Open Access Article
Open Access ArticleFreed from iron: easy release of a stable ketene from the reaction of CO with di-iron bis-μ2-alkylidenes†
Sandip
Munshi‡
 a,
Raphaël
Ravel-Massol‡
a,
Raphaël
Ravel-Massol‡
 a,
Ricardo
Garcia-Serres
a,
Ricardo
Garcia-Serres
 b,
Nicolas
Suaud
b,
Nicolas
Suaud
 c,
Rémi
Maurice
c,
Rémi
Maurice
 d,
Nathalie
Saffon-Merceron
d,
Nathalie
Saffon-Merceron
 e,
Nicolas
Mézailles
e,
Nicolas
Mézailles
 *a and
Marie
Fustier-Boutignon
*a and
Marie
Fustier-Boutignon
 *a
*a
aUniversité de Toulouse, CNRS, LHFA - UMR 5069, 118 Route de Narbonne, 31062 Toulouse, France. E-mail: marie.fustier-boutignon@univ-tlse3.fr
bUniversité Grenoble Alpes, CNRS, CEA, IRIG, Laboratoire de Chimie et Biologie des Métaux, 17 rue des Martyrs, 38000 Grenoble, France
cUniversité de Toulouse, CNRS, LCPQ, 118 Route de Narbonne, 31062 Toulouse, France
dUniv Rennes, CNRS, ISCR (Institut des Sciences Chimiques de Rennes) – UMR 6226, F-35000 Rennes, France
eUniversité de Toulouse, CNRS, Institut de Chimie de Toulouse ICT - UAR2599, 118 Route de Narbonne, 31062 Toulouse, France
First published on 7th May 2025
Abstract
Iron bis-μ2-alkylidene complexes were shown to perform CO/alkylidene coupling and liberate a stable ketene derivative. The easy release of the ketene formed from CO incorporation is usually the prerogative of terminal iron carbene complexes, while dimetallic, bridging carbenes tend to retain bridging acyl ligands after 1,1 migratory insertion of CO. Observations at −40 °C showed that an acyl intermediate could evolve into a ketene and form a stable μ2-alkylidene di-iron hexacarbonyl complex. Ketene release was shown to depend on the redox state of the iron byproducts. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond formation is reversible, with instant hydration–decarboxylation upon water addition at room temperature.
C bond formation is reversible, with instant hydration–decarboxylation upon water addition at room temperature.
The interaction of carbon monoxide with organometallic complexes of early transition metals, and particularly iron, has been a topic of abundant research over the years, from the understanding of the reaction mechanisms in heterogeneous Fischer–Tropsch synthesis1,2 to the development of sustainable hydroformylation reaction catalysts.3,4 These somewhat distant catalytic processes are related to each other by the co-existence of a CO ligand with alkyl- and/or alkylidene-type ligands at the surface or in the coordination sphere of iron. In homogeneous conditions, terminal and bridging alkylidenes are differentiated by their reactivity, especially toward CO. Terminal carbenes/alkylidenes are classically known to lead to the formation of ketenes by direct carbonylation via 1,1 insertion at an sp2 carbon (Scheme 1A, eqn (1)).5 This reactivity is often used to illustrate the carbenic character of ligands or intermediates6–9 (Scheme 1A, eqn (2)). Although catalytic versions of in situ generated carbene and CO coupling have been developed for the synthesis of ketenes,10 iron-based catalysts are presently unknown. Conversely, bridging iron nucleophilic alkylidenes follow different pathways.11 They primarily react as (double) alkyl ligands, undergoing CO migratory insertion. The resulting acyl ligands are then transformed into a variety of compounds by migratory insertion of alkenes and alkynes, and oxidation. Upon solvolysis of the acyl with alcohols, esters are obtained (Scheme 1B, eqn (3)12 and (4)13), and ketene-type intermediates have been proposed. This reactivity of bridging alkylidenes with CO was mostly described with dimetallic ironI compounds, generating iron0 byproducts. In the absence of appropriate ligand(s) on the iron, the reverse reaction is observed, and ketene derivatives would be decarbonylated by iron0 complexes to generate dimetallic ironI alkylidene (Scheme 1B, eqn (5)).14 Further stabilization by conjugation of the ketene would hamper this process, as exemplified by the abundance of iron0 vinylketene complexes in the literature.15 The reluctance of bridging iron alkylidenes to liberate ketenes contrasts with the ability of cobalt analogues to catalyze the coupling of CO and diazo compounds, via bridging alkylidene intermediates.6,10,16
 | ||
| Scheme 1 Usual reactivity of CO to (A) terminal vs. (B) bridging iron alkylidene, and (C) bridging iron bis-alkylidene (this work). | ||
Our group has been using P-stabilized geminal dianions to build terminal and bridging alkylidene complexes of a variety of transition metals.17 Most recently, we explored the reactivity of sulfur and carbon ligated di-iron bis-alkylidene complexes,18 with the aim of developing synthetic nitrogenase mimics.19,20 This platform effectively provided S- and C-based coordination sites to the iron, while introducing charge at the carbon centre. Its structural flexibility allowed for the stabilization of redox-stable poly-iron species, where the (S∼C∼S)2− ligand adopted a bridging alkylidene configuration. In this work, we explore the ability of carbonyl to promote a terminal iron alkylidene reactivity in di-iron bis-alkylidene complexes, i.e., fast and quantitative CO/alkylidene coupling resulting in the formation and release of a ketene, and the role of iron oxidation states in promoting ketene decoordination in non-coordinating media.
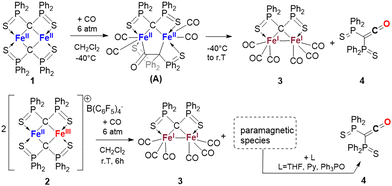
Complexes 1 and 2 were synthesized following the reported procedure.20 Subjecting a CH2Cl2 solution of complex 1 to 6 bar CO resulted instantaneously in a colour change from green to dark red (Scheme 1C). 1H and 31P{1H} NMR monitoring proved complete conversion in 6 h at this pressure (3 days at 1 atm) to two diamagnetic compounds in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, as illustrated by the appearance of two singlets in 31P{1H} NMR, at 72.1 and 38.0 ppm (37.4 ppm in THF). The signal at 72.1 ppm could be attributed to (1κS1,2κS5-μ2)-bis-(diphenylthiophosphinoyl)-methanediide di-iron hexacarbonyl complex 3, as determined by X-Ray diffraction on crystals grown from the reaction medium. Complex 3 shows C1–Fe bond lengths in the range of C-bridged Fe alkylidene dimers (Fig. 1). The Fe–Fe bond length (2.626(2) Å) is very close to those in (CF3)2C alkylidene,14 Ph2C
1 ratio, as illustrated by the appearance of two singlets in 31P{1H} NMR, at 72.1 and 38.0 ppm (37.4 ppm in THF). The signal at 72.1 ppm could be attributed to (1κS1,2κS5-μ2)-bis-(diphenylthiophosphinoyl)-methanediide di-iron hexacarbonyl complex 3, as determined by X-Ray diffraction on crystals grown from the reaction medium. Complex 3 shows C1–Fe bond lengths in the range of C-bridged Fe alkylidene dimers (Fig. 1). The Fe–Fe bond length (2.626(2) Å) is very close to those in (CF3)2C alkylidene,14 Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C allenic alkylidene,21 carbene substituted alkylidene,22 or conjugated alkylidenes.23 In 13C{1H} NMR, the alkylidene signal is seen as the expected high field triplet at 39.1 ppm (Jc–P = 21.7 Hz), attesting to the high residual negative charge on C. Five infra-red vibrations of CO ligands were seen at 2040 cm−1, 1992 cm−1, 1965 cm−1, 1954 cm−1 and 1937 cm−1. Complexes 1 and 3 obviously display quite different electronic structures, notably due to the change in the oxidation states of the Fe centres. To describe these in a more detailed way, we have performed multiconfigurational electronic structure theory calculations (see ESI† for details). In complex 1, we have confirmed the high-spin characters of the FeII centres, the antiferromagnetic coupling between these while locked in their ground orbital configurations, and the potential importance of near-orbital degeneracies on the magnetism of this compound. Complex 3 is a closed-shell system, best described as a σ-bonded di-iron complex, with an effective Fe–Fe bond order24 of 0.77, which is indicative of a single bond. This depiction is in line with the Mössbauer spectra (see ESI†), which can be simulated with a unique set of parameters. The low isomer shift of 0.07 mm s−1 is consistent with an FeI coordinated to strong π-acceptor ligands (the three CO molecules),25 while the magnetic Mössbauer spectra elicit simulation with an S = 0 Hamiltonian, consistent with the covalent coupling described earlier.
C allenic alkylidene,21 carbene substituted alkylidene,22 or conjugated alkylidenes.23 In 13C{1H} NMR, the alkylidene signal is seen as the expected high field triplet at 39.1 ppm (Jc–P = 21.7 Hz), attesting to the high residual negative charge on C. Five infra-red vibrations of CO ligands were seen at 2040 cm−1, 1992 cm−1, 1965 cm−1, 1954 cm−1 and 1937 cm−1. Complexes 1 and 3 obviously display quite different electronic structures, notably due to the change in the oxidation states of the Fe centres. To describe these in a more detailed way, we have performed multiconfigurational electronic structure theory calculations (see ESI† for details). In complex 1, we have confirmed the high-spin characters of the FeII centres, the antiferromagnetic coupling between these while locked in their ground orbital configurations, and the potential importance of near-orbital degeneracies on the magnetism of this compound. Complex 3 is a closed-shell system, best described as a σ-bonded di-iron complex, with an effective Fe–Fe bond order24 of 0.77, which is indicative of a single bond. This depiction is in line with the Mössbauer spectra (see ESI†), which can be simulated with a unique set of parameters. The low isomer shift of 0.07 mm s−1 is consistent with an FeI coordinated to strong π-acceptor ligands (the three CO molecules),25 while the magnetic Mössbauer spectra elicit simulation with an S = 0 Hamiltonian, consistent with the covalent coupling described earlier.
Along with red crystals of complex 3, colourless needles formed spontaneously in the crude mixture, corresponding to the 38.0 ppm signal as shown by 31P{1H} NMR of the redissolved crystals. Compound 4 is an unusual example of a room temperature stable ketene. According to X-Ray diffraction (Fig. 1), its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond distances of 1.280(6) Å and 1.184(6) Å are close to those observed in typical examples of stable ketenes, such as Mes2CCO and (3,5-Br2-2,4,6-Me3Ph)2CCO (1.29(1) Å and 1.25(3) Å for C
O bond distances of 1.280(6) Å and 1.184(6) Å are close to those observed in typical examples of stable ketenes, such as Mes2CCO and (3,5-Br2-2,4,6-Me3Ph)2CCO (1.29(1) Å and 1.25(3) Å for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond lengths, and 1.18(1) Å and 1.17(3) Å for C
C bond lengths, and 1.18(1) Å and 1.17(3) Å for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths, respectively),26 and those in the only rare examples of Ph2P(S)-substituted ketenes 5-E27 (Scheme 2 eqn (7), dC
O bond lengths, respectively),26 and those in the only rare examples of Ph2P(S)-substituted ketenes 5-E27 (Scheme 2 eqn (7), dC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C being 1.304(2) Å, E = TMS, 1.314(5) Å, E = Ph3C, and dC
C being 1.304(2) Å, E = TMS, 1.314(5) Å, E = Ph3C, and dC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O being 1.162(2) Å, R = TMS, and 1.166(5) Å, R = Ph3C). The 13C{1H} NMR chemical shifts for CCO and PCP (181.9 ppm, resp. 38.1 ppm, t, 1JPC = 106 Hz) in 4 are very close to those of 5-CPh3 (182.0 ppm, resp. 45.3 ppm). A sharp and intense absorption at 2112 cm−1 is seen in IR spectroscopy for the stretching of the CO bond. We evaluated its reactivity as an electrophilic species. In stark contrast with what was described for 5-CPh3, which needed prolonged heating in presence of water (3.5 months at 60 °C) to be converted to the corresponding carboxylic acid 6 (Scheme 2, eqn (7)), ketene 4 proved to undergo instantaneous decarboxylation upon the addition of traces of water, to generate the neutral, doubly protonated bis (thiophosphinoyl)-methane ligand precursor (Scheme 2, eqn (8)). CO2 evolution was confirmed by gas chromatography (see ESI†). If the effect of steric hindrance on the kinetics of hydration/decarboxylation of α-ketoketene has been documented (Scheme 2, eqn (9)),28 the electronic effects of a second strongly electron withdrawing “Ph2PS” moiety should be considered as well to explain the impressive difference in reactivity between 5-E and 4. The lability of the newly formed C
O being 1.162(2) Å, R = TMS, and 1.166(5) Å, R = Ph3C). The 13C{1H} NMR chemical shifts for CCO and PCP (181.9 ppm, resp. 38.1 ppm, t, 1JPC = 106 Hz) in 4 are very close to those of 5-CPh3 (182.0 ppm, resp. 45.3 ppm). A sharp and intense absorption at 2112 cm−1 is seen in IR spectroscopy for the stretching of the CO bond. We evaluated its reactivity as an electrophilic species. In stark contrast with what was described for 5-CPh3, which needed prolonged heating in presence of water (3.5 months at 60 °C) to be converted to the corresponding carboxylic acid 6 (Scheme 2, eqn (7)), ketene 4 proved to undergo instantaneous decarboxylation upon the addition of traces of water, to generate the neutral, doubly protonated bis (thiophosphinoyl)-methane ligand precursor (Scheme 2, eqn (8)). CO2 evolution was confirmed by gas chromatography (see ESI†). If the effect of steric hindrance on the kinetics of hydration/decarboxylation of α-ketoketene has been documented (Scheme 2, eqn (9)),28 the electronic effects of a second strongly electron withdrawing “Ph2PS” moiety should be considered as well to explain the impressive difference in reactivity between 5-E and 4. The lability of the newly formed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is promising for a post-functionalisation of the carbon centre originating from CO. The easy formation of ketene 4 from the bis-μ2-alkylidene complex 2 also contrasts with the known reactivity of the free, dilithiated (S∼C∼S)2− ligand toward CO, which was shown to undergo CO insertion into one CP bond29 (Scheme 2, eqn (10)). In a similar way, related ylide species27 lead to ketenyl anion 7-Kvia breaking of one C–P bond. With the aim of identifying intermediates of the conversion of 1 to 3 and 4, low temperature NMR experiments were carried out. At the early stage of the reaction between 2 and CO at −40 °C, two sets of two doublets were observed in a 1
C bond is promising for a post-functionalisation of the carbon centre originating from CO. The easy formation of ketene 4 from the bis-μ2-alkylidene complex 2 also contrasts with the known reactivity of the free, dilithiated (S∼C∼S)2− ligand toward CO, which was shown to undergo CO insertion into one CP bond29 (Scheme 2, eqn (10)). In a similar way, related ylide species27 lead to ketenyl anion 7-Kvia breaking of one C–P bond. With the aim of identifying intermediates of the conversion of 1 to 3 and 4, low temperature NMR experiments were carried out. At the early stage of the reaction between 2 and CO at −40 °C, two sets of two doublets were observed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (75.6 ppm, 56.2 ppm, JPP = 14 Hz, 65.8 ppm, 30.4 ppm, JPP = 58 Hz) in 31P{1H} NMR. Such displacements are consistent with cyclometallated and carbonylated diphenyl-thiophosphinoyl-ylide ligands.30–33 A 2D experiment as well as selective decoupling allowed the signals at 75.6 and 56.2 ppm in 31P{1H} NMR to be correlated with a carbon resonance at 34.3 ppm in 13C{1H} NMR, close to the displacement of the alkylidene carbon in 3. The signals at 65.8 and 30.4 ppm in 31P{1H} NMR correlated with a doublet of doublets at 87.2 ppm and two multiplets at 206 and 224 ppm in 13C{1H} NMR. Similar 13C NMR displacement at 226 ppm was already observed for a conjugated acyl coordinated to iron(II) carbonyl complexes.34 This 13C-NMR signature is consistent with the product of 1,1 migratory insertion of CO in one Fe–C bond. Three non-31P-coupled, coordinated CO resonances were also seen. These observations are thus in accordance with structure A (Scheme 3). Evolution of complex A directly leads to the formation of complex 3 and the ketene compound 4 upon warming to room temperature.
1 ratio (75.6 ppm, 56.2 ppm, JPP = 14 Hz, 65.8 ppm, 30.4 ppm, JPP = 58 Hz) in 31P{1H} NMR. Such displacements are consistent with cyclometallated and carbonylated diphenyl-thiophosphinoyl-ylide ligands.30–33 A 2D experiment as well as selective decoupling allowed the signals at 75.6 and 56.2 ppm in 31P{1H} NMR to be correlated with a carbon resonance at 34.3 ppm in 13C{1H} NMR, close to the displacement of the alkylidene carbon in 3. The signals at 65.8 and 30.4 ppm in 31P{1H} NMR correlated with a doublet of doublets at 87.2 ppm and two multiplets at 206 and 224 ppm in 13C{1H} NMR. Similar 13C NMR displacement at 226 ppm was already observed for a conjugated acyl coordinated to iron(II) carbonyl complexes.34 This 13C-NMR signature is consistent with the product of 1,1 migratory insertion of CO in one Fe–C bond. Three non-31P-coupled, coordinated CO resonances were also seen. These observations are thus in accordance with structure A (Scheme 3). Evolution of complex A directly leads to the formation of complex 3 and the ketene compound 4 upon warming to room temperature.
 | ||
| Scheme 2 Comparative reactivity of ketenes obtained from methanediide, yldiide, or 2-diazo-1,3-diketone, and the reactivity of dilithiated methanediide with CO. | ||
To our surprise, when complex 2 was reacted with CO in CH2Cl2, compound 3 proved to be the only diamagnetic compound in solution. Its isolated yield was measured to represent ca. 50% of the starting iron, suggesting the concomitant formation of paramagnetic Fe complex(es). This was confirmed by Evan's method (see ESI†). As only one (S∼C∼S)2− ligand is bound to complex 3, the other S∼C∼S moiety has to be bound to the paramagnetic Fe centre(s). Consistently, changing the solvent from non-coordinating CD2Cl2 to coordinating solvents such as THF-d8 or CD3CN led to the appearance of the 31P NMR diamagnetic signal of 4 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio compared to 3. Multiple solvent change cycles (THF-d8 to CD2Cl2, then CD2Cl2 to THF-d8) demonstrated the reversibility of this decoordination/coordination process of compound 4. Classical L-type ligands such as pyridine and triphenylphosphine oxide were also able to displace 4. The by-product(s) of this reaction were reluctant to crystallise and difficult to analyse by NMR due to their highly paramagnetic nature. The IR (ATR) spectrum revealed a sharp absorption at 2132 cm−1 corresponding to CCO bond stretching, demonstrating coordination of the ketene species 4 to the Fe centre. Coordinated CO absorption bands at 1981, 2036 and 2072 cm−1 were also seen. This last high value of νCO is reminiscent of cationic, thioether bonded FeII carbonyl complexes,35 and other FeII coordinated by poor donor ligands.36,37 Intense signals for the B(C6F5)4− anion were also present. No active species was seen in X-band EPR spectroscopy, excluding the presence of FeIII centres. These findings are consistent with the low-field Mössbauer spectrum of the by-products (see ESI†), which shows a very broad main doublet with a 0.95 mm s−1 isomer shift characteristic of adventitiously-bound FeII accompanied by a minor doublet (∼30% in Fe content) with a low isomer shift of 0.11 mm s−1, which can be assigned to an FeI-carbonyl species different from 3. These elements pointed to polymetallic, cationic iron oligomers featuring both several CO and ketene ligands.
1 ratio compared to 3. Multiple solvent change cycles (THF-d8 to CD2Cl2, then CD2Cl2 to THF-d8) demonstrated the reversibility of this decoordination/coordination process of compound 4. Classical L-type ligands such as pyridine and triphenylphosphine oxide were also able to displace 4. The by-product(s) of this reaction were reluctant to crystallise and difficult to analyse by NMR due to their highly paramagnetic nature. The IR (ATR) spectrum revealed a sharp absorption at 2132 cm−1 corresponding to CCO bond stretching, demonstrating coordination of the ketene species 4 to the Fe centre. Coordinated CO absorption bands at 1981, 2036 and 2072 cm−1 were also seen. This last high value of νCO is reminiscent of cationic, thioether bonded FeII carbonyl complexes,35 and other FeII coordinated by poor donor ligands.36,37 Intense signals for the B(C6F5)4− anion were also present. No active species was seen in X-band EPR spectroscopy, excluding the presence of FeIII centres. These findings are consistent with the low-field Mössbauer spectrum of the by-products (see ESI†), which shows a very broad main doublet with a 0.95 mm s−1 isomer shift characteristic of adventitiously-bound FeII accompanied by a minor doublet (∼30% in Fe content) with a low isomer shift of 0.11 mm s−1, which can be assigned to an FeI-carbonyl species different from 3. These elements pointed to polymetallic, cationic iron oligomers featuring both several CO and ketene ligands.
To illustrate the relative reluctancy of ketene release in the case of FeIFeII compounds, preliminary DFT calculations were performed. The H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketene was chosen to simplify the calculations. As the starting complexes are not known, we based our analysis on initial FeIFeII and FeIFeI complexes derived from 3 by substituting one CO ligand by the model ketene (see ESI†). From this study, we confirm that ketenes have a better affinity for FeIFeII complexes than for FeIFeI ones. Note that while the ketene binds on localized FeII sites in the initial FeIFeII complex, the final FeIFeII complex is valence delocalized; the ketene thus acts here as a “localization enforcer”.
O ketene was chosen to simplify the calculations. As the starting complexes are not known, we based our analysis on initial FeIFeII and FeIFeI complexes derived from 3 by substituting one CO ligand by the model ketene (see ESI†). From this study, we confirm that ketenes have a better affinity for FeIFeII complexes than for FeIFeI ones. Note that while the ketene binds on localized FeII sites in the initial FeIFeII complex, the final FeIFeII complex is valence delocalized; the ketene thus acts here as a “localization enforcer”.
In conclusion, a bridging iron alkylidene complex featuring (S∼C∼S)2− ligands was able to release a stable ketene upon reaction with CO, under moderate pressure and at room temperature. This transformation can be performed from the neutral, bis-ferrous alkylidene complex 1, generating the μ2-alkylidene di-iron hexacarbonyl complex 3 as a by-product, or its mixed-valence analogue 2. In the latter case, retention of the ketene moiety highlighted the impact of the oxidation state of iron on its release. The facile and complete cleavage of the newly formed C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond upon hydrolysis opens up new perspectives for the development of further reactivity, using the gem-dianion as a mediator for CO incorporation.
C bond upon hydrolysis opens up new perspectives for the development of further reactivity, using the gem-dianion as a mediator for CO incorporation.
SM and RRM thank the ANR for financial support (ANR-21-CE07-0007, ENigM). CalMip is acknowledged by MFB for a generous grant of computing time. The ICT NMR service members are gratefully acknowledged for their technical support. RGS thanks Labex Arcane, CBH-EUR-GS (ANR-17-EURE-0003) for financial support.
Data availability
X-ray crystallographic data have been deposited in the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) with reference numbers: CCDC 2424974 (3) and 2424975 (4).† Experimental data and computational details have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- W. A. Herrmann, Angew. Chem., Int. Ed. Engl., 1982, 21, 117–130 CrossRef.
- X. Yin and J. R. Moss, Coord. Chem. Rev., 1999, 181, 27–59 CrossRef CAS.
- J. Pospech, I. Fleischer, R. Franke, S. Buchholz and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 2852–2872 CrossRef CAS PubMed.
- S. Pandey, K. V. Raj, D. R. Shinde, K. Vanka, V. Kashyap, S. Kurungot, C. P. Vinod and S. H. Chikkali, J. Am. Chem. Soc., 2018, 140, 4430–4439 CrossRef CAS PubMed.
- T. W. Bodnar and A. R. Cutler, J. Am. Chem. Soc., 1983, 105, 5926–5928 CrossRef CAS.
- Z. Zhang, Y. Zhang and J. Wang, ACS Catal., 2011, 1, 1621–1630 CrossRef CAS.
- S. K. Russell, J. M. Hoyt, S. C. Bart, C. Milsmann, S. C. Stieber, S. P. Semproni, S. DeBeer and P. J. Chirik, Chem. Sci., 2014, 5, 1168–1174 RSC.
- S. Takebayashi, M. A. Iron, M. Feller, O. Rivada-Wheelaghan, G. Leitus, Y. Diskin-Posner, L. J. W. Shimon, L. Avram, R. Carmieli, S. G. Wolf, I. Cohen-Ofri, R. A. Sanguramath, R. Shenhar, M. Eisen and D. Milstein, Nat. Catal., 2022, 5, 494–502 CrossRef CAS.
- Q. Wang, R. A. Manzano, H. Tinnermann, S. Sung, B. Leforestier, T. Krämer and R. D. Young, Angew. Chem., Int. Ed., 2021, 60, 18168–18177 CrossRef CAS PubMed.
- T. R. Roose, D. S. Verdoorn, P. Mampuys, E. Ruijter, B. U. W. Maes and R. V. A. Orru, Chem. Soc. Rev., 2022, 51, 5842–5877 RSC.
- R. Mazzoni, M. Salmi and V. Zanotti, Chem. – Eur. J., 2012, 18, 10174–10194 CrossRef CAS PubMed.
- M. Röper, H. Strutz and W. Keim, J. Organomet. Chem., 1981, 219, C5–C8 CrossRef.
- B. Denise, D. Navarre, H. Rudler and J. C. Daran, J. Organomet. Chem., 1989, 375, 273–289 CrossRef CAS.
- M. Wiederhold and U. Behrens, J. Organomet. Chem., 1994, 476, 101–109 CrossRef CAS.
- S. E. Gibson and M. A. Peplow, In Advances in Organometallic Chemistry, ed R. West, A. F. Hill, Academic Press, 1999, vol. 44, pp. 275–355 Search PubMed.
- T. Kegl and F. Ungvary, Lett. Org. Chem., 2010, 7, 634–644 CrossRef CAS.
- M. Fustier-Boutignon, N. Nebra and N. Mézailles, Chem. Rev., 2019, 119, 8555–8700 CrossRef CAS PubMed.
- M. Fustier-Boutignon, H. Heuclin, X. F. Le Goff and N. Mézailles, Chem. Commun., 2012, 48, 3306–3308 RSC.
- S. Yogendra, D. W. N. Wilson, A. W. Hahn, T. Weyhermüller, C. Van Stappen, P. Holland and S. DeBeer, Inorg. Chem., 2023, 62, 2663–2671 CrossRef CAS PubMed.
- R. Ravel-Massol, S. Munshi, A. Pujol, R. Garcia-Serres, N. Saffon-Merceron, N. Mézailles and M. Fustier-Boutignon, Chem. – Eur. J., 2023, 29, e202302130 CrossRef CAS PubMed.
- O. S. Mills and A. D. Redhouse, J. Chem. Soc. A, 1968, 1282–1292 RSC.
- J. C. Daran, B. Heim, M. Gouygou and Y. Jeannin, J. Organomet. Chem., 1994, 479, 109–116 CrossRef CAS.
- S. Bernès, R. A. Toscano, A. C. Cano, O. G. Mellado, C. Alvarez-Toledano, H. Rudler and J.-C. Daran, J. Organomet. Chem., 1995, 498, 15–24 CrossRef.
- B. O. Roos, A. C. Borin and L. Gagliardi, Angew. Chem., Int. Ed., 2007, 46, 1469–1472 CrossRef CAS PubMed.
- S. A. Stoian, C.-H. Hsieh, M. L. Singleton, A. F. Casuras, M. Y. Darensbourg, K. McNeely, K. Sweely and C. V. Popescu, J. Biol. Inorg. Chem., 2013, 18, 609–622 CrossRef CAS PubMed.
- S. E. Biali, M. Gozin and Z. Rappoport, J. Phys. Org. Chem., 1989, 2, 271–280 CrossRef CAS.
- M. Jörges, F. Krischer and V. H. Gessner, Science, 2022, 378, 1331–1336 CrossRef PubMed.
- H. Meier, H. Wengenroth, W. Lauer and V. Krause, Tetrahedron Lett., 1989, 30, 5253–5256 CrossRef CAS.
- M. Xu, T. Wang, Z.-W. Qu, S. Grimme and D. W. Stephan, Angew. Chem., Int. Ed., 2021, 60, 25281–25285 CrossRef PubMed.
- E. Lindner, A. Rau and S. Hoehne, Angew. Chem., 1979, 91, 568–569 CrossRef CAS.
- J. Weismann and V. H. Gessner, Eur. J. Inorg. Chem., 2015, 4192–4198 CrossRef CAS.
- P. Oulié, N. Nebra, S. Ladeira, B. Martin-Vaca and D. Bourissou, Organometallics, 2011, 30, 6416–6422 CrossRef.
- J.-Y. Guo, Y. Li, R. Ganguly and C.-W. So, Organometallics, 2012, 31, 3888–3893 CrossRef CAS.
- C. P. Casey, L. K. Woo, P. J. Fagan, R. E. Palermo and B. R. Adams, Organometallics, 1987, 6, 447–454 CrossRef CAS.
- D. Sellmann, G. Mahr, F. Knoch and M. Moll, Inorg. Chim. Acta, 1994, 224, 45–59 CrossRef CAS.
- T. S. Piper, F. A. Cotton and G. Wilkinson, J. Inorg. Nucl. Chem., 1955, 1, 165–174 CrossRef.
- F. A. Cotton and B. F. G. Johnson, Inorg. Chem., 1967, 6, 2113–2115 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental and computational details and cif file for X-ray diffraction analysis. CCDC 2424974 and 2424975. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc02019k |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |