the fabrication simplicity of stimuli-responsive nanocomposites and tunable EC properties of MOFs.
P. H. and F.-Y. Wu designed the experiments. M. Z. and S. H. performed research and analysed data. All authors wrote and reviewed the manuscript.
We thank the support from the National Natural Science Foundation of China (22164013), the Graduate Innovation Special Fund Project of Jiangxi Province (YC2023-S034), and the Open Sharing Fund for the Large Instruments and Equipments of Nanchang University (Grant No. 2024CX15).
Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Nie, T. Brown and Y. Zhang, Chem. Commun., 2016, 52, 7454 RSC.
- Z. Farka, T. Jurik, D. Kovar, L. Trnkova and P. Skladal, Chem. Rev., 2017, 117, 9973 CrossRef CAS.
- J. Liu, M. Li, Q. Man, L. Huang, J. Wang, M. Gao and X. Zhang, Anal. Chem., 2023, 95, 8011 CrossRef CAS PubMed.
- Y. Chen, Y. Xianyu and X. Jiang, Acc. Chem. Res., 2017, 50, 310 CrossRef CAS PubMed.
- R. Xu, S. Zhang, P. Wang, R. Zhang, P. Lin, Y. Wang, L. Gao, H. Wei, X. Zhang, D. Ling, X. Yan and K. Fan, Coord. Chem. Rev., 2024, 501, 215519 CrossRef CAS.
- L. Gao, H. Wei, S. Dong and X. Yan, Adv. Mater., 2024, 36, 2305249 CrossRef CAS.
- M. A. F. Nejad, S. Ranjbar, C. Parolo, E. P. Nguyen, R. ÁLvarez-Diduk, M. R. Hormozi-Nezhad and A. Merkoçi, Mater. Today, 2021, 50, 476 CrossRef.
- P. M. Aller and F. J. del Campo, Curr. Opin. Electrochem., 2019, 15, 66 CrossRef.
- R. Yuan, H. Ma, H. Hong, L. Xiao, B. Li and K. Wang, Biosens. Bioelectron., 2024, 246, 115900 CrossRef CAS.
- Z. Yu, G. Cai, X. Liu and D. Tang, Anal. Chem., 2021, 93, 2916 CrossRef CAS PubMed.
- D. Capoferri, R. Alvarez-Diduk, M. Del Carlo, D. Compagnone and A. Merkoçi, Anal. Chem., 2018, 90, 5850 CrossRef CAS PubMed.
- S. Ranjbar, M. A. F. Nejad, C. Parolo, S. Shahrokhian and A. Merkoçi, Anal. Chem., 2019, 91, 14960 CrossRef CAS PubMed.
- N. Hao, Y. Zuo, Z. Dai, M. Xiong, J. Wei, J. Qian and K. Wang, Anal. Chem., 2021, 93, 14053 CrossRef CAS PubMed.
- Q. Yang, Q. Hao, J. Lei and H. Ju, Anal. Chem., 2018, 90, 3703 CrossRef CAS PubMed.
- Z. Yu, G. Cai, R. Ren and D. Tang, Analyst, 2018, 143, 2992 RSC.
- X. Sun, H. Zhang, S. Hao, J. Zhai and S. Dong, ACS Sens., 2019, 4, 2631 CrossRef CAS.
- M. A. Pellitero, A. Guimera, M. Kitsara, R. Villa, C. Rubio, B. Lakard, M.-L. Doche, J.-Y. Hihn and F. J. del Campo, Chem. Sci., 2017, 8, 1995 RSC.
- C.-a Tao, Y. Li and J. Wang, Coord. Chem. Rev., 2023, 475, 214891 CrossRef CAS.
- C. R. Wade, M. Li and M. Dinca, Angew. Chem. Int. Ed., 2013, 52, 13377 CrossRef CAS PubMed.
- A. Kumar, J. Li, A. K. Inge and S. Ott, ACS Nano, 2023, 17, 21595 CrossRef.
- T. Fu, Y.-L. Wei, C. Zhang, L.-K. Li, X.-F. Liu, H.-Y. Li and S.-Q. Zang, Chem. Commun., 2020, 56, 13093 RSC.
- G. Cai, P. Cui, W. Shi, S. Morris, S. N. Lou, J. Chen, J.-H. Ciou, V. K. Paidi, K.-S. Lee, S. Li and S. P. Lee, Adv. Sci., 2020, 7, 1903109 CrossRef CAS.
- J. Liu, X. Y. Daphne Ma, Z. Wang, L. Xu, T. Xu, C. He, F. Wang and X. Lu, ACS Appl. Mater. Interfaces, 2020, 12, 7442 CrossRef CAS.
- S. Feng, J. Wang, Z. Tong and H.-Y. Qu, Chem. Eng. J., 2022, 442, 136158 CrossRef CAS.
- J. Feng, X. Wang, Y. Luo, J. Wang, Z. Wang, C. Wei and G. Cai, ACS Appl. Mater. Interfaces, 2024, 16, 1170 CrossRef CAS.
- L. Shupletsov, S. Topal, A. Schieck, S. Helten, R. Gruenker, A. Deka, A. De, M. Werheid, V. Bon, I. Weidinger, A. Pöppl, I. Senkovska and S. Kaskel, J. Am. Chem. Soc., 2024, 146, 25477 CrossRef CAS.
- S. Huang, Y. Liu, P. Huang, F.-Y. Wu and L. Mao, Chem. – Eur. J., 2023, 29, e202300263 CrossRef CAS PubMed.
- Q.-L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468 RSC.
- X. Jia, J. Li and E. Wang, Nanoscale, 2012, 4, 5572 RSC.
- Y. Sun, T. Shu, J. Ma, Q. Dai, P. Peng, Z. Zhou, X. Zhou, L. Su and X. Zhang, Anal. Chem., 2022, 94, 3408 CrossRef CAS.
- Y. Wang, L. Lu, H. Peng, J. Xu, F. Wang, R. Qi, Z. Xu and W. Zhang, Chem. Commun., 2016, 52, 9247 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc05997b |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |