Metal–oxygen bonding characteristics dictate activity and stability differences of RuO2 and IrO2 in the acidic oxygen evolution reaction†
Longdan
Tang
ab,
Xia
Chen
ab,
Zhuoyang
Xie
ab,
Qiong
Xiang
ab,
Jin
Liu
ab,
Li
Li
 *ab and
Zidong
Wei
*ab and
Zidong
Wei
 ab
ab
aState Key Laboratory of Advanced Chemical Power Sources (Chongqing University), Chongqing, 400044, China. E-mail: liliracial@cqu.edu.cn
bSchool of Chemistry and Chemical Engineering, Chongqing University, Chongqing, 400044, China
First published on 2nd April 2025
Abstract
Ruthenium dioxide (RuO2) and iridium dioxide (IrO2) serve as benchmark electrocatalysts for the acidic oxygen evolution reaction (OER), yet their intrinsic activity–stability relationships remain elusive. Herein, we employ density functional theory (DFT) calculations to systematically investigate the origin of divergent OER catalytic behaviors between RuO2 and IrO2 in acidic media. Mechanistic analyses reveal that RuO2 follows the adsorbate evolution mechanism with superior activity (theoretical overpotential: 0.698 V vs. 0.909 V for IrO2), while IrO2 demonstrates enhanced stability due to a higher dissolution energy change (>2.9 eV vs. −0.306 eV for RuO2). Electronic structure analysis reveals that RuO2 exhibits ionic-dominated metal–oxygen bonds with delocalized electron distribution, facilitating intermediate desorption but promoting detrimental RuO42− dissolution. In contrast, IrO2 features covalent bonding characteristics with more electron filling in Ir–oxygen bonds (2.942 vs. 2.412 for RuO2), thereby stabilizing surface intermediates against dissolution at the expense of higher OER barriers. This work establishes a clear correlation between the bonding nature and electrocatalytic performance metrics, offering fundamental insights for the rational design of acid-stable OER electrocatalysts with optimized activity–stability relationships.
1. Introduction
Electrochemical water splitting technology is widely regarded as an ideal method for utilizing renewable electricity to produce clean hydrogen (H2) fuel.1,2 Acidic proton exchange membrane water electrolyzers (PEMWEs), with outstanding proton conductivity, lower ohmic losses, and more compact system design, have been widely regarded as one of the most efficient technological devices for green hydrogen production, which has sparked extensive research interest.3–5 However, the commercialization of PEMWEs is significantly hindered by the sluggish acidic oxygen evolution reaction (OER) kinetics, a bottleneck primarily attributed to the current lack of active, stable, and cost-effective OER catalysts.6–10 To date, ruthenium (Ru)-based catalysts, particularly RuO2, and iridium (Ir)-based catalysts, notably IrO2, are commonly regarded as the preferred materials for anode catalysts due to their excellent durability and good activity in the commercial application of PEMWEs.11–14Nevertheless, when it comes to large-scale applications, the two catalysts, RuO2 and IrO2, confront three pivotal constraints. Firstly, their scarcity and high cost pose a significant barrier, as the price of Ru can reach up to $9523 per kg, and Ir can soar to $60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 per kg.15,16 More importantly, Ru-based catalysts still grapple with formidable challenges in terms of long-term stability during the OER in acidic environments or PEM reactors.10,17,18 Additionally, Ir exhibits a relatively high overpotential during the OER, resulting in lower energy efficiency.14,19,20 These intertwined limitations collectively hinder the achievement of the Department of Energy's (DOE's) goal of reducing hydrogen production costs to below $2 per kg by 2025.21,22 In recent years, great efforts have been made to improve the Ru or Ir acidic OER performance via strategies such as multimetal oxides or doping (Mn-doped RuO2,23 Ta-RuO2,24 Hf-doped IrO2,25 and Re-doped IrO226), morphology and structure tuning (ultra-thin RuO2 nanosheets,27 single-atom Ru–N–C,28 and Au@AuIr220), strain effect (Ru/RuO2@NCS29 and Ta0.1Tm0.1Ir0.8O2−δ14), surface reconstruction (CaCu3Ru4O12,30 A2Ru2O7, A = Y, Nd, Gd and Bi,31 and SrCo0.9Ir0.1O3−δ32) and so on, resulting in enhanced OER activity and stability compared with commercial RuO2 and IrO2 nanoparticles in acids.
670 per kg.15,16 More importantly, Ru-based catalysts still grapple with formidable challenges in terms of long-term stability during the OER in acidic environments or PEM reactors.10,17,18 Additionally, Ir exhibits a relatively high overpotential during the OER, resulting in lower energy efficiency.14,19,20 These intertwined limitations collectively hinder the achievement of the Department of Energy's (DOE's) goal of reducing hydrogen production costs to below $2 per kg by 2025.21,22 In recent years, great efforts have been made to improve the Ru or Ir acidic OER performance via strategies such as multimetal oxides or doping (Mn-doped RuO2,23 Ta-RuO2,24 Hf-doped IrO2,25 and Re-doped IrO226), morphology and structure tuning (ultra-thin RuO2 nanosheets,27 single-atom Ru–N–C,28 and Au@AuIr220), strain effect (Ru/RuO2@NCS29 and Ta0.1Tm0.1Ir0.8O2−δ14), surface reconstruction (CaCu3Ru4O12,30 A2Ru2O7, A = Y, Nd, Gd and Bi,31 and SrCo0.9Ir0.1O3−δ32) and so on, resulting in enhanced OER activity and stability compared with commercial RuO2 and IrO2 nanoparticles in acids.
However, the longest lifetime achieved so far for these novel catalysts is only 2000 hours under electrochemical testing conditions for Y2Ru1.2Ir0.5O7.33 This falls far short of meeting the needs of practical applications, especially when compared to the target lifetime of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–120
000–120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hours by 2050.34 Conversely, studying and understanding the fundamental processes occurring in typical noble metal-based oxide catalysts (RuO2 and IrO2) is expected to have a high degree of universality and contribute to the development of low-cost and high-efficiency materials. Mechanistically, the reaction pathways of RuO2 and IrO2, especially the involvement of lattice oxygen, remain widely controversial. Macounova et al.35 investigated the lattice oxygen participation of nanocrystalline RuO2-based catalysts, revealing that lattice oxygen contributed roughly 9% in pure RuO2 and even up to 50% in Ru0.9Ni0.1O2. However, this finding was later challenged by a study that found no evidence of oxygen exchange occurring on any of the four orientation surfaces of RuO2, regardless of whether the electrolyte is acidic or alkaline.36 Similarly, researchers have also expressed differing views on the participation of lattice oxygen in IrO2. Kasian et al.37 considered the participation of lattice oxygen to be ubiquitous in the Ir-based oxide system, and the specific structural characteristics of the catalyst significantly influence the evolution pathway of lattice oxygen. Conversely, Scott et al.38 concluded that lattice oxygen is, at most, a negligible contribution to the overall OER activity for Ir-based catalysts in acidic electrolytes. Furthermore, the specific dissolution path and dissolved products of RuO2 and IrO2, which will inevitably suffer corrosion due to the harsh acidic environment, have been widely concerned.9,39–41 It is suggested that the over-oxidation of RuO2, occurring at elevated temperatures and under high O2 partial pressures or as a consequence of anodic polarization, leads to the production of volatile and water-soluble RuO42−.39,42 Alexandrov et al.41 discovered the presence of two pivotal intermediates (RuO2(OH) and RuO2(OH)2) on the RuO2(110) surface, which couple the Ru dissolution process with the catalytic process at the atomic scale. Interestingly, it was computationally revealed that the dissolution of IrO2 exhibits potential dependence, transforming into IrO3 at high anodic potentials and into Ir(OH)3 at low anodic potentials.43 Nevertheless, other authors have experimentally discovered that IrO2 ultimately dissolves in the form of IrO42− at high anodic potentials.44 Evidently, the widespread controversies surrounding the catalytic activity and deactivation mechanisms of the two catalysts stem primarily from the lack of consensus in understanding their underlying mechanisms.
000 hours by 2050.34 Conversely, studying and understanding the fundamental processes occurring in typical noble metal-based oxide catalysts (RuO2 and IrO2) is expected to have a high degree of universality and contribute to the development of low-cost and high-efficiency materials. Mechanistically, the reaction pathways of RuO2 and IrO2, especially the involvement of lattice oxygen, remain widely controversial. Macounova et al.35 investigated the lattice oxygen participation of nanocrystalline RuO2-based catalysts, revealing that lattice oxygen contributed roughly 9% in pure RuO2 and even up to 50% in Ru0.9Ni0.1O2. However, this finding was later challenged by a study that found no evidence of oxygen exchange occurring on any of the four orientation surfaces of RuO2, regardless of whether the electrolyte is acidic or alkaline.36 Similarly, researchers have also expressed differing views on the participation of lattice oxygen in IrO2. Kasian et al.37 considered the participation of lattice oxygen to be ubiquitous in the Ir-based oxide system, and the specific structural characteristics of the catalyst significantly influence the evolution pathway of lattice oxygen. Conversely, Scott et al.38 concluded that lattice oxygen is, at most, a negligible contribution to the overall OER activity for Ir-based catalysts in acidic electrolytes. Furthermore, the specific dissolution path and dissolved products of RuO2 and IrO2, which will inevitably suffer corrosion due to the harsh acidic environment, have been widely concerned.9,39–41 It is suggested that the over-oxidation of RuO2, occurring at elevated temperatures and under high O2 partial pressures or as a consequence of anodic polarization, leads to the production of volatile and water-soluble RuO42−.39,42 Alexandrov et al.41 discovered the presence of two pivotal intermediates (RuO2(OH) and RuO2(OH)2) on the RuO2(110) surface, which couple the Ru dissolution process with the catalytic process at the atomic scale. Interestingly, it was computationally revealed that the dissolution of IrO2 exhibits potential dependence, transforming into IrO3 at high anodic potentials and into Ir(OH)3 at low anodic potentials.43 Nevertheless, other authors have experimentally discovered that IrO2 ultimately dissolves in the form of IrO42− at high anodic potentials.44 Evidently, the widespread controversies surrounding the catalytic activity and deactivation mechanisms of the two catalysts stem primarily from the lack of consensus in understanding their underlying mechanisms.
In our work, we explored the OER and the deactivation mechanisms of RuO2 and IrO2 in detail to understand the intrinsic differences in their activity and stability by density functional theory (DFT) calculations. Initially, we evaluated three distinct OER pathways for each catalyst to determine the thermodynamically favorable reaction routes. Subsequently, we analyzed potential deactivation mechanisms for both catalysts, with a focus on dissolution pathways, the species formed upon dissolution, and the resultant activity following the dissolution of active sites. Furthermore, we conducted a comprehensive assessment of their intrinsic electronic structures, examining orbital states and bonding characteristics, and correlated these elements with OER activity and stability. Our findings indicate that variations in metal–oxygen (M–O) bonding characteristics are the fundamental reason for the observed differences in performance. This research not only deepens our understanding of the differing performances between OER catalysts but also offers a robust theoretical framework to guide future optimization efforts in catalyst performance.
2. Computational details
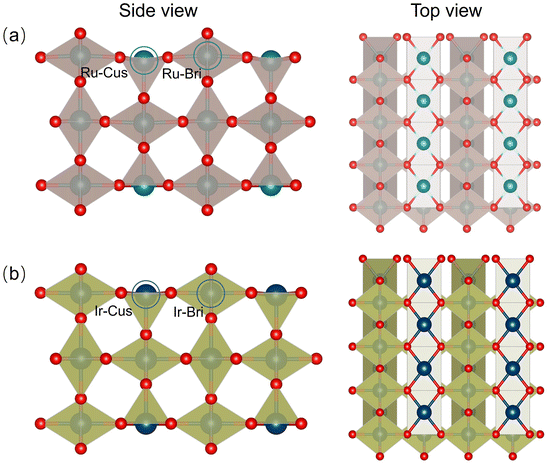
Spin-polarized DFT calculations were conducted using the Vienna ab initio simulation package (VASP),45,46 in which the ionic cores of all atoms are described by projector augmented wave (PAW) pseudopotentials.47 The generalized gradient approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE)48 exchange-functional was applied, along with D3-dispersion correction (BJ)49,50 to account for long-range van der Waals interactions. During the optimization of geometric structures using a force-based conjugate gradient method, the convergence thresholds for force and energy were set to 0.02 eV Å−1 and 10−5 eV, respectively. The cut-off energy for plane-wave basis was set as 450 eV.The lattice parameters of the rutile structure were optimized to give a = 4.490 Å, c = 3.118 Å for RuO2, and a = 4.527 Å, c = 3.173 Å for IrO2, which is in agreement with a previous experimental prediction.51 The Brillouin zone was sampled using the Monkhorst–Pack scheme with a 6 × 6 × 8 k-point mesh for RuO2 and IrO2 cells. Detailed information regarding the optimized structure of the cell model is provided in Fig. S1 and Tables S1, S2 (ESI†). In our calculations, we selected the MO2(110) (M = Ru, Ir) surfaces, the most stable structure that have been extensively studied.36,38,41,43,52–54 It is noteworthy that the rutile (110) surface comprises two distinct metal positions: a coordinatively unsaturated site (Cus) that is not capped by oxygen and is bound to five O atoms, and a bridge site (Bri) that is bound to six O atoms. We utilized the slab model consisting of 48 M atoms and 96 O atoms, where the first layer was fixed during geometry optimization, as depicted in Fig. 1. Tables S1 and S2 (ESI†) present the relevant parameters of the optimized geometric structures of RuO2(110) and IrO2(110). In subsequent discussions, we will uniformly adopt RuO2 and IrO2 to refer to the corresponding surface materials, respectively. For the calculations of the slab, the k-point mesh was set to 3 × 3 × 1. A 20 Å thick vacuum layer was introduced perpendicular to the surface to minimize any potential interactions between periodic replicas. Due to the strong correlation of d electrons in Ru and Ir, a U–J value of 2.0 eV was adopted and spin polarization was considered in our study.55,56 More details regarding the calculation formulas and derivation process can be found in the ESI.†
3. Results and discussion
3.1 OER mechanism and activity of RuO2 and IrO2
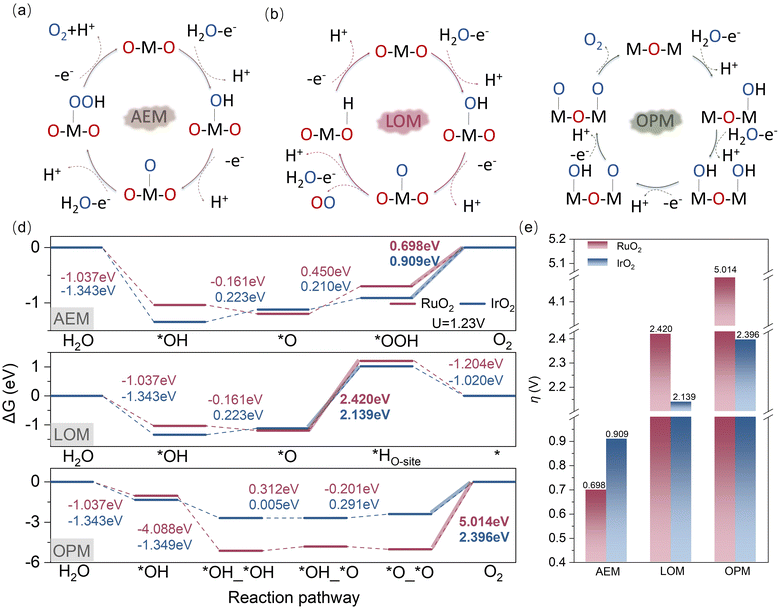
Three general mechanisms of OER in an acid medium, including the adsorbate evolution mechanism (AEM), lattice oxygen oxidation mechanism (LOM), and oxidation pathway mechanism (OPM), are described in Fig. 2a–c.57–60 In the complete crystalline structure, we select the Cus site as the active site (Fig. S2, ESI†). When defects occur, the Bri site is also exposed to a five-coordinate environment and serves as the active site (Fig. S3, ESI†). For traditional AEM, water firstly deprotonates to a hydroxyl anion (OH−), oxidized to an *OH intermediate that adsorbs on the active site (M). Afterward, the *OH deprotonates generate an *O intermediate, which is subsequently attacked by OH− yielding an *OOH intermediate. Finally, The O2 dissociates from the catalyst surface via deprotonation, freeing up the active site and enabling the continuation of the reaction cycle. For the LOM, while the initial two steps closely resemble the AEM, divergence emerges from the third step where *O interacts with lattice oxygen to produce O2, leaving an oxygen vacancy site (OV). Subsequently, the vacancy is occupied by *OH, which ultimately undergoes deprotonation to drive the reaction cycle forward. Different from the single-site mechanism of the AEM and LOM, OPM demonstrates a dual-site mechanism. After an *OH intermediate is generated at a metal site, a water molecule, acting as a nucleophile, attacks the adjacent active site once again, leading to the formation of *OH adsorption at that location, thereby achieving a dual-site-adsorbed *OH state (*OH_*OH). Subsequently, dehydrogenation reactions occur sequentially at the two adsorption sites, first transitioning into an *OH_*O structure and then into an *O_*O structure, ultimately culminating in O2 production and the perpetuation of the reaction cycle.The reaction thermodynamics for these three mechanisms on the bare RuO2 and IrO2 facets are compared. The free energy diagrams for the OER via the AEM, LOM, and OPM on the RuO2 and IrO2 surfaces are respectively presented in Fig. 2d. For the AEM, forming *OH and *O on RuO2 is inherently spontaneous, while generating *OOH and O2 poses relatively more challenges. Specifically, the transition from *OOH to O2 generation serves as the potential-determining step (PDS). A similar trend is observed for IrO2, consistent with a previous result.61 Under the thermodynamic equilibrium potential of 1.23 V (vs. RHE), the theoretical OER overpotential (η) of RuO2 is 0.698 V, significantly lower than that of IrO2, which is 0.909 V. This demonstrates that RuO2 has higher activity and a distinct advantage over IrO2 from a thermodynamic perspective. It is attributed to the fact that the single active site of RuO2 shows a weaker interaction with *OOH than IrO2. Moreover, the reaction trends of both catalysts following the AEM mechanism exhibited nearly identical behaviour under both solvation and vacuum conditions (Fig. S4, ESI†). This consistency strongly supports the conclusion that solvation effects play a minimal role in the catalytic system under study.
In the case of LOM, the difficulty in forming oxygen vacancies (Table S3, ESI†) poses a significant challenge for the production of O2, resulting in an exceptionally high theoretical overpotential, which ultimately hinders both oxides from effectively undergoing this mechanism. For the OPM featuring dual active sites, when the phenomenon of dual-site adsorption is observed, it is found that the species adsorption is too strong on both RuO2 and IrO2, resulting in significant difficulties in the desorption process. Therefore, the OPM is not applicable. Fig. 2e compares the theoretical overpotential to the AEM, LOM, and OPM pathways on RuO2 and IrO2, respectively. The results suggest that the OER on the RuO2 and IrO2 is thermodynamically more likely to follow the AEM rather than the LOM or OPM, in good agreement with previous investigations.51,62,63
3.2 The OER deactivation mechanism of RuO2 and IrO2
Fig. 3a illustrates the potential deactivation pathways during the OER process. The LOM pathway is less likely to occur due to the inherent presence of vacancies, while the oxygen-containing intermediates in both AEM and OPM may disrupt the structure of active sites, leading to dissolution. For instance, the adsorption of intermediates like *OH and *O combined with lattice oxygen results in the leaching of MO3 from the active site and the formation of tetra-coordinated MO42− through further oxidation of MO3 by the electrolyte. When *OOH adsorbed, or a dual-site adsorption phenomenon occurs (i.e., the adsorbed species are *OH_*OH, *OH_*O, or *O_*O), the dissolution of tetra-coordinated MO42− can be triggered directly.The dissolution of active sites is closely related to the stability of the RuO2 and IrO2 structures and their defect configurations after dissolution. The thermodynamic stability of the defect configurations after the dissolution of catalysts has been thoroughly evaluated, as shown in Fig. S5 and S6 (ESI†). Table S4 (ESI†) presents the formation energies for all defect configurations. A smaller formation energy indicates a more stable defect structure, making it more likely to occur during dissolution. In the case of RuO2, defect configuration 6 is the most stable, formed by releasing a five-coordinated Ru and two neighbouring bridge O atoms. On the other hand, for IrO2, defect configuration 4 is the most stable and is more readily formed by the interaction of a six-coordinated Ir atom with two neighbouring bridge O atoms.
Fig. 3b and Fig. S7 (ESI†) show that the dissolution of the Ru site in RuO2 mainly produces the four-coordinate RuO42− species rather than the three-coordinate RuO3 species, being more thermodynamically favoured in the AEM, with distinct negative free energy changes. In comparison, the dissolution of RuO3 requires an uphill free energy of over 1.5 eV. Specifically, all the intermediates in the AEM can spontaneously induce the dissolution of the Ru sites into the RuO42−. As the valence state of the Ru site increases, the more negative free energy changes become, indicating the easier dissolution of active sites. During the OPM, the dissolution pathway of RuO2 is non-spontaneous, with a free energy requirement for the leaching of RuO42− exceeding 3.5 eV. These findings indicate that RuO2 has a strong tendency to dissolve in the AEM of the OER. Regarding the dissolution behaviour of RuO2 catalysts, the influence of solvation effects has been further investigated. By coupling the dissolution process with AEM, the dissolution pathway and trend show excellent agreement with the results obtained under vacuum conditions (Fig. S9, ESI†). This finding indicates that solvation effects have negligible impact on the dissolution behaviour of RuO2 catalysts in our studied system. In addition, once the Ru defects formed on the RuO2, as illustrated in Fig. S10 (ESI†), the theoretical overpotential for the OER increases to over 1.5 V, showing a significant depression in OER intrinsic activity. It means that the dissolution of the Ru site during OER catalysis is the main reason for the deactivation of the RuO2 catalyst. Preserving the integrity of the crystal structure of RuO2 as much as possible is an effective strategy to maintain its high intrinsic OER activity.
In comparison to RuO2, the dissolution pathways of IrO2 during AEM and OPM, as shown in Fig. 3c, are thermodynamically non-spontaneous. The free energy changes of the dissolution step, whether dissolved as IrO42− or IrO3, are higher than 2.9 eV, indicating considerable thermodynamic non-feasible. This highlights the remarkable stability of IrO2 throughout the entire OER process. Interestingly, once IrO2 has a defect, it exhibits enhanced intrinsic activity in both AEM and OPM compared to the IrO2 surface (Fig. S10, ESI†). This indicates that pre-introducing oxygen vacancies in the IrO2 surface is an effective way to improve the OER activity of IrO2. Upon comparing the dissolution performance of IrO2 and RuO2, it is evident that IrO2 exhibits higher stability during the OER process.
3.3 The descriptor of activity and stability for the OER
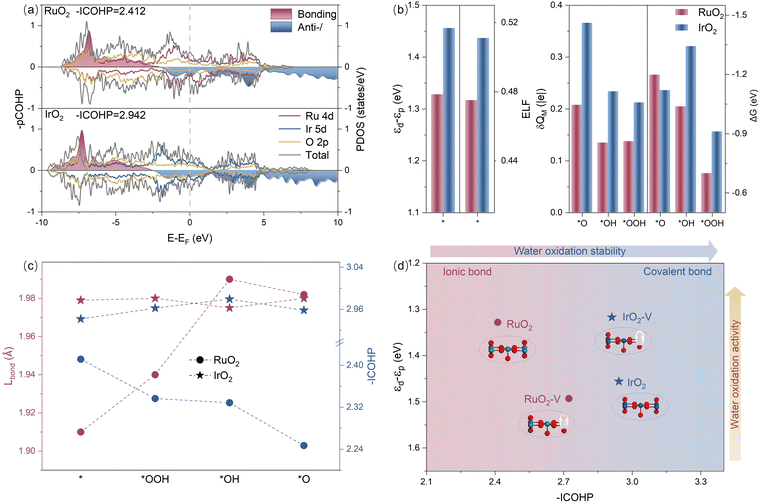
An investigation of the electronic structure reveals that the bonding properties of RuO2 and IrO2 are attributed to the contrasting trends in their activity and stability. Fig. 4a displays the projected density of states (PDOS), illustrating the overlap of the orbitals from metal and oxygen atoms and how electrons are distributed in both compounds. Notably, the energy level of the bonding orbitals in IrO2 is lower than that in RuO2, whereas the energy level of the antibonding orbitals is higher in IrO2. This observation indicates that the overlap of the Ir-d orbitals with the O-p orbitals is more significant than that of the Ru-d orbitals with the O-p orbitals, suggesting that the Ir–O bonding has a greater covalency than Ru–O bonding. Further confirmation comes from the projected crystal orbital Hamilton overlap population (pCOHP) analysis, which shows that the electron filling in the Ir–O bonds (2.942) is notably higher than in RuO2 (2.412), indicating stronger covalency in the Ir–O bond. Additionally, the energy difference between the d-band of Ru and the p-band of O (εd − εp) is smaller for RuO2 (1.328 eV) compared to IrO2 (1.456 eV), indicating more ionic bonding nature in the Ru–O bond. This is also supported by the electronic localization function (ELF) analysis, which reveals that the Ru–O bonds exhibit a lower electronic delocalization value (0.475). In contrast, the Ir–O bonds have a higher value (0.511), suggesting a more covalency bonding nature due to increased electronic localization.64,65 Furthermore, the electronic distributions of RuO2–V and IrO2–V exhibit significant changes after vacancy formation (Fig. S11, ESI†). For RuO2–V, εd shifts positively, and εp shifts negatively, resulting in an increase in εd − εp. In contrast, for IrO2–V, εd shifts negatively and εp shifts positively, leading to a decrease in εd − εp, showing an opposite trend. Analysis of the pCOHP of the defective catalysts reveals that the bonding orbital interaction of RuO2–V (−ICOHP = 2.723) is enhanced compared to the intact catalyst, while that of IrO2–V (−ICOHP = 2.911) is weakened. Therefore, it can be concluded that the nature of the metal–oxygen bonds may have changed after defect formation, leading to the observed changes in catalytic activity.The covalent Ir–O bonds contribute to the stability of IrO2, even after the adsorption of OER intermediates. However, these bonds with more localized electrons also impede the release of O2 by enhancing the adsorption strength of the intermediates. In contrast, the greater ionic bonding characteristics of RuO2 with more delocalized electrons facilitate O2 formation and desorption; nonetheless, the Ru–O structure is more susceptible to disruption by the adsorption of intermediates. Fig. 4b illustrates significant variations in the charge transfer number (δQM) associated with active sites upon the adsorption of three intermediate species: *O, *OH, and *OOH. During this adsorption process, the metal active sites of the oxides interact with the intermediates primarily through covalent interaction (orbital overlap) and electrostatic interactions. A higher degree of orbital overlap correlates with increased charge transfer and enhanced binding strength. In RuO2, its greater ionic bonding leads to the adsorption of intermediate species mainly through electrostatic interactions, resulting in lower electron transfer and relatively weaker binding. In contrast, the covalent bonding nature of IrO2 promotes stronger overlap between the Ir orbitals and those of the intermediate species, leading to more significant electron transfer and stronger adsorption.
As shown in Fig. 4c, while the Ir–O bond length in IrO2 (1.979 Å) is notably longer than the Ru–O bond length in RuO2 (1.910 Å) due to the larger radius of Ir, the Ru–O bond length of RuO2 significantly elongates upon the adsorption of reaction intermediates, whereas that of IrO2 remains largely unchanged. This geometric behavior highlights the tendency of RuO2 to undergo deformation, indicating its lower stability. Furthermore, the −ICOHP value for RuO2 decreases considerably, while that of IrO2 remains relatively stable (Fig. S12, ESI†).
Fig. 4d illustrates the complex relationship between the bonding properties of RuO2 and IrO2 and their catalytic performance. It demonstrates that electron filling in the M–O bonds contributes to the stability of these oxides. Specifically, a higher degree of electron filling in the M–O bonds makes the catalyst less susceptible to external disturbances, as evidenced by the highly stable IrO2. In contrast, the difference between εd and εp in an oxide can indicate its OER activity. Smaller εd − εp values correlate with more delocalized electrons and weaker adsorption of intermediates at reactive sites, which is favorable for the release of oxygen. RuO2 serves as a prime example of a catalyst with excellent OER activity. To enhance the stability of RuO2, it is essential to increase the electron filling degree in Ru–O bonds to maintain structural integrity. This can be achieved by doping electron-rich atoms, as demonstrated by Zhang et al.,66 where La doping in RuO2 forms a La–O–Ru local structure, optimizes Ru–O bond strength, enhances RuO2 stability, and suppresses Ru dissolution, enabling stable operation for 450 hours at 100 mA cm−2. On the other hand, improving IrO2 activity requires increasing the delocalized electrons on Ir, which can be realized by either reducing Ir–O orbital overlap or introducing oxygen vacancies. Wang et al.67 exemplified this approach through their Gd-doped IrO2 catalyst, synthesized via template-free ammonia complexation. The Gd doping optimized OER performance by regulating the Ir4+/Ir3+ ratio and increasing oxygen vacancy concentration, leading to enhanced H2O adsorption and reduced *OH dissociation energy.
4. Conclusions
Our comprehensive thermodynamic analysis reveals the dominant reaction pathways governing both OER mechanisms and dissolution processes in RuO2 and IrO2 catalysts. The AEM emerges as the predominant OER pathway for both oxides, with RuO2 demonstrating superior catalytic activity compared to IrO2. Crucially, this AEM pathway drives the thermodynamically spontaneous dissolution of RuO2 into soluble RuO42− species, while maintaining IrO2's exceptional stability through non-spontaneous dissolution characteristics. Electronic structure analysis through εd − εp energy gaps, ELF, and −ICOHP values uncovers fundamental bonding differences: RuO2 exhibits ionic-dominated bonding with significant electron delocalization, whereas IrO2 displays covalent-dominated interactions with higher electron filling. This contrast creates an intrinsic activity–stability trade-off; the ionic character in RuO2 weakens intermediate adsorption to enhance OER activity but sacrifices structural integrity through labile metal–oxygen bonds. Conversely, IrO2's covalent nature stabilizes the catalyst framework but strengthens intermediate binding, thereby limiting catalytic performance.These atomistic insights establish a fundamental structure–activity–stability relationship. The revealed electronic origins of the RuO2 and IrO2 difference provide critical design principles for next-generation OER catalysts: strategic engineering of metal–oxygen bonding character through alloying, strain modulation, or heterostructure construction could potentially decouple the activity–stability correlation, enabling simultaneous optimization of both essential properties for practical water electrolysis applications.
Author contributions
L. T. performed the theoretical calculations and the interpretation. L. T., X. C. and Z. X. were responsible for data collection and analysis. L. L. and L. T. conducted the data analysis and wrote this manuscript. Q. X. and J. L. contributed to the revision. L. L. and Z. W. directed the project and finalized the manuscript. All authors discussed the results and contributed to the final manuscript.Data availability
The data supporting the findings of this study are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2021YFB4000300) and the National Natural Science Foundation of China (21822803).References
- J. A. Turner, Sustainable Hydrogen Production, Science, 2004, 305, 972–974 CrossRef CAS PubMed
.
- I. Dincer and M. I. Aydin, New paradigms in sustainable energy systems with hydrogen, Energy Convers. Manage., 2023, 283, 116950 CrossRef CAS
.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, A comprehensive review on PEM water electrolysis, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS
.
- C. Spöri, J. T. H. Kwan, A. Bonakdarpour, D. P. Wilkinson and P. Strasser, The Stability Challenges of Oxygen Evolving Catalysts Towards a Common Fundamental Understanding and Mitigation of Catalyst Degradation, Angew. Chem., Int. Ed., 2017, 56, 5994–6021 CrossRef PubMed
.
- S. Pan, H. Li, D. Liu, R. Huang, X. Pan, D. Ren, J. Li, M. Shakouri, Q. Zhang, M. Wang, C. Wei, L. Mai, B. Zhang, Y. Zhao, Z. Wang, M. Graetzel and X. Zhang, Efficient and stable noble-metal-free catalyst for acidic water oxidation, Nat. Commun., 2022, 13, 2294 CrossRef CAS PubMed
.
- J. Song, C. Wei, Z.-F. Huang, C. Liu, L. Zeng, X. Wang and Z. J. Xu, A review on fundamentals for designing oxygen evolution electrocatalysts, Chem. Soc. Rev., 2020, 49, 2196–2214 RSC
.
- Q. Wang, X. Huang, Z. L. Zhao, M. Wang, B. Xiang, J. Li, Z. Feng, H. Xu and M. Gu, Ultrahigh-Loading of Ir Single Atoms on NiO Matrix to Dramatically Enhance Oxygen Evolution Reaction, J. Am. Chem. Soc., 2020, 142, 7425–7433 CrossRef CAS PubMed
.
- Y.-R. Zheng, J. Vernieres, Z. Wang, K. Zhang, D. Hochfilzer, K. Krempl, T.-W. Liao, F. Presel, T. Altantzis, J. Fatermans, S. B. Scott, N. M. Secher, C. Moon, P. Liu, S. Bals, S. Van Aert, A. Cao, M. Anand, J. K. Nørskov, J. Kibsgaard and I. Chorkendorff, Monitoring oxygen production on mass-selected iridium–tantalum oxide electrocatalysts, Nat. Energy, 2021, 7, 55–64 CrossRef
.
- L. An, C. Wei, M. Lu, H. Liu, Y. Chen, G. G. Scherer, A. C. Fisher, P. Xi, Z. J. Xu and C. Yan, Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment, Adv. Mater., 2021, 33, 2006328 CrossRef CAS PubMed
.
- Y. Wen, P. Chen, L. Wang, S. Li, Z. Wang, J. Abed, X. Mao, Y. Min, C. T. Dinh, P. D. Luna, R. Huang, L. Zhang, L. Wang, L. Wang, R. J. Nielsen, H. Li, T. Zhuang, C. Ke, O. Voznyy, Y. Hu, Y. Li, W. A. Goddard III, B. Zhang, H. Peng and E. H. Sargent, Stabilizing Highly Active Ru Sites by Suppressing Lattice Oxygen Participation in Acidic Water Oxidation, J. Am. Chem. Soc., 2021, 143, 6482–6490 CrossRef CAS PubMed
.
- L. An, F. Yang, C. Fu, X. Cai, S. Shen, G. Xia, J. Li, Y. Du, L. Luo and J. Zhang, A Functionally Stable RuMn Electrocatalyst for Oxygen Evolution Reaction in Acid, Adv. Funct. Mater., 2022, 32, 2200131 CrossRef CAS
.
- K. Wang, Y. Wang, B. Yang, Z. Li, X. Qin, Q. Zhang, L. Lei, M. Qiu, G. Wu and Y. Hou, Highly active ruthenium sites stabilized by modulating electron-feeding for sustainable acidic oxygen-evolution electrocatalysis, Energy Environ. Sci., 2022, 15, 2356–2365 RSC
.
- Q. Shi, C. Zhu, D. Du and Y. Lin, Robust noble metal-based electrocatalysts for oxygen evolution reaction, Chem. Soc. Rev., 2019, 48, 3181–3192 RSC
.
- S. Hao, H. Sheng, M. Liu, J. Huang, G. Zheng, F. Zhang, X. Liu, Z. Su, J. Hu, Y. Qian, L. Zhou, Y. He, B. Song, L. Lei, X. Zhang and S. Jin, Torsion strained iridium oxide for efficient acidic water oxidation in proton exchange membrane electrolyzers, Nat. Nanotechnol., 2021, 16, 1371–1377 CrossRef CAS PubMed
.
- Z. Shi, J. Li, Y. Wang, S. Liu, J. Zhu, J. Yang, X. Wang, J. Ni, Z. Jiang, L. Zhang, Y. Wang, C. Liu, W. Xing and J. Ge, Customized reaction route for ruthenium oxide towards stabilized water oxidation in high-performance PEM electrolyzers, Nat. Commun., 2023, 14, 843 CrossRef CAS PubMed
.
- D. A. Kuznetsov, M. A. Naeem, P. V. Kumar, P. M. Abdala, A. Fedorov and C. R. Müller, Tailoring Lattice Oxygen Binding in Ruthenium Pyrochlores to Enhance Oxygen Evolution Activity, J. Am. Chem. Soc., 2020, 142, 7883–7888 CrossRef CAS PubMed
.
- Y. Yao, S. Hu, W. Chen, Z.-Q. Huang, W. Wei, T. Yao, R. Liu, K. Zang, X. Wang, G. Wu, W. Yuan, T. Yuan, B. Zhu, W. Liu, Z. Li, D. He, Z. Xue, Y. Wang, X. Zheng, J. Dong, C.-R. Chang, Y. Chen, X. Hong, J. Luo, S. Wei, W.-X. Li, P. Strasser, Y. Wu and Y. Li, Engineering the electronic structure of single atom Ru sites via compressive strain boosts acidic water oxidation electrocatalysis, Nat. Catal., 2019, 2, 304–313 CrossRef CAS
.
- S. Hao, M. Liu, J. Pan, X. Liu, X. Tan, N. Xu, Y. He, L. Lei and X. Zhang, Dopants fixation of Ruthenium for boosting acidic oxygen evolution stability and activity, Nat. Commun., 2020, 11, 5368 CrossRef CAS PubMed
.
- S. Cherevko, S. Geiger, O. Kasian, N. Kulyk, J.-P. Grote, A. Savan, B. R. Shrestha, S. Merzlikin, B. Breitbach, A. Ludwig and K. J. J. Mayrhofer, Oxygen and hydrogen evolution reactions on Ru, RuO2, Ir, and IrO2 thin film electrodes in acidic and alkaline electrolytes: A comparative study on activity and stability, Catal. Today, 2016, 262, 170–180 CrossRef CAS
.
- H. Wang, Z. Chen, D. Wu, M. Cao, F. Sun, H. Zhang, H. You, W. Zhuang and R. Cao, Significantly Enhanced Overall Water Splitting Performance by Partial Oxidation of Ir through Au Modification in Core–Shell Alloy Structure, J. Am. Chem. Soc., 2021, 143, 4639–4645 CrossRef CAS PubMed
.
- H. Over, Fundamental Studies of Planar Single-Crystalline Oxide Model Electrodes (RuO2, IrO2) for Acidic Water Splitting, ACS Catal., 2021, 11, 8848–8871 CrossRef CAS
.
- J. Yu, Q. He, G. Yang, W. Zhou, Z. Shao and M. Ni, Recent Advances and Prospective in Ruthenium-Based Materials for Electrochemical Water Splitting, ACS Catal., 2019, 9, 9973–10011 CrossRef CAS
.
- S. Chen, H. Huang, P. Jiang, K. Yang, J. Diao, S. Gong, S. Liu, M. Huang, H. Wang and Q. Chen, Mn-Doped RuO2 Nanocrystals as Highly Active Electrocatalysts for Enhanced Oxygen Evolution in Acidic Media, ACS Catal., 2020, 10, 1152–1160 Search PubMed
.
- J. Zhang, X. Fu, S. Kwon, K. Chen, X. Liu, J. Yang, H. Sun, Y. Wang, T. Uchiyama, Y. Uchimoto, S. Li, Y. Li, X. Fan, G. Chen, F. Xia, J. Wu, Y. Li, Q. Yue, L. Qiao, D. Su, H. Zhou, W. A. Goddard and Y. Kang, Tantalum-stabilized ruthenium oxide electrocatalysts for industrial water electrolysis, Science, 2025, 387, 48–55 Search PubMed
.
- F. Zhao, B. Wen, W. Niu, Z. Chen, C. Yan, A. Selloni, C. G. Tully, X. Yang and B. E. Koel, Increasing Iridium Oxide Activity for the Oxygen Evolution Reaction with Hafnium Modification, J. Am. Chem. Soc., 2021, 143, 15616–15623 CrossRef CAS PubMed
.
- W. Huo, X. Zhou, Y. Jin, C. Xie, S. Yang, J. Qian, D. Cai, Y. Ge, Y. Qu, H. Nie and Z. Yang, Rhenium Suppresses Iridium (IV) Oxide Crystallization and Enables Efficient, Stable Electrochemical Water Oxidation, Small, 2023, 19, 2207847 Search PubMed
.
- Z. L. Zhao, Q. Wang, X. Huang, Q. Feng, S. Gu, Z. Zhang, H. Xu, L. Zeng, M. Gu and H. Li, Boosting the oxygen evolution reaction using defect-rich ultra-thin ruthenium oxide nanosheets in acidic media, Energy Environ. Sci., 2020, 13, 5143–5151 RSC
.
- L. Cao, Q. Luo, J. Chen, L. Wang, Y. Lin, H. Wang, X. Liu, X. Shen, W. Zhang, W. Liu, Z. Qi, Z. Jiang, J. Yang and T. Yao, Dynamic oxygen adsorption on single-atomic Ruthenium catalyst with high performance for acidic oxygen evolution reaction, Nat. Commun., 2019, 10, 4849 Search PubMed
.
- Y. Qiu, Y. Rao, Y. Zheng, H. Hu, W. Zhang and X. Guo, Activating ruthenium dioxide via compressive strain achieving efficient multifunctional electrocatalysis for Zn-air batteries and overall water splitting, InfoMat, 2022, 4, e12326 Search PubMed
.
- X. Miao, L. Zhang, L. Wu, Z. Hu, L. Shi and S. Zhou, Quadruple perovskite ruthenate as a highly efficient catalyst for acidic water oxidation, Nat. Commun., 2019, 10, 3809 CrossRef PubMed
.
- M. A. Hubert, A. M. Patel, A. Gallo, Y. Liu, E. Valle, M. Ben-Naim, J. Sanchez, D. Sokaras, R. Sinclair, J. K. Nørskov, L. A. King, M. Bajdich and T. F. Jaramillo, Acidic Oxygen Evolution Reaction Activity–Stability Relationships in Ru-Based Pyrochlores, ACS Catal., 2020, 10, 12182–12196 Search PubMed
.
- Y. Chen, H. Li, J. Wang, Y. Du, S. Xi, Y. Sun, M. Sherburne, J. W. Ager, A. C. Fisher and Z. J. Xu, Exceptionally active iridium evolved from a pseudo-cubic perovskite for oxygen evolution in acid, Nat. Commun., 2019, 10, 572 Search PubMed
.
- H. Liu, Z. Zhang, M. Li, Z. Wang, X. Zhang, T. Li, Y. Li, S. Tian, Y. Kuang and X. Sun, Iridium Doped Pyrochlore Ruthenates for Efficient and Durable Electrocatalytic Oxygen Evolution in Acidic Media, Small, 2022, 18, 2202513 CrossRef CAS PubMed
.
- S. Cherevko, A. R. Zeradjanin, A. A. Topalov, N. Kulyk, I. Katsounaros and K. J. J. Mayrhofer, Dissolution of Noble Metals during Oxygen Evolution in Acidic Media, ChemCatChem, 2014, 6, 2219–2223 Search PubMed
.
- K. Macounova, M. Makarova and P. Krtil, Oxygen evolution on nanocrystalline RuO2 and Ru0.9Ni0.1O2−δ electrodes – DEMS approach to reaction mechanism determination, Electrochem. Commun., 2009, 11, 1865–1868 CrossRef CAS
.
- K. A. Stoerzinger, O. Diaz-Morales, M. Kolb, R. R. Rao, R. Frydendal, L. Qiao, X. R. Wang, N. B. Halck, J. Rossmeisl, H. A. Hansen, T. Vegge, I. E. L. Stephens, M. T. M. Koper and Y. Shao-Horn, Orientation-Dependent Oxygen Evolution on RuO2 without Lattice Exchange, ACS Energy Lett., 2017, 2, 876–881 Search PubMed
.
- O. Kasian, S. Geiger, T. Li, J.-P. Grote, K. Schweinar, S. Zhang, C. Scheu, D. Raabe, S. Cherevko, B. Gault and K. J. J. Mayrhofer, Degradation of iridium oxides via oxygen evolution from the lattice: correlating atomic scale structure with reaction mechanisms, Energy Environ. Sci., 2019, 12, 3548–3555 RSC
.
- S. B. Scott, J. E. Sørensen, R. R. Rao, C. Moon, J. Kibsgaard, Y. Shao-Horn and I. Chorkendorff, The low overpotential regime of acidic water oxidation part II: trends in metal and oxygen stability numbers, Energy Environ. Sci., 2022, 15, 1988–2001 RSC
.
- F. Hess, S. Rohrlack, M. Knapp and H. Over, Evidence of a Tetrahedrally Coordinated RuO4 Surface Complex on RuO2 (100): Density Functional Theory and Beyond, J. Phys. Chem. C, 2022, 126, 946–956 CrossRef CAS
.
- F. Hess and H. Over, Coordination Inversion of the Tetrahedrally Coordinated Ru4f Surface Complex on RuO2 (100) and Its Decisive Role in the Anodic Corrosion Process, ACS Catal., 2023, 13, 3433–3443 CrossRef CAS
.
- K. Klyukin, A. Zagalskaya and V. Alexandrov, Role of Dissolution Intermediates in Promoting Oxygen Evolution Reaction at RuO2 (110) Surface, J. Phys. Chem. C, 2019, 123, 22151–22157 CrossRef CAS
.
- A. Goryachev, M. Etzi Coller Pascuzzi, F. Carlà, T. Weber, H. Over, E. J. M. Hensen and J. P. Hofmann, Electrochemical stability of RuO2(110)/Ru(0001) model electrodes in the oxygen and chlorine evolution reactions, Electrochim. Acta, 2020, 336, 135713 CrossRef CAS
.
- A. Zagalskaya and V. Alexandrov, Mechanistic Study of IrO2 Dissolution during the Electrocatalytic Oxygen Evolution Reaction, J. Phys. Chem. Lett., 2020, 11, 2695–2700 CrossRef CAS PubMed
.
- A. Lončar, D. Escalera-López, S. Cherevko and N. Hodnik, Inter-relationships between Oxygen Evolution and Iridium Dissolution Mechanisms, Angew. Chem., Int. Ed., 2022, 61, e202114437 CrossRef PubMed
.
- G. Kresse and J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS
.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed
.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed
.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed
.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed
.
- A. Zagalskaya and V. Alexandrov, Role of Defects in the Interplay between Adsorbate Evolving and Lattice Oxygen Mechanisms of the Oxygen Evolution Reaction in RuO2 and IrO2, ACS Catal., 2020, 10, 3650–3657 CrossRef CAS
.
- Y. Ping, W. A. Goddard and G. A. Galli, Energetics and Solvation Effects at the Photoanode/Catalyst Interface: Ohmic Contact versus Schottky Barrier, J. Am. Chem. Soc., 2015, 137, 5264–5267 CrossRef CAS PubMed
.
- R. R. Rao, M. J. Kolb, N. B. Halck, A. F. Pedersen, A. Mehta, H. You, K. A. Stoerzinger, Z. Feng, H. A. Hansen, H. Zhou, L. Giordano, J. Rossmeisl, T. Vegge, I. Chorkendorff, I. E. L. Stephens and Y. Shao-Horn, Towards identifying the active sites on RuO2 (110) in catalyzing oxygen evolution, Energy Environ. Sci., 2017, 10, 2626–2637 RSC
.
- D. González, J. Heras-Domingo, S. Pantaleone, A. Rimola, L. Rodríguez-Santiago, X. Solans-Monfort and M. Sodupe, Water Adsorption on MO2 (M = Ti, Ru, and Ir) Surfaces. Importance of Octahedral Distortion and Cooperative Effects, ACS Omega, 2019, 4, 2989–2999 CrossRef PubMed
.
- Q. Liang, A. Bieberle-Hütter and G. Brocks, Anti-Ferromagnetic RuO2: A Stable and Robust OER Catalyst over a Large Range of Surface Terminations, J. Phys. Chem. C, 2022, 126, 1337–1345 CrossRef CAS
.
- L. F. Mattheiss, Electronic structure of RuO2, OsO2, and IrO2, Phys. Rev. B, 1976, 13, 2433–2450 CrossRef CAS
.
- J. Song, C. Wei, Z.-F. Huang, C. Liu, L. Zeng, X. Wang and Z. J. Xu, A review on fundamentals for designing oxygen evolution electrocatalysts, Chem. Soc. Rev., 2020, 49, 2196–2214 RSC
.
- A. Grimaud, O. Diaz-Morales, B. Han, W. T. Hong, Y.-L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper and Y. Shao-Horn, Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution, Nat. Chem., 2017, 9, 457–465 CrossRef CAS PubMed
.
- F. Song, M. M. Busch, B. Lassalle-Kaiser, C.-S. Hsu, E. Petkucheva, M. Bensimon, H. M. Chen, C. Corminboeuf and X. Hu, An Unconventional Iron Nickel Catalyst for the Oxygen Evolution Reaction, ACS Cent. Sci., 2019, 5, 558–568 CrossRef CAS PubMed
.
- H. Jin, X. Liu, P. An, C. Tang, H. Yu, Q. Zhang, H.-J. Peng, L. Gu, Y. Zheng, T. Song, K. Davey, U. Paik, J. Dong and S.-Z. Qiao, Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution, Nat. Commun., 2023, 14, 354 CrossRef CAS PubMed
.
- W. Huo, X. Zhou, Y. Jin, C. Xie, S. Yang, J. Qian, D. Cai, Y. Ge, Y. Qu, H. Nie and Z. Yang, Rhenium Suppresses Iridium (IV) Oxide Crystallization and Enables Efficient, Stable Electrochemical Water Oxidation, Small, 2023, 19, 2207847 CrossRef CAS PubMed
.
- Z.-Y. Wu, F.-Y. Chen, B. Li, S.-W. Yu, Y. Z. Finfrock, D. M. Meira, Q.-Q. Yan, P. Zhu, M.-X. Chen, T.-W. Song, Z. Yin, H.-W. Liang, S. Zhang, G. Wang and H. Wang, Non-iridium-based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis, Nat. Mater., 2023, 22, 100–108 CrossRef CAS PubMed
.
- X. Wang, X. Wan, X. Qin, C. Chen, X. Qian, Y. Guo, Q. Xu, W.-B. Cai, H. Yang and K. Jiang, Electronic Structure Modulation of RuO2 by TiO2 Enriched with Oxygen Vacancies to Boost Acidic O2 Evolution, ACS Catal., 2022, 12, 9437–9445 CrossRef CAS
.
- L. Tian and C. Fei-Wu, Meaning and Functional Form of the Electron Localization Function, Acta Phys.-Chim. Sin., 2011, 27, 2786–2792 Search PubMed
.
- A. D. Becke and K. E. Edgecombe, A simple measure of electron localization in atomic and molecular systems, J. Chem. Phys., 1990, 92, 5397–5403 CrossRef CAS
.
- X. Zhang, H. Yin, C. Dang, H. Nie, Z. Huang, S. Zheng, M. Du, Z. Gu, J. Cao and X. Wu, Unlocking Enhanced Catalysis Stability in Acidic Oxygen Evolution: Structural Insights for PEM Applications under High-Current Density, Angew. Chem., Int. Ed., 2025, e202425569 Search PubMed
.
- Y. Wang, S. Hou, R. Ma, J. Jiang, Z. Shi, C. Liu, J. Ge and W. Xing, Modulating Crystallinity and Surface Electronic Structure of IrO2 via Gadolinium Doping to Promote Acidic Oxygen Evolution, ACS Sustainable Chem. Eng., 2021, 9, 10710–10716 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cp00666j |
| This journal is © the Owner Societies 2025 |