Utilising triplet–triplet annihilation upconversion for overall photocatalytic water splitting†
E.
Madbak
 a,
D. J.
Osborn
a,
D. J.
Osborn
 a,
T.
Small
a,
T.
Ishwara
a,
T.
Small
a,
T.
Ishwara
 b,
T. W.
Schmidt
b,
T. W.
Schmidt
 b,
K.
Domen
b,
K.
Domen
 c and
G. F.
Metha
c and
G. F.
Metha
 *a
*a
aDepartment of Chemistry, University of Adelaide, Adelaide, SA 5005, Australia. E-mail: greg.metha@adelaide.edu.au
bSchool of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia
cOffice of University Professors, The University of Tokyo, Japan
First published on 3rd December 2024
Abstract
An organic triplet–triplet annihilation upconversion system consting of Ir(coumarin-6)2(acac) and TIPS-naphthalene is used to drive overall water-splitting using the UV-active photocatalyst Rh/Cr2O3/CoOOH/Al:SrTiO3. Light from 455 and 470 nm LEDs was upconverted to 355–410 nm at 6.1% efficiency. Hydrogen production doubled at 455 nm and tripled at 470 nm, compared to controls.
Green hydrogen will be instrumental to achieving a more environmentally friendly future,1 as it is capable of acting as a fuel source without releasing harmful CO2.2 However, the costly production methods of green hydrogen via electrolysis cannot compete with fossil fuel-based production methods. Photocatalytic water splitting as a means of green hydrogen production has the potential to produce hydrogen cheaply, meeting industry requirements.3 For photocatalysts to become viable in a commercial/industrial setting using solar energy, the solar-to-hydrogen (STH) efficiency of photocatalytic systems needs to increase.4 In order to function the photocatalyst needs enough energy to excite electrons from the valence band to the conduction band. This can only be done by providing wavelengths of light with high enough energy to bridge the band gap of the material.5,6 One way to increase the STH is by increasing the number of solar photons with high enough energy available to the photocatalyst. This can be achieved with triplet–triplet annihilation upconversion (TTA-UC), as it is a process by which two photons of lower energy are used to produce a single photon of higher energy. In doing so, such a system would enable wavelengths that are otherwise not accessible for photocatalysis (i.e. too low in energy), to become usable, thus utilising more of the solar spectrum and increasing the STH of the photocatalyst.7,8
Photocatalytic water splitting from upconverted near-infrared light using rare earth ions has been previously reported. Wang et al., used Gd2MoO6:0.04Er3+/0.10Yb3+ to upconvert light from a filtered Xe lamp to generate H2 using a sacrificial reagent (TEOA).9,10 Acosta-Mora et al. upconverted 980 nm laser light into UV-blue emission from crystals of K2YbF5:Tm3+ fluoride to split water using the same photocatalyst used here (vide infra).11
Herein, we demonstrate overall water splitting, into hydrogen and oxygen without sacrificial agents, upconverting visible photons into UV using a transition metal–organic-based upconversion system, which has never been demonstrated to the best of our knowledge. The combined upconversion-photocatalysis system uses a well-known and studied photocatalyst, Rh/Cr2O3/CoOOH/Al:SrTiO3 (hereafter Al:STO),12 coupled with a TTA-UC system of TIPS-nph and Ir(C6)2(acac) that has been previously reported.13 This combination of photocatalyst and upconversion system were chosen due to the overlap between the produced upconverted photons and the bandgap of the photocatalyst. The Al:STO photocatalyst has a bandgap of 3.2 eV, or 387 nm, and has a very high internal quantum efficiency. The selected upconversion system has been shown to absorb light at visible wavelengths and emit across the UV in the range of 350–410 nm, which overlaps with the bandgap of the Al:STO photocatalyst. The downside of this upconversion system is its short lifetime. This work demonstrates that an upconversion system can be simply combined with photocatalytic water splitting to increase overall photon to chemical conversion by opening up the visible part of the solar spectrum. Furthermore, this can be achieved at photon fluxes corresponding to solar intensities. In combination, this could increase the STH efficiency towards 10%, which is needed for commercial viability and industrial implementation of photocatalytic hydrogen production.
The emitter and sensitiser, TIPS-naphthalene and Ir(C6)2(acac), system (hereafter TIPS-Ir) was synthesised following the procedure used by Harada et al. (further details in ESI†).13 Upon synthesis of each component, the solution using a combination of 10 mM/100 μM emitter/sensitiser was tested in a custom-built spectrometer using a 455 nm LED (see Methods for more information and Fig. S1 in ESI†). As shown in Fig. 1, although some of the emitted LED radiation is detected at 450 nm, it is clear that TTA-upconversion is occurring between 350–420 nm.
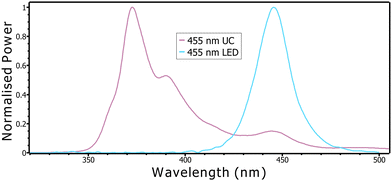 | ||
| Fig. 1 Normalised emission spectra of the 455 nm LED and the upconversion system, showing output of the upconverted photons between 350–420 nm. | ||
Having established upconversion, the system was optimised using different concentrations and molar ratios of sensitiser and emitter in order to maximise the total upconverted power output. This procedure involved changing the concentrations of the materials and combining them in different molar ratios. The output power was re-measured with the spectrometer and the measured power outputs are shown below in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sensitiser molar ratios
sensitiser molar ratios
| [Stock solution] | Emitter![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sensitiser molar ratio sensitiser molar ratio |
|||
|---|---|---|---|---|
| Emitter (acceptor) (mM) | Sensitiser (donor) (μM) | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Maximum power output (W m−2) | ||||
| 5 | 50 | 0.466 | 0.5359 | 0.265 |
| 10 | 100 | 2.589 | 2.230 | 1.388 |
| 20 | 200 | 4.460 | 4.723 | 5.810 |
The optimisation process showed that a molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (emitter
1 (emitter![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sensitiser) of TIPS-Ir using concentrations of 20 mM and 200 μM yielded the highest output power, and a general trend could be observed that higher output power was achieved at higher concentrations. Additionally, the half-life of the material was determined to be ∼90 s (see Fig. S2 in ESI†) with a useful lifetime of 10 minutes that could be used for the upconversion-photocatalysis experiments, before completely degrading the UC material.
sensitiser) of TIPS-Ir using concentrations of 20 mM and 200 μM yielded the highest output power, and a general trend could be observed that higher output power was achieved at higher concentrations. Additionally, the half-life of the material was determined to be ∼90 s (see Fig. S2 in ESI†) with a useful lifetime of 10 minutes that could be used for the upconversion-photocatalysis experiments, before completely degrading the UC material.
Upconversion quantum yield (UCQY) was measured at the University of New South Wales (UNSW) using a 450 nm CW laser with a 425 nm shortpass filter. A higher upconverted emission was observed for upconversion samples prepared with anhydrous ZerO2 THF (Sigma-Aldrich) compared to samples prepared with deaerated THF. Details about the UCQY breadboard setup have previosuly been reported.14 In order to correct for the shortpass filter spectrum loss, a neat emitter (TIPS-Nph) solution spectrum was obtained with a 405 nm CW laser and 425 nm long-pass filter to block the excitation light. The UCQY measurement takes into account 4π steradian collection and the shortpass filter to give an efficiency of 6.1% (see Fig. S3 and S4 in ESI†). The upconversion spectrum shown in Fig. S3 (ESI†) shows self-absorption which is not considered here for wavelengths below 425 nm, and so this would further increase the UCQY.
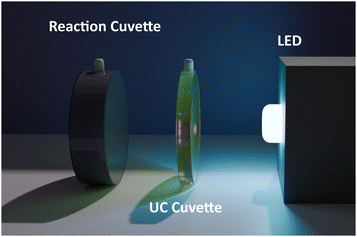
Hydrogen production was tested using the photocatalyst Al:STO, which was synthesised at the University of Tokyo.12 The photocatalyst was suspended in water using a bespoke apparatus shown in Fig. 2. The apparatus consists of two cuvettes adjacent to each other; a 1 mm pathlength cuvette containing the UC solution and a 10 mm pathlength cuvette containing the photocatalyst solution. Gas produced by the photocatalyst was extracted from the headspace of the cuvette and analysed using a μGC. Further information is provided in the Methods section and the ESI† (Fig. S7 and S8).
Upconversion-photocatalytic runs were completed using 365, 455, and 470 nm LEDs. The 365 nm LED was used to determine a standard baseline for the amount of hydrogen and oxygen produced in the cuvette reactor by direct irradiation through the 1 mm UC cuvette containing solvent (deaerated THF). At a power of 150 mW cm−2 (14.1 × 1017 photons) impinging on the side of the 1 mm cuvette, 13.297 μmol of H2 was produced after 10 min, equating to an efficiency of 1.88%. Note that this is much lower than the reported apparent quantum yield (AQY) of ∼50% for this photocatalysis due to several reasons. Firstly, this is a very high photon flux, equivalent to ∼30 suns of UV, and it is known that particulate photocatalysts saturate or become less efficient under high illumination conditions.15,16 Secondly, the side illumination is not the optimum way to irradiate the photocatalyst suspension. Thirdly, the cuvette is sealed so any build up of hydrogen and oxygen pressure will slow down the rate of further gas production.
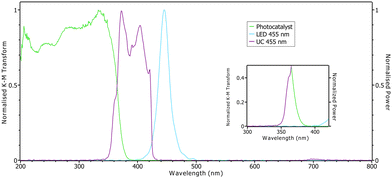
Fig. 3 shows the emission of the 455 nm LED and the TIPS-Ir upconversion system superimposed on the absorption curve of the Al:STO photocatalyst. It is clear that only ∼14% of the upconverted photons overlap with the photocatalyst, between between 350–390 nm. Furthermore, the upconversion efficiency is ∼6%, and since the upconversion process is isotropic the double cuvette setup has a maximum photon collection efficiency of 50%. Therefore, it is expected that the amount of gas produced by the photocatalysis will be quite low. This gives a theoretical best efficency of 0.42% relative to the power of the illuminating LED and so quite low amounts of gas are expected.
The power from the 455 and 470 nm LEDs is 24.7 and 16.5 mW cm−2, respectively, an order of magnitude lower than the 365 nm LED. For each wavelength, photocatalysis runs were repeated three times over 10 minutes. In addition, three control runs were performed with only solvent in the UC cuvette (i.e. no TIPS-Ir). The amount of hydrogen produced from these runs is summarised in Table 2. Given the relatively small amounts of hydrogen produced in these runs, it was not possible to quantity the amount of O2 because of background signal from ambient air in the μGC.
Although the amount of H2 produced is relatively low compared to the 365 nm reference, the data clearly shows that the hydrogen generated with the UC system present is higher than the control runs without TIPS-Ir. Furthermore, the H2 production runs using the 455 nm LED are also higher than that using the 470 nm LED. Indeed, it is surprising that the control runs produce any hydrogen since the Al:STO photocatalyst does not absorb at wavelengths longer than 400 nm.12 However, careful inspection of the 455 and 470 nm LED emission shows a long tail that extends towards 400 nm (see inset of Fig. 3 and Fig. S8 in ESI†) which has slight overlap with the absorption profile of Al:STO. The overlap is less for the 470 nm LED and so the background level of the control is less than for the 455 nm LED.
After correcting for the background signal of the control runs, the overall efficiencies for the 455 and 470 nm LEDs are 0.00143% and 0.00157% (Table 2), respectively, compared to the theoretical maximum of 0.42% calculated above. There are an additional two contributing factors that would decrease the efficiency. Firstly, the short lifetime of the TIPS-Ir system, which has almost completely degraded after the 10 minutes of the photocatalysis runs. Assuming the maximum UC output is consistent with no decay over 10 minutes, then the actual observed UC output would be ∼42% of that. Secondly, it is unknown what the efficiency of the photocatalyst system is running under these low light intensities, therefore the observed low efficiencies would appear reasonable.
In summary, it is clear that the upconversion photocatalyst runs are producing more hydrogen than the controls and demonstrates that non-sacrificial photocatalytic hydrogen production is possible through the use of an upconversion system. The number of available upconversion systems that are capable of emitting upconverted UV photons are limited,17 and this in turn restricts the visible wavelengths that can be utilised for upconversion for UV active photocatalysts such as Al:STO. However, as visible light active photocatalysts are developed, there will be stronger overlap with known triplet–triplet annihilation upconversion systems which would further increase the STH of the photocatalyst operating under solar irradiation. This would also require technical developments to better couple the upconverted light with the photocatalyst.
Methods: the synthesis of TIPS-naphthalene (TIPS-Nph) and Ir(C6)2(acac) followed the procedure outline by Harada et al.;13 full details are described in the ESI.†
UV-Vis absorption spectra for the upconversion system compounds were obtained using a Cary UV-Vis compact spectrophotometer, with a 1 cm path length quartz cuvette (Starna). Fluorescence spectra were obtained using the PS Shimadzu RF-6000 fluorescence spectrophotometer and a 1 cm path length quartz fluorescence cuvette (Starna) was used. The concentrations used for both measurements were 0.0037 mM and 1 μM for TIPs-naphthalene and Ir(C6)2(acac), respectively.
The upconversion material was prepared by mixing stock solutions of the upconversion materials in deaerated THF at different molar ratios of emitter:sensitiser. For the UC optimisation measurements at the University of Adelaide, a 4-sided quartz cuvette (10 mm × 1 mm) containing the upconversion material was illuminated by a 455 nm (Thorlabs, M455L4) within a light-proof box, and the emission collected with a spectroradiometer (Apogee Instruments CXR-SR-25) at 90° via a fibre optic.
The Al:STO photocatalyst was provided by the University of Tokyo and has been reported before.12 22 mg of the photocatalyst powder was sonicated in 2.4 mL of water and placed into a round 10 mm path length quartz reaction cuvette of 19 mm diameter (FireflySci Type 32S). The suspension of the photocatalyst was stirred constantly with a magnetic stirring bar while illumination took place, to keep the photocatalyst material uniformly distributed. The reaction cuvette is sealed with a septum that allows an airtight GC syringe to take gas samples after each run. Prior to each experiment, the photocatalyst suspension was purged for 20 minutes with a steady flow of argon gas and sealed. The UC system was loaded by syringe into a round 1 mm path length quartz cuvette (FireflySci Type 32S) and purged with Ar. The cuvette containing the UC was placed adjacent to the reaction cuvette containing the suspended photocatalyst and side illuminated by either a 365 nm (UVET, NSC4), 455 nm LED or 470 nm LED (Thorlabs, M455L4/M470L5) for 10 minutes after which time the upconversion material is no longer active. A 400 μL sample of gas was extracted from the headspace of the cuvette using a 500 μL gas syringe and analysed using a GC-MS (Agilent 7890B GC-MS using Ar carrier gas).
We acknowledge the assistance of Prof Nobuhiro Yanai (formerly Kyushu University, now University of Tokyo) for providing additional instructions on the synthesis of the TIPS-naphthalene and Ir(coumarin 6)2(acac).
Data availability
An ESI† file is available.Conflicts of interest
There are no conflicts to declare by the authors.References
- Q. Hassan, S. Algburi, A. Z. Sameen, H. M. Salman and M. Jaszczur, Int. J. Hydrogen Energy, 2024, 50, 310–333 CrossRef CAS
.
- D. Voiry, H. S. Shin, K. P. Loh and M. Chhowalla, Nat. Rev., 2018, 2, 0105 CAS
.
- Z. Kuspanov, B. Bakbolat, A. Baimenov, A. Issadykov, M. Yeleuov and C. Daulbayev, Sci. Total Environ., 2023, 885, 163914 CrossRef CAS
.
- Q. Wang and K. Domen, Chem. Rev., 2020, 120, 919–985 CrossRef CAS PubMed
.
- J. Chen, T. Tang, W. Feng, X. Liu, Z. Yin, X. Zhang, J. Chen and S. Cao, ACS Appl. Nanomater., 2022, 5, 1296–1307 CrossRef CAS
.
- P. Makuła, M. Pacia and W. Macyk, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef PubMed
.
- V. Gray, K. Moth-Poulsen, B. Albinsson and M. Abrahamsson, Coord. Chem. Rev., 2018, 362, 54–71 CrossRef CAS
.
- R. Enomoto, M. Hoshi, H. Oyama, H. Agata, S. Kurokawa, H. Kuma, H. Uekusa and Y. Murakami, Mater. Horiz., 2021, 8, 3449–3456 RSC
.
- C. Wang, P. Du, L. Luo, Y. Tian and W. Li, Ind. Eng. Chem. Res., 2021, 60, 16245–16257 CrossRef CAS
.
- J. Schneider and D. W. Bahnemann, J. Phys. Chem. Lett., 2013, 4, 3479–3483 CrossRef CAS
.
- P. Acosta-Mora, K. Domen, T. Hisatomi, H. Lyu, J. Méndez-Ramos, J. C. Ruiz-Morales and N. M. Khaidukov, Chem. Commun., 2018, 54, 1905–1908 RSC
.
- T. Takata, J. Jiang, Y. Sakata, M. Nakabayashi, N. Shibata, V. Nandal, K. Seki, T. Hisatomi and K. Domen, Nature, 2020, 581, 411–414 CrossRef CAS PubMed
.
-
N. Harada, Y. Sasaki, M. Hosoyamada, N. Kimizuka and N. Yanai, Angew. Chem., Int. Ed., 2021, 60, 142–147 Search PubMed
.
- T. Ishwara, J. Feng, D. M. de Clercq, R. Geng, J. Alves, D. R. McCamey, M. P. Nielsen and T. W. Schmidt, ACS Energy Lett., 2023, 8, 4078–4084 CrossRef CAS
.
- T. Hisatomi, K. Maeda, K. Takanabe, J. Kubota and K. Domen, J. Phys. Chem. C, 2009, 113, 21458–21466 CrossRef CAS
.
- J. Z. Bloh, Front. Chem., 2019, 7, 128 CrossRef CAS
.
- W. Ahmad, J. Wang, H. Li, Q. Ouyang, W. Wu and Q. Chen, Coord. Chem. Rev., 2021, 439, 213944 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc03961k |
| This journal is © The Royal Society of Chemistry 2025 |